Abstract
Background:
Polycystic ovarian syndrome (PCOS) is one of the most common metabolic disorders seen in women of the reproductive age group, with the majority of them having insulin resistance. There is a need to identify sensitive markers of insulin resistance. CC chemokine ligand 18 (CCL 18) secreted from white adipose tissue is upregulated in individuals with insulin resistance.
Objectives:
To study the correlation between serum CCL 18 levels and insulin resistance in PCOS.
Materials and Methods:
This case-control study included 45 PCOS women and an equal number of age and body mass index (BMI) matched controls. Estimation of serum CCL 18, serum testosterone, fasting plasma glucose, fasting insulin, HbA1c, and ultrasonography of abdomen and pelvis was done and HOMA IR was calculated.
Results:
Serum CCL 18 level was higher in women with PCOS when compared to controls. The mean level of serum CCL 18 (ng/mL) in the PCOS group and control group was 28.32 ± 4.17 and 11.90 ± 4.91, respectively (P < 0.001). Blood pressure, waist circumference, waist-hip ratio, modified Ferriman Gallway score (FG) score serum total testosterone, fasting serum insulin, and HOMA IR showed a relationship with serum CCL 18 levels. Serum CCL 18 was an independent predictor of PCOS (P < 0.05). A serum CCL 18 cutoff level of 18.84 ng/mL showed 93.3% sensitivity and 91.7% specificity in distinguishing PCOS subjects from healthy individuals.
Conclusion:
There is a significant correlation of serum CCL 18 level with insulin resistance in PCOS subjects and serum CCL levels can be considered as a marker of PCOS.
Keywords: CCL 18, insulin resistance, polycystic ovarian syndrome, HOMA IR
INTRODUCTION
Globally type 2 diabetes mellitus has reached epidemic proportions with 424.9 million people affected globally and more than 50% being unaware of their disease or undiagnosed.[1] People with insulin resistance syndrome have more than seven times the risk of developing diabetes, compared to those with no cardiometabolic risk factors.[2] It has been estimated that 20%–25% of South Asians have insulin resistance and many more may be prone to it,[3] and the prevalence of the insulin resistance syndrome in Asian Indian women is 1.5–2 times higher as compared with men.[4]
Polycystic ovarian syndrome (PCOS) is one of the most common metabolic disorders. Globally, the prevalence of PCOS varies from 2.2% to 26%,[5] while in India, the prevalence ranges from 9.13% to 36%.[6] Severe degree of insulin resistance or impaired glucose tolerance (IGT) is more common in obese PCOS.[7] About two-thirds of females with PCOS are insulin resistant[8] and the degree of insulin resistance exceeds that of women without PCOS matched for body mass index (BMI) or the degree of adiposity. Studies have reported the prevalence of insulin resistance in Indian women with PCOS to be 75%[9] and also more severe than their white counterparts. There is consistent evidence that a large proportion of women with PCOS develop diabetes mellitus later in life.[10] IGT or frank diabetes is evident in approximately 45% of women with PCOS by their fourth decade.[11] Both insulin resistance and type 2 diabetes mellitus are linked to disturbances in white adipose tissue (WAT)[12] with mechanisms involving altered secretion of adipokines, peptides, which exert autocrine, paracrine, and or endocrine effects on metabolism.[13]
CC chemokine ligand 18 (CCL18) is a chemokine constitutively expressed in the lung and is endowed with chemotactic properties.[14] The main producers of CCL18 are antigen-presenting cells like alveolar macrophages and follicular dendritic cells in vivo[15] and in vitro, several human cells “spontaneously” secrete CCL18, such as monocyte-derived dendritic cells.[16] Using gene microarray data from subcutaneous WAT of obese and nonobese women, it was shown that CCL18 expression was significantly increased in obesity and decreased upon weight loss. In a study by Eriksson Hogling et al.[17] in obese women before undergoing bariatric surgery, it has been found that CCL 18 was significantly enriched in macrophages and its secretion reflects adipose gene expression and constitutes a marker of inflammation. CCL 18 was highly expressed by M2 macrophages and showed a correlation with insulin resistance and adiposity[17,18] and may be an indirect measurement of subcutaneous WAT.
Though insulin resistance can be estimated by various methods, most of them are difficult to employ in clinical practice. Absolute values of plasma insulin levels from different laboratories cannot be compared as analytic methods for measurements are not standardized. Of the surrogate indices for insulin sensitivity or insulin resistance that have been developed such as Tumor Necrosis Factor (TNF) alpha,[19,20] IL-6,[21,22,23] and resistin,[24,25] contrasting results have been observed. Hence, there is a need to identify more reliable markers of insulin resistance. Studies have shown a correlation of serum CCL 18 levels to insulin resistance in the Caucasian population. However, it is not known whether a similar correlation holds good for the Asian population. We hypothesized that since PCOS patients represent a group that has a high prevalence of insulin resistance, it would be interesting to observe the relationship between CCL 18 levels and insulin resistance in them. The present study was undertaken to study the correlation between insulin resistance and serum CCL 18 levels in PCOS women.
MATERIALS AND METHODS
This study was undertaken at Ramaiah Medical College and hospitals in Bangalore, South India, during the period 2017–2018 starting from Jan 2017 to September 2018, after due clearance by the institutional ethics committee. Based on a study conducted by Eriksson Hogling et al. in which serum CCL 18 levels in insulin-resistant patients were estimated, a sample size of 45 in each group was required, calculated by N master software developed by the department of biostatistics, CMC Vellore. Forty-five consecutive patients of PCOS in the age group of 18 to 40 years visiting endocrinology outpatient were enrolled in the study and the diagnosis of PCOS was based on Rotterdam criteria. The control group was chosen from the family welfare clinic and included an equal number of age and BMI matched healthy women with regular menstrual cycles and with no clinical evidence of hyperandrogenism, such as hirsutism or acne. Informed consent was taken from all the subjects. The exclusion criteria included other causes of irregular menstrual cycles and or androgen excess such as Cushing's syndrome, hyperprolactinemia, congenital adrenal hyperplasia, other adrenal disorders, thyroid disorders, galactorrhea, lactating women, pregnancy, diabetes mellitus, hypertension, coronary artery disease, acute or chronic infection, known malignancy, on oral contraceptive agents and/or antiandrogen therapy (within the preceding six months), lipid-lowering agents, metformin, pioglitazone, or any other oral antidiabetic agent. A complete clinical examination which included anthropometric indices like, height, weight, waist circumference, waist-hip ratio was obtained. Blood pressure measurement and modified FG scoring, acne, acanthosis nigricans, skin tags were also examined. Patients were called on a separate day and 2 mL of a blood sample, after an overnight fast for 8 h, was collected from all the subjects, sera separated and stored at −80°C. Serum testosterone levels, HbA1c (HPLC), and ultrasound examination of abdomen and pelvis for ovaries and adnexa were done as part of routine evaluation. CCL 18 level was estimated by the ELISA method (Ray Biotech). Fasting blood glucose level was estimated by the hexokinase method. Serum insulin level was estimated with Chemiluminescence Immunoassay (CLIA) technique and serum testosterone was processed with Electro chemiluminescence Immunoassay (ECLIA). Insulin sensitivity was estimated by HOMA IR model from fasting plasma glucose and fasting insulin levels by using the formula
HOMA IR = [Fasting insulin (miU/mL) × Fasting blood glucose (mg/dL)] ÷ 405
Statistical methods
The statistical analyses were done using Statistical Package for Social Sciences version 18.0 (SPSS, Inc). All the quantitative parameters such as age, BMI, and CCL 18 values are expressed as a mean with standard deviation and median with interquartile range. Differences in the mean values were tested for statistical significance by Student's t-test/Mann–Whitney's test. Association of categorical variables was tested for statistical significance by Chi-square test of significance. Pearson's correlation analyses were used to examine the relationship between serum CCL 18 levels and other parameters. Logistic regression analysis was done and P < 0. 05 was considered statistically significant.
RESULTS
There were 45 patients each in the PCOS group and the age, BMI matched control group. The baseline characteristics are shown in Table 1. The mean age of the subjects was 24.00 years (SD 5.240). The mean BMI in the PCOS and control groups were 28.82 kg/m2 (SD 4.78) and 27.55 kg/m2 (SD 2.72), respectively. The mean HOMA IR in the PCOS group and control group was 3.49 (SD 3.19) and 2.01 (SD 1.32), respectively. The mean serum CCL 18 levels in the PCOS group were 28.32 ± 4.17 ng/mL with the levels in the control group being 11.90 ± 4.91 ng/mL.
Table 1.
Baseline characteristics of cases and controls
| Parameters | Cases (n=45) | Controls (n=45) | P |
|---|---|---|---|
| Age (years) | 24.00±5.240 | 25.18±3.01 | 0.195 |
| Weight (kg) | 74.18±9.52 | 73.59±8.20 | 0.875 |
| BMI (kg/m2) | 28.82±4.78 | 27.55±2.72 | 0.77 |
| Waist Circumference (cm) | 94.62±9.37 | 87.26±6.51 | 00.03 |
| Waist-Hip Ratio | 0.92±0.03 | 0.90±0.04 | 0.004 |
| SBP (mmHg) | 120.62±5.78 | 115.96±6.775 | 0.001 |
| DBP (mmHg) | 80.44±3.01 | 78.53±4.77 | 0.026 |
| Modified FG score | 10.09±4.97 | 0.56±1.307 | 0.000 |
| HbA1C (%) | 5.34±0.33 | 5.10±0.91 | 0.100 |
| FBS (mg/dL) | 92.5 (8.25)* | 89.00 (10)* | 0.044 |
| Fasting Insulin (mIU/L) | 12.12 (12.45)* | 7.72 (4.97)* | 0.017 |
| HOMA IR | 3.49±3.19 | 2.01±1.32 | 0.005 |
| CCL 18 (ng/mL) | 28.32±4.17 | 11.90±4.91 | <0.001 |
| Serum testosterone (ng/dL) | 44 (31.07)* | 13.5 (14.75)* | <0.001 |
*Median (IQR). Bold: P<0.05
The correlation of insulin resistance with serum CCL 18 level is shown in Figure 1.
Figure 1.
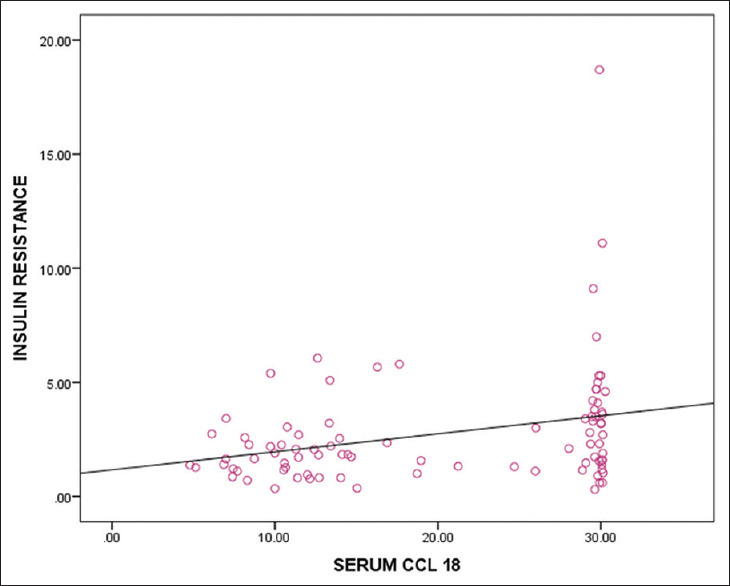
Correlation of insulin resistance with serum CCL 18
In the present study, a cutoff for HOMA IR is 2.06, which has been calculated by applying the Receiver Operating Characteristic (ROC) curve [Figure 2 and Table 2].
Figure 2.
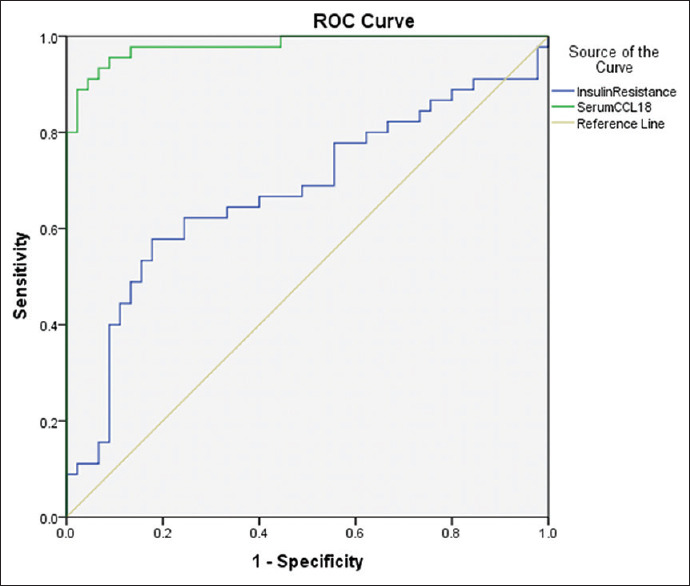
ROC curve for serum CCL 18 and HOMA IR as a predictor of PCOS
Table 2.
Insulin resistance by HOMA IR
| HOMA IR | Number (%) | Cases (n, %) | Controls (n, %) |
|---|---|---|---|
| Insulin Resistant (≥2.06) | 45 (50%) | 29 (64.4%) | 16 (35.6%) |
| Insulin Sensitive(<2.06) | 45 (50%) | 16 (35.6%) | 29 (64.4%) |
| Total | 90 | 45 | 45 |
P=0.05
The following parameters showed a relationship with serum CCL 18 levels: systolic blood pressure, diastolic blood pressure, waist circumference, waist-hip ratio, modified FG score, serum total testosterone, fasting insulin, and HOMA IR [Table 3].
Table 3.
Correlation of CCL 18 with different parameters
| Parameter | Correlation coefficient | P |
|---|---|---|
| HOMA IR | 0.286 | 0.00 (<0.01) |
| Weight (kg) | 0.012 | 0.914 |
| SBP (mmHg) | 0.338 | 0.001 (<0.01) |
| DBP (mmHg) | 0.294 | 0.005 (<0.01) |
| BMI (kg/m2) | 0.150 | 0.158 |
| Waist circumference (cm) | 0.392 | 0.00 (<0.01) |
| Waist-Hip Ratio | 0.286 | 0.006 (<0.05) |
| Modified FG score | 0.748 | 0.00 (<0.01) |
| Fasting Insulin (mIU/L) | 0.256 | 0.015 (<0.05) |
| FBS (mg/dL) | 0.102 | 0.337 |
| HbA1c (%) | 0.151 | 0.155 |
| S. testosterone (ng/dL) | 0.541 | 0.00 (<0.01) |
Bold: P<0.05
In order to identify independent predictors, logistic regression analysis was employed. Those factors which were significant at univariate analysis (systolic blood pressure, diastolic blood, waist circumference, waist-hip ratio, modified FG score, fasting insulin, HOMA IR, serum CCL 18 levels, and serum testosterone) were included in the logistic regression analysis to identify the independent predictors for PCOS. It was noted that serum CCL 18 levels (OR 1.71, 95% CI 1.09–2.67) were found to be the independent factor for predicting PCOS after adjusting for other factors [Table 4].
Table 4.
Logistic Regression analysis for independent predictors of PCOS
| Factor | Odds Ratio | 95% confidence interval | Significance | |
|---|---|---|---|---|
| Lower | Upper | |||
| SBP | 1.287 | 0.852 | 1.943 | 0.23 |
| DBP | 0.835 | 0.549 | 1.27 | 0.401 |
| Waist circumference | 1.06 | 0.80 | 1.40 | 0.688 |
| Waist-Hip Ratio | 1.07 | 0.01 | 1.73 | 0.216 |
| Serum CCL 18 | 1.71 | 1.09 | 2.67 | 0.018 |
| Serum Testosterone | 1.12 | 0.986 | 1.27 | 0.082 |
| FBS | 1.30 | 0.904 | 1.89 | 0.154 |
| Fasting Insulin | 0.987 | 0.585 | 1.66 | 0.962 |
| HOMA IR | 0.93 | 0.076 | 11.40 | 0.954 |
A ROC curve was constructed to determine the sensitivity and specificity of serum CCL 18 levels in distinguishing between PCOS subjects and normal individuals. The area under the curve was 0.981. A serum CCL 18 cutoff level of 18.84 ng/mL showed 93.3% sensitivity and 91.7% specificity in distinguishing between PCOS subjects and normal individuals.
DISCUSSION
There is a renewed interest to identify readily available sensitive markers to detect insulin resistance and the current study was aimed to establish the relevance of serum CCL 18 levels and its association with insulin resistance in women with PCOS, the major underlying pathophysiological factor.
Studies undertaken previously on serum CCL 18 levels were mainly in inflammatory conditions, and a positive relation has been established between the two. However, recently the role of serum CCL 18 levels as a marker of white adipose tissue inflammation is gaining importance. On reviewing the literature, the present study is the first to examine the relationship between serum CCL 18 levels and insulin resistance in PCOS.
In the present study, there was a significant correlation with insulin resistance and also a significant difference in serum CCL 18 levels between PCOS subjects and age, BMI matched healthy controls. Besides, serum CCL 18 level showed a relationship with waist circumference, waist-hip ratio, blood pressures, modified FG score, fasting serum insulin level, and serum total testosterone level. Logistic regression analysis proved that serum CCL 18 level could act as an independent predictor of PCOS.
In the present study, there was no significant correlation noted between serum CCL 18 levels and age. Previous studies have shown contrasting relationships.[26,27] In the present study, there was no correlation of serum CCL 18 levels with the weight although the study by Hagg et al.[18] has shown a positive correlation with weight.
The finding of the significant difference in the serum CCL 18 levels between PCOS women and normal controls is in agreement with the study done by Eriksson Hogling et al.,[17] where it was demonstrated that the mean serum CCL 18 levels were 63.5 ng/mL in insulin-resistant subjects and 41.0 ng/mL in the insulin-sensitive cases, whereas in our study, the mean serum CCL 18 levels were 28.32 ng/mL and 11.90 ng/mL in cases and controls, respectively. The relationship between BMI and serum CCL 18 levels has been disputed with studies showing conflicting relationships.[26,27] In our study, the relationship between serum CCL 18 and BMI was not significant, whereas a significant relationship was shown between serum CCL 18 and waist circumference and waist-hip ratio. The waist circumference in the study done by Eriksson Hogling et al.[17] showed a significant difference in the insulin-resistant group and insulin-sensitive group, and a similar difference was present in our study also. We feel that the greater waist circumference, a marker of insulin resistance, seen in the earlier study, as well as in our study, could be responsible for the greater serum CCL 18 levels. Serum CCL 18 was shown to be a marker of WAT inflammation and alternatively activated M2 macrophages, which are abundant in fibrotic areas of insulin-resistant subjects, act as a source of CCL 18. This could account as a possible mechanism for the significant relationship between insulin resistance and serum CCL18 levels in PCOS subjects as a majority of them have insulin resistance.
Given the higher visceral obesity and increased insulin resistance levels being consistently associated with the South East Asian population, we expected higher levels of serum CCL 18 levels in our study group as compared to the Caucasian population. However, the values obtained by us are lower than those in Caucasian population studies. These findings make us think that there could be other factors that could affect the levels of serum CCL 18 in the Indian population.
In our study, there was a positive correlation between serum CCL 18 levels and blood pressure. To our knowledge, this is the first time that a positive correlation has been found between blood pressure and serum CCL 18 levels in both PCOS subjects and controls, even after taking into note that none of our cases and controls had ever been diagnosed or treated for hypertension. This further strengthens the fact of association of high blood pressure with insulin resistance syndrome and, hence, serum CCL 18 levels in both the conditions. An earlier study done by Hagg et al.[18] has not shown any positive correlation between CCL 18 levels and blood pressures.
Testosterone is one of the hormones implicated in the pathophysiology of PCOS. In the present study, a very strong correlation was noted between serum total testosterone levels and serum CCL 18 levels and this correlation persists even after adjusting for other confounding variables.
In our study, there was no correlation noted between serum CCL 18 levels and HbA1c or fasting blood glucose. This is in agreement with the studies done by Khanian et al.[28] and Hagg et al.[18] This could reflect that serum CCL 18 levels are elevated in subjects with insulin resistance and not in those with established diabetes mellitus.
Often the diagnosis of PCOS remains inconclusive and also there are no reliable biochemical markers that can be of use in the follow-up of these patients. The outcomes of the treatment in PCOS patients are mainly assessed on clinical parameters like regularization of menstrual cycles, decreases in features of hyperandrogenism, and have limitations of being subjective and may not be reproducible. Our study has shown that a serum CCL 18 level of 18.84 ng/mL has 93.3% sensitivity and 91.7% specificity in PCOS subjects and this can be of use as a diagnostic marker. Further, it may be an additional tool in follow-up to assess the progress added to clinical parameters.
Our study has a few limitations. First, even though dyslipidemia is a known component of insulin resistance, the estimation of lipid profiles, which could have established the relationship between these parameters and serum CCL 18 levels, could not be done. Second, no investigations have been carried out to detect any asymptomatic, occult conditions which could have affected the serum CCL 18 levels and hence a limitation in the study. However, this is very unlikely. Third, insulin resistance in our study was measured by HOMA IR and was not correlated with the gold standard clamp studies of insulin resistance.
CONCLUSION
There is a significant correlation of serum CCL 18 levels with insulin resistance in PCOS subjects and this can act as a marker of PCOS. There is also a significant difference in serum CCL 18 levels in PCOS subjects with age and BMI matched healthy controls. Logistic regression analysis showed that serum CCL 18 level could act as an independent predictor of PCOS. Construction of a ROC curve has proved that a serum CCL 18 cutoff level of 18.84 ng/mL showed 93.3% sensitivity and 91.7% specificity in distinguishing between PCOS subjects and normal individuals.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Grant from Research Society for the Study of Diabetes in India (RSSDI)
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.International Diabetes Federation (IDF). Eighth Edition 2017. IDF Diabetes Atlas. 8th ed. 2017. pp. 1–150. [PubMed] [Google Scholar]
- 2.Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 3.Nestel P, Lyu R, Low LP, Sheu WH-H, Nitiyanant W, Saito I, et al. Metabolic syndrome: Recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr. 2007;16:362–7. [PubMed] [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome in urban Asian Indian adults--A population study using modified ATP III criteria. Diabetes Res Clin Pract. 2003;60:199–204. doi: 10.1016/s0168-8227(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 5.Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol. 1999;51:779–86. doi: 10.1046/j.1365-2265.1999.00886.x. [DOI] [PubMed] [Google Scholar]
- 6.Nair MKC, Pappachan P, Balakrishnan S, Leena ML, George B, Russell PS. Menstrual irregularity and poly cystic ovarian syndrome among adolescent girls--a 2 year follow-up study. Indian J Pediatr. 2012;79(Suppl 1):S69–73. doi: 10.1007/s12098-011-0432-y. [DOI] [PubMed] [Google Scholar]
- 7.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 8.Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Kalra A, Nair S, Rai L. Association of obesity and insulin resistance with dyslipidemia in Indian women with polycystic ovarian syndrome. Indian J Med Sci. 2006;60:447–53. [PubMed] [Google Scholar]
- 10.Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Odén A, Janson PO, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: A long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57:505–13. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- 11.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metabol. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 12.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leal V de O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94. doi: 10.1016/j.cca.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Chenivesse C, Tsicopoulos A. CCL18-Beyond chemotaxis. Cytokine. 2018;109:52–6. doi: 10.1016/j.cyto.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159:1140. [PubMed] [Google Scholar]
- 16.Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, et al. A dendritic-cell-derived C–C chemokine that preferentially attracts naive T cells. Nature. 1997:713–7. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson Hogling D, Petrus P, Gao H, Bäckdahl J, Dahlman I, Laurencikiene J, et al. Adipose and circulating CCL18 levels associate with metabolic risk factors in women. J Clin Endocrino Metabol. 2016;101:4021–9. doi: 10.1210/jc.2016-2390. [DOI] [PubMed] [Google Scholar]
- 18.Hägg DA, Olson FJ, Kjelldahl J, Jernås M, Thelle DS, Carlsson LMS, et al. Expression of chemokine (C-C motif) ligand 18 in human macrophages and atherosclerotic plaques. Atherosclerosis. 2009;204:e15–20. doi: 10.1016/j.atherosclerosis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Swaroop J, Naidu J, Rajarajeswari D. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2012;135:127–30. doi: 10.4103/0971-5916.93435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruun J, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003:535–42. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 21.El-Byoumy I. Interleukin 6 as inflammatory marker and insulin resistance in obese Kuwaiti adolescents. Integr Obesity Diabetes [Internet] 2017. [Last cited on 2020 May 10]. p. 3. Available from: http://www.oatext.com/interleukin-6-as-inflammatory-marker-and-insulin-resistance-in-obese-kuwaitiadolescents.php .
- 22.Fernandez-Real J-M, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, et al. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metabol. 2001;86:1154–9. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 23.Hossain M, Faruque MO, Kabir G, Hassan N, Sikdar D, Nahar Q, et al. Association of serum TNF-α and IL-6 with insulin secretion and insulin resistance in IFG and IGT subjects in a Bangladeshi population. Int J Diabetes Mellitus. 2010;2:165–8. [Google Scholar]
- 24.Tsiotra PC, Tsigos C, Anastasiou E, Yfanti E, Boutati E, Souvatzoglou E, et al. Peripheral mononuclear cell resistin mRNA expression is increased in type 2 diabetic women. Mediators Inflamm 2008. 2008:892864. doi: 10.1155/2008/892864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: Cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metabol. 2003;88:4848–56. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 26.De Sutter J, Struyf S, de Veire NR Van, Philippé J, De Buyzere M, Van Damme J. Cardiovascular determinants and prognostic significance of CC Chemokine Ligand-18 (CCL18/PARC) in patients with stable coronary artery disease. J Mol Cell Cardiol. 2010;49:894–6. doi: 10.1016/j.yjmcc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Sin DD, Miller BE, Duvoix A, Man SFP, Zhang X, Silverman EK, et al. Serum PARC/CCL-18 concentrations and health outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1187–92. doi: 10.1164/rccm.201008-1220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanian M S, Ardekani AA, Khosropanah S, Doroudchi M. Correlation of Early and Late Ejection Fractions with CCL5 and CCL18 Levels in Acute Anterior Myocardial Infarction. Iran J Immunol. 2016;13:100–13. [PubMed] [Google Scholar]


