Abstract
Background:
Central neurocytomas are rare, mostly benign neuroectodermal tumors of the central nervous system typically located within the lateral and third ventricles of cerebrum. No consensus guidelines for the management of central neurocytoma available due to the rarity of the disease.
Case Description:
We report a case of right ventricular central neurocytoma of a 28-year-old lady who had a subtotal resection and ventriculoperitoneal shunting. Postoperatively, she was treated with concomitant chemotherapy with oral temozolomide and radiotherapy, followed by add-on chemotherapy with same drug. Imaging, microscopic evaluation, treatment modalities, and outcome of treatment are presented.
Conclusion:
Subtotal resection of tumor through transcallosal approach and ventriculoperitoneal shunt was performed. Imaging done 2 weeks postsurgery confirmed residual disease. Concurrent chemoradiotherapy (54 Gy in 30 fractions +Oral Temozolomide 75 mg/m2 daily), followed by six cycles of 5-day chemotherapy with temozolomide (150 mg/m2 in Cycle 1, and 175 mg/m2 in subsequent cycles) at 28-day intervals, was given. No major toxicities encountered. Follow-up scan after 36 months showed complete remission.
Keywords: Central neurocytoma, Immunohistochemistry, Radiotherapy, Temozolomide

INTRODUCTION
Central neurocytoma, a rare tumor composed of mature neuronal cells of the central nervous system, was originally described by Hassoun in 1982.[8] The total number of reported cases of central neurocytoma world over is less than 1000, stated in an article in 2014.[14] The term central neurocytoma refers to a slow growing neoplasm occurring in the ventricular system typically arising from the septum pellucidum, fornix, or the walls of the lateral ventricles (subependymal layer).[1] About 50% of central neurocytomas are in the anterior portion of a lateral ventricle, 15% in both lateral and third ventricles and 15% are bilateral.[19] Essentially a benign neoplasm, it is mostly seen in 2nd to 3rd decades of life and can cause obstructive hydrocephalus.[6] These slow growing tumors are classified as the WHO Grade II, although malignant variants have been reported.[17] Higher than 2% MIB-1 labeling index is linked to the aggressive behavior of the neoplasm.[4] About 50% of central neurocytomas are in the anterior portion of a lateral ventricle, 15% in both lateral and third ventricles and 15% are bilateral.[19]
CASE REPORT
A 25-year-old otherwise healthy woman presented to the OPD with complaints of persistent headache and defective vision of 6 months duration. She was investigated and identified with an intracranial space occupying lesion in the right lateral ventricle. She was scheduled for right parieto-occipital craniotomy and resection the mass. Tumor was accessed through an interhemispheric transcallosal approach and debulked. Preoperatively, there was a significant tumor bleed, and resultant brain swelling due to intraventricular hemorrhage. She recovered with symptomatic ventriculomegaly, subsequently a ventriculoperitoneal shunt was placed by using the Chhabra “Slit n Spring” hydrocephalus shunt system. Histopathology with immunohistochemical correlation confirmed central neurocytoma with an MIB-1 labeling index 4%. Residual tumor confirmed on a postoperative imaging and she was subjected to 3D conformal radiotherapy to a total dose of 54 Gy in 30 fractions on a daily dose of 180 cGy over 6 weeks. She was given oral temozolomide for all 6 weeks (75 mg/m2), followed by six cycles of temozolomide at 28 day intervals. Each cycle was for 5 days (150 mg/m2 in 1st cycle, subsequent cycles, 175 mg/m2). She tolerated therapy well and are on regular follow-up. No recurrence observed in the MRI scan after 3 years post therapy [Figures 1 and 2].
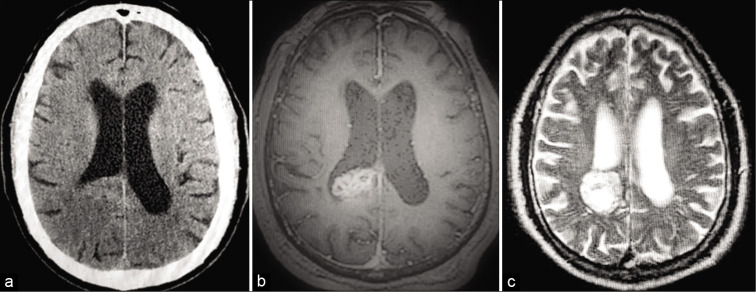
Figure 1:
Preoperative imaging. (a) NCCT - Non contrast CT image showing low-density mass lesion with irregular margins in the right lateral ventricle. (b) MRI T1W - MRI T1-weighted axial image showing heterogeneous signal intensity mass in the right lateral ventricle. (c) MRI T2W - MRI T2-weighted image showing well circumscribed lesion in right lateral ventricle with cystic changes and heterogeneous signal intensity.
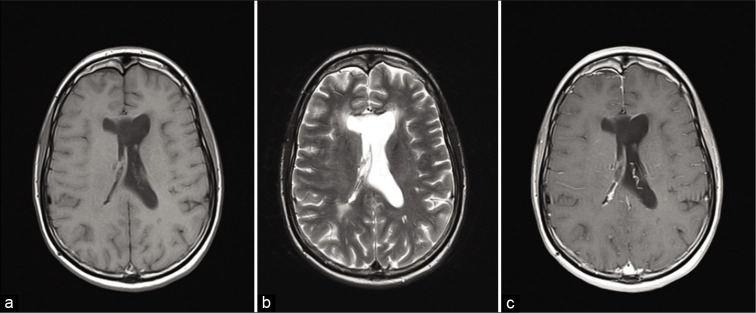
Figure 2:
Postoperative imaging. (a) MRIT1W at 36 months - MRI T1-weighted axial image at 36 months post therapy. (b) MRI T2Wat 36 months - MRI T1-weighted with contrast at 36 months post therapy. (c) MRI T1W with contrast - MRI T2-weighted axial image at 36 months post therapy complete remission.
DISCUSSION
Central neurocytomas are typically a tumor growing supratentorially filling the lateral ventricles and/or third ventricle. Neurological symptoms are related to rise in intracranial pressure resulting in headache and visual disturbances. Tumor usually affects young age patients with no gender preponderance.[3]
These tumors appear in computerized tomography as hyper attenuating to white matter with calcification.[28] MRI findings on T1-weighted images of central neurocytoma is a heterogeneous well-circumscribed, lobulated mass, isointense or slightly hypointense to gray matter and hyperintense in T2-weighted images. Multiple small cysts, coarse, or punctate calcification are also observed. Hydrocephalus and intratumoral hemorrhage are seen in some. MR spectroscopy of the central nervous system shows elevated choline, decreased creatine, and N-acetyl-aspartate.[10]\
Histopathology of the central neurocytoma mimics that of oligodendroglioma or ependymoma. All three neoplasms have small uniform cells with rounded nuclei and scanty cytoplasm with perinuclear halos.[25] H&E staining shows isomorphic cells with clear cytoplasm, speckled chromatin, and fibrillar matrix. IHC studies are essential to diagnose central neurocytoma. Synaptophysin and neuron specific enolase positivity are seen in the central neurocytoma. Tumor is negative for glial fibrillary acidic protein [Figure 3].[24]
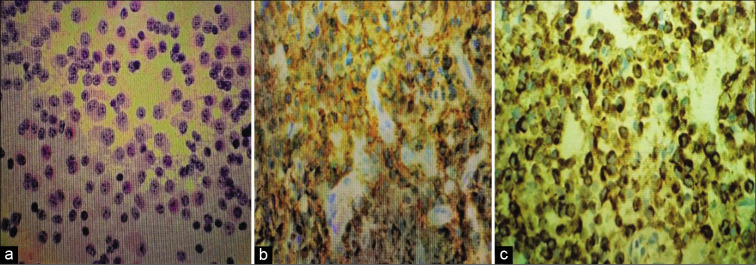
Figure 3:
Histology and immunohistochemistry. (a) Eosin hematoxylin stain - Hematoxylin and eosin stained film showing uniform round cells with salt and pepper speckled chromatin. (b) Neuron-specific enolase - Immunohistochemistry showing Neuron Specific Enolase positivity, confirming the pure neuronal origin of tumor. (c) Synaptophysin - Immunohistochemistry showing synaptophysin positivity, the neuronal marker.
The most important end point of the therapy of the central neurocytoma is achievement of long-term local control. Recurrent or residual disease can result in malignant transformation, craniospinal dissemination of tumor, and intracerebral hemorrhage.
Conventionally, surgery has been the primary treatment of choice for the central neurocytoma.[9] Gross resection has better outcome than a subtotal resection.[18] Extent of resection is considered as the most important prognostic factor in the central neurocytoma.[27] Recurrence rate of central neurocytoma, even after complete resection has been shown in long-term follow- up studies.[16] Stereotactic radiosurgery in residual or recurrent central neurocytoma was found to be effective in a series of patients – 15 out of 22 cases – in a 2011 study.[6]
Ten-year local control after incomplete resection was estimated at only 35 % in a meta-analysis done on 91 centers on a population of 109 patients.[23] Role of adjuvant radiotherapy in the central neurocytoma has contradictory results in many studies.[7,20,21] However, recent studies confirm that addition of adjuvant radiotherapy to a total dose of 54Gy in cases of incompletely resected tumors has similar outcome as in case of gross tumor resection.[2] Concerns about the post radiotherapy neurotoxicity is largely derived from the experience on other low grade gliomas-such toxicity has not been established in central neurocytoma.[5] Preoperative tumor volume and a 2-cm margin, as in case of other low- grade gliomas gives the optimal target volume.[22]
The management of central neurocytoma after stereotactic biopsy followed by radical external beam therapy or radiosurgery alone does not have enough evidence base as the reported follow-up times and duration was brief.[16]
Being a low grade tumor, the time- and dose-dependent effect of chemotherapy in the central neurocytoma is theoretically minimal.[15] Temozolomide, an oral alkylating agent, has been in use for central neurocytoma since 2008.[11] It serves as a radiosensitizer as well as anti-neoplastic agent. Radiotherapy induces the DNA double strand break and subsequent cell death. This DNA break initiates DNA damage response through repair proteins. Temozolomide inhibits those proteins and ensures cell death.[13] Being a rare tumor, randomized trials are lacking in the management of the central neurocytoma, so a consensus guideline for the chemotherapy of these tumor that is lacking. Hence, the use of temozolomide in the management of the central neurocytoma is not evidence based, but mostly anecdotal.[12]
CONCLUSION
Central neurocytoma is a rare, slow growing tumor is best managed with surgery. Compared to initial tumor size and MIB1 tagging index the extent of tumor resection is the most important prognosticator. Incomplete resection must be complimented with adjuvant radiotherapy to a total dose of 54 Gy 30 fractions over a period of 6 weeks. There are no consensus guidelines for systemic chemotherapy; however, oral chemotherapy with temozolomide is gaining popularity. We report a residual neurocytoma of lateral ventricle, s/p VP shunt treated with temozolomide concurrently with radiotherapy at a dose of 75 mg/M2 on days of radiotherapy, followed by six 5 day cycles of temozolomide at intervals of 28 days (150 mg/m2 for first cycle, and 175 mg/m2 for subsequent cycles). Patient got complete radiological resolution of tumor and no major toxicities encountered after 36 months of adjuvant therapy.
Footnotes
How to cite this article: Narayanan V, Julius K, Mbogo J. Long-term follow-up of lateral ventricular central neurocytoma treated with subtotal resection followed by concurrent chemoradiotherapy and add on chemotherapy – Case report from a Tertiary Kenyan Cancer Hospital. Surg Neurol Int 2020;11:272.
Contributor Information
Vijayakumar Narayanan, Email: vijayakumarnarayan@hotmail.com.
Kiboi Julius, Email: j_kiboi@yahoo.com.
James Mbogo, Email: jamesmbogo7@gmail.com.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ashkan K, Casey AT, D’Arrigo C, Harkness WF, Thomas DG. Benign central neurocytoma. Cancer. 2008;89:1111–20. [PubMed] [Google Scholar]
- 2.Chen YD, Li WB, Feng J, Qiu XG. Long-term outcomes of adjuvant radiotherapy after surgical resection of central neurocytoma. Radiat Oncol. 2014;9:242. doi: 10.1186/s13014-014-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhari KA, Kaliaperumal C, Jain A, Sarkar C, Soo MY, Rades D, et al. Central neurocytoma: A multi-disciplinary review. Br J Neurosurg. 2009;23:585–95. doi: 10.3109/02688690903254350. [DOI] [PubMed] [Google Scholar]
- 4.Christov C, Adle-Biassette H, Le Guerinel C. Recurrent central neurocytoma with marked increase in MIB-1 labelling index. Br J Neurosurg. 1999;13:496–9. [PubMed] [Google Scholar]
- 5.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009;8:810–8. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 6.Genc A, Bozkurt SU, Karabagli P, Seker A, Bayri Y, Konya D, et al. Gamma knife radiosurgery for cranial neurocytomas. J Neurooncol. 2011;105:647–57. doi: 10.1007/s11060-011-0635-0. [DOI] [PubMed] [Google Scholar]
- 7.Hallock A, Hamilton B, Ang LC, Tay KY, Meygesi JF, Fisher BJ, et al. Neurocytomas: Long-term experience of a single institution. Neuro Oncol. 2011;13:943–9. doi: 10.1093/neuonc/nor074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982;56:151–6. doi: 10.1007/BF00690587. [DOI] [PubMed] [Google Scholar]
- 9.Imber BS, Braunstein SE, Wu FY, Nabavizadeh N, Boehling N, Weinberg VK, et al. Clinical outcome and prognostic factors for central neurocytoma: Twenty year institutional experience. J Neurooncol. 2016;126:193–200. doi: 10.1007/s11060-015-1959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiuchi S, Tamura M. Central neurocytoma: An immunohistochemical, ultrastructural and cell culture study. Acta Neuropathol. 1997;94:425–35. doi: 10.1007/s004010050729. [DOI] [PubMed] [Google Scholar]
- 11.Jakacki RI, Hamilton M, Gilbertson RJ, Blaney SM, Tersak J, Krailo MD, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: A children’s oncology group Phase I consortium study. J Clin Oncol. 2008;26:4921–7. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MO, Kirkpatrick JP, Patel MP, Desjardins A, Randazzo DM, Friedman HS, et al. The role of chemotherapy in the treatment of central neurocytoma. CNS Oncol. 2019;8:CNS41. doi: 10.2217/cns-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesari S, Advani SJ, Lawson JD, Kahle KT, Ng K, Carter B, et al. DNA damage response and repair: Insights into strategies for radiation sensitization of gliomas. Future Oncol. 2011;7:1335–46. doi: 10.2217/fon.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CY, Kim DG, Joo JD, Kim YH. Clinical outcome and quality of life after treatment of patients with Central neurocytoma. Neurosurg Clin N Am. 2015;26:83–90. doi: 10.1016/j.nec.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni V, Rajshekhar V, Haran RP, Chandi SM. Long-term outcome in patients with central neurocytoma following stereotactic biopsy and radiation therapy. Br J Neurosurg. 2002;16:126–32. doi: 10.1080/02688690220131714. [DOI] [PubMed] [Google Scholar]
- 16.Leenstra JL, Rodriguez FJ, Frechette CM, Giannini C, Stafford SL, Pollock BE, et al. Central neurocytoma: Management recommendations based on a 35-year experience. Int J Radiat Oncol Biol Phys. 2007;67:1145–54. doi: 10.1016/j.ijrobp.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattar MA, Badry AE. Clinical outcome and prognostic factors for central neurocytoma, a study of 14 cases. Rom Neurosurg. 2018;32:73–84. [Google Scholar]
- 19.Nishio S, Morioka T, Suzuki S, Fukui M. Tumours around the foramen of Monro: Clinical and neuroimaging features and their differential diagnosis. J Clin Neurosci. 2002;9:137–41. doi: 10.1054/jocn.2000.0910. [DOI] [PubMed] [Google Scholar]
- 20.Rades D, Fehlauer F, Lamszus K, Schild SE, Hagel C, Westphal M, et al. Well-differentiated neurocytoma: What is the best available treatment? Neuro Oncol. 2005;7:77–83. doi: 10.1215/S1152851704000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rades D, Fehlauer F. Treatment options for central neurocytoma. Neurology. 2002;59:1268–70. doi: 10.1212/wnl.59.8.1268. [DOI] [PubMed] [Google Scholar]
- 22.Rades D, Schild SE, Ikezaki K, Fehlauer F. Defining the optimal dose of radiation after incomplete resection of central neurocytomas. Int J Radiat Oncol Biol Phys. 2003;55:373–7. doi: 10.1016/s0360-3016(02)03918-4. [DOI] [PubMed] [Google Scholar]
- 23.Rades D, Schild SE. Treatment recommendations for the various subgroups of neurocytomas. J Neurooncol. 2006;77:305–9. doi: 10.1007/s11060-005-9047-3. [DOI] [PubMed] [Google Scholar]
- 24.Rai P, Nayak R, Anand D, Menon G. Central neurocytoma in the posterior fossa. BMJ Case Rep. 2019;12:e231626. doi: 10.1136/bcr-2019-231626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravikanth R. Neuroradiological and histopathological findings of intraventricular Central neurocytoma. Chrismed J Health Res. 2017;4:125–7. [Google Scholar]
- 26.Sgouros S, Carey M, Aluwihare N, Barber P, Jackowski A. Central neurocytoma: A correlative clinicopathologic and radiologic analysis. Surg Neurol. 1998;49:197–204. doi: 10.1016/s0090-3019(97)00017-7. [DOI] [PubMed] [Google Scholar]
- 27.Vasiljevic A, François P, Loundou A, Fèvre-Montange M, Jouvet A, Roche PH, et al. Prognostic factors in central neurocytomas: A multicenter study of 71 cases. Am J Surg Pathol. 2012;36:220–7. doi: 10.1097/PAS.0b013e31823b8232. [DOI] [PubMed] [Google Scholar]
- 28.Wichmann W, Schubiger O, von Deimling A, Schenker C, Valavanis A. Neuroradiology of central neurocytoma. Neuroradiology. 1991;33:143–8. doi: 10.1007/BF00588253. [DOI] [PubMed] [Google Scholar]