Abstract
Areal bone mineral density (aBMD) is currently the gold standard for the diagnosis of osteoporosis, however, it has its own pitfalls. Trabecular bone score (TBS), a novel tool in the evaluation of osteoporosis is an indirect indicator of bone microarchitecture. It is a textural index that evaluates pixel gray-level variations in the lumbar spine DXA (dual energy X-ray absorptiometry) image. Both cross-sectional and longitudinal studies have demonstrated that TBS may independently predict fragility fractures. TBS can also be used to adjust FRAX probabilities of fracture, though data available till date doesn’t support any additional benefit. TBS also shows an improving trend with anti-osteoporotic treatment; however, the least significant change (LSC) is high that it takes more than 2 years for the change to manifest. TBS is also used in the evaluation of bone strength in cases of secondary osteoporosis. Though TBS predicts fracture risk independently in both genders, with the currently available data, it cannot be recommended as a standalone tool for decision regarding treatment of osteoporosis. TBS can be used as a tool to complement BMD in assessment of bone health. Additional studies are needed to assess its utility in clinical practice.
Keywords: Bone microarchitecture, fragility fracture, osteoporosis, trabecular bone score
INTRODUCTION
Osteoporosis, which is reported to occur in about 25–60% of Indian postmenopausal women, is a common, yet under recognized public health problem.[1,2] The lifetime risk of osteoporotic fracture is around 40–50% in women and the mortality rate following fragility fractures is as high as 25% in the first year.[3]
Osteoporosis is a disease characterized by low bone mass, microarchitectural deterioration of bone tissue leading to enhanced bone fragility, and a consequent increase in fracture risk.[4] Thus the definition itself brings forth the concept that not only bone mass, but also microarchitectural quality is an important determinant of bone strength. However, areal bone mineral density (aBMD) assessment by DXA (dual energy X-ray absorptiometry) being the gold standard for non-invasive diagnosis of osteoporosis doesn’t provide information on bone microarchitecture. Also, around 50% individuals with fragility fractures can have aBMD value in the osteopenic/normal range, which suggests that in addition to bone mass, there are other factors that determine bone strength.[5]
Microarchitecture of the bone can be measured by histomorphometric analysis of the transiliac crest bone biopsy, quantitative computed tomography (QCT), high-resolution peripheral QCT (HRpQCT), high-resolution magnetic resonance imaging (HRMRI), microcomputed tomography (mCT), and trabecular bone score (TBS). Among these, TBS appears to be a non-invasive, readily available technology that permits efficient and accurate clinical evaluation of skeletal microarchitecture.[6,7] Moreover, it has minimal radiation exposure and can be retrieved retrospectively through previously available lumbar spine aBMD images.[8]
A study on cadaveric vertebrae to determine the level of correlation between mCT and TBS showed a good correlation (0.77 ≤ r2≤ 0.96).[9] In the study by Silva et al. TBS positively correlated with LS trabecular volumetric BMD (vBMD) (r = 0.664) and cortical thickness (r = 0.540) assessed by QCT.[10]
WHAT IS TRABECULAR BONE SCORE?
TBS is a texture index that evaluates pixel gray-level variations in the lumbar spine DXA image, providing an indirect measure of bone microarchitecture. A dense trabecular microstructure projected onto a plane generates an image containing a large number of pixel-to-pixel gray-level variations of small amplitude. Conversely, a 2D projection of a porous trabecular structure produces an image with a low number of pixel-to-pixel gray-level variations, but of higher amplitude. A variogram of these projected images, calculated as the sum of squared gray-level differences between pixels, can estimate a 3D structure from the existing variations on the 2D projected images. TBS is calculated as the slope of the log–log transform of the variogram, where the slope characterizes the rate of gray-level amplitude variations [Figure 1].[8]
Figure 1.
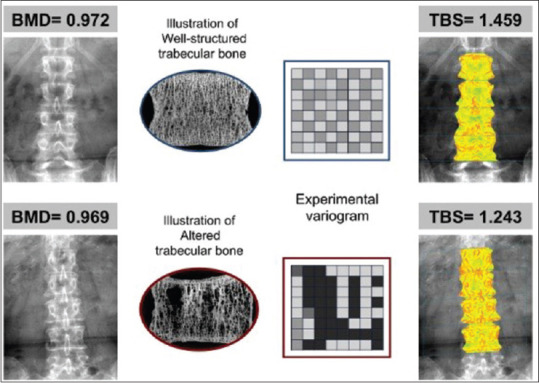
An illustration of the basic TBS principle and relationship to BMD. The upper panel shows BMD and TBS of a 73-year-old woman with BMI of 24.2 kg/m2 and the lower panel shows BMD and TBS of a 74-year-old woman with BMI of 24.3 kg/m2. The images of the bone architecture and the experimental variogram demonstrate TBS principles: the bone with greater number of trabeculae are associated with high TBS and vice-versa (8)
DXA is based on the variation in absorption of X-ray by the different body components and uses high and low energy X-ray photons. Over the past years, DXA components have undergone advancement that information regarding fractures (vertebral fracture analysis), bone stiffness (finite element analysis of X-ray), and mineral distribution at proximal femur (hip structural analysis) can be obtained from the DXA images. TBS is one such advance, which provides an indirect measurement of bone microarchitecture that relates to 3D bone characteristics such as the trabecular number, the trabecular separation, and the connectivity density. High TBS represents strong, fracture-resistant microarchitecture, while a low TBS reflects weak, fracture-prone microarchitecture. A brief comparison of DXA and TBS is provided in Table 1. Currently certain cutoffs [Table 2] are proposed by the working group of TBS users from different countries.[8]
Table 1.
Comparison of aBMD and TBS
| aBMD | TBS | |
|---|---|---|
| Bone site | Total body | Lumbar spine |
| Lumbar spine | ||
| Hip | ||
| Forearm | ||
| Effective radiation exposure | Adult spine DXA 0.013 mSv | No additional radiation in addition to DXA |
| Adult hip DXA 0.009 mSv | ||
| Scan time | 10-20 mins | Obtained in less than a minute from spine DXA image |
| Advantages | More published literature in different ethnicities and disease cohorts | Indirect measure of bone micro-architecture |
| It's a complementary tool to aBMD assessment by DXA | ||
| Can be obtained retrospectively by re-analysis of DXA images | ||
| Disadvantages | No data on microarchitecture | Not widely available |
| Cannot separate cortical and trabecular bone | Soft tissue interference, syndesmophytes may falsely increase TBS | |
| Fracture of spine may give falsely high values | Relatively novel tool, hence cannot be used as a stand-alone tool for diagnosing/treating osteoporosis |
Table 2.
TBS cut-offs proposed for postmenospausal women
| TBS score (no units) | Bone status |
|---|---|
| >1.350 | Normal |
| 1.200 and 1.350 | partially degraded bone |
| ≤1.200 | degraded bone |
FACTORS AFFECTING TBS
The current recommendations for the use of TBS in clinical practice are within a BMI range of 15–37 kg/m2 in order to mitigate the artifactual effects of extreme variations in tissue thickness.[11] The original TBS algorithm (version-1) was optimized for women of average body size. Limitations were identified when used in men or extremes of BMI (< 15 kg/m2, >37 kg/m2). The increase in soft tissue thickness, in both groups, falsely decreases the TBS.[12] The updated TBS algorithm (version 3, 4) is less affected by BMI, gives higher mean results for men than women. Thus it seems to overcome the residual negative correlation of TBS with body size.[13] TBS has been shown in various studies to have a negative correlation with advancing age. This is due to the bone microarchitectural changes that occur with aging.[14,15] The change in TBS with age in both genders is depicted in Figure 2.[16] Age and puberty have been found to be significant determinants of TBS in children.[17] A study in Caucasian and African–American population has reported TBS to be less discriminatory with regard to fracture risk in African–Americans.[18] However, this difference in various ethnic groups needs further validation.
Figure 2.
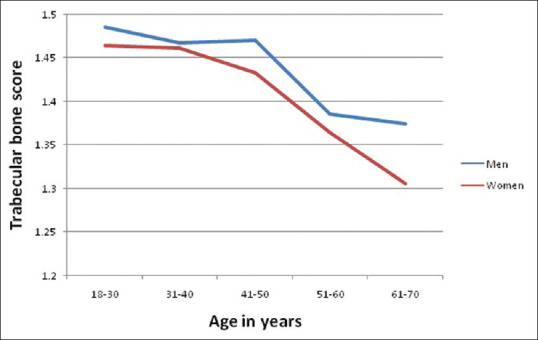
Trabecular bone score (TBS) and age-related changes. The blue and red lines represent the male and female normative TBS curves for age respectively. Adapted from Simonelli et al.[15]
Trabecular bone score measured by different DXA manufacturers showed that, although there was a good correlation (r = 0.73; P < 0.05) between scanners, there was a significant difference in precision error.[19] TBS also depends on DXA scan acquisition mode, differences between densitometer manufacturers, and scanner resolution. Krueger et al. reported differences in TBS measurements between densitometers, suggesting a need to evaluate TBS thresholds by each model and limiting serial comparisons to scans obtained on the same instrument.[20] Pencil beam devices are not compatible with TBS software. A certain threshold of image quality has been determined to maintain the correlation between TBS score and quality of trabecular bone assessed (trabecular spacing, trabecular number, and connectivity density). Below a certain threshold, this correlation is lost and hence pencil beam devices are not compatible.
Several studies have shown that TBS is lower in patients with vertebral fractures compared to controls.[21,22,23,24,25,26,27,28,29,30,31,32,33] However, vertebrae with fractures have to be excluded from TBS measurements, just as for BMD.[34] For example, if one patient has a fracture in L3, TBS will be computed on L1, L2, and L4. However, at least two vertebrae are necessary to compute a TBS value. As per International Society for Clinical Densitometry (ISCD) recommendations, while obtaining TBS, all evaluable vertebrae must be taken into account and those that are affected by local structural change or artifact should be excluded.[34]
TBS AS A TOOL TO PREDICT FRACTURES
Several cross-sectional studies have looked at how TBS predicted fragility fractures.[21,22,23,24,25,26,27] The findings of these studies are summarized in Table 3. All studies have found that TBS could potentially complement aBMD in predicting fragility fractures. Longitudinal studies have also looked at how TBS predicts incident fractures.[28,29,30,31,32,33] The findings of these studies are summarized in Table 4. Most studies that have been summarized were conducted in postmenopausal women. It was found that those with low TBS were associated with 1.5 times higher risk of fracture compared with those with normal TBS. Also, it was found that TBS predicted vertebral as well as major osteoporotic fracture (MOF) with an area under the curve of 0.6–0.7 [Table 4].
Table 3.
List of cross-sectional studies that looked at the utility of TBS to predict fragility fractures
| References | Study population Study design | Fragility fractures (prevalent) | Results OR for TBS per SD decrease and AUC to predict fractures with 95% CI |
|---|---|---|---|
| Pothuaud et al. 2009[21] | Postmenopausal women (n=200- cases 45, controls 155) | 45 (all sites) | OR per SD 1.95 (1.31-2.89), AUC 0.685 (0.599-0.762) |
| Retrospective case-control | |||
| Winzenreith et al. 2010[22] | Postmenopausal women with T score between -2.5 and -1 (n=243- cases 81, controls 162) | 81 (vertebral) | OR per SD 2.52 (1.82-3.53), AUC 0.721 (0.660-0.777) |
| Retrospective case-control | |||
| Rabier et al. 2010[23] | Postmenopausal women with T score <-1 (n=168- cases 42, controls 126) | 42 (vertebral) | OR per SD 3.20 (2.01-5.08), AUC 0.746 |
| Retrospective case control | |||
| Del Rio et al. 2012[24] | Postmenopausal women >50 years (n=191- cases 83, controls 108) | 83 (femur) | OR per SD 2.05 (1.45-2.89), AUC 0.668 (0.597-0.734) |
| Retrospective case-control | |||
| Krueger et al. 2014[25] | Postmenopausal women (n=429- cases 158, controls 271) | 158 (vertebral on VFA/fragility non vertebral fracture) | Any fracture:, OR per SD 2.46 (1.9-3.1), AUC 0.74, Vertebral fracture:, OR per SD 2.49 (1.9-3.3), AUC O.73 |
| Retrospective case-control | |||
| Choi et al. 2017[26] | Postmenopausal women with rheumatoid arthritis (n=279) | 34 (vertebral) | OR per SD 2.86 (1.34-6.09) |
| Cross-sectional study | |||
| Kim et al. 2019[27] | Postmenopausal women with polymyalgia rheumatic (n=212 - cases 106, controls 106) | 45 (vertebral)- 31 in cases, 14 in controls | AUC 0.759 (0.601-0.918) |
| Case-control study |
OR: Odds ratio. AUC: Area under curve
Table 4.
List of longitudinal studies that assessed the utility of TBS in detecting incident fragility fractures
| References | Study population, mean follow up (years) | Fragility fractures (Incident) | Results |
|---|---|---|---|
| Hans et al. 2011 Manitoba study[28] | Women >50 years old, n=29,407, 4.7 years | 1668- MOF (vertebral 439, hip 293) | MOF: AUC 0.63 (0.61-0.64), HR 1.35 (1.20-1.42), Vertebral: AUC 0.66 (0.64-0.69), HR 1.22 (1.10-1.34), Hip: AUC 0.68 (0.65-0.71), HR 1.46 (1.13-1.46) |
| Boutroy et al. 2013 OFELY study[29] | Postmenopausal women, n=560, 7.8±1.3 years | 112 (vertebral 32, hip 8, wrist 35, others 37) | OR 1.57 (1.25-1.98), AUC 0.63 (0.57-0.68), 35% fractures in osteopenic women- however was within lowest quartile of TBS |
| Briot et al. 2013 OPUS study[30] | Women >55 years of age, n=1007, 6 years | 82 clinical fractures, 46 radiological vertebral | Clinical fracture: AUC 0.62 (0.56-0.69), OR 1.62 (1.3-2.01), Radiographic vertebral: AUC 0.63 (0.54-0.72), OR 1.54 (1.17-2.03) |
| Iki et al. 2015 JPOS study[31] | Women >50 years of age, n=665, 10 years | 92 vertebral fracture by VFA | OR 1.98 (1.56-2.51), AUC 0.682 (0.662-0.773) |
| Popp et al. 2016[32] | Women with mean age 76 years, n=556, 2.7 years | 52 clinical fragility | HR 2.01 (1.54-2.63), AUC 0.69 (0.62-0.77) |
| McCloskey et al. 2015[33] | Men and women with a mean age of 72 years n=17809, 6.7 years | 1109 (298 hip fracture) | OR for MOF 1.44 (1.35-1.53) |
MOF: Major osteoporotic fracture, AUC: Area under curve
HOW DOES TBS MODIFY FRAX (FRACTURE RISK ASSESSMENT TOOL)?
FRAX adjusted for TBS is an algorithm derived from the online FRAX calculation tool to adjust probability of fracture from clinical risk factors, aBMD and TBS. A meta-analysis by McCloskey et al. found that though TBS predicted fracture risk independently, addition of TBS to FRAX didn’t improve the fracture prediction significantly (area under the ROC curve for FRAX + aBMD Vs FRAX + aBMD + TBS - 0.74 vs. 0.79).[33]
Similarly in another study by Mirzaei et al., it was found that addition of TBS to FRAX didn’t make any difference to the fracture prediction (area under the ROC curve for FRAX + aBMD vs. FRAX + aBMD + TBS – 0.765 vs. 0.781, P = 0.19).[35] Holloway et al. also noted that addition of TBS didn’t improve the fracture prediction by FRAX (area under ROC curve for MOF : FRAX + aBMD vs. FRAX + aBMD + TBS – 0.740 vs. 0.738).[36]
UTILITY OF TBS IN PATIENTS WITH DIABETES MELLITUS
Glucose tolerance and aBMD are negatively affected by advancing age and quite often they coexist. Assessments of bone quantity based on aBMD underestimates the risk of fracture in patients with type 2 diabetes mellitus (T2DM),[37] suggesting that bone fragility in these patients is caused by poor bone quality.[38] A study by Yamamoto et al. found that in T2DM, low TBS significantly correlated with vertebral fractures irrespective of BMD in both genders.[39] Similarly in a study by Lin et al., it was found that TBS had a higher AUC for detecting vertebral fractures as compared to aBMD in T2DM.[40] Thus, bone microarchitectural deterioration by TBS may be a better clinical indicator of poor bone health in T2DM compared to aBMD.[41] However, further studies would be needed to confirm this finding.
A study on patients with type 1 diabetes mellitus (T1DM) looked at the TBS values in T1DM (n = 119) and controls (n = 68) and didn’t find any significant difference (1.357 vs. 1.389, P = 0.075). However, in those with prevalent fractures (n = 24), TBS was significantly lower as compared to those without (1.309 vs. 1.370, P = 0.04). Considering the scarce data available, more studies are needed to reach a definite conclusion.[42]
IMPACT OF DEGENERATIVE SPINE DISEASE ON TBS
In older men, aBMD measured at the lumbar spine shows an apparent increase with advancing age.[43] This apparent increase is often attributed to degenerative changes of the spine. Hence there is a need for other methods to assess bone quality in those with degenerative changes of the spine. Anderson et al. found that TBS was less affected by degenerative changes compared to aBMD. TBS in those with and without degenerative changes was 1.219 and 1.196, however the corresponding BMD was 1.317 g/cm2 and 1.198 g/cm2.[44] Similarly in a study by Buehring et al., it was found that in patients with rheumatoid arthritis (n = 143), about 20% had vertebral fractures in the presence of normal lumbar spine aBMD. However, their TBS was low correlating with poor bone microarchitecture.[45]
EFFECT OF HYPERPARATHYROIDISM ON TBS
Patients with primary hyperparathyroidism (PHPT) (asymptomatic) usually show low bone strength that is often under estimated by aBMD measurement. Torres et al. found that a higher number of subjects with PHPT showed microarchitectural deterioration by TBS, compared to osteoporosis as assessed by aBMD (51.7% vs. 37.5%). The AUC for TBS performed better than the combination of femoral, hip, and spine BMD for prevalent fractures (0.714 vs. 0.679). Thus TBS may be a useful tool to identify increased fracture risk in patients with PHPT when under-diagnosed by aBMD.[46]
HOW TBS PREDICTS FRACTURES IN GLUCOCORTICOID INDUCED OSTEOPOROSIS (GIO)?
Vertebral fractures are the most common fractures associated with glucocorticoid (GC) treatment and the risk of vertebral fracture increases within 3 months after initiation of treatment and peaks at 12 months.[47] In a study by Florez et al., the utility of TBS was compared with aBMD to assess fracture risk in GC treated patients. It was found that the prevalence of vertebral fractures was more among those with degraded microarchitecture (low TBS) compared to those with osteoporosis (76% vs. 38%). Thus TBS may be a better discriminant tool compared to aBMD for fracture assessment in GC treated patients.[48]
TBS AS A TOOL TO MONITOR TREATMENT RSPONSE
The LSC for TBS is estimated to be about 5.4%.[49] aBMD has a better LSC compared to TBS. With pharmacologic treatment studies for 1.5–2 years, the lumbar spine aBMD changes were consistently greater than TBS changes, with 4.1%–8.8% increase of LS aBMD versus 1.4%–3.6% increase of TBS.[49] This suggests a longer time interval required to achieve a statistically significant change with TBS than with LS aBMD.
A recent study from southern India found that TBS didn’t show any significant decline (over a period of 3 years) following yearly Zoledronic acid infusion in the cohort studied. Thus bisphosphonates lead to preservation of bone mass in contrast to the normal decline in bone mass with advancing age.[6] A review of the best available evidence at the 2019 ISCD Position Development Conference concluded that the role of TBS in monitoring anti-resorptive therapy is unclear and that TBS is potentially useful for monitoring anabolic therapy.[50] In another study done to assess bone health in recipients of allogeneic stem cell transplant, it was found that TBS measurements provided similar information as the lumbar spine aBMD and did not differ significantly between cases and controls.[7]
TBS IN CLINICAL PRACTICE
Although TBS predicts fracture risk independently in both genders, it cannot be recommended as a standalone tool for decision regarding treatment of osteoporosis. It can be used along with aBMD in assessing risk for fragility fractures. The LSC of TBS is high and hence it takes more than 2 years of anti-resorptive therapy for the change to reflect in TBS. Hence, though TBS changes with treatment, at present there is not enough evidence to recommend it as a tool to monitor response to anti-resorptive treatment. It may be useful to assess risk of fracture in patients with T2DM, hyperparathyroidism, and degenerative spine disorders as described before.[51]
Despite its utility in clinical practice, there are certain limitations in the use of TBS. DXA and derived parameters including TBS are subject to the deleterious effects of image noise. Winzenrieth et al. has reported that the effect of adding noise to DXA images resulted in a reduction in TBS.[9] It is well established that variations in soft-tissue density can result in significant errors in aBMD measurements and a similar effect is present with TBS, with increases in soft-tissue thickness overlying the spine resulting in lower TBS values.[12] Being a relatively novel tool, its availability as well as lack of normative data also adds to its limitations.
CONCLUSION
TBS is a textural index from spine DXA images that predicts the risk of fracture independent of aBMD and clinical risk factors. TBS is associated with incident vertebral, hip and major osteoporotic fractures in postmenopausal women and in men greater than 50 years of age. TBS may be used to adjust FRAX probabilities of fracture, though data available till date doesn’t support any additional benefit. Although TBS improves on various anti-osteoporotic treatments, these changes are usually smaller than improvements in LS aBMD and many times do not exceed the least significant change. However, TBS may play a role in the evaluation of fracture risk in diverse conditions, such as T2DM or PHPT. Thus, TBS is an emerging technology and future work will add to the existing data, confirming and extending its clinical utility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Thakur P, Kuriakose C, Cherian KE, Asha HS, Kapoor N, Paul TV. Knowledge gap regarding osteoporosis among medical professionals in Southern India. J Eval Clin Pract. 2020;26:272–80. doi: 10.1111/jep.13164. [DOI] [PubMed] [Google Scholar]
- 2.Binu AJ, Cherian KE, Kapoor N, Jebasingh FK, Asha HS, Paul TV. Bone health after fifth decade in rural ambulatory south Indian postmenopausal women. Indian J Community Med. 2019;44:205–8. doi: 10.4103/ijcm.IJCM_161_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl 2):S3–7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 4.Consensus development conference: Prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–10. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- 5.Miller PD, Siris ES, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: Evidence from the national osteoporosis risk assessment. J Bone Miner Res. 2002;17:2222–30. doi: 10.1359/jbmr.2002.17.12.2222. [DOI] [PubMed] [Google Scholar]
- 6.Sooragonda B, Cherian KE, Jebasingh FK, Dasgupta R, Asha HS, Kapoor N, et al. Longitudinal changes in bone mineral density and trabecular bone score following yearly zoledronic acid infusion in postmenopausal osteoporosis-a retrospective-prospective study from southern India. Arch Osteoporos. 2019;14:79. doi: 10.1007/s11657-019-0630-1. [DOI] [PubMed] [Google Scholar]
- 7.Cherian KE, Kapoor N, Devasia AJ, Mathews V, Srivastava A, Thomas N, et al. Do bone density, bone microarchitecture, and body composition differ in recipients of allogeneic hematopoietic stem cell transplant? A cross-sectional study from southern India. Biol Blood Marrow Transplant. 2020;26:540–5. doi: 10.1016/j.bbmt.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, et al. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518–30. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 9.Winzenrieth R, Michelet F, Hans D. Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: Effects of resolution and noise. J Clin Densitom. 2013;16:287–96. doi: 10.1016/j.jocd.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Silva BC, Walker MD, Abraham A, Boutroy S, Zhang C, McMahon DJ, et al. Trabecular bone score is associated with volumetric bone density and microarchitecture as assessed by central QCT and HRpQCT in Chinese American and white women. J Clin Densitom. 2013;16:554–61. doi: 10.1016/j.jocd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martineau P, Leslie WD. The utility and limitations of using trabecular bone score with FRAX. Curr Opin Rheumatol. 2018;30:412–9. doi: 10.1097/BOR.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 12.Amnuaywattakorn S, Sritara C, Utamakul C, Chamroonrat W, Kositwattanarerk A, Thamnirat K, et al. Simulated increased soft tissue thickness artefactually decreases trabecular bone score: A phantom study. BMC Musculoskelet Disord. 2016;17:17. doi: 10.1186/s12891-016-0886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevroja E, Aubry-Rozier B, Hans G, Rodriguez EG, Stoll D, Lamy O, et al. Clinical performance of the updated trabecular bone score (TBS) algorithm, which accounts for the soft tissue thickness: The OsteoLaus study. J Bone Miner Res. 2019;34:2229–37. doi: 10.1002/jbmr.3851. [DOI] [PubMed] [Google Scholar]
- 14.Leslie WD, Krieg M-A, Hans D, Manitoba bone density program. Clinical factors associated with trabecular bone score. J Clin Densitom. 2013;16:374–9. doi: 10.1016/j.jocd.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Simonelli C, Leib E, Mossman N, Winzenrieth R, Hans D, McClung M. Creation of an age-adjusted, dual-energy x-ray absorptiometry-derived trabecular bone score curve for the lumbar spine in non-Hispanic US White women. J Clin Densitom. 2014;17:314–9. doi: 10.1016/j.jocd.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Bazzocchi A, Ponti F, Diano D, Amadori M, Albisinni U, Battista G, et al. Trabecular bone score in healthy ageing. Br J Radiol. 2015;88:20140865. doi: 10.1259/bjr.20140865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shawwa K, Arabi A, Nabulsi M, Maalouf J, Salamoun M, Choucair M, et al. Predictors of trabecular bone score in school children. Osteoporos Int. 2016;27:703–10. doi: 10.1007/s00198-015-3255-2. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK, Narang DK, Hans D, Vokes TJ. Ethnic differences in trabecular bone score. J Clin Densitom. 2017;20:172–9. doi: 10.1016/j.jocd.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Fan B, Lewiecki M, Miller PD, Genant HK, Shepherd JA. TBS precision and comparison between the Hologic and GE-Lunar DXA scanners. J Clin Densitom. 2015;18:424. [Google Scholar]
- 20.Krueger D, Libber J, Binkley N. Spine trabecular bone score precision, a comparison between GE lunar standard and high-resolution densitometers. J Clin Densitom. 2015;18:226–32. doi: 10.1016/j.jocd.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Pothuaud L, Barthe N, Krieg M-A, Mehsen N, Carceller P, Hans D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: A preliminary spine BMD-matched, case-control study. J Clin Densitom. 2009;12:170–6. doi: 10.1016/j.jocd.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Winzenrieth R, Dufour R, Pothuaud L, Hans D. A retrospective case-control study assessing the role of trabecular bone score in postmenopausal caucasian women with osteopenia: Analyzing the odds of vertebral fracture. Calcif Tissue Int. 2010;86:104–9. doi: 10.1007/s00223-009-9322-y. [DOI] [PubMed] [Google Scholar]
- 23.Rabier B, Héraud A, Grand-Lenoir C, Winzenrieth R, Hans D. A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone. 2010;46:176–81. doi: 10.1016/j.bone.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Del Rio L, Winzenrieth R, Cormier C, Digregorio S. Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture. A Spanish case-control study? Osteoporos Int. 2012;24:991–8. doi: 10.1007/s00198-012-2008-8. [DOI] [PubMed] [Google Scholar]
- 25.Krueger D, Fidler E, Libber J, Aubry-Rozier B, Hans D, Binkley N. Spine trabecular bone score subsequent to bone mineral density improves fracture discrimination in women. J Clin Densitom. 2014;17:60–5. doi: 10.1016/j.jocd.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Choi YJ, Chung Y-S, Suh C-H, Jung J-Y, Kim H-A. Trabecular bone score as a supplementary tool for the discrimination of osteoporotic fractures in postmenopausal women with rheumatoid arthritis. Medicine (Baltimore) 2017;96:e8661. doi: 10.1097/MD.0000000000008661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H-A, Lee HY, Jung J-Y, Suh C-H, Chung Y-S, Choi YJ. Trabecular bone score is a useful parameter for the prediction of vertebral fractures in patients with polymyalgia rheumatica? J Clin Densitom. 2019 doi: 10.1016/j.jocd.2019.05.006. doi: 10.1016/j.jocd.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Hans D, Goertzen AL, Krieg M-A, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J Bone Miner Res. 2011;26:2762–9. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 29.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: The OFELY study. Osteoporos Int. 2013;24:77–85. doi: 10.1007/s00198-012-2188-2. [DOI] [PubMed] [Google Scholar]
- 30.Briot K, Paternotte S, Kolta S, Eastell R, Reid DM, Felsenberg D, et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: The OPUS study. Bone. 2013;57:232–6. doi: 10.1016/j.bone.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: The Japanese population-based osteoporosis (JPOS) cohort study. J Bone Miner Res. 2014;29:399–407. doi: 10.1002/jbmr.2048. [DOI] [PubMed] [Google Scholar]
- 32.Popp AW, Meer S, Krieg M-A, Perrelet R, Hans D, Lippuner K. Bone mineral density (BMD) and vertebral trabecular bone score (TBS) for the identification of elderly women at high risk for fracture: The SEMOF cohort study. Eur Spine J. 2016;25:3432–8. doi: 10.1007/s00586-015-4035-6. [DOI] [PubMed] [Google Scholar]
- 33.McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31:940–8. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- 34. [Last cited on 2020 Apr 30]. 2019 ISCD Official Positions-Adult-International Society for Clinical Densitometry (ISCD) Available from: https://www.iscd.org/officialpositions/2019-iscd-official-positions-adult/
- 35.Mirzaei A, Jahed SA, Nojomi M, Rajaei A, Zabihiyeganeh M. A study of the value of trabecular bone score in fracture risk assessment of postmenopausal women. Taiwan J Obstet Gynecol. 2018;57:389–93. doi: 10.1016/j.tjog.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Holloway KL, Mohebbi M, Betson AG, Hans D, Hyde NK, Brennan-Olsen SL, et al. Prediction of major osteoporotic and hip fractures in Australian men using FRAX scores adjusted with trabecular bone score. Osteoporos Int. 2018;29:101–8. doi: 10.1007/s00198-017-4226-6. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24:702–9. doi: 10.1359/jbmr.081207. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto M. Insights into bone fragility in diabetes: The crucial role of bone quality on skeletal strength. Endocr J. 2015;62:299–308. doi: 10.1507/endocrj.EJ15-0129. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Yamauchi M, Sugimoto T. Prevalent vertebral fracture is dominantly associated with spinal microstructural deterioration rather than bone mineral density in patients with type 2 diabetes mellitus. PLoS One. 2019;14:e0222571. doi: 10.1371/journal.pone.0222571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin YC, Wu J, Kuo SF, Cheung YC, Sung CM, Fan CM, et al. Vertebral fractures in type 2 diabetes patients: Utility of trabecular bone score and relationship with serum bone turnover biomarkers. J Clin Densitom. 2020;23:37–43. doi: 10.1016/j.jocd.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Jackuliak P, Kužma M, Killinger Z, Payer J. Good long-term glycemic compensation is associated with better trabecular bone score in postmenopausal women with type 2 diabetes. Physiol Res. 2019;68(Suppl 2):S149–56. doi: 10.33549/physiolres.934304. [DOI] [PubMed] [Google Scholar]
- 42.Neumann T, Lodes S, Kästner B, Lehmann T, Hans D, Lamy O, et al. Trabecular bone score in type 1 diabetes--a cross-sectional study. Osteoporos Int. 2016;27:127–33. doi: 10.1007/s00198-015-3222-y. [DOI] [PubMed] [Google Scholar]
- 43.Henry MJ, Pasco JA, Korn S, Gibson JE, Kotowicz MA, Nicholson GC. Bone mineral density reference ranges for Australian men: Geelong osteoporosis study. Osteoporos Int. 2010;21:909–17. doi: 10.1007/s00198-009-1042-7. [DOI] [PubMed] [Google Scholar]
- 44.Anderson KB, Holloway-Kew KL, Mohebbi M, Kotowicz MA, Hans D, Pasco JA. Is trabecular bone score less affected by degenerative-changes at the spine than lumbar spine BMD? Arch Osteoporos. 2018;13:127. doi: 10.1007/s11657-018-0544-3. [DOI] [PubMed] [Google Scholar]
- 45.Buehring B, Thomas J, Wittkämper T, Baraliakos X, Braun J. [Evaluation of the trabecular bone score (TBS) in routine clinical care of patients with inflammatory rheumatic and non-inflammatory diseases: Correlation with conventional bone mineral density measurement and prevalence of vertebral fractures] Z Rheumatol. 2020 doi: 10.1007/s00393-020-00764-9. doi: 10.1007/s00393-020-00764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muñoz-Torres M, Manzanares Córdova R, García-Martín A, Avilés-Pérez MD, Nieto Serrano R, Andújar-Vera F, et al. Usefulness of trabecular bone score (TBS) to identify bone fragility in patients with primary hyperparathyroidism. J Clin Densitom. 2019;22:162–70. doi: 10.1016/j.jocd.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547–56. doi: 10.1056/NEJMcp1800214. [DOI] [PubMed] [Google Scholar]
- 48.Florez H, Hernández-Rodríguez J, Muxi A, Carrasco JL, Prieto-González S, Cid MC, et al. Trabecular bone score improves fracture risk assessment in glucocorticoid-induced osteoporosis. Rheumatol Oxf Engl. 2019:kez464. doi: 10.1093/rheumatology/kez464. doi: 10.1093/rheumatology/kez464. [DOI] [PubMed] [Google Scholar]
- 49.Bandirali M, Poloni A, Sconfienza LM, Messina C, Papini GDE, Petrini M, et al. Short-term precision assessment of trabecular bone score and bone mineral density using dual-energy X-ray absorptiometry with different scan modes: Anin vivo study. Eur Radiol. 2015;25:2194–8. doi: 10.1007/s00330-015-3606-6. [DOI] [PubMed] [Google Scholar]
- 50.Krohn K, Schwartz EN, Chung Y-S, Lewiecki EM. Dual-energy X-ray absorptiometry monitoring with trabecular bone score: 2019 ISCD official position. J Clin Densitom. 2019;22:501–5. doi: 10.1016/j.jocd.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: The 2015 ISCD official positions part 2: Trabecular bone score. J Clin Densitom. 2015;18:309–30. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]


