Abstract
Objective:
We built a novel mock pharmacogenomics web portal to deliver pharmacogenomic information and results to patients. Utilizing a patient focus group, we then sought to understand patient insights on desired features of an effective pharmacogenomics patient portal.
Methods:
The mock YourPGx Portal delivered four sample pharmacogenomic results (omeprazole, simvastatin, clopidogrel, and codeine). Patients from our existing institutional, prospective pharmacogenomics implementation study were recruited to pilot the mock portal and then asked to participate in a focus group discussion led by two facilitators. All patients had been previously genotyped, but none had been directly provided access to their own genotyping results and none had previously used the YourPGx portal. The focus group discussion explored nine domains: (i) factors influencing drug response, (ii) concerns about drug effects, (iii) understanding of genomics and pharmacogenomics, (iv) reasons to undergo pharmacogenomic testing, (v) sources of pharmacogenomic information for patient education, (vi) attributes of pharmacogenomic sources of information, (vii) considerations about privacy and personal pharmacogenomic information, (viii) sharing of pharmacogenomic information, and (ix) features of an effective patient portal.
Results:
The median age of patients (n=10) was 65.5 years old (range 38–72), 70% female, 50% Caucasian/30% Black, and 60% held a bachelor/advanced degree. When asked about resources for seeking pharmacogenomic information, patients preferred consulting their providers first, followed by self-education, then using information provided by university research organizations. A theme emerged regarding attributes of these sources, namely a desire for understandability and trust. Patients said that the effectiveness of a pharmacogenomics patient portal is improved with use of symbolisms/graphics and clear and concise content. Effective use of colors, quantifying information, consistency, and use of layperson’s language were additional important facets. Patients communicated the appeal of secured phone/app-enabled access and said that they would desire linking to their electronic medical records to allow sharing of information with different members of their healthcare team.
Conclusion:
Patients named providers as their primary source of pharmacogenomic information, but a pharmacogenomics patient portal that is carefully constructed to incorporate desired features may be a favorable tool to effectively deliver pharmacogenomic information and results to patients.
Keywords: pharmacogenomics, patient portal, patient education, precision medicine
INTRODUCTION
With recent advances in health information technologies and a growing workforce of data scientists, there is an emergent interest in developing patient web portals to engage patients in self-management of their own healthcare [1–4]. This is driving institutions to increasingly develop and incorporate patient portals to provide patients convenient and reliable access to their medical records online. The features, content, and functionalities of these portals vary substantially, but they must be dynamic and carefully constructed to address the differing needs of diverse patient subpopulations [3].
Nevertheless, studies have shown that there exists a “digital divide”, that is, differences in patient portal use across patient subpopulations by age, race/ethnicity, educational background, and income [5–9]. This digital divide may further expand the existing disparities in access to health care [5]. Barron et al. explored perspectives on feasibility of a patient portal for older adults and found that readability, simplicity, and clearly explained medical concepts were valued [10]. It is therefore important to carefully design patient portals that meet the needs of a diverse population with varying levels of health and computer literacy.
Pharmacogenomics is the study of how genetic variability affects individual responses to medications. Several institutions and commercial entities currently offer clinical pharmacogenomic services and deliver genotyping test results using a variety of mechanisms, including reports within existing institutional electronic health records, in-house developed software and phone apps, safety-code cards, and printed paper reports mailed directly to the patient [11–16]. There are currently few known existing patient portals dedicated to delivering pharmacogenomic results to patients. Because of the complexity of genomics, an electronic pharmacogenomics portal may pose additional challenges for effectively delivering pharmacogenomic test results that are easily understandable by the layperson [17].
We previously described the development and beta-testing of a mock pharmacogenomics patient results web portal, the YourPGx Portal, and, using pre- and post-tests, assessed patient knowledge and perceptions of pharmacogenomics before and after its access [18]. That study, however - while showing positive effects on patient knowledge and perceptions of pharmacogenomics - did not allow a more in-depth qualitative understanding of the desired features of an effective portal. In this current study, we utilized a focus group to understand patient insights on features of an effective pharmacogenomics patient portal with the goal of incorporating these desired features to improve upon and deploy a live functional version.
MATERIALS AND METHODS
Development of the YourPGx Portal
The YourPGx Portal user interface was modeled after the design of our institutional pharmacogenomic results delivery tool used for providers, the Genomic Prescribing System (GPS), which has been previously described [18–19]. The patient portal delivered four sample drug results (omeprazole, clopidogrel, simvastatin, and codeine) in a compact accordion view, which could be expanded to provide more information for each medication by clicking on its name without leaving the page (Figure 1). The portal employed our prototypical traffic light iconography, with a legend box to define each light – red “warning”, yellow “caution”, and green “favorable” lights (Figure 1). In addition to the traffic light signal, each drug result could be expanded to include a quick summary of the drug-gene patient risk, the medication indication(s), population and patient estimates for efficacy or adverse drug events, and graphics to illustrate mechanism of drug metabolism and side effects. We purposely provided varying depth of information and different display formats for each of the four mock summaries with the intent of assessing patient preferences for each (Figure 1).
Figure 1.
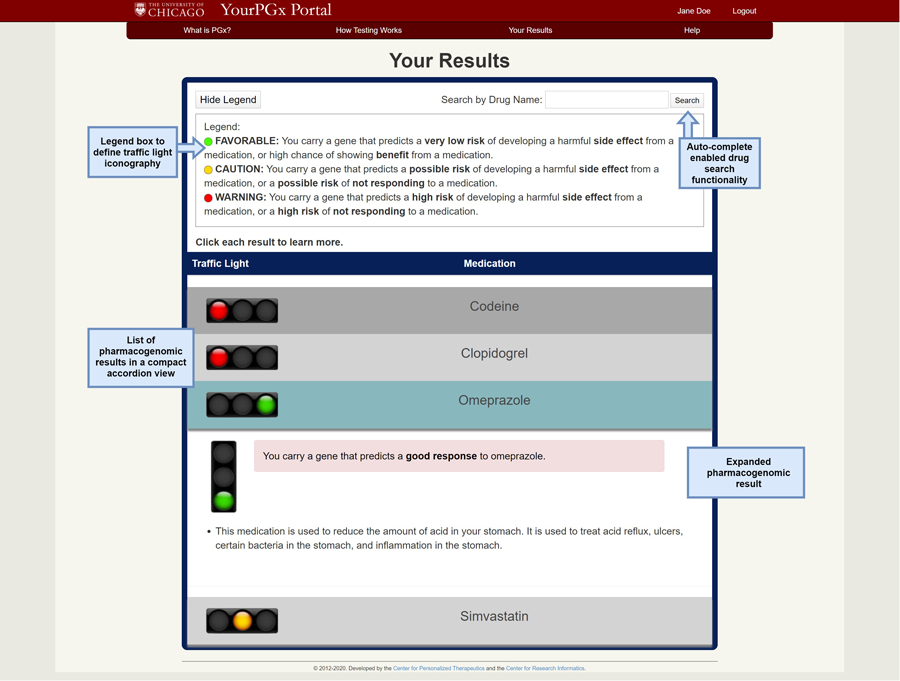
The Your Results page of the mock YourPGx Portal, showing an auto-complete enabled drug search functionality, a collapsible legend box to define the traffic light iconography, and a list of pharmacogenomic results in a compact accordion view which can be expanded for more details.
Participants
A sample size of 10–15 total participants for the focus group was predetermined in consensus with published literature that recommends an ideal size of 4–12 participants per group (this included the expectation that there would be dropout or “no show rate” on the day of the actual focus group amongst those who agreed to participate) [20–21]. Participants from our existing institutional, prospective pharmacogenomics implementation study (The 1200 Patients Project; clinicaltrials.gov NCT01280825) [22–23] were recruited by phone using a transcript to explain the purpose of the focus group: to gather patients’ ideas and opinions to build a pharmacogenomics patient results portal. A confirmation letter was mailed to all individuals who agreed to participate. All patients who participated in person were given a $50 Visa gift card as an incentive for partaking. This study was approved by the University of Chicago Institutional Review Board.
Study design and data collection
A patient focus group using a semi-structured interview guide allowed us to examine research questions inferred from published literature as well as explore themes that emerged during the discussion. The focus group convened for a single 180-minute session in August 2018. At the start of the session, participants were asked to complete a short demographic questionnaire, then given 15–20 minutes to navigate the YourPGx Portal, and finally participated in a discussion led by two facilitators (T.M.T. and P.H.O.) using a semi-structured interview guide (Supplementary Materials). The discussion explored nine themes: (i) factors influencing drug response, (ii) concerns about drug effects, (iii) understanding of genomics and pharmacogenomics, (iv) reasons to get pharmacogenomic testing, (v) sources of pharmacogenomic information for patient education, (vi) attributes of pharmacogenomic sources of information, (vii) considerations about privacy and personal pharmacogenomic information, (viii) sharing of pharmacogenomic information, and (ix) features of an effective patient portal. The discussion was audio-recorded with participants’ full knowledge and the transcript was formulated with identifiers removed.
Data analysis
A combined deductive and inductive method was used for thematic analysis of the de-identified transcript [24]. This approach allowed for establishing a priori codes on the basis of research questions and developing de novo codes on the basis of emergent themes. A conceptual framework using themes derived from the previous literature on patients’ knowledge and attitudes towards pharmacogenomics was used for the deductive portion [25]. ATLAS.ti 8.3.20 (ATLAS.ti; GmbH, Berlin, Germany) was used to facilitate coding. Two investigators (T.M.T. and P.H.O.) independently reviewed portions of the transcript to develop the initial coding scheme, followed by a comparative analysis to establish inter-rater reliability (satisfactory K score ≥ 0.75) until thematic saturation was achieved. When both investigators agreed on the final coding framework, it was then applied to the entire transcript by one of the investigators (T.M.T.). The primary outcome was to identify themes, subthemes, and representative quotations for each.
RESULTS
Patient demographics
We contacted 130 participants by phone: 66 did not answer, 48 refused or were unable to participate, and 20 agreed to participate. The commonly cited reasons for declining participation (in descending order) were schedule conflict, no means of transportation to get to the session, and lack of interest. Ten participants who initially agreed to participate by phone did not show up to the focus group, leaving 10 participants total on the day of the actual focus group (Table 1).
Table 1.
Demographics of the participants (n=10).
| (n=10) | |
|---|---|
| Age (mean ± SD) | 57.7 ± 13.7 |
| Female [n(%)] | 7 (70) |
| Race | |
| White | 5 (50) |
| African-American | 3 (30) |
| Other | 2 (20) |
| Education [n(%)] | |
| High school or less | 2 (20) |
| Some college | 2 (20) |
| Bachelor’s degree | 2 (20) |
| Advanced degree | 4 (40) |
| Number of prescription medications [n(%)] | |
| 0 | 1 (10) |
| 1–4 | 6 (60) |
| 5–8 | 2 (20) |
| 9 or more | 1 (10) |
| Self-reported history of medication-related side effects [n(%)] | 5 (50) |
| Self-reported medication management [n(%)] | |
| Self | 9 (90) |
| Family | 1 (10) |
| Caretaker | 0 (0) |
| Other | 0 (0) |
| Recalls stopping a medication due to a side effect or because felt like it was ineffective [n(%)] | 5 (50) |
| Recalls having a genetic test done [n(%)] | 3 (30) |
| Recalls receiving a PGx-determined prescription [n(%)] | 3 (30) |
Participants’ views on pharmacogenomics, testing, and other related themes.
Each emergent major theme is discussed in detail below and summarized in Table 2.
Table 2.
Leading themes identified from the focus group, with subthemes and representative quotations.
| THEME | SUBTHEMES AND REPRESENTATIVE QUOTATIONS |
|---|---|
|
1.
Factors influencing drug response a) Age ᠅ “I think age plays a role too.” b) Lifestyle ᠅ “Whether or not you drink, you smoke, if you’re active, sedentary.” c) Genetics ᠅ “.. give someone else medication might not work the same, but I would think DNA would have a big part of you know the reason why it doesn’t work for everybody the same way.” ᠅ “You don’t know the whole back story. I’m not a one size fits all.” d) Disease states ᠅ “So both me and my mother, she was recently prescribed carvedilol. Her dose was just 3.125 mg twice a day whereas mine is 25 mg twice a day. So again, I’m taller than she is, I’m more bulkier than she is, I’m younger than she is, but again the severity of the condition as well.” e) Body mass/size ᠅ “Height, weight.” f) Medications and dosage ᠅ “What gets prescribed and the dosage.” | |
|
2.
Concerns about drug effects a) Drug effect/response ᠅ “Realizing that some medications that I’m given are to avoid, but I have no idea whether they’re any better than sugar pills.” ᠅ “It turns out that one of my water pills was too strong for me. So they cut that in half, they say that was a cause.” b) Drug allergies ᠅ “And the only way you can know that is if when they prescribe you medication, if you have a reaction.” c) Drug toxicity/adverse effects ᠅ “My doctor prescribed me Lisinopril and about a couple months later I developed a persistent dry cough. A part of the population develops that reaction, so they changed the medication for me.” ᠅ “My husband had a severe reaction to a statin medication. And because of the side effects of the statin drug, he’s had deterioration of his muscles.” d) Drug interactions ᠅ “The medication cocktail that some of us have. I mean you have so many different types of medications.” ᠅ “So it kind of feels like the dose of medications are kind of contradicting you know not really working in his favor.” e) Drug costs ᠅ “Also with my doctor, they try to find the generic version of the drug instead of the brand or the more expensive version.” f) Limited knowledge ᠅ “The doctors are just looking at effects, not how it’s actually acting. They don’t know how it’s acting at this point. At least that’s my opinion as a consumer.” g) Non-adherence ᠅ “The doctor would prescribe a medication and he just wouldn’t take it. Which in result he had a stroke because he’s a diabetic and had high cholesterol.” ᠅ “Our patients or members are not necessarily taking their medications, if they’re compliant, if they have missed three months of prescriptions refills and stuff like that.” | |
|
3.
Understanding of genomics and pharmacogenomics ᠅ “DNA has to do with each of our individual selves. It’s our own person.” ᠅ “Pharmacy based on genetics.” ᠅ “It’s the ultimate personalized medicine.” | |
|
4.
Reasons to get PGx testing a) Benefit own care ᠅ “And had we known that prior to him taking the drug that he was going to react so poorly to it, we could have avoided it, as a result and make our lives better.” ᠅ “.. and so I was able to adjust what I was taking and take less, because it was working.” ᠅ “I’d like it for every time I get prescribed a new medicine or change.” b) Help other patients ᠅ “If they can study my DNA and that can in turn help somebody else.” ᠅ “I think it would help the children of today incredibly. I think medicine will be revolutionized over a period of time.” c) Desire for knowledge ᠅ “I just want to learn until, as long as I’m on the upside of the earth.” ᠅ “So if we don’t come in and help out, how are you guys gonna you know further research, if we don’t participate.” | |
|
5.
Sources of PGx information for patient education a) Self-education ᠅ “You should be reading on your own.” ᠅ “I really don’t have any problems with it. I don’t understand something, I look it up. It’s so simple.” b) Providers ᠅ “They also like to educate you. I come here because I feel like they educate me and they go through all my options, and then they make a decision based on what’s going to be appropriate.” ᠅ “Well if you don’t understand, it would be advantageous to reach out to your physician, you have to have a dialogue with the physician.” c) University research organizations ᠅ “Some of the web portals from research organizations, you can actually get into the research database if you want to try and decipher what in the world their paper is about. But you can get tons of information if you want to go and dive really deep into it.” d) Pharmaceutical companies ᠅ “So sometimes you have to go back through the pharmaceutical companies and try to find some of the studies, you know somewhat ridiculous.” e) Research studies ᠅ “There’s also new studies that come out all the time, so if anything is published you need to be able to see the latest because in science there’s always questioning whatever research was done.” | |
|
6.
Attributes of PGx sources of information a) Understandability ᠅ “But you also have a medical dictionary besides you.” ᠅ “The private language in medicine is just unlike any other thing. You try to make it as complicated as you can to keep us out.” ᠅ “If you ever read the cut sheet that comes with the medication, it’s going to do everything except start World War III. Because you know, I think it’s all for the lawyers, not for us.” b) Trust vs. non-trust ᠅ “I think sometimes too if you google if you don’t know if it’s good information.” ᠅ “I read a bunch of universities with doctors who generally want to help as oppose to say a pharmaceutical company because pharmaceutical companies make their business by people being sick. I mean if you’re cured you have no customers.” ᠅ “And was the study done ethically too or was it done just to sell more drugs that you’re trying to bring to the market.” | |
|
7.
Considerations about privacy and personal pharmacogenomic information a) Right to own results/information ᠅ “If you get the test why wouldn’t you get the results?” ᠅ “They should give you information.” b) Pharmacogenomic information is not a concern ᠅ “Even with the security thing, what can they do with your information on your DNA. I mean can they do anything harmful to me, knowing what kind of medications I take. I don’t think so.” ᠅ “I figure if they want to clone my DNA then there’s another of me running around, great!” c) Information security ᠅ “I at least would want my information to be as secured as possible, to my doctors, to my wife, because I don’t think probably myChart is all that security conscious. I’d want at least a two-factor.” ᠅ “Well I’m not so worried about my social security number I’m more worried about all the medical files.” d) Who has access to pharmacogenomic information ᠅ “I think though, one of the things though that we also have to keep in mind is nowadays, they have these 23andme that’s spitting in a tube and sending off your DNA, and so it’s amazing how many people do that. And I would be far more concerned about that type of thing where I don’t even know where my stuff is going versus knowing that this is where it’s rooted in. I know that what it’s for. I know. I mean if you send your saliva who knows how many people can get that or what they’re doing with it.” e) How pharmacogenomic information is used ᠅ “You know science is either moral or immoral, it’s just amoral. It depends on how you use the information. My biggest concern about the whole genomic area is, at some point our government will decide hey lets go back to eugenics. Let’s see what kind of master race we can breed, having all everybody’s genetic information. That’s frightening to me. You know, if the government can get to them.” | |
|
8.
Sharing of PGx information a) Patient determines ᠅ “I would say whoever you feel comfortable sharing it with. And it might be different for different people.” b) Government/military ᠅ “The military they can help a whole lot trying to identify remains.” ᠅ “I don’t really mind the policemen. I mean, I trust them.” c) Family ᠅ “I think I put down my physician, my spouse, my siblings, my children, and I think that was it.” d) Providers ᠅ “I think you have to be careful with who you allow it to be shared with, but it would be the physician for sure.” e) Research universities ᠅ “I don’t mind certain universities, medical universities like the University of Chicago, or the Mayo Institute.. [ ].. they do have really cutting edge research.” f) Employers ᠅ “Do you want your respective employers to have your information? Oh you might get diabetes, we don’t want you on our health plan.” g) Insurance companies ᠅ “And the insurance companies, they get a hold of your information.. [ ].. it’ll make premiums and everything go up.” h) Social media ᠅ “Didn’t put down the one about the social media.” | |
1. Factors influencing drug response
Participants discussed the many different factors that may influence drug response, with lifestyle often coming up throughout the discussion as one of the major factors.
2. Concerns about drug effects
Patients expressed concerns about the effect of providers’ limited knowledge on certain medications, resulting in ‘trial and error’ of many medications prescribed to them. Participants discussed the desire to know ahead of time whether a medication was going to work for them or whether they would experience an allergic reaction or toxicity to a medication in order to avoid this ‘trial and error’.
3. Understanding of genomics and pharmacogenomics
Participants mentioned that genetics could help providers pre-determine whether a medication is going to be effective for an individual, as opposed to ‘trial and error’ which was perceived as being due to providers’ lack of knowledge. One participant defined pharmacogenomics as “pharmacy based genetics” and another said, “it’s the ultimate personalized medicine”. One participant shared a story of a provider prescribing a medication which they did not take, and stated, “I’m not a one size fits all”, alluding to the concept of personalized medicine.
4. Reasons to get pharmacogenomic testing
Participants stated that the primary reasons to get testing are to benefit their own care, to help other patients, and a desire for knowledge. When discussing benefit to their own care as a reason for pharmacogenomic testing, one participant said, “If there’s a point where I need morphine, and if it doesn’t work, then I would want my doctor to know that ahead of time so they don’t give it to me”. One participant shared a story of her husband experiencing detrimental deterioration of his muscles due to a statin medication, and said, “had we known that prior to him taking the drug, that was he was going to react so poorly to it, we could have avoided it and as a result make our lives better”.
5. Sources of pharmacogenomic information for patient education
Patients listed providers as the most prominent source for seeking pharmacogenomic information, followed by self-education, university research organizations, and lastly, pharmaceutical companies.
6. Attributes of pharmacogenomic sources of information
Two themes emerged regarding attributes of these sources of information, specifically a desire for understandability and trust. Regarding understandability of pharmacogenomic sources of information, participants discussed the challenges of comprehending the scientific and medical language, and mentioned the need for a ‘medical dictionary’ to understand the jargon. Although the participants listed self-education as important, one person stated “I think sometimes too if you google it you don’t know if it’s good information”. This led to the theme of trust vs. non-trust for pharmacogenomic sources of information, which included whether the source of information is reliable, as well as whether the source of information has the patients’ best interest in mind.
7. Considerations about privacy and personal pharmacogenomic information
Some participants expressed the importance of security for their medical information. However, there were also a few participants that disagreed and said “what can they do with your information on your DNA?” This sparked a discussion about the importance of how pharmacogenomic information is used and who has access to this information. There was general agreement that patients should ultimately decide who has access to their information.
8. Sharing of pharmacogenomic information
Participants agreed that their providers should have access to their pharmacogenomic results; however, opinions differed on which members of their family should have this information, varying from spouse, to parents, siblings, and/or children. They agreed that they did not want employers or insurance companies to have this information due to the risk of increasing premiums or not having access to health plans (this is despite the portal describing the Genetic Information Nondiscrimination Act [GINA] of 2008 law protecting patients from discrimination based on their genetic information in both health insurance and employment) [26].
Patient insights on features of an effective patient portal.
Participants were then asked to discuss the mock patient web portal and provide their insights and preferences for desired features. Each feature is discussed in detail below, and some of the features are illustrated in Figure 2.
Figure 2.
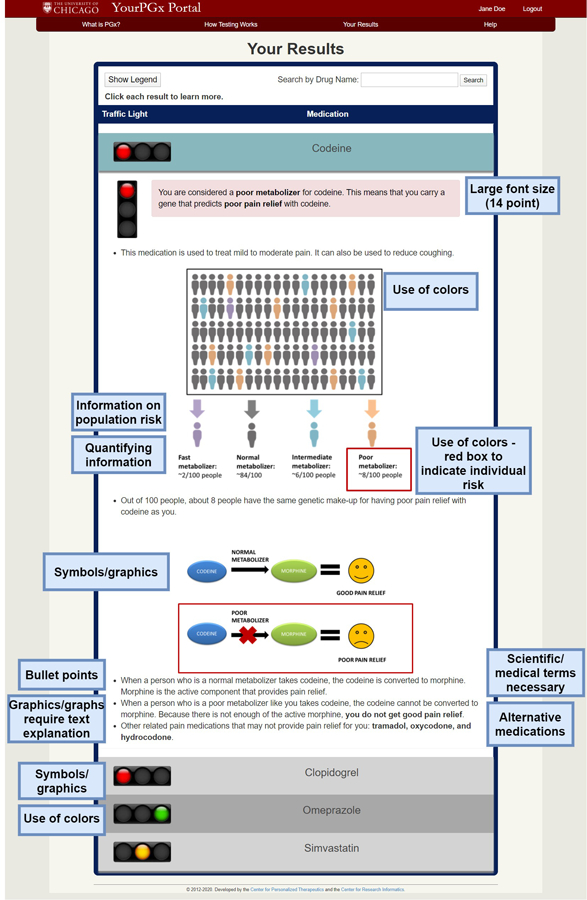
Patient insights on desired features of an effective pharmacogenomics patient web portal.
1. Clear layout/easy to navigate
Participants expressed their desire for the portal to have a clear layout and be easy to navigate. Patients liked the ability to ‘click each result to learn more’ from the compact accordion view to expand each drug result for more information, without having to leave the page.
2. Consistency
Participants expressed the importance of maintaining consistency on different pages of the portal.
3. Font size
Participants liked the large font size (14 point) of the portal, with one patient saying, “I thought the printing was large enough because I left my reading glasses at home”.
4. Clear and concise content
Patients wanted the portal to clearly communicate the information in a concise manner, particularly noting that they liked being able to quickly read the results in a few minutes. When asked about the very succinct content for omeprazole, one participant said, “it was enough. Sometimes a lot can be said with a few words”. Participants said that including brand names of medications would be useful to help patients understand the information better.
5. Simple layperson’s language
All participants agreed that it was important to deliver the information in simple layperson’s language, with one participant suggesting a 5th grade reading level. Some patients did not like the term “poor metabolizer” and stated “do I need to know that I’m a poor metabolizer? I mean now you’re going to send me to the dictionary to find out what ‘metabolizer’ means.” Other patients disagreed and one said, “most people understand what metabolism is. What are you going to replace it with?”. Although patients agreed on using simple layperson’s language throughout the portal, many also said that some scientific and medical terms were necessary, such as “pharmacogenomics” and the names of drugs.
6. Use of symbols/graphics
In general, patients liked the use of graphics throughout the portal, with one person saying, “I think [a] picture of showing people with different ratios of where it falls is really beneficial to someone who is more visual, to see it versus just reading it”. Patients liked the picture of statin-induced muscle pain to help visualize exactly where one may experience the pain. Participants also liked the graphics to illustrate population risk. Patients disagreed regarding the graphic to illustrate the metabolism of codeine, with one person saying, “I don’t know if you need the picture, in this particular instance [it] really doesn’t do anything for me because you already have said that I’m a poor metabolizer and it’s not going to work”. Participants mentioned that the pictures were not understandable without the bottom legends, with one person saying, “I got more from the words than from the picture”.
7. Use of colors
Participants liked the use of the traffic light colors because they were basic universal colors that everyone could recognize. However, one patient brought up an interesting thought regarding people with red/green color blindness, and their inability to differentiate these colors in the portal.
8. Use of bullet points
A few patients thought that the results page contained too much text and said that they preferred the bullet point format.
9. Quantifying information
Participants said that quantifying information gives a patient a good sense of their true risk. One patient wanted statistical information even for a green light result, stating, “if you’re giving all this for the bad stuff, it’s kind of nice to know how much of a chance there is of it working for you.”
10. Alternative medications
Patients suggested including alternative medications. For the codeine example, one person said, “I think it’s helpful because it list[s] other things that are probably not going to work/help because of how you don’t metabolize it.” However, one person suggested to include “talk to your doctor” when including alternative medications because “you don’t want someone taking their neighbor’s drug or something”.
11. Information on population risk
Patients liked information on their risk relative to the overall population risk, with one person saying, “I do like the fact that it tells you how many people have the same genetic makeup as you, for every 100 people about 5 people have the same genetic makeup”. They suggested putting the images in color order instead of scattered (see Figure 2). Participants also liked the graphs depicting population risk and the red box to indicate to which risk group they belonged.
12. Inform/educate but not give medical advice
Participants debated on including additional medical advice outside the realm of pharmacogenomics. Some suggested including more information on other factors that could affect response to medications, such as food and drug interactions, lifestyle, etc. This led to the discussion of the purpose of the portal to deliver only pharmacogenomic information. Although the mock portal contained a tab discussing precautions and disclaimers, one patient said it was important to repeat “talk to your doctor” for each result.
13. Information sharing and linking to electronic health records (EHR)
Participants wanted to know if the results from the patient portal could be merged with electronic health records, such as EPIC myChart. When the moderator asked the group if they would want pharmacogenomic information to be a part of this information sharing, they all unanimously agreed that they would.
14. Phone/app-enabled access
Participants expressed interest in being able to access the portal and their pharmacogenomic results on their mobile device, with one person saying, “I think more people are accessing the internet through their phone than through a PC.”
15. Information Security
One participant was concerned about information security with linking their pharmacogenomic results to EHR such as myChart and said they would want at least two-factor authentication. Another participant said, “I used to work with myChart here at the university, and they are extremely security conscious for people.”
DISCUSSION
To our knowledge, this is the first study to assess patients’ insights on desired features of a pharmacogenomics patient results web portal. In this study, we explored the attitudes and perceptions of patients regarding several themes about medications, pharmacogenomics, and sharing of pharmacogenomic information. Patients named providers as the primary source of pharmacogenomic information, followed by self-education and university research organizations. The desire for understandability and trust of these sources was most important to patients. Given that a patient-access portal would offer an increased chance for self-education about pharmacogenomics, it follows that the thoughtful design of such a portal with a focus on clarity of information and pre-testing in a university research setting may be viewed as valuable steps before deployment to ensure eventual effectiveness.
Patients named specific features that were desired for an effective patient portal. Many features were focused on visual appeal, and visual aids to enhance clarity and understandability, including use of symbols/graphics and effective use of colors. Consistent, concise, lay-accessible wording that provided quantifying contextual information was also a desired feature. This is consistent with a previous study that explored three perspectives (patients, caregivers, and informatics experts) on feasibility of a patient portal for older adults - namely that features of readability, simplicity, and clearly explained medical concepts were desired [17]. Patients expressed interest in linking the patient portal to their EHR. Pharmacogenomic test results stored in EHRs can maximize their consideration for new treatments across the members of the patients’ health care teams at the point of care [27]. The idea of having secured phone/app-enabled access to the portal to facilitate information sharing between different members of their providers was also mentioned as important. The World Wide Web Consortium has created different accessibility standards that apply to Web content and Web browsers to improve accessibility [28]. One of these primary design principles is that the Web site has to be operable on a robust and wide range of browsers and devices. This is especially important considering certain population subgroups are more likely to own and access specific types of devices such as solely using their mobile phone for Internet access [29].
This study had several limitations. First, the mock YourPGx Portal only provided sample results for four medications, not actual personalized results for each individual patient. Second, patients were given ~15 minutes to view the portal and then the focus group was conducted. While the portal was again shown during the focus group, it is possible that some feedback/features might have been additionally cited if patients had unlimited time to explore the portal. Additionally, individual opinions, particularly minor or comprehension problems, could be lost or suppressed during discussions. Furthermore, as previously discussed, in future contexts we will formally test the readability of the portal by using the Fry and SMOG methods to determine the average reading grade level of the contents. The findings from this focus group will also aid in optimizing the understandability and actionability of the portal which subsequently will be formally evaluated using the Patient Education Materials Assessment Tool (PEMAT) [30–32]. Finally, it is possible that the feedback received in this focus group may not be representative of the views of a more general population, given that all participants in our focus group had previously voluntarily enrolled in a pharmacogenomics testing program at our institution, and our participants were highly educated (60% Bachelor’s or more advanced degree). To address this, additional pre-testing in other populations is underway at our institution. The findings from this study have been utilized to build a secured, protected-access, live functional version of the YourPGx Portal which has been deployed to a limited cohort of patients at our institution. This live version of the portal delivers actual patient-specific pharmacogenomic results to patients for numerous medications beyond the four sample medications in this study. Our ultimate goal is to provide all patients from our various institutional pharmacogenomic implementation studies access to the YourPGx Portal, with permission from their treating providers (the latter is an institutional process decision so that treating providers have an opportunity to review results and provide appropriate advance education and consultation, if desired).
CONCLUSION
Patient access to genomic results is likely to increase in the years ahead. Thoughtful design of patient-access portals is therefore critical. The results of this study could serve as a useful framework to others who are designing platforms for pharmacogenomic results delivery as pharmacogenomics continues to become more widespread.
Supplementary Material
Table 3.
Patient insights on features of an effective patient portal with representative quotations.
| THEME | REPRESENTATIVE QUOTATIONS |
|---|---|
|
1. Clear layout/easy to navigate ᠅ “And it wasn’t like you had to go through some very long thing to get to everything.. it’s like boom boom boom.” ᠅ “I liked it, it was easy to navigate.” 2. Consistency ᠅ “I’m not sure that if you have a very simple first two pages, you don’t need to at least do something similar on the third page.” ᠅ “I think it would be consistent, you would use that language where, replace where it says favorable, so that the language is consistent across the top of the ledger.” 3. Font size ᠅ “I thought the printing was large enough because I left my reading glasses at home.” 4. Clear and concise content ᠅ “I thought it was straightforward, which I appreciated.” ᠅ “It was enough. Sometimes a lot can be said with a few words.” ᠅ “I like this because it’s not much but it tells you everything. It’s much better than trying to read the back of a prescription bottle.” 5. Simple layperson’s language ᠅ “I do believe in having it in layman’s terms so that everyone can understand I think when you make it very simple for people, it keeps them interested.” ᠅ “You’re also not doing it necessarily for academics and scientists, you’re doing it for lay people.” ᠅ “No because when it comes to the med, to the biologies or, there’s always going to be large terms, and I think it’s important for us to learn it instead of dumb down.” ᠅ “I think too like some of those words are just part of what you do, like you know, pharmaco.. pharmacy. That is just something you can’t not have on there. And you know, like the name of the drug, if that’s something that’s hard for you, but it’s what you’re taking. So I think some of those things, you can’t really get rid of.” ᠅ “I think it helps, most people know what metabolism is this day and age. What are you going to replace it with? You know? And then it says this means you carry a gene that predicts poor pain relief.” ᠅ Why not have a thing about you know, this discipline of what is then, and people can read that if they want to.” ᠅ “Can you have like a simple thing, and then go deeper. Like I mean kind of a link to a little bit more of a robust, you know, explanation.” 6. Use of symbolism/graphics ᠅ “I do like the fact that you had the green light, red light, yellow light. That was pretty good because that actually let you know, everyone knows what stop lights are even if you don’t drive.” ᠅ “No I think it’s good because it’s showing you exactly where you might develop the pain.” ᠅ “It’s perspective. It shows you, you visually how, how the break down is. And for someone who’s visual I think that’s very helpful.” ᠅ “I got more form the words than from the picture, I got more from the top part where it shows areas of the body than the bottom.” ᠅ “Your graphic in itself is not complete. You have to read the bullet points down below to understand it.” 7. Use of colors ᠅ “Like as soon as I saw it, ok green is like ok to take, it’s good to take. Yellow is like, take it, but take it with caution. And then, you know, the red was like, do not take” ᠅ “But for me, it’s easy to look at that right away because based on the darker color, those are the low risk, but then look at the very pale all the way to the right, you know, you don’t see many, so basically the same type of thing” 8. Use of bullet points ᠅ “The bullet points are helpful.” ᠅ “I love the bullet points.” 9. Quantifying information ᠅ “If it says 2% chance of having a stroke that’s different, but 12% is a lot” ᠅ “You know how you were talking about how the green one just says it’s good? Is there any way to like put more like statistical stuff, like you know like you did with the one that was a red light? Like the green light, just something else under that says it’s a good thing? I mean sometimes it’s nice, you know, if you’re giving all this for the bad stuff, it’s kind of nice to know how much of a chance there is of it working for you.” 10. Alternative medications ᠅ “But that second point, the way it gives you alternatives, that’s very important.” ᠅ “Because I think a lot of times, at least with those types of drugs, like there are a lot of options out there. There’s omeprazole, there’s lansoprazole, pantoprazole, there’s lots of them. And just to see where you would fall on the category of different medications might be beneficial?” 11. Information on population risk ᠅ “I do like the fact it tells you how many people have the same genetic makeup as you for that particular, for every 100 people about 5 people have the same genetic makeup. I think that’s just really interesting information, and I like that.” 12. Inform/educate but not give medical advice ᠅ “Put a little disclaimer, you know, talk to your physician.” ᠅ “But the point that was just raised more things you should put on there. You’re making an affirmative statement about something, and the idea of liability gets greater and greater.” ᠅ “That’s why I think you should have on there, ‘if you’re having muscle pain, consult your doctor’. That way they’re the ones giving the advice, not here.” 13. Information sharing and linking to electronic health records (HER) ᠅ “And I think it would be good if it was with myChart, because you can go in and see your medications, see your refills. And so if it’s all in one instead of having to go to separate portals. I think it would be easier for the use and for us to be educated as patients, and also as doctors so they can see it all in one.” ᠅ “Aren’t the major hospitals like, University of Chicago, Northwestern, Loyola, already linked through myChart? Yeah, they already talk to one another.” 14. Phone/app enabled access ᠅ “Let’s say you’re seeing someone else, and they don’t have access to that information, it would be nice to be able to pull it up and be like ‘here I’m in this study, and here is what this is saying’. I think I’m also just visual, and so being able to have that information accessible while I’m somewhere else could be beneficial.” ᠅ “If you can build an app and it has the graphics that will allow you to visualize this stuff, that would be great. A lot of people do now access this information through a PC, because they don’t have one or they don’t have the internet through that thing.” 15. Information security ᠅ “the only bad thing about having a direct link is security, because I don’t think probably myChart is all that security conscious. I’d want at least two factor [authentication]”. | |
ACKNOWLEDGEMENTS
We thank Ms. Deborah Stoit for her assistance with organizing the logistics of the focus group.
SOURCES OF FUNDING
This research was supported by National Institutes of Health (NIH) grants 5T32GM007019-41 (T.M.T.), 1R01HG009938-01A1 (P.H.O.), and the Benjamin McAllister Research Fellowship (T.M.T.). The funding sources had no involvement in the conduct of this research and/or preparation of this article.
Footnotes
CONFLICTS OF INTEREST
Dr. Ratain is a co-inventor on patents related to pharmacogenetic diagnostics and has received royalties related to UGT1A1 genotyping. No royalties were received from this research. All other authors declare no competing interests for this work.
REFERENCES
- 1.Ricciardi L, Mostashari F, Murphy J, Daniel JG, Siminerio EP. A national action plan to support consumer engagement via e-health. Health Aff (Millwood) 2013;32:376–384. [DOI] [PubMed] [Google Scholar]
- 2.Seiver A Critical care computing. Past, present, and future. Crit Care Clin 2000;16:601–621. [DOI] [PubMed] [Google Scholar]
- 3.Aziz O, Lo B, Pansiot J, Atallah L, Yang GZ, Darzi A. From computers to ubiquitous computing by 2010: health care. Philos Trans A Math Phys Eng Sci 2008;366:3805–3811. [DOI] [PubMed] [Google Scholar]
- 4.Garmire LX, Gliske S, Nguyen QC, Chen JH, Nemati S, VAN Horn JD, et al. The Training of Next Generation Data Scientists in Biomedicine. Pac Symp Biocomput 2017;22:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med 2010;363:501–504. [DOI] [PubMed] [Google Scholar]
- 6.Graetz I, Gordon N, Fung V, Hamity C, Reed ME. The Digital Divide and Patient Portals: Internet Access Explained Differences in Patient Portal Use for Secure Messaging by Age, Race, and Income. Med Care 2016;54:772–779. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar U, Karter AJ, Liu JY, Adler NE, Nguyen R, Lopez A, et al. Social disparities in internet patient portal use in diabetes: evidence that the digital divide extends beyond access. J Am Med Inform Assoc 2011;18:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu J, Huang J, Kinsman J, Fireman B, Miller R, Selby J, et al. Use of e-Health services between 1999 and 2002: a growing digital divide. J Am Med Inform Assoc 2005;12:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrido T, Kanter M, Meng D, Turley M, Wang J, Sue V, et al. Race/ethnicity, personal health record access, and quality of care. Am J Manag Care 2015;21:e103–113. [PubMed] [Google Scholar]
- 10.Kim EH, Stolyar A, Lober WB, Herbaugh AL, Shinstrom SE, Zierler BK, et al. Challenges to using an electronic personal health record by a low-income elderly population. J Med Internet Res 2009;11:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sample RightMed Gene Report. (Accessed January 23, 2020, at oneome.com.)
- 12.Boston Children’s Hospital Clinical Pharmacogenomics Service (Accessed January 23, 2020, at http://www.childrenshospital.org/centers-and-services/programs/a-_-e/clinical-pharmacogenomics-service-program/frequently-asked-questions.)
- 13.Translational Software Sample PGx Report. (Accessed January 23, 2020, at https://cdn2.hubspot.net/hubfs/4110056/Premium%20Content/Translational_Software_Sample_PGx_Report.pdf?__hssc=218920582.1.1579809900782&__hstc=218920582.dfbb602bef6f936cc633ecb211043a02.1579809900781.1579809900781.1579809900781.1&__hsfp=3311241238&hsCtaTracking=7db08eca-9f65-4fa0-9b9d-63cc757e6bd9%7C3372f468-b633-4f76-bd7b-ac623ec78c29.)
- 14.Safety-code. (Accessed January 23, 2020, at http://safety-code.org/patients/.)
- 15.Pharmacogenomics for Patients. (Accessed February 3, 2020, at https://www.medstarhealth.org/mhs/our-services/pharmacogenomics/for-patients/.)
- 16.Jones LK, Kulchak Rahm A, Gionfriddo MR, Williams JL, Fan AL, Pulk RA, et al. Developing Pharmacogenomic Reports: Insights from Patients and Clinicians. Clin Transl Sci 2018;11:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barron J, Bedra M, Wood J, Finkelstein J. Exploring three perspectives on feasibility of a patient portal for older adults. Stud Health Technol Inform 2014;202:181–184. [PubMed] [Google Scholar]
- 18.Truong TM, Lipschultz E, Danahey K, Schierer E, Ratain MJ, O’Donnell PH. Assessment of patient knowledge and perceptions of pharmacogenomics before and after using a mock results patient web portal. Clin Transl Sci 2020;13(1):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danahey K, Borden BA, Furner B, Yukman P, Hussain S, Saner D, et al. Simplifying the use of pharmacogenomics in clinical practice: Building the genomic prescribing system. J Biomed Inform 2017;75:110–121. [DOI] [PubMed] [Google Scholar]
- 20.Tang KC, Davis A. Critical factors in the determination of focus group size. Fam Pract 1995;12:474–475. [DOI] [PubMed] [Google Scholar]
- 21.Halcomb EJ, Gholizadeh L, DiGiacomo M, Phillips J, Davidson PM. Literature review: considerations in undertaking focus group research with culturally and linguistically diverse groups. J Clin Nurs 2007;16:1000–1011. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell PH, Danahey K, Jacobs M, Wadhwa NR, Yuen S, Bush A, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care--initial results of the University of Chicago “1,200 Patients Project”. Am J Med Genet C Semin Med Genet 2014;166C:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell PH, Bush A, Spitz J, Danahey K, Saner D, Das S, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther 2012;92:446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval 2006;27:237–246. [Google Scholar]
- 25.Lee YM, McKillip RP, Borden BA, Klammer CE, Ratain MJ, O’Donnell PH. Assessment of patient perceptions of genomic testing to inform pharmacogenomic implementation. Pharmacogenet Genomics 2017;27:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genetic Discrimination. (Accessed February 3, 2020, at https://www.genome.gov/about-genomics/policy-issues/Genetic-Discrimination.)
- 27.Haga SB, Kawamoto K, Agans R, Ginsburg GS. Consideration of patient preferences and challenges in storage and access of pharmacogenetic test results. Genet Med 2011;13:887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Introduction to understanding WCAG 2.0.2016. (Accessed January 23, 2020, at https://www.w3.org/TR/UNDERSTANDING-WCAG20/intro.html.)
- 29.Mobile Fact Sheet. (Accessed January 23, 2020, at https://www.pewresearch.org/internet/fact-sheet/mobile/.)
- 30.Friedman DB, Hoffman-Goetz L. A systematic review of readability and comprehension instruments used for print and web-based cancer information. Health Educ Behav 2006;33:352–373. [DOI] [PubMed] [Google Scholar]
- 31.Toolkit for Making Written Material Clear and Effective. (Accessed January 10, 2019, at https://www.cms.gov/Outreach-and-Education/Outreach/WrittenMaterialsToolkit/index.)
- 32.Shoemaker SJ, Wolf MS, Brach C. Development of the Patient Education Materials Assessment Tool (PEMAT): a new measure of understandability and actionability for print and audiovisual patient information. Patient Educ Couns 2014;96:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


