Abstract
Objective:
To investigate the association of left ventricular end-diastolic volume (LVEDV) and the response to cell therapy in patients with nonischemic dilated cardiomyopathy (NICM).
Patients and Methods:
Five-year registry data from 133 consecutive patients with NICM who underwent CD34+ cell treatment were analyzed. All patients received granulocyte-colony stimulating factor; CD34+ cells were collected by apheresis and delivered by transendocardial injections. Patients with baseline LVEDV less than 200 mL (group A; n=72) and patients with LVEDV 200 to 370 mL (group B; n=54) were included. Patients with LVEDV greater than 370 mL were excluded (n=7). Favorable ejection fraction response was pre-defined by improvement in left ventricular ejection fraction (LVEF) greater than or equal to 5% at 1 y post-cell therapy.
Results:
At baseline, groups A and B were comparable with regards to age (52±11 y in group A vs 53±10 y in group B; P=.95), sex (male: 79% vs 83%, respectively; P=.55), creatinine (1.07±0.28 mg/dL vs 1.03±0.21 mg/dL, respectively; P=.21), or N-terminal probrain natriuretic peptide (1454±1658 pg/mL vs 1589±1338 pg/mL, respectively; P=.80). Baseline LVEF was higher in group A (32.8±8.7%) than in group B (30.2±8.7%; P=.03). During follow-up, there were four deaths in group A (6%), and 2 in group B (4%, P=.63). At 1-y post-cell therapy, LVEDV decreased significantly in group B (−56±30 mL; P=.003), but not in group A (+12±97 mL; P=.13). On multivariate analysis, baseline LVEDV was an independent correlate of favorable response in LVEF to therapy (P=.02).
Conclusion:
Larger LVEDV was associated with more pronounced increase in LVEF after transendocardial CD34+ cell therapy in NICM patients, informing target individuals with the highest likelihood of regenerative response.
Trial Registration:
clinicaltrials.gov identifier: NCT02445534
Nonischemic dilated cardiomyopathy (NICM) is a leading cause of advanced heart failure accounting for more than half of all heart transplantations.1 This evolving trend underscores an unmet need in managing NICM, warranting the exploration of treatment strategies that would enhance current best practices. In this context, accumulated evidence suggests that cell therapy may be of benefit in NICM leading to improved ventricular remodeling, augmented exercise tolerance, and better outcomes when administered as adjunct to standard-of-care.2–4 Despite these encouraging findings, there is significant individual variation in the clinical response to cell therapy. A substantial portion of the NICM population does not respond to cell intervention.5 Delineating the criteria that define most responsive patients would optimize regenerative therapy regimens.6
To date, the majority of clinical trials assessing cell therapy have focused on patients with ischemic heart disease.7 Most trials enrolled a relatively small number of patients, which makes it difficult to adequately define the patient responder pro-files.8 The largest regenerative study to date is the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) clinical trial, which investigated cardiopoiesis-based cell therapy in 271 ischemic heart failure patients with moderate-to-severe disease.9,10 Although the primary endpoint was neutral, exploratory analyses suggested a significant benefit of cell treatment on the primary composite in patients with baseline left ventricular end-diastolic volume (LVEDV) between 200 and 370 mL.9,11 Because heart failure progression in ischemic heart failure and NICM may be based on similar mechanisms,12 the aim of this study was to investigate a potential association of LVEDV and response to cell therapy in patients with NICM.
PATIENTS AND METHODS
Patient Population
This study is based on the analysis of a single-center, 5-y cumulative registry of NICM patients who were treated with cell therapy at the Advanced Heart Failure and Transplantation Center at University Medical Center Ljubljana between September 1, 2012, and September 1, 2017, and followed thereafter. Patient inclusion criteria consisted of the following: age 18 to 70 y, diagnosis of NICM according to the European Society of Cardiology,13 optimal medical management for 6 mo or longer, and left ventricular ejection fraction (LVEF) less than 40%. Patients with left ventricular aneurysm or thrombus, acute multi-organ failure, or a history of hematologic neoplasms were excluded from the study. Informed consent was obtained in all patients before participation in the study and the protocols were approved by the National Ethics Committee of the Republic of Slovenia, Agency for Medicinal Products and Medical Devices of the Republic of Slovenia, and the European Medicines Agency. The trial was registered with Clinicaltrials.gov (NCT02445534).
Study Design
At baseline, all patients received granulocyte-colony stimulating factor (10 μg/kg, 5 d). Thereafter, CD34+ cells were collected via apheresis and injected transendocardially guided by electro-anatomical mapping. Patients were followed for 1 y. At the time of enrollment and 12 mo thereafter, comprehensive clinical evaluation that included echocardiography and the 6-min walk test (6MWT) were performed, and plasma levels of N-terminal probrain natriuretic peptide (NT-proBNP) measured. Patients with baseline LVEDV less than 200 mL were included in group A; patients with LVEDV 200 to 370 mL were included in group B; patients with LVEDV greater than 370 mL were excluded. The cutoff values for LVEDV were based on unbiased subpopulation delineation by median baseline LVEDV from the CHART-1 clinical study.9
Clinical Evaluation
Acquired echocardiography data sets were analyzed at the end of the study by an independent echocardiographer who was blinded to the study groups and timing of the recordings. Left ventricular end-systolic and enddiastolic dimensions were measured in the parasternal long-axis view. Left ventricular end-systolic volume, LVEDV, and LVEF were estimated using the Simpson biplane method. Indexed LVEDV was defined as LVEDV divided by body surface area. The degree of mitral regurgitation was quantified by the Doppler echocardiography multipara-metric approach d by measuring vena contracta, calculating the effective regurgitant orifice area by the proximal isovelocity surface area method, and calculating the regurgitant volume. Significant mitral regurgitation was defined as grade greater than or equal to 1. All echocardiographic measurements were averaged over 5 cycles. In all patients, the 6MWT was performed by a blinded observer according to a standardized protocol.14 NT-proBNP assays were performed at a central independent laboratory, blinded to the patient’s clinical data using an established diagnostic kit (Roche Diagnostics, Mannheim, Germany).
Peripheral Blood Stem Cell Mobilization and Collection
All patients underwent stem cell mobilization and collection.15 Peripheral blood stem cells were mobilized by daily subcutaneous injections of granulocyte-colony stimulating factor (10 μg/kg daily) for 5 d, then collected with a Miltenyi cell separator (Miltenyi Biotech, Germany). The magnetic cell separator Isolex 300i (Nexell Therapeutics Inc, Irvine, CA) was used for immunomagnetic positive selection of CD34+ cells. All of the recovered CD34+ cells were used for transendocardial injection.
Electroanatomical Mapping and Transendocardial Cell Delivery
Electroanatomical mapping was performed using the Biosense NOGA system (Bio-sense-Webster, Diamond Bar, CA), which allows point-by-point analysis of left ventricular viability and local contractility. Using this technique, three-dimensional maps of color-coded myocardial viability (unipolar voltage [UV]) and regional myocardial contraction (local linear shortening [LLS]) and their corresponding 9-segment bull’s-eye maps, consisting of greater than or equal to 150 sampling points were generated for each patient before stem cell transplantation. In accordance with previous studies in NICM,16 segments with electromechanical mismatch (EMM) were defined as areas with average UV greater than or equal to 8.27 mV and average LLS less than 6%, scarred myocardium was defined as areas with UV less than 8.27 mV and LLS less than 6%, and normal myocardium was defined as areas with UV greater than or equal to 8.27 mV and LLS greater than or equal to 6%. After completion of electroanatomical mapping, cells were injected in EMM target zones using the Myo-star catheter (Biosense-Webster) applying pre-specified criteria of UV greater than or equal to 8.27 mV and LLS less than 6%. Each patient received 20 injections of stem cell suspension (0.3 mL each).
Follow-up and Endpoints
Patients were followed for 12 months. Favorable response to cell therapy was pre-defined by improvement in LVEF greater than or equal to 5% at 1-y post-cell therapy. The cutoff of 5% was chosen based on meta-analysis of heart failure trials showing that a 5% increase in the mean LVEF change corresponds to a relative odds ratio of 0.86 (95% CI, 0.77 to 0.96) for mortality.17 Theprimary endpoint was the intergroup difference in favorable response at 12 mo. Secondary endpoints included changes in LVEDV, NT-proBNP levels, and 6MWT distance. In exploratory analysis, intergroup differences in heart failure hospitalizations, transplantation-free survival, and electroanatomical parameters of the left ventricle were also evaluated.
Statistical Methods and Analysis
Continuous variables were expressed as mean ± SD and categorical variables were expressed as a number and a percentage. Continuous variables were explored for normal distribution with the ShapiroeWilk test. Differences within the groups were analyzed using a Student t test for continuous variables with correction for unequal variance when appropriate, and with χ2 or Fisher exact test when appropriate. Differences between groups A and B were analyzed using linear mixed models. Correlation between baseline LVEDV and change in LVEF within 1 y was evaluated by the Pearson correlation coefficient. In an exploratory analysis, we aimed to better define potential predictors of favorable response by performing backward method multiple regression analysis with an inclusion criteria P less than .05 and exclusion criteria of P greater than .10. A value of P less than .05 was considered significant. All statistical analyses were performed with the SPSS software (version 20.0).
RESULTS
Patient Characteristics
Of 133 consecutive NICM patients undergoing CD34+ cell therapy, 72 (54%) had baseline LVEDV less than 200 mL (group A), 54 (40%) presented with LVEDV between 200 and 370 mL (group B), and 7 (5%) had LVEDV greater than 370 mL and were thus excluded from the study. At baseline, groups A and B did not differ in demographic parameters, NT-proBNP, exercise capacity, plasma creatinine, bilirubin, sodium and hemoglobin, or heart failure medications; however, patients in group A had higher baseline LVEF, and lower incidence of significant mitral regurgitation (Table).
TABLE.
| Group A (LVEDV <200 mL) (n=72) |
Group B (LVEDV 200–370 mL) (n=54) |
P | |
|---|---|---|---|
| Age, y | 52±11 | 53±10 | .95 |
| Male sex | 57 (79) | 45 (83) | .55 |
| BMI, kg/m2 | 24.7±4.1 | 25.8±3.5 | .60 |
| DCM etiology | |||
| History of viral infection | 55 (76) | 43 (80) | .67 |
| Familial | 3 (4) | 3 (6) | |
| Idiopathic | 14 (19) | 8 (15) | |
| Creatinine, mg/dL | 1.07±0.28 | 1.03±0.21 | .2l |
| Bilirubin, mg/dL | 0.98±0.55 | 1.00±0.51 | .58 |
| Hemoglobin, g/dL | 13.5 ± 1.2 | 13.2±1.1 | .2l |
| Sodium, mmol/L | 138±7 | 137±6 | .62 |
| NT-proBNP, pg/mL | 1454±1658 | 1589±1338 | .80 |
| LVEF, % | 32.8±8.7 | 30.2±8.6 | .03 |
| Significant FMR | 60 (83) | 52 (96) | .02 |
| 6-minute walk, m | 355±87 | 345±92 | .72 |
| Drug therapy | |||
| ACEI/ARB | 70 (97) | 53 (98) | .74 |
| Beta blockers | 64 (89) | 50 (93) | .48 |
| MRA | 55 (76) | 45 (83) | .34 |
| Digoxin | 10 (14) | 6 (11) | .64 |
| Loop diuretics | 68 (94) | 52 (96) | .63 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; DCM = dilated cardiomyopathy; FMR = functional mitral regurgitation; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NT-pro BNP = N-terminal probrain natriuretic peptide.
Values are presented as mean SD or n (%).
Outcomes
Comparison of clinical outcomes of groups A and B is presented in Figure 1. At 1 y following cell therapy, a favorable response (LVEF increase ≥ 5%) was found in 31 of 72 patients (43.1%) in group A versus 35 of 54 (64.8%) in group B (P=.02). When directly comparing groups A and B at 1 y post-cell therapy, there was a significant difference in change in LVEF (from 32.8±8.7% to 35.2±7.9% in group A and from 30.2±8.6% to 38.1±6.5% in group B; P=.01). The continuous association between LVEDV at baseline, indexed LVEDV at baseline, and changes in LVEF within 1 y is presented in Figure 2. In this analysis, patients with larger baseline LVEDV appeared to have more pronounced increase in LVEF (Pearson r=0.25, P=.005). A similar association was found when correlating indexed LVEDV and LVEF changes (Pearson r=0.35, P=.001).
FIGURE 1.
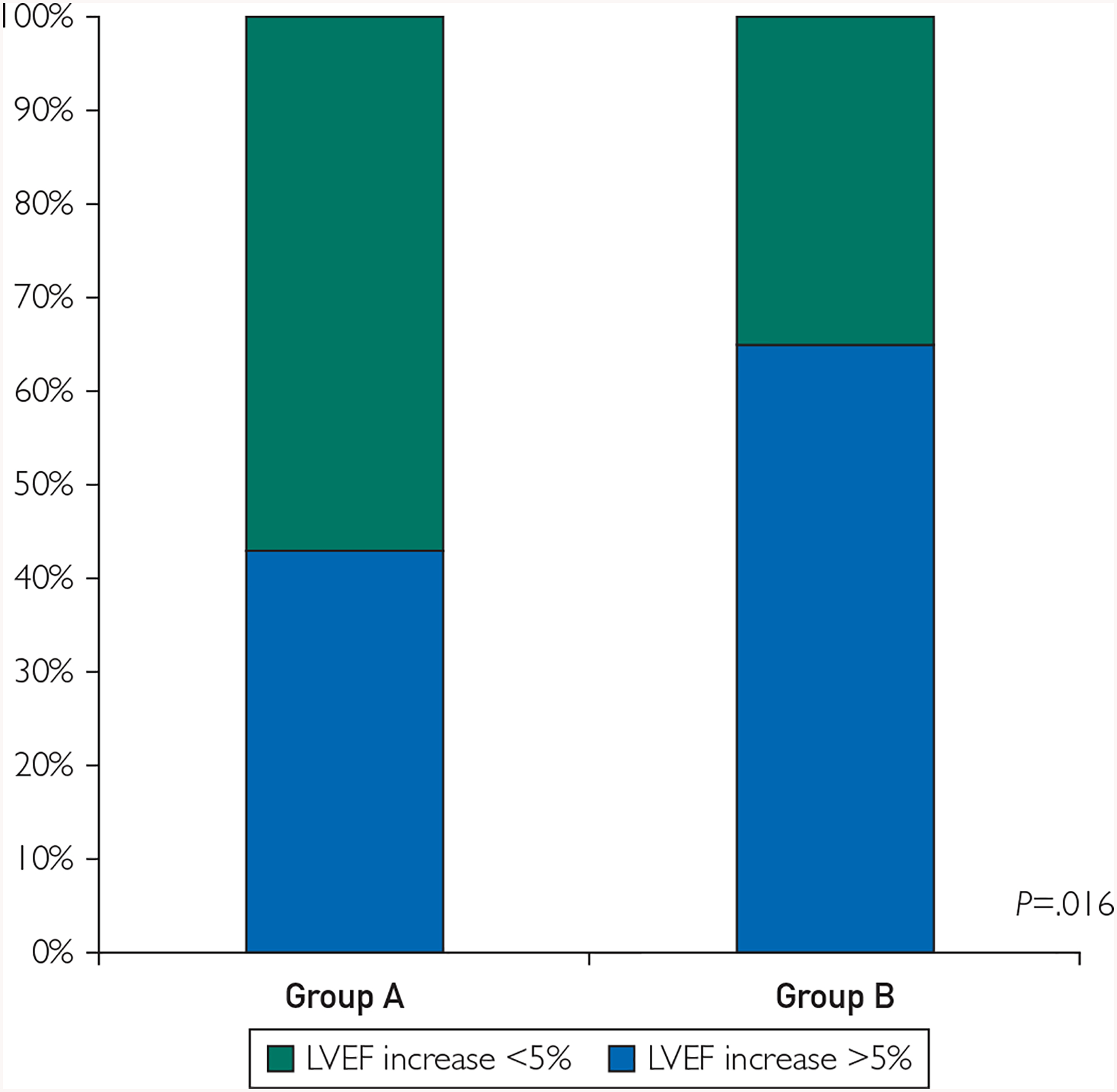
Changes in left ventricular ejection fraction (LVEF) at 1 y. Improvement in LVEF by at least 5% was present in a significantly higher proportion in nonischemic dilated cardiomyopathy patients with baseline left ventricular end-diastolic volume (LVEDV) between 200 and 370 mL (group B) when compared to patients with baseline LVEDV less than 200 mL (group A).
FIGURE 2.
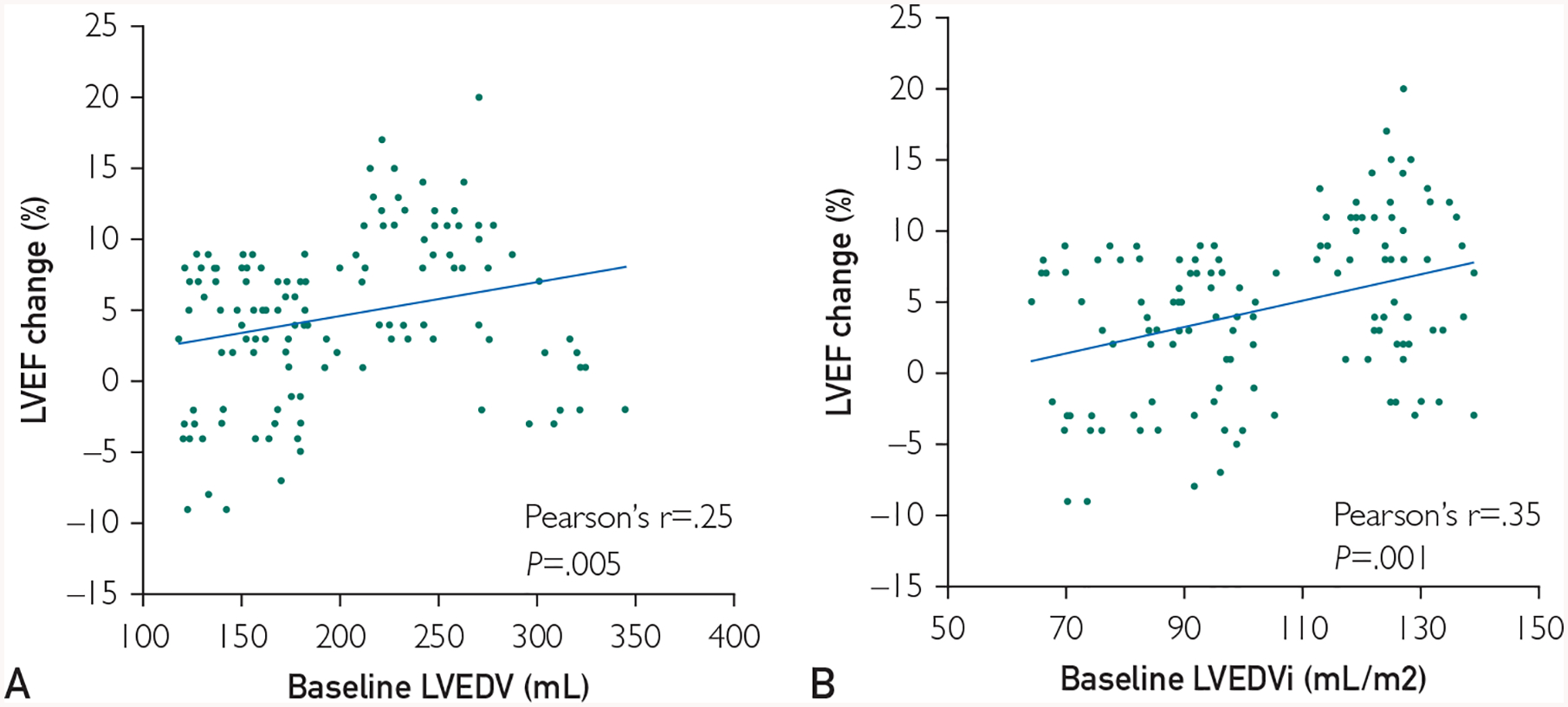
The association of baseline left ventricular end-diastolic volume (LVEDV) and changes in left ventricular ejection fraction (LVEF) within 1 y. In our cohort, patients with lower baseline LVEDV had less improvement in LVEF when compared to patients with higher LVEDV (A). A similar correlation was observed when investigating the association between indexed LVEDV at baseline and changes in LVEF within 1 y (B). LVEDVi = indexed left ventricular end-diastolic volume.
Within 1-y follow-up, we found a significant decrease in LVEDV in group B but not in group A (from 153±31 mL to 165±45 mL in group A and from 256±36 mL to 200±33mL in group B; P=.01). The changes in NT-proBNP levels and 6MWT distance were comparable in both groups. The degree of mitral regurgitation decreased by at least 1 grade in 12 of 72 patients (16.7%) in group A and in 29 of 54 (53.7%) in group B (P=.001). On multivariate analysis, baseline LVEDV was an independent correlate of 1-y clinical outcome (P=.02).
Survival and Heart Failure Hospitalization Rates
One-year survival in groups A and B was comparable (Figure 3). There were four deaths in group A (5.6%), and two in group B (3.7%, P=.63); heart transplantation was performed in four patients from group A(5.6%) and in five patients in group B(9.3%, P=.42). However, the number of patients requiring at least one hospitalization for worsening of heart failure was significantly higher in group A (50%) than in group B (31%, P=.03), underscoring the impact of baseline LVEDV in forecasting re-hospitalization in this NICM cell-treated patient population.
FIGURE 3.
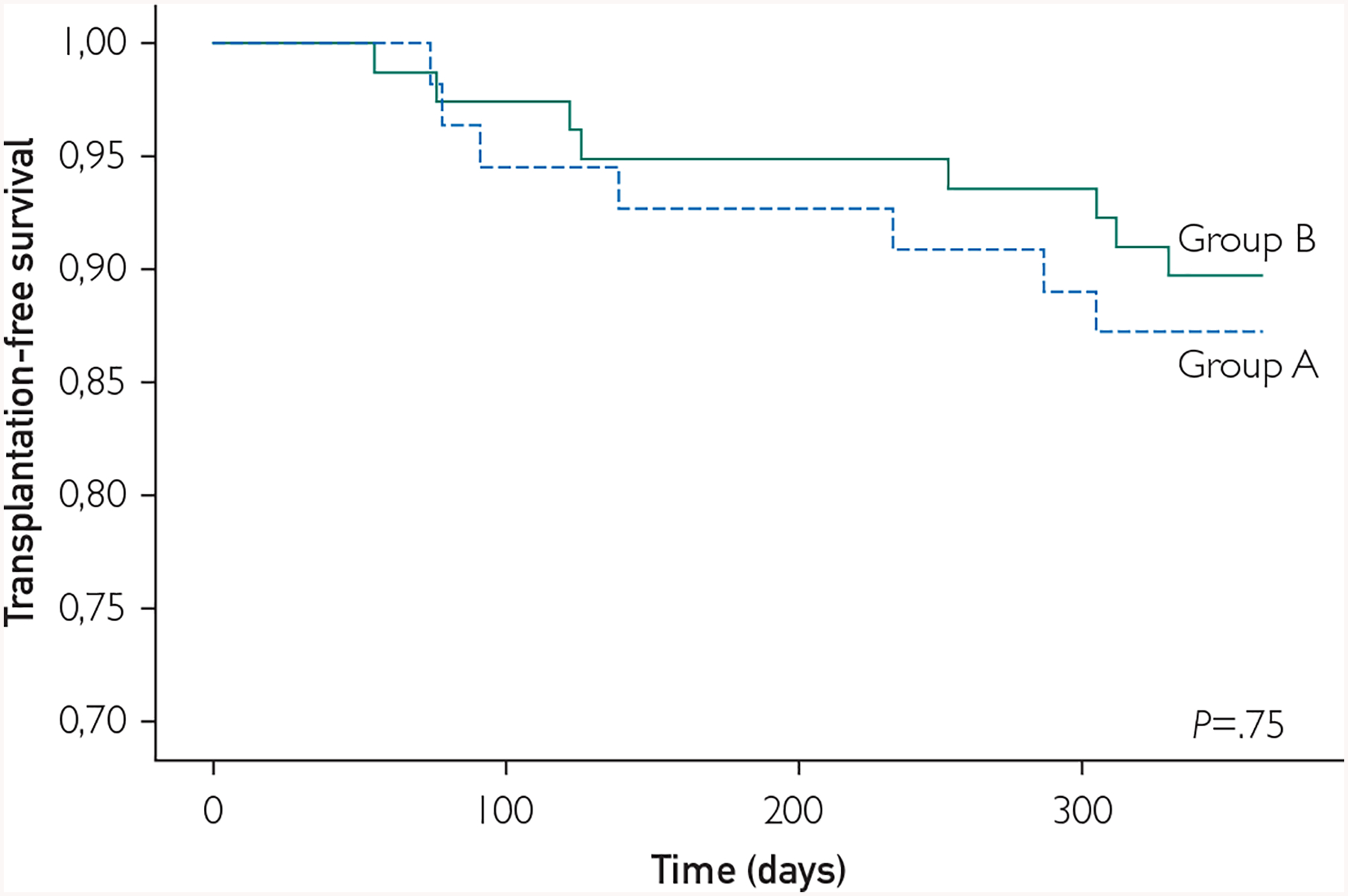
Transplantation-free survival. Within 1 y, we found no significant differences in transplantation-free survival between the groups with baseline left ventricular end-diastolic volume (LVEDV) between 200 and 370 mL (group B, solid line) and LVEDV less than 200 mL (group A, dashed line).
Electroanatomical Parameters
A representative bull’s eye map with data on electroanatomical mapping of the left ventricle performed before the cell injection is presented in Figure 4. Although the scar area was comparable in both groups, group B displayed significantly more segments with EMM and less segments of normal myocardium when compared with group A, suggesting larger areas of potentially reversible myocardial dysfunction.
FIGURE 4.
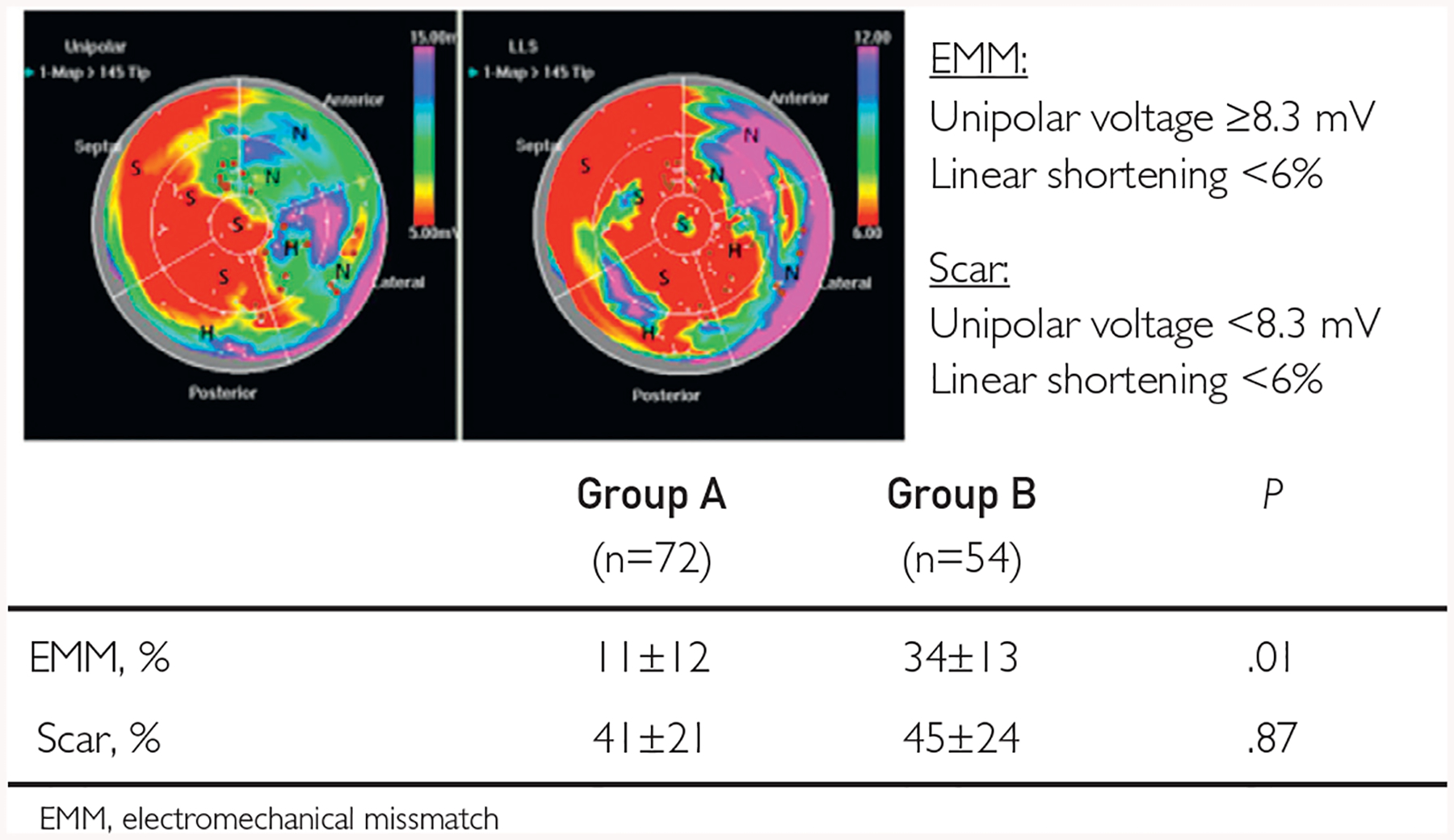
Electroanatomical properties at baseline. A representative quantitative bull’s eye unipolar voltage map (upper left panel) and local linear shortening (LLS) map (upper right panel) before cell injection. Segments with predominance of high unipolar voltage and high LLS (purple, blue, or green color on both panels) are defined as normal myocardium(N); segments with predominance of low unipolar voltage and low LLS (red and yellow color on both panels) are defined as scarred myocardium (S); and segments with a predominance of high unipolar voltage (purple, blue, or green on left panel) and low LLS (red or yellow on right panel) are defined as areas of electromechanical mismatch (EMM), which represents hibernating myocardium (H). Brown dots represent the sites of cell injections. Patients with baseline left ventricular end-diastolic volume (LVEDV) between 200 and 370 mL (group B) displayed larger areas of EMM when compared with patients with LVEDV less than 200 mL (group B).
DISCUSSION
This is the first study that tests for association of LVEDV with response to cell therapy in patients with NICM. In a single-center heart failure cohort, larger LVEDV at baseline (range, 200 mL to 370 mL) was associated with greater benefit at 1 y following CD34+ cell therapy. The apparent superiority in cell therapy outcome that correlated with baseline LVEDV was demonstrable in both cardiac functional and structural readouts, as well as in re-hospitalization rate. Although underlying mechanisms remain speculative, patients with larger LVEDV exhibited larger areas of potentially reversible myocardial dysfunction, evidenced by a higher number of EMM segments. Patients with larger baseline LVEDV also showed a more pronounced decrease in mitral regurgitation after cell therapy, which may further contribute to favorable outcome. In the setting of NICM, baseline LVEDV thus appears integral in determining cell response.
Achieving reverse remodeling is recognized as an important therapeutic goal in the management of patients with NICM.18 The present study implicates the added benefit of cell therapy as adjunct to standard of care. Although it has been established that normalization of both LVEF and left ventricular diameters may be attained in a sizeable portion of the general NICM patient population on standard of care, only approximately 10% show long-term improvement.19 Lack of reverse remodeling is especially pronounced in NICM patients with larger left ventricular end-diastolic dimensions and volumes.20 In line with this notion, the present study shows that a decrease in LVEDV is associated with improvement in LVEF, decrease in mitral regurgitation, and decrease in heart failure hospitalizations in patients with NICM. Therapy-induced reverse remodeling, as a multifactorial global myocardial process, is collectively implicated in determining outcomes in NICM.
In our established registry of CD34+ cell therapy treated patients with NICM, we report more pronounced evidence of reverse remodeling in patients with baseline LVEDV between 200 and 370 mL, which is in line with the results of cardiopoietic cell therapy in patients with ischemic heart failure.11 Together, these data suggest that the efficacy of cell therapy in chronic heart failure may be titrated by the cardiomyopathic stage, with patients with less severe disease having an inferior response compared to counterparts with more advanced disease. Although speculative, the reasons for this finding may be multiple. In our NICM cohort, patients with more pronounced left ventricular enlargement had more segments with EMM, which suggests that they had more areas of dysfunctional, yet viable (hibernating) myocardium. Previously, in a study of repeated cell therapy in NICM,21 we have shown that transendocardial injection of CD34+ cells in the areas of hibernating myocardium improves myocardial viability at the cell injection sites. Similarly, in patients with ischemic heart failure undergoing transendocardial transplantation of mesenchymal cells, ventricular functional responses preferentially occurred at the cell injection sites; improvement was greatest when segmental left ventricular dysfunction was severe.22 Based on these findings, one might speculate that patients with LVEDV between 200 and 370 mL have more target areas suitable for cell therapy and thus may have more potential to improve regional and global left ventricular performance when compared with patients with LVEDV below 200 mL. In our cohort, patients with higher and lower LVEDV had comparable NT-proBNP levels and exercise capacity, which suggests that innate properties of the failing myocardium may be more indicative than global hemodynamics when ascertaining response to cell therapy. Taken together, these data suggest that LVEDV could represent an important tool to guide and refine patient selection in future clinical trials assessing the validity of cell therapy.
A potential alternative mechanism for the findings of this study could be associated with the decrease in functional mitral regurgitation, which was significantly more frequent in patients with LVEDV between 200 and 370 mL. In patients with chronic heart failure, mitral regurgitation effective regurgitant orifice area and regurgitation volume both depend on LVEDV,23 and reduction of left ventricular volumes leads to a decrease of functional mitral regurgitation.24,25 Accordingly, reverse remodeling of the left ventricle with a reduction of LVEDV after cell therapy could partly be responsible for the improvement of functional mitral regurgitation seen in the present study.
Current data in NICM suggest that mitral regurgitation may be considered as an independent predictor of progressive adverse remodeling. Namely, some patients with NICM have mitral regurgitation that cannot be explained solely by left ventricular dilation and could thus benefit from mitral valve interventions.26 Although studies of mitral valve repair in heart failure patients have yielded inconsistent results, it appears that patients who benefit most from this approach are those with lower LVEDV and severe mitral regurgitation that is not proportional to the degree of left ventricular dilation.27,28 In our study, NICM patients with baseline LVEDV between 200 and 370 mL displayed a significant decrease in left ventricular volumes after cell therapy; however, an improvement of mitral regurgitation occurred only in approximately half of patients from this group. Thus, it appears that cell therapy may also serve to better define candidates appropriate for mitral valve repair by outlining the subgroup of patients who have persistent mitral regurgitation despite the reduction in LVEDV.
Study Limitations
The results of the present study are subject to several limitations. First, the results represent the analysis of a single-center registry data, which limits the impact of the evaluation of the cell therapy effects. The patient population included patients with NICM, but no biopsies were performed to exclude secondary cardiomyopathies, although we obtained careful clinical history, detailed echocardiography, and coronary angiogram in all patients. The sample size was relatively small, which makes the survival analysis underpowered to be definitive. Finally, we recognize that patients with NICM are a heterogeneous patient population and dynamic changes in ventricular function may be multifactorial. However, to minimize the potential effects of medical management, all patients were followed in our heart failure outpatient clinic for at least 3 mo before inclusion, which allowed for adequate optimization of medical therapy. During the 1-y follow-up, no additional changes in heart failure therapy were performed.
CONCLUSION
In a single-center registry of an NICM patient population, larger LVEDV was associated with more favorable response to transendocardial CD34+ cell therapy. Further studies are warranted to better define the underlying mechanisms and to investigate whether or not LVEDV can be used to inform target individuals with the highest likelihood of regenerative response.
Grant Support:
This work was supported by Slovenian Research Agency grant # J3-9283. The work was also supported through collaboration with Stanford Cardiovascular Institute. Dr Terzic is supported sby the National Institutes of Health (R01HL134664), Marriott Foundation, MichaelS. and Mary Sue Shannon Family, VanCleve Cardiac Regenerative Medicine Program, and Mayo Clinic Center for Regenerative Medicine.
Footnotes
Potential Competing Interests: The authors report no competing interests.
REFERENCES
- 1.Lund LH, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016;35(10):1158–1169. [DOI] [PubMed] [Google Scholar]
- 2.Hamshere S, Arnous S, Choudhury T, et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE- DCM clinical trial. Eur Heart J. 2015;36(44):3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrtovec B, Poglajen G, Lezaic L, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128(11 suppl 1):S42–S49. [DOI] [PubMed] [Google Scholar]
- 4.Vrtovec B, Poglajen G, Lezaic L, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013; 112(1):165–173. [DOI] [PubMed] [Google Scholar]
- 5.Vrtovec B Cell Therapy for Nonischemic cardiomyopathy: current status and future perspectives. Circ Res. 2018;122(1):28–30. [DOI] [PubMed] [Google Scholar]
- 6.Behfar A, Terzic A. Regeneration for all: an odyssey in bio-therapy. Eur Heart J. 2019;40(13):1033–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menasché P Cell therapy trials for heart regeneration - lessons learned and future directions. Nat Rev Cardiol. 2018;15(11): 659–671. [DOI] [PubMed] [Google Scholar]
- 8.Afzal MR, Samanta A, Shah ZI, et al. Adult bone marrow cell therapy for ischemic heart disease: evidence and insights from randomized controlled trials. Circ Res. 2015;117(6):558–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartunek J, Terzic A, Davison BA, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38(9):648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teerlink JR, Metra M, Filippatos GS, et al. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail. 2017;19(11):1520–1529. [DOI] [PubMed] [Google Scholar]
- 11.Bartunek J, Terzic A, Behfar A, Wijns W. Clinical experience with regenerative therapy in heart failure: advancing care with cardiopoietic stem cell interventions. Circ Res. 2018;122(10): 1344–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemljic G, Poglajen G, Sever M, et al. Electroanatomic properties of the myocardium predict response to CD34+ cell therapy in patients with ischemic and nonischemic heart failure. J Card Fail. 2017;23(2):153–160. [DOI] [PubMed] [Google Scholar]
- 13.Kaski JP, Elliott P; ESC Working Group. The classification concept of the ESC Working Group on myocardial and pericardial diseases for dilated cardiomyopathy. Herz. 2007;32(6): 446–451. [DOI] [PubMed] [Google Scholar]
- 14.Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JG. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;26(8):778–793. [DOI] [PubMed] [Google Scholar]
- 15.Dreger P, Haferlach T, Eckstein V, et al. G-CSFemobilized peripheral blood progenitor cells for allogeneic transplantation: safety, kinetics of mobilization, and composition of the graft. Br J Haematol. 1994;87(3):609–613. [DOI] [PubMed] [Google Scholar]
- 16.Campos B, Jauregui ME, Park KM, et al. New unipolar electrogram criteria to identify irreversibility of nonischemic left ventricular cardiomyopathy. J Am Coll Cardiol. 2012;60(21):2194–2204. [DOI] [PubMed] [Google Scholar]
- 17.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56(5):392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merlo M, Cannatà A, Gobbo M, Stolfo D, Elliott PM, Sinagra G. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail. 2018;20(2):228–239. [DOI] [PubMed] [Google Scholar]
- 19.Merlo M, Caiffa T, Gobbo M, Adamo L, Sinagra G. Reverse remodeling in dilated cardiomyopathy: Insights and future perspectives. Int J Cardiol Heart Vasc. 2018;18:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNamara DM, Starling RC, Cooper LT, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011; 58(11):1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrtovec B, Poglajen G, Sever M, et al. Effects of repetitive transendocardial CD34(+) cell transplantation in patients with nonischemic dilated cardiomyopathy. Circ Res. 2018;123(3):389–396. [DOI] [PubMed] [Google Scholar]
- 22.Suncion VY, Ghersin E, Fishman JE, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114(8):1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grayburn PA, Carabello B, Hung J, et al. Defining “severe” secondary mitral regurgitation: emphasizing an integrated approach. J Am Coll Cardiol. 2014;64(25):2792–2801. [DOI] [PubMed] [Google Scholar]
- 24.Nasser R, Van Assche L, Vorlat A, et al. Evolution of functional mitral regurgitation and prognosis in medically managed heart failure patients with reduced ejection fraction. J Am Coll Cardiol HF. 2017;5(9):652–659. [DOI] [PubMed] [Google Scholar]
- 25.Stolfo D, Merlo M, Pinamonti B, et al. Early improvement of functional mitral regurgitation in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2015;115(8):1137–1143. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin MM, Smith RL, Grayburn PA. Ischemic and functional mitral regurgitation in heart failure: natural history and treatment. Curr Cardiol Rep. 2014;16(8):517. [DOI] [PubMed] [Google Scholar]
- 27.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379(24):2307–2318. [DOI] [PubMed] [Google Scholar]
- 28.Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379(24):2297–2306. [DOI] [PubMed] [Google Scholar]


