Abstract
Objectives
The outbreak of the 2019 coronavirus disease (COVID-19) pandemic in Wuhan, China, has subsided after being hard hit by the disease and subsequent city lockdown. Information on the number of people involved in Wuhan is still inadequate. This study aimed to describe the screening results of 61 437 community members in Wuchang District, Wuhan.
Methods
In mid-May 2020, Wuhan launched a population-scale city-wide SARS-CoV-2 testing campaign, which aimed to perform nucleic acid and viral antibody testing for citizens in Wuhan. Here we show the screening results of cluster sampling of 61 437 residents in Wuchang District, Wuhan, China.
Results
A total of 1470 (2.39%, 95% CI 2.27–2.52) individuals were detected positive for at least one antiviral antibody. Among the positive individuals, 324 (0.53%, 95% CI 0.47–0.59) and 1200 (1.95%, 95% CI 1.85–2.07) were positive for immunoglobulin IgM and IgG, respectively, and 54 (0.08%, 95% CI 0.07–0.12) were positive for both antibodies. The positive rate of female carriers of antibodies was higher than those of male counterparts (male-to-female ratio of 0.75), especially in elderly citizens (ratio of 0.18 in 90+ age subgroup), indicating a sexual discrepancy in seroprevalence. In addition, viral nucleic acid detection using real-time PCR had showed 8 (0.013%, 95% CI 0.006–0.026) asymptomatic virus carriers.
Discussion
The seroprevalence of SARS-CoV-2 in Wuhan was low. Most Wuhan residents are still susceptible to this virus. Precautions, such as wearing mask, frequent hand hygiene and proper social distance, are necessary before an effective vaccine or antiviral treatments are available.
Keywords: 2019 novel coronavirus, colloidal gold-based immunochromatographic strip, COVID-19, immunoglobulin antibody, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related coronavirus disease (COVID-19) is a respiratory transmissible disease that may cause critical illness to death [1,2]. Various control measures against SARS-CoV-2 were implemented in Wuhan, the first city hit by the coronavirus. After months of endeavour, viral transmission was largely contained [3]. However, sporadically infected cases and asymptomatic carriers were still detected. Hence, Wuhan launched a population-scale, massive SARS-CoV-2 testing campaign for detecting viral nucleic acid and antibodies in residents to further prevent viral transmission, screen out infected patients who were in the incubation period or were asymptomatic virus carriers, and map the epidemiological serodistribution of this infectious disease in the epicentre. Wuchang District, one of the 13 administrative divisions in Wuhan, is located in the central urban area and is adjacent to the Yangtze River. According to the Wuhan Statistical Yearbook 2018, the total population of Wuchang District is 1.28 million, accounting for 11.7% of the Wuhan population. The present study describes the screening results of 61 437 community members in Wuchang District, Wuhan.
Materials and methods
Participants and ethics
The study followed the guidance of Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases [4]. Using the cluster sampling method for residential communities, a total of 61 437 residents in Wuchang District, Wuhan, were enrolled from May 15 to May 26, 2020. Hospitalized patients, including confirmed patients with COVID-19 and other diseases, and individuals under quarantine, were excluded. The demographic data, including age, sex and residential area of each participant, were collected. Participants were screened for SARS-CoV-2 infection using a real-time reverse transcription polymerase chain reaction (RT-PCR) test for nucleic acid detection and a serological test for immunoglobulin (Ig)G and IgM antibodies against a recombinant antigen of the virus. The medical ethics committees of Zhongnan Hospital of Wuhan University approved this study. The ethics committee waived written informed consent because of the retrospective nature of this study.
Serological test against SARS-CoV-2 IgM and IgG antibodies
Individual blood samples were collected at community sampling stations. The serum samples were obtained by centrifugation at 3500 rpm for 8 min. The SARS-CoV-2 IgM and IgG antibodies were detected using a 2019-nCoV IgG/IgM detection kit (catalogue number: C6603C; Colloidal Gold-Based; Nanjing Vazyme Medical Technology Co., Ltd, Nanjing, China). The kit was approved for detecting SARS-CoV-2 IgM and IgG antibodies by the National Medical Products Administration. Specifically, one drop (about 20 μL) of the serum sample was added to the sample-loading position, followed by two to three drops (about 60 μL) of sample dilution buffer. After 10–15 min, the appearance of the control line (C line) was considered to be a valid test. The presence of the T1 and T2 lines indicated positivity for IgM and IgG, respectively.
Real-time RT-PCR
Throat swab samples were collected at community sampling stations and subjected to SARS-CoV-2 nucleic acid detection using a detection kit for 2019 novel coronavirus (2019-nCoV) RNA (catalogue number: DA0932; Da An Gene Co., Ltd of Sun Yat-sen University, Guangzhou, China). ORF1ab and N genes of the virus were amplified following the manufacturer's protocol.
Statistical analysis
The graphs of age and sex distribution were depicted for the entire tested population. For seropositive populations, the positive rates in each age and sex group were calculated by dividing the corresponding entire tested population. The 95% confidence interval (CI) was presented. The male-to-female ratio (MFR) was calculated as the male positivity rate divided by the female positivity rate.
Results
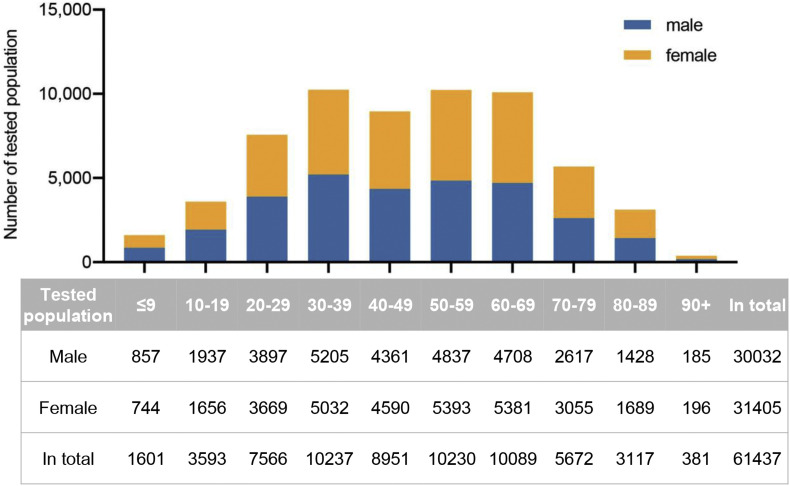
Among 61 437 community members included in the study, 30 032 (48.88%) were male. The age range is from several months to 101 years old and the median age was 48 (interquartile range (IQR) 32–64) years. The sex and age distribution is depicted in Fig. 1 .
Fig. 1.
Age and sex distribution of the tested population. Distribution of 61 437 residents with different age and sex is depicted as bar graphs. The table shows the corresponding number in each group.
As shown in Tables 1 and 1470 people tested positive for at least one SARS-CoV-2 antibody, accounting for 2.39% (95% CI 2.27–2.52) of the whole tested population. Among these seropositive people, 616 (41.88%) were male. The subgroup data showed that 324 (0.53%, 95% CI 0.47–0.59) and 1200 (1.95%, 95% CI 1.85–2.07) residents were positive for IgM and IgG antibodies, respectively. A 3.7-fold positivity rate was observed in IgG groups compared with IgM groups.
Table 1.
Information of antibodies positive populations
| IgM+ |
IgG+ |
IgM+ and/or IgG+ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| variables | subgroups | tested No. | No. | % (95% CI) | MFR | No. | % (95% CI) | MFR | No. | % (95% CI) | MFR |
| Age | ≤9 | 1601 | 3 | 0.19% (0.05–0.60) | — | 21 | 1.31% (0.83–2.03) | 1.41 | 24 | 1.50% (0.98–2.26) | 1.74 |
| 10–19 | 3593 | 6 | 0.17% (0.07–0.38) | — | 40 | 1.11% (0.81–1.53) | 1.42 | 44 | 1.22% (0.90–1.66) | 1.65 | |
| 20–29 | 7566 | 9 | 0.12% (0.06–0.23) | 0.27 | 57 | 0.75% (0.58–0.98) | 0.98 | 65 | 0.86% (0.67–1.10) | 0.81 | |
| 30–39 | 10237 | 25 | 0.24% (0.16–0.37) | 0.45 | 132 | 1.29% (1.08–1.53) | 0.73 | 156 | 1.52% (1.30–1.79) | 0.69 | |
| 40–49 | 8951 | 32 | 0.36% (0.25–0.51) | 1.35 | 169 | 1.89% (1.62–2.20) | 0.88 | 196 | 2.19% (1.90–2.52) | 0.95 | |
| 50–59 | 10230 | 69 | 0.67% (0.53–0.86) | 0.63 | 238 | 2.33% (2.05–2.64) | 0.85 | 298 | 2.91% (2.60–3.26) | 0.77 | |
| 60–69 | 10089 | 89 | 0.88% (0.71–1.09) | 0.50 | 296 | 2.93% (2.62–3.29) | 0.77 | 371 | 3.68% (3.32–4.07) | 0.68 | |
| 70–79 | 5672 | 65 | 1.15% (0.89–1.47) | 0.56 | 173 | 3.05% (2.63–3.54) | 0.91 | 221 | 3.90% (3.41–4.44) | 0.79 | |
| 80–89 | 3117 | 24 | 0.77% (0.51–1.16) | 0.71 | 68 | 2.18% (1.71–2.77) | 0.69 | 88 | 2.82% (2.28–3.48) | 0.68 | |
| 90+ | 381 | 2 | 0.52% (0.09–2.09) | 0.00 | 6 | 1.57% (0.64–3.57) | 0.21 | 7 | 1.84% (0.81–3.92) | 0.18 | |
| Sex | Male | 30032 | 119 | 0.4% (0.33–0.48) | 525 | 1.75% (1.60–1.90) | 615 | 2.05% (1.89–2.22) | |||
| Female | 31405 | 205 | 0.65% (0.57–0.75) | 675 | 2.15% (1.99–2.32) | 855 | 2.72% (2.55–2.91) | ||||
| Total | 61437 | 324 | 0.53% (0.47–0.59) | 0.61 | 1200 | 1.95% (1.85–2.07) | 0.81 | 1470 | 2.39% (2.27–2.52) | 0.75 | |
Dash symbols in MFR column represent zero positive cases in the corresponding female groups. CI, confidence interval; MFR, male-to-female ratio.
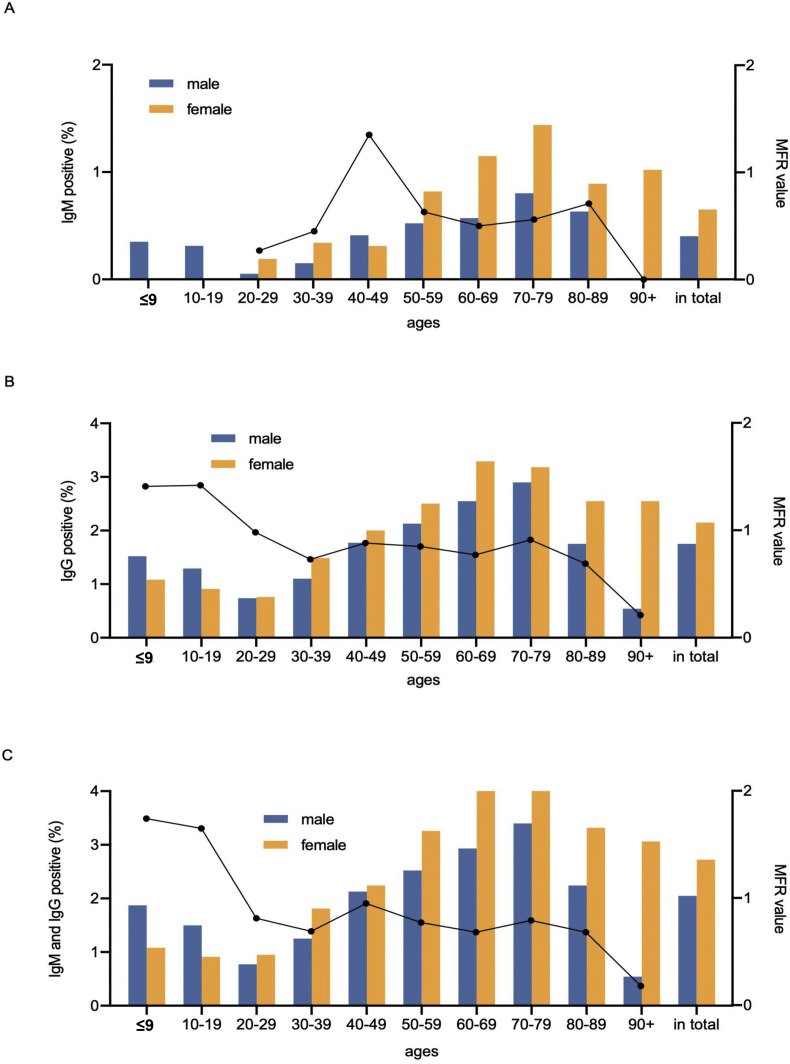
Further, a discrepancy in seroprevalence was observed in age and sex subgroups (Table 1 and Fig. 2 ). The positivity rates of IgM, IgG or their combination gradually increased with the increase in age, with the peak in the subset of people aged 70–79 years (Fig. 2). The lowest positivity rates (IgM, 0.12%; IgG, 0.75%; and IgM and IgG, 0.86%) were observed in groups of people aged 20–29 years. When considering subgroups by sex, the MFR, was 0.75 in IgM- and IgG-seropositive populations (Table 1 and Fig. 2), suggesting a significantly higher frequency in women than in men. In age subgroups, a trend of the female positivity rate higher than the male positivity rate (MFR <1) was captured in most age groups, except for the ≤19 subgroups. Additionally, the MFR values decreased when age increased, and the lowest MFR (0.18) was observed in 90+ age subgroup, indicating an increased probability of the female antibody carrier with the increase in age.
Fig. 2.
Age and sex distribution of the antibody-positive population. (A) Distribution of 324 IgM-positive individuals. (B) Distribution of 1200 IgG-positive individuals. (C) Distribution of 1470 IgM- and IgG-positive individuals. Vertical bars indicate the positive rates, and dot lines represent the MFR. MFR, male-to-female ratio.
The real-time RT-PCR identified eight (0.013%, 95% CI 0.006–0.026) asymptomatic virus carriers. Five of them were male and three were female. The age range was 45–77 years. Among them, four were negative for both IgM and IgG antibodies, and four were positive for IgG antibody only.
Discussion
A total of 1470 individuals tested positive for at least one SARS-CoV-2 IgM or IgG antibody, with an overall seropositivity rate of 2.39%. Only healthy members were included in the campaign, while confirmed patients with COVID-19, people under quarantine and hospitalized inpatients were excluded to reduce possible cross-infection. This group of people was more susceptible to SARS-CoV-2 infection than other citizens, thus decreasing the seropositivity rates. In addition, most non-local workers who left Wuhan before city shutdown because of the Chinese New Year in January returned to Wuhan by the date of sampling. However, the universities still remained closed, and hence a majority of college students were not included in the study. Wuchang District had many colleges; therefore, exclusion of this population had a significant effect on the positive rates. The lack of college students, who were mainly around 20 years old, might have led to elevated seropositivity rates because the 10–19 and 20–29 age groups had the lowest antibody positivity rates (Fig. 2 and Table 1). Furthermore, the participants were voluntarily recruited, and hence it was reasonable to speculate that a small proportion of mobility-impaired individuals were reluctant to participate, although they performed fewer activities during the outbreak and had fewer chances to be infected.
The present study was conducted in Wuchang District, while data in different districts in Wuhan hit by COVID-19 might vary. However, the observed rate was comparable with that reported in other similar studies. A recent study detected 17 368 individuals from different geographic regions in China, including 1993 residents from different Wuhan sub-cohorts. It suggested a seropositivity rate of 3.8%, 3.2% and 3.8% in healthcare workers, their family members and their staff members, respectively, from hotels designated for the accommodation of healthcare workers during city lockdown in Wuhan between 30 March and 10 April 2020 [5]. As the exposure of healthcare workers and their close contacts to SARS-CoV-2 was relatively higher than that of most other citizens, it was reasonable that the seropositivity from these subgroups was higher than that from populations with massive testing. Another study testing 452 asymptomatic Hong Kong residents evacuated from Hubei province in early March 2020 indicated a 3.76% (17/452) seropositivity rate [6]. All these seropositivity rates indicated that the prevalence of the population carrying the antibody in Wuhan was low.
Among 61437 community members included in the study, eight (0.013%), 324 (0.53%) and 1200 (1.95%) individuals were detected positive for nucleic acid, IgM and IgG, respectively. As IgM is regarded as the first class of immunoglobulins in response to initial exposure, the presence of IgM antibody represents an early exposure to the antigen [7]. The anti-SARS-CoV-2 IgM antibody could be detected in patients after 4 days of onset, peaking at 2–3 weeks after the onset of symptoms before its level started to decline [8,9]. However, IgM positivity alone may not be a good diagnostic indicator because not all of the people develop a detectable IgM antibody [8]. In addition, the IgM antibody may still be detectable after several months, although it is considered as an ‘early infection’. Most of the recent studies showed detectable SARS-CoV-2 anti-IgM antibodies after 1 to 2 months [10,11]. IgG represents the most robust and long-duration antibody against the virus [12]. The positivity of the IgG antibody indicates a ‘past infection’ of the virus. Wuhan reported approximately 50 000 patients with confirmed symptomatic COVID-19. The result suggested that Wuhan, a city with 11 million people, had proportionally 269 200 (2.39%) asymptomatic individuals infected with SARS-CoV-2 in the last few months, besides confirmed patients. A more accurate seropositivity rate in Wuhan may need further analysis, including multidistrict testing results.
Remarkably, the present study detected more female carriers of asymptomatic antibodies compared with male carriers in most age subgroups and a reverse correlation trend of MFR with the increase in age. Another study in Wuhan also reported similar findings [13]. Indeed, it was suggested that SARS-CoV-2 affected women less than men due to different innate immunity, steroid hormones and factors related to sex chromosomes [14]. However, this female over male trend in asymptomatic carriers was not captured in other areas and countries, such as South Korea, Thailand, Iran, Spain and California in the USA [[15], [16], [17], [18]]. Whether this trend is observed only in Wuhan or can be observed in other parts of China or other countries too needs further investigation.
This study had several limitations. First, several groups of people were not included in the study, which might have had different impacts on detecting real seropositivity. Second, the rates were affected by the quality of the kit. As mentioned in the kit specifications, the sensitivity and specificity of this commercially available IgM/G detection kit were 91.54% (86.87–94.65) and 97.02% (94.74–98.33), respectively. The sensitivities were comparable with those of other brands [19]. These intrinsic shortcomings of the rapid immunochromatographic kit might inevitably cause false-positive and false-negative results. Third, massive tests were conducted within 12 days, and hence the possibility of higher false-positive or false-negative rate due to the labour-intensive work was unavoidable. Fourth, a rapid and ready-to-use method, the immune colloidal gold technique, was adopted for the screening test because a large number of samples were needed for handling and the technicians had some limitations. The test provided only a qualitative positive or negative result. A more quantitative result may be obtained using the chemiluminescence enzyme immunoassay.
In summary, the majority of the residents in Wuhan are still immunologically naive to SARS-CoV-2, far from herd immunity. Although Wuhan re-opened on 8 April 2020, proper control measures, such as frequent hand hygiene, wearing face masks and keeping proper social distance, are necessary before effective vaccines or antiviral drugs are available.
Transparency declaration
All the authors have nothing to disclose. This work was supported by the Key Project for Anti-2019 Novel Coronavirus Pneumonia from the National Key Research and Development Program of China (2020YFC0845500) and the National Key Research and Development Program of China (2018YFE0204500).
Authors' contributions
Conceptualization: P.Y. and L.Y. Methodology: P.Y., Y.G., F.J., T.Y., H.X., G.S., and L.J. Formal Analysis: P.Y. and L.X. Investigation: P.Y., M.D., C.Z., Y.Y. L.Y. and W.X. Data curation: P.Y., Y.G. and L.Y. Writing – Original Draft: P.Y. and L.X. Writing – Review and Editing: P.Y. and L.X. Supervision: Y.Y., L.Y. and W.X.
Acknowledgements
We acknowledge all health-care workers involved in the diagnosis and treatment of patients in Wuhan.
Editor: E. Bottieau
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraemer M.U.G., Yang C.H., Gutierrez B., Wu C.H., Klein B., Pigott D.M. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368:493–497. doi: 10.1126/science.abb4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field N., Cohen T., Struelens M.J., Palm D., Cookson B., Glynn J.R. Strengthening the reporting of molecular Epidemiology for infectious diseases (STROME-ID): an extension of the STROBE statement. Lancet Infect Dis. 2014;14:341–352. doi: 10.1016/S1473-3099(13)70324-4. [DOI] [PubMed] [Google Scholar]
- 5.Xu X., Sun J., Nie S., Li H., Kong Y., Liang M. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 6.To K.K.-W., Cheng V.C.-C., Cai J.-P., Chan K.-H., Chen L.-L., Wong L.-H. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: a multicohort study. Lancet Microbe. 2020;1:e111–e118. doi: 10.1016/S2666-5247(20)30053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. 2000;37:1141–1149. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 8.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020;81:e28–e32. doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X., Wang J., Xu X., Liao G., Chen Y., Hu C.H. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbe. Infect. 2020;9:1269–1274. doi: 10.1080/22221751.2020.1773324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu J., Wu C., Li X., Zhang G., Jiang Z., Li X. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;Online ahead of print doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padoan A., Cosma C., Sciacovelli L., Faggian D., Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin Chem Lab Med. 2020;58:1081–1088. doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- 12.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10:1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling R., Yu Y., He J., Zhang J., Xu S., Sun R. Seroprevalence and epidemiological characteristics of immunoglobulin M and G antibodies against SARS-CoV-2 in asymptomatic people in Wuhan, China. medRxiv. 2020 2020.06.16.20132423. [Google Scholar]
- 14.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34:339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 15.Song S.K., Lee D.H., Nam J.H., Kim K.T., Do J.S., Kang D.W. IgG seroprevalence of COVID-19 among individuals without a history of the coronavirus disease infection in daegu, Korea. J Korean Med Sci. 2020;35:e269. doi: 10.3346/jkms.2020.35.e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nopsopon T., Pongpirul K., Chotirosniramit K., Hiransuthikul N. COVID-19 antibody in Thai community hospitals. medRxiv. 2020 doi: 10.1136/bmjopen-2020-046676. 2020.6.24.20139188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakiba M., Hashemi Nazari S.S., Mehrabian F., Rezvani S.M., Ghasempour Z., Heidarzadeh A. Seroprevalence of COVID-19 virus infection in Guilan province, Iran. medRxiv. 2020 doi: 10.3201/eid2702.201960. 2020.04.26.20079244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendavid E., Mulaney B., Sood N., Shah S., Ling E., Bromley-Dulfano R. COVID-19 antibody seroprevalence in santa clara county, California. medRxiv. 2020 doi: 10.1093/ije/dyab010. 2020.04.14.20062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida S., Ono C., Hayashi H., Shiraishi S., Tomono K., Arase H. SARS-CoV-2-induced humoral immunity through B cell epitope analysis and neutralizing activity in COVID-19 infected individuals in Japan. bioRxiv. 2020 doi: 10.1038/s41598-021-85202-9. 2020.07.22.212761. [DOI] [PMC free article] [PubMed] [Google Scholar]