Abstract
As an emerging global health crisis, coronavirus disease 2019 (COVID-19) has been labeled a worldwide pandemic. Growing evidence is revealing further pathophysiological mechanisms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Amongst these dysregulated pathways inflammation seems to play a more critical role toward COVID-19 complications. In the present study, precise inflammatory pathways triggered by SARS-CoV-2, along with potential therapeutic candidates have been discussed.
Prevailing evidence has indicated a close correlation of inflammatory cascades with severity, pathological progression, and organ damages in COVID-19 patients. From the mechanistic point of view, interleukin-6, interleukin-1β receptor, interferon-gamma, tumor necrosis factor-alpha receptor, toll-like receptor, receptor tyrosine kinases, growth factor receptor, Janus kinase/signal transducers and transcription pathway, mammalian target of rapamycin, cytokine storm and macrophage activation have shown to play critical roles in COVID-19 complications. So, there is an urgent need to provide novel mechanistic-based anti-inflammatory agents. This review highlights inflammatory signaling pathways of SARS-CoV-2. Several therapeutic targets and treatment strategies have also been provided in an attempt to tackle COVID-19 complications.
Keywords: COVID-19, Inflammatory pathways, Cytokines, Interleukin, TLR, JAK/STAT
Highlights
-
•
Inflammatory pathways are in a close linkage with COVID-19 complications.
-
•
Targeting IL-6, IL-1β receptor, TLR, IFN-γ, TNF-α and mTOR could pave the road in combating COVID-19.
-
•
JAK/STAT and related interconnected pathways are of pivotal pathways to be targeted in COVID-19.
-
•
PI3K/Akt/mTOR, Ras/Raf/MEK/ERK, and TRAF/TRADD/caspase could be promising targets in combating COVID-19.
1. Introduction
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) happened at the end of 2019 Wuhan, Hubei, China, and resulted in a disease named coronavirus disease 2019 (COVID-19) (Lai et al., 2020a; Ruan et al., 2020.). The outbreak was declared a Public Health Emergency of International Concern by World Health Organization on March 11, 2020. Based on the data released from clinical trials of China, about 20% of the patients infected with SARS-CoV-2 ended with severe illness, while possessing common symptoms (Zhang et al., 2020c). The patients showed exacerbated symptoms suddenly within 1–2 weeks after the disease onset, accompanied by low peripheral blood lymphocyte, particularly natural killer cells and low lymphocyte count in lymphoid organs. The atrophy of spleen and lymph nodes with bilateral involvement of lungs in computed tomography (CT) of the chest were also shown in COVID-19 patients (Jin et al., 2020.). From a histological perspective, the alveolar damage was associated with a huge number of eosinophil, formation of hyaline membrane, high mono- and poly-nucleated cells, pneumocyte hyperplasia, and thickening of interstitial fluid. Additionally, high levels of monocytes and macrophages were obvious in lung lesions (Peiris et al., 2010.).
In terms of pharmacological mechanisms, oxidative stress, inflammation, and post-virus entry apoptosis seem to play destructive roles in the aforementioned pathological conditions during COVID-19 (Fakhri et al., 2020). Following the virus entry, the first defense line is the innate immune system, that recognizes the pathogen-associated molecular patterns (PAMPS) by pathogen recognition receptors (PRR), including NOD-like receptors (NLR), toll-like receptors (TLR-3,4,7,8,9) (Ky and Mann, 2020), RIG like receptors (RLR) (Channappanavar and Perlman, 2017) and angiotensin receptor (South et al., 2020). After that, the inflammatory pathways will be activated, followed by dendritic cells maturation and macrophage activation (Lampropoulou et al., 2016.). Accordingly, pro-inflammatory agents will be produced and released by the stimulation of T-helper cells (Chen et al., 2016), and complement system compartments will take part in the war against the virus. The defense strategy of the coronavirus against T cells is based on apoptosis induction in these cells. Consequently, overactivaton of the immune system leads to the production of huge amounts of inflammatory and apoptotic agents and their increased local levels, finally resulting in organ damage (Mathern and Heeger, 2015; Traggiai et al., 2004). Enhanced levels of pro-inflammatory receptors along with their downstream mediators, including interleukin (IL)-6, IL-1β, IL-18, and tumor necrosis factor-alpha (TNF-α) (Peiris et al., 2010.; Wan et al., 2020), Janus kinase/signal transducers and activator of transcription (JAK/STAT), c-reactive protein (CRP), as well as ferritin (Perricone et al., 2020) have been observed during the disease. COVID-19 patients have also shown dysregulated levels of IL-2,7,10, granulocyte-colony stimulating factor (G-CSF), interferon-γ (IFN-γ), induced protein-10 (IP-10), macrophage inflammatory protein 1-α (MIP-1α), and monocyte chemo attractant protein-1 (MCP-1) (Ky and Mann, 2020).
A recent study focused on the blockade of IL-6 to tackle COVID-19 cytokine release (Liu et al., 2020). Some other studies have also reported inflammation as a promising target in combating COVID-19 (Cancio et al., 2020; Jose and Manuel, 2020; Stebbing et al., 2020b). There is yet a deep need to reveal all the inflammatory receptors/pathways involved in the pathogenesis of COVID-19. This is the first review that highlights the predominant inflammatory pathways, as well as potential targets, and therapeutic candidates for the management of COVID-19.
2. Virology and pathogenesis of SARS-CoV-2
Since now, efforts to find the origin of the SARS-CoV-2 have not achieved a final outcome and investigations are going on. Although based on the evolutionary and genome sequencing analyses, the primary natural host of the novel virus is bat with unknown intermediate hosts responsible for transmission (Yi et al., 2020).
Based on their differences in genotype and serological parameters, coronaviruses have been divided into four types, including α, β, γ, and δ-CoVs (de Wilde et al., 2019.; Weiss and Leibowitz, 2005). After isolation of the novel virus from alveolar fluid samples of the patient’s lungs, analyses showed that α and β types of the coronaviruses are the main causes of the disease (Zhu et al., 2019). SARS-CoV-2 consists of a nucleocapsid containing RNA and phosphorylated protein. It possesses a positive-sense single-stranded and wrapped RNA with a size of about 29.9 kb. A bilayer phospholipid is wrapped around the nucleocapsid and two kinds of proteins exist in the bilayer phospholipid called spike proteins (Khailany et al., 2020), including S and hemagglutinin esterase. In the viral envelope, membrane protein (M) and envelope protein are also located on the virus with 60–100 nm size and round/oval shape (Fehr and Perlman, 2015). This novel virus entitled “Public enemy number 1” by World Health Organization, which is the third type of crisis after SARS and Middle East Respiratory Syndrome (MERS) (Yan et al., 2020).
From another point of view, a wide range of complications are caused by this virus, including enteric, respiratory, neurological, and hepatic ones (Jin et al., 2020.; Pan et al., 2020.). More common clinical manifestations in patients infected with SARS-CoV-2 are fever, dyspnea, cough, myalgia, fatigue, sputum production, shortness of breath, sore throat, headache, decreased leukocytes count and radiographic lung with pneumonia. Besides, some patients presented gastrointestinal symptoms like diarrhea and vomiting. Overall, fever and respiratory complications are the most common symptoms of COVID-19 (Chen et al., 2019.; Ren et al., 2020). There were also patients who showed no respiratory symptoms but kidney failure, heart and liver injuries, as well as testicular involvement could lead to infertility in young patients (Huang et al., 2020.). Most of the adults and children showed mild flu-like symptoms without any serious complication (Yi et al., 2020).
The exact pathogenesis of SARS-CoV-2 is not fully understood owing to its novelty but the pathogenicity of SARS-CoV and MERS-CoV gives us a lot of clues. The main routes of transmission are contact and respiratory droplets, but recent studies have reported the existence of the virus in stool and urine samples of the patients, suggesting the fecal-oral way of transmission (Bellani et al., 2016.). In general, revealing the mechanisms of SARS-CoV-2 pathogenesis could pave the road for finding promising agents in combating COVID-19.
2.1. SARS-CoV-2 entry and replication
Some receptors and mediators have been found to be involved in facilitating the entrance of SARS-CoV-2. Among those, renin-angiotensin (Ang) system (RAS) and its regulator angiotensin-converting enzyme 2 (ACE2) seem to play critical roles. The RAS and ACE2 has shown an important role in maintaining proper physiological functions of kidneys, heart, and lungs. Any malfunction of this system may lead to the pathogenicity of these organs (Zou et al., 2020). ACE2 is most abundantly expressed in kidneys, heart, lungs, and endothelium (Donoghue et al., 2000). Moreover, ACE2 plays regulatory roles in the hemostasis of the local concentrations of Ang II and Ang1-7 in cardiovascular system (Michot et al., 2020). Ang II is a potent vasoconstrictor and pro-inflammatory agent which enhances fibrosis and is one of the main constituents of RAS. Besides, Ang I is the main substrate of ACE1 which is degraded to Ang II by a carboxypeptidase enzyme. Its carboxypeptidase activity is different from dipeptidase activity of ACE. Therefore, ACE inhibitors (ACEI) have no antagonizing effect on ACE2 (Yong et al., 2016). Virus entry into the cell is initiated by binding of S protein-ligand on the virus and ACE2 receptors on the tissue cell. The spike protein on the virus membrane binds to its receptor and direct membrane fusion of host cell and virus is the first step of viral entrance (Zhou et al., 2020). After virus entrance, its RNA genome and translation products including two polyprotein/structural proteins will be released. The first site of virus replication is epithelial cells of the upper respiratory tract and further proliferation occurs at the lower respiratory tract and gastrointestinal mucosa (Li et al., 2003). Besides, transmembrane serine protease type 2 (TMPRSS2) cleaves the S protein of the virus and exposes the fusion component of the virus. After this step, fusion of the virus envelope with the host cell membrane will be facilitated and virus entry takes place (McCreary et al., 2020). Besides, AP2 associated protein kinase 1 (AAK1) is one of the other regulators of SARS-CoV-2 endocytosis into the host cell. Several inflammatory receptors and downstream mediators, as well as ACE and AAK1 are also gateways involved in the pathogenesis of COVID-19 (Ziai et al., 2020). Thus, their targeting would be of great importance.
3. General therapeutics used in COVID-19
Several strategies are examined to manage SARS-CoV-2, including supportive care, and those targeting of several destructive pathways (Zhang et al., 2020b). From the therapeutic point of view, at first, agents like IFN-α, broad-spectrum antibiotics, and antivirals were used. Among the applied agents, just remdesivir alone or in combination with chloroquine or IFN-β showed satisfying results (Holshue et al., 2020). During a clinical trial in Shanghai, clinicians also used the plasma of recovered patients which yielded promising results (Ky and Mann, 2020). As previously described, several host factors are also involved in the pathogenesis of COVID-19. In this line, transmembrane serine protease type 2 (TMPRSS2) cleaves the S protein of the virus, thereby exposing the fusion component of the virus. Afterwards, fusion of the virus envelope with the host cell membrane will be facilitated and virus entry takes place. Based on the aforementioned role of TMPRSS2, this component could be considered a reasonable therapeutic target of serine protease inhibitors, like camostate mesylate (McCreary et al., 2020). Camostate mesylate has already shown promising efficacy in inhibiting the replication of influenza and parainfluenza viruses. The function of cysteine protease cathepsin L (which activates S protein) is pH-dependent. Hence, altering the endosomal pH could be used to inhibit the activity of cysteine protease beside the agents that directly block cathepsin L (Ky and Mann, 2020). Consequently, chloroquine and hydroxychloroquine (anti-malaria agents) have been used as antiviral agents by the ability to increase the endosomal pH via a rapid protonation inside the cell. Increased pH inhibits cathepsin to process S protein of the virus. These two agents also showed the potency to inhibit the proliferation of the novel virus in cultured cells, although hydroxychloroquine was more potent than chloroquine. In addition to suppressing the production and release of TNF-α and IL-6, chloroquine also inhibits autophagy. In a clinical trial, it was observed that viral clearance rate is significantly increased compared with the control group of patients not taking hydroxychloroquine (Gao et al., 2020). Based on another study, adding azithromycin to hydroxychloroquine therapy also increased the rate of virus elimination. It was proven that the combination therapy with chloroquine and remdesivir, inhibited the novel virus, in vitro (Wang et al., 2020). Remdesivir, a nucleoside analog, is one of the broad-spectrum antiviral agents. It inhibits viral RNA production by interfering with RNA polymerase (McCreary et al., 2020). First American COVID-19 patients were treated by remdesivir (Gordon et al., 2020). In the line of using antiviral agents, favipiravir is another antiviral drug that inhibits RNA polymerase of the virus. Favipiravir and remdesivir are both prodrugs that are converted to active metabolites in body. Although favipiravir is not FDA approved, studies have shown that it is more potent than lopinavir/ritonavir, as virus protease inhibitors (Du and Chen, 2020). On the other hand, a prompt decrease of viral load by adding anti-retroviral drugs avoided immunosuppression and macrophage activation syndrome (MAS) which is vital for disease treatment (Choy et al., 2020). Lopinavir in a fixed-dose combination with another protease inhibitor, ritonavir, is used for the treatment of HIV. In a clinical trial, combination of these two drugs as a 14-days regimen containing 400 mg lopinavir and 100 mg ritonavir (daily) was used. The viral load of COVID-19 in Korean patients following the use of lopinavir/ritonavir was significantly reduced (Kim et al., 2016). On the contrary, in a randomized, controlled, open-label trial in hospitalized adult patients with severe COVID-19, no benefit was attained with lopinavir/ritonavir beyond standard-care groups (Cao et al., 2020). Another protease inhibitor that could be added to ritonavir is darunavir, which is under investigation Chu et al., 2004; Gordon et al., 2020). Besides, in Shanghai Public Health Clinical Center, treatment with lopinavir/ritonavir, and arbidol in COVID-19 patients with pneumonia showed promising results (de Wilde et al., 2014). Abidol is one of the antiviral drugs that inhibits replication of virus. It is used as treatment and prophylaxis in SARS and MERS (McCreary et al., 2020). As another nucleoside analog, ribavirin is a prodrug similar to the two already mentioned drugs. The metabolites of this agent resemble adenosine and guanine nucleosides that merge with the virus RNA and prevent viral RNA replication. Ribavirin (FDA approved) in combination with peginterferon α-2a is used for the treatment of chronic hepatitis type C (McCreary et al., 2020).
Consistently, after viral infection, IFNs activate the innate immune system. There are two types of IFNs. Type 1 IFNs (α and β) are produced in almost all the cells in response to a viral infection and type 2 (γ) is produced by cells of the immune system after activation by antigens (Morgenstern et al., 2005). Both types of IFNs have shown antiviral effects through the inhibition of mononuclear macrophage-mediated pro-inflammation, reducing the immigration of the neutrophils to the site of inflammation, and blocking the activation of epithelial cells IFN-γ (Mustafa et al., 2018). IFN also exerts antiviral effects without overactivation of the immune system which is ideal for therapeutic purposes. Using IFNs at the early phase of the disease lowers the viral burden, hence reducing symptoms of the disease. However, the reduction in mortality has not been proven as the benefit of IFN therapy in COVID-19 (McCreary et al., 2020).
As another complication in COVID-19, thromboembolism could cause disseminated intravascular coagulation and denote a primary causes of death during COVID-19. Prevailing results suggest that thrombotic events in COVID-19 is in a near link with multiple dysregulated pathway of the coagulation system, including a significant increment of D-dimer. Cavalli et al. reported that the thromboembolic events and disseminated intravascular coagulation (DIC) provoked by SARS-CoV-2 may reveal a secondary anti-phospholipid antibody syndrome (Cavalli et al., 2020a). Fogarty et al. represented that using heparin in COVID-19 patients significantly reduced DIC (Fogarty et al., 2020) and lowered their mortality rate (Tang et al., 2020).
Besides the aforementioned strategies used for controlling COVID-19 and related pathogenic mechanisms, a deep appreciation of the inflammatory mechanisms responsible for the disease pathogenesis seems valuable with the aim of developing more efficient therapies.
4. Inflammatory pathways involved in the pathogenesis of COVID-19: advances in related anti-inflammatory treatments
Multiple dysregulated pathways are responsible for the pathogenesis of COVID-19, among which several inflammatory receptors/mediators/pathways, including receptor tyrosine kinases (RTK), growth factor receptors (GFRs), IL-6 receptor, IFN-γ, TNF-α receptor, TLR, JAK/STAT pathway, cytokine storm, macrophage activation and mammalian target of rapamycin (mTOR) play pivotal destructive roles.
4.1. Receptor tyrosine kinases and growth receptors
Essentially, viruses are parasites that use the intracellular machinery to live and proliferate. After the lysis of host cells viruses invade other neighboring cells (Walls et al., 2020). RTKs family are responsible for cell multiplication, apoptosis, and migration (Robinson et al., 2000). Enzyme activation is the first consequence of ligand binding to the extracellular part of the receptor. Following the activation of these receptors, the downstream signaling cascades will be triggered by the signaling molecules, resulting in the dysregulation of downstream events that finally lead to biological responses including cell growth, apoptosis inhibition, stimulation of angiogenesis, and cell motility (Yamaoka et al., 2018).
GFRs belonging to transmembrane proteins bind extracellular growth factors with a high affinity. Consequently, a chain of reactions takes place with the leading role of protein kinases that results in the growth modulation. GFRs have been noted as golden gates for some kinds of viruses, like coronaviruses to enter cells and take part in viral proliferation (Hondermarck et al., 2020). Currently, drugs that target GFRs and their downstream signaling pathways are used in the treatment of malignancies. They also could be of potential benefits in fighting viral infections but require more clinical trials to be approved (Hondermarck et al., 2020). In this line, heparan sulfate has been shown to play a crucial role in the activation of fibroblast growth factor receptors (FGFRs). Besides, it can be combined with proteoglycan complexes to form heparan sulfate proteoglycans (HSPGs) thereby creating entry points for virus entry into the cells (Madu et al., 2007). This mechanism of invasion was first observed in herpes simplex viruses (WuDunn and Spear, 1989). HSPGs employment to attach cell membrane is also proven for coronaviruses in several studies. Based on these data, it is also reasonable to assume this way of cell attachment for SARS-CoV-2 (Milewska et al., 2018).
In addition to GFRs, RTKs show the potential of being targeted by therapeutic agents in combating COVID-19. Blockade of tyrosine kinase activity inside the cells is achieved through applying tyrosine kinase inhibitors (TKIs) to shut down the downstream signaling pathways. First studies on these agents were conducted to survey their ability in targeting EGFR family. Therefore, they were initially used in oncology trials (Ciardiello and Tortora, 2008). Just to mention the names of those agents, first-generation EGFR TKIs, gefitinib and erlotinib, as well as second-generation EGFR TKIs, afatinib and dacomitinib have been widely used in the treatment of malignancies (Sim et al., 2018). Considering their close link with downstream inflammatory mediators, RTKs could be promising targets in fighting COVID-19.
Overall, the role of GFRs, RTKs, and the co-receptors like HSPGs in the entrance and proliferation of coronaviruses is undeniable and their targeting could envisage a bright future in combating COVID-19 (Hondermarck et al., 2020).
4.2. IL-6 receptor, and IFN-γ
IL-6 is a glycoprotein, secreted by various cells, including B and T cells, endothelial cells, monocytes, keratinocytes, and adipose cells. It plays a key role in many processes of the body like inflammation, synthesis of acute phase proteins, immune responses against antigens, and hematopoiesis (Hirano et al., 1986). IL-6 receptor is also expressed in many cells like B and T cells, monocytes, neutrophils, and hepatocytes. There are two types of IL-6 receptors (Hibi et al., 1990), including trans-membrane (mIL-6 receptor), which is located on the cell surface, and soluble (sIL-6 receptor) which exists in circulation and is synthesized either by the enzymatic cleavage of mIL-6 receptor through ADAM-17 (a disintegrin and metalloproteinase enzyme) activity or by alternative splicing (McGonagle et al., 2020). Due to their short cytoplasmic domains, glycoprotein 130 (gp130) is needed for proper functioning of these two types of receptors to transduce intracellular signals. After ligation of gp130 to IL-6/IL-6 receptor complex, it forms a high-affinity homo-dimer complex responsible for the activation of tyrosine kinases that result in the phosphorylation of transcription factors (Mihara et al., 2011). Soluble gp130 (sgp130) binds to IL-6–sIL-6 receptor complex and inhibits the binding of IL-6–sIL-6 receptor complex to membrane-bound gp130 (mgp130), thereby playing a key role as a natural inhibitor of the IL-6 signaling pathway (McGonagle et al., 2020).
Stimulation of the IL-6 receptor with IL-6 leads to the activation of JAK/STAT3 pathway. STAT3 enters the nucleus following activation. Controlling of STAT3 could be achieved via the inhibition of cytokine signaling components and inhibitors of STAT proteins (Martens et al., 2000). Another signaling pathway governed by IL-6 is phosphoinositol-3 kinase (PI3K)/protein kinase B (PKB)/Akt. Activation of PI3K is performed by JAK, after which the phosphorylated PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3). Then, PIP3 phosphorylates PKB/Akt serine/threonine kinase, as a modulator of cell survival genes expression (Narazaki et al., 1993).
Viral infection results in increased levels of IL-6 and elimination of the inhibitory sources of IL-6. IL-6 dysregulation is also observed in many autoimmune and chronic inflammatory diseases. In the presence of any inflammatory damage, synthesized IL-6 is transported to the liver and causes extensive production of acute phase proteins like CRP, serum amyloid A, fibrinogen, haptoglobin, and a1-antichymotrypsin. IL-6 also regulates iron and zinc transporters (Bettelli et al., 2006.). By inducing hepcidin which is an inhibitor of the iron transporter, IL-6 exerts its effects on chronic inflammation associated hypoferremia. Inflammation-induced hypozincemia is due to the over expression of zinc importer ZIP14 on hepatocytes. Hematopoietic and platelet-producing effects of IL-6 is used as a diagnostic tool to assess inflammation severity. Additionally, IL-6 bridges innate and acquired immunity by inducing differentiation of naive CD4+ T cells to effector helper T cells, CD8+ T cells into cytotoxic T cells, and activated B cells to plasma cells (B-cell differentiation) (Scheller et al., 2006).
It was shown that after IL-6 injection, owing to its myelopoiesis activity, causes neutrophilia in a biphasic pattern. The first peak was observed at 1.5 h after administration and second peak occurred following 4–12 h. Based on this information and the knowledge on the overexpression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecules (ICAM-1) in inflamed sites and endothelial cells, blockade of IL-6 leads to the inhibition and controlling of inflammation and preventing associated deteriorating effects (Okabayashi et al., 2006).
The uncontrolled and sudden burst of inflammatory responses in severe patients, along with lack of any proven medication or vaccine, sparkled the idea of anti-inflammatory treatment to mitigate overwhelming immune responses such as MAS and secondary haemophagocytic lymphohistocytosis (sHLH). During MAS, macrophages produce IL-6 that could be controlled by therapeutic interventions (Ye et al., 2020). Since IL-6 is a key player of inflammation, its inhibition seems to be an important step in ameliorating COVID-19 inflammatory cascades/complications (Lee et al., 2014). A meta-analysis showed 2.9-fold higher levels of IL-6 in compromised COVID-19 patients in comparison to uncompromised patients confirming the benefit of targeting IL-6 for therapeutic aims (Jones et al., 2005; McCreary et al., 2020). The major inflamed organ is lung rather than other organs. IL-6 plays a key role in lungs immunopathogenesis and leads to acute respiratory distress syndrome (ARDS). Any doubt about IL-6 involvement in the COVID-19 associated MAS can be answered by presence of the aforementioned biomarkers (Lu, 2019). High levels of other inflammatory biomarkers like CRP and ferritin are also among common features of COVID-19 patients. In patients with MAS/HLH, the levels of these markers raised similar to what is observed in patients with COVID-19 pneumonia. Another similarity between COVID-19 pneumonia and MAS/HLH is increased levels of cytokines, including TNF, IL-1β,2,6,8,17 and CCL2 (de Wit et al., 2016). These common features of COVID-19 pneumonia and MAS/HLH enforced the idea of immunosuppressive therapeutic strategies for COVID-19 pneumonia. Besides, previous studies have shown the efficacy of IL-6 antagonist therapy in patients suffering from SARS coronavirus (Scheller et al., 2006). Patients with all types of ARDS exhibited high levels of IL-6 with a poor survival rate. As augmented IL-6 level is a common feature in ARDS, MAS, and HLH, and cannot be considered as a reliable marker for differential diagnosis in COVID-19 pneumonia. In patients with COVID-19 pneumonia, severe ARDS and lymphopenia-induced immunosuppression with profound down-regulation of IFNs are present. After any irritation of the lung tissue by viral infections or chemical irritants, IL-6 takes part in lung remodeling. This feature of IL-6 is a key point to be taken into consideration for defining the exact timing of anti-IL-6 therapy. On the other hand, a prompt decrease of viral load by anti-retroviral drugs prevented immunosuppression and MAS, which are vital in the treatment course (Kennedy et al., 2014). According to the current data, it is not possible to precisely clarify whether raised levels of IL-6 are beneficial or harmful. The dual function of IL-6 in suppressing or facilitating virus replication necessitates more research to exactly determine IL-6 effects in COVID-19. A recent study showed that early administration of anti-IL-6 may decrease the clearance of the virus and conducting further clinical trials to assess the proper timing of medication are mandatory (Mihara et al., 2011).
Tocilizumab (TCZ) is a recombinant anti-human IL-6 monoclonal antibody. It binds to IL-6 (soluble and membrane-bound) and hence blocks the related downstream signaling pathways. Its main indication is for rheumatic diseases like rheumatoid arthritis and cytokine storm in patients treated with chimeric antigen receptor (CAR) T cell therapy (McCreary et al., 2020; Tanaka et al., 2016). Accordingly, a study was carried out on the efficacy of TCZ in the management of critical patients infected with SARS-CoV-2. In their study, 20 patients were taken 400 mg of TCZ (i.v. Once daily). After a few days, fever and other symptoms vanished, oxygenation improved in 75% of patients, lung CT scans of 90% of patients got better, and 52.6% of patients experienced return of peripheral lymphocyte counts to normal. So, the results indicated a good therapeutic response in COVID-19 patients treated with TCZ (Fu et al., 2020). Additionally, sarilumab is a human monoclonal antibody against IL-6 with a labeled indication for rheumatoid arthritis. This antibody has also been evaluated in the treatment of COVID-19 (McCreary et al., 2020). As a humanized murine chimeric monoclonal antibody which directly binds IL-6, siltuximab is used for the treatment of cytokine storm, although it has not hitherto received FDA approval. It has been studied as an option in COVID-19 patients (Chen et al., 2020).
Due to the major role IL-6 plays in inflammatory responses, it could be considered a reasonable target for the treatment of COVID-19 patients. Although the role of IL-6 in inflammation associated pathogenesis is more focused, the roles of other cytokines including IFN-γ, IL-18, IL-1α, IL-1β and their related pathways should be also taken into consideration (Ye et al., 2020). Canakinumab is a monoclonal antibody against human IL-1β that is used in MAS (Ruperto et al., 2012). Besides, rilonacep has been also used in MAS which neutralizes IL-1β and IL-1α (Ilowite et al., 2014). In this regard, taking alfa, as a recombinant human IL-18-binding protein, has also shown beneficial results in MAS (Canna et al., 2017.).
In addition to the critical role of ILs in COVID-19, IFN-γ seems to be another key mediator. IFN-γ is another player of immune and inflammatory responses with dual roles in viral complications. Receptors of IFN-γ are expressed on both malignant and non-malignant cells. Following the activation of IFN-γ receptors, JAK1 and JAK2 are activated, leading to homodimerization of STAT1, nuclear translocation, and activation of various genes. Its role in combating pathogens, including viruses, has been also established (Davidson et al., 2016). Increased levels of IFN-γ in COVID-19 patients with ARDS have also been reported. In addition to its beneficial effects as a protective agent in COVID-19, it has also been attributed to deteriorating effects with respect to cytokine storm and induction of IL-6 production. Accordingly, trials on using anti-IFN-γ emapalumab in combination with the IL-1 receptor antagonist anakinra for COVID-19 is ongoing (Lu). Additionally, ponatinib by inhibiting breakpoint cluster region-Abelson kinase and regulating type I IFNs, is in the preclinical stage to counteract cytokine storms in influenza infection . Consistently, emapalumab, an antibody that binds to soluble and receptor-bound forms of IFN-γ, is approved for HLH treatment (Locatelli et al., 2020).
In general, antagonizing inflammatory ILs and modulating IFN-γ could be of potential importance in reducing SARS-CoV-2 complication and suppressing COVID-19.
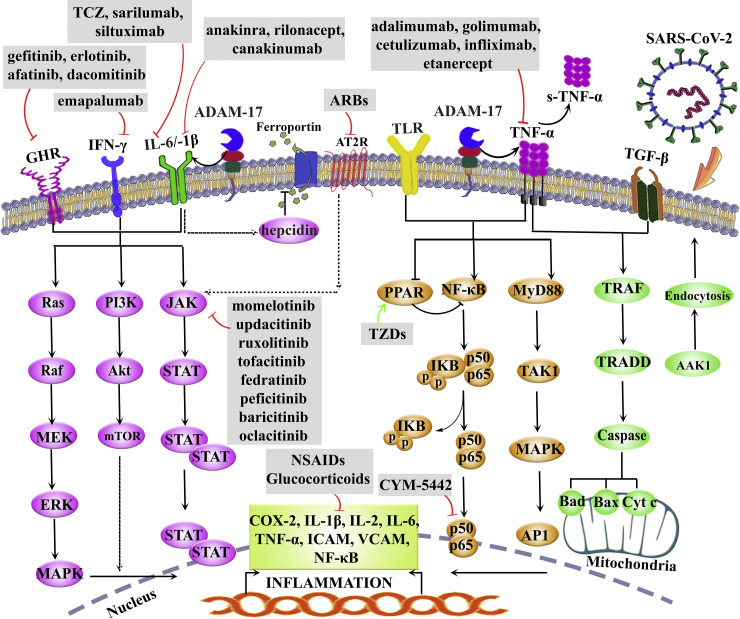
Fig. 1 shows the dysregulated inflammatory pathways in COVID-19, as well as selective anti-inflammatory drugs.
Fig. 1.
Selected dysregulated inflammatory pathways in COVID-19: Selective anti-inflammatory drugs. AAK1: AP2 associated protein kinase 1, ADAM: a disintegrin and metalloproteinase, AP1: activator protein 1, ARBs: angiotensin II receptor blocker, AT2R: angiotensin II receptor, COX: cyclooxygenase, Cyt c: cytochrome c, GHR: growth hormone receptor, ICAM: intercellular adhesion molecules, IL: interleukin, IFN: interferon, JAK: Janus kinase, mTOR: mammalian target of rapamycin, MAPK: mitogen-activated protein kinase, NF-κB: nuclear factor-κB, PI3K: phosphoinositol-3 kinase, PPARs: peroxisome proliferative activator receptors, STAT: signal transducer and activator of transcription, TCZ: tocilizumab, TGF-β: transforming growth factor-β, TNF-α: tumor necrosis factor-alpha, TLR: toll-like receptor, TZDs: thiazolidinediones, VCAM: vascular cell adhesion molecule.
4.3. TNF-α receptor and TLR
Growing evidence is focusing on the importance of TNF-α receptor, and TLRs in inflammatory cascades. In this regard, TNF-α plays key roles in immune responses and tissue homeostasis. Despite the role of TNF-α signaling in combating viral infections, high levels of TNF-α in some diseases like influenza and COVID-19 are associated with lung injuries. Studies have demonstrated that high levels of TNF-α are observed in plasma and alveolar fluid lavage of patients with ARDS. Elevated levels of cytokines lead to increased endothelial permeability and decreased alveolar fluid clearance caused by down-regulation of sodium channels in the epithelium (), and the main sources of TNF-α are activated monocytes, fibroblasts, and endothelial cells. The initial form of TNF-α is a membrane protein which turns into a soluble form via ADAM17 (Ma et al., 2020).
Several monoclonal antibodies against TNF-α are recommended for the treatment of COVID-19. Infliximab is a TNF-α antibody with the main indication in inflammatory bowel diseases, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis (Qiu et al., 2013). Etanercept is a TNF-α receptor blocker fused to immunoglobulin G (IgG) Fc, which is indicated for the treatment of ankylosing spondylitis, rheumatoid arthritis, plaque psoriasis and psoriatic arthritis (Chadwick et al., 2018; Murdaca et al., 2018).
As other anti-TNF-α antibodies, adalimumab and golimumab were indicated for the treatment of rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, psoriatic arthritis and plaque psoriasis (Choquette et al., 2019). Certulizumab pegol is another anti-TNF-α antibody conjugated to a 40 kDa polyethylene glycol indicated for decreasing symptoms and signs in Crohn’s disease and the treatment of ankylosing spondylitis, rheumatoid arthritis and psoriatic arthritis (Felsenstein et al., 2020; Tosi et al., 2019). Along with the beneficial role of anti-TNF-α agents, there are also some concerns regarding their usage. In China, studies have demonstrated limited benefits from anti-TNF-α therapy in the management of SARS-CoV-2 infected patients which underscores targeting other alternative pathways like TNF-α downstream signaling pathways as potential therapeutic options (Channappanavar et al., 2016; Ma et al., 2020; McDermott et al., 2016). Of other concerns related to using anti-TNF-α agents is secondary infections caused by bacterial and fungal germs. Thus, patients with latent tuberculosis are not recommended to take such medications (Channappanavar and Perlman, 2017; Ma et al., 2020; McDermott et al.).
Among other compounds with inhibitory effects on TNF-α, ulinastatin, as a glycoprotein naturally occurring in the body inhibits the release of the pro-inflammatory mediators such as TNF-α, IL-6, and IFN-γ, while increasing levels of the anti-inflammatory cytokine IL-10, thereby protecting the vascular system. Its main clinical use is in pancreatitis and circulatory failure. The advantage of ulinastatin usage over glucocorticoids is that it doesn’t suppress the immune system, hence it can be widely used in COVID-19 patients (Ju et al., 2019). But currently, it is not included in the treatment of COVID-19 patients and studies are going on (Ye et al., 2020).
In addition to the critical role of TNF-α in COVID-19 complications, TLRs have been shown to play key roles, as well. TLRs play an important role in the induction of immune responses through recognizing PAMPs. They are present in a wide range of creatures from primitive nematodes to the most advanced creatures namely human being (Vijay, 2018). TLRs are PRRs with critical roles in the initiation of the innate immune response through recognizing potentially harmful pathogens. PRRs initiate multiple signaling pathways in the host cell. TLRs signaling trigger the release of IFNs, chemokines, and host defense peptides leading to macrophages and neutrophils activation (Medzhitov et al., 1997). TLRs dysregulation, which causes the secretion of cytokines and chemokines, occurs in autoimmune disorders. From the therapeutic view, TLRs could be targeted in two ways. The first is applying TLRs agonists that bind TLRs and activate them hence acting like a vaccine. Another way is the inhibition of TLRs by specific antibodies. But, so far not enough research has been carried out on TLRs targeted therapeutic modalities in COVID-19 patients and studies are going on (Arora et al., 2019). We have previously shown the role of TLR modulators in the prevention/treatment of viral diseases (Pour et al., 2019). Consistently, diacerein, a slow-acting medicine of the anthraquinone class for joint diseases, has been hypothesized to be a potent multi-target agent through various mechanisms, including TLR-4 suppression (de Oliveira et al., 2020). Consequently, TAK-242 was a TLR-4 non-competitive inhibitor (Ii et al., 2006) in a phase III clinical trial on sepsis patients; however, it was failed (Rice et al., 2010). Besides, calixarene (Saluja and Sekhon, 2013), alpinetin (He et al., 2016), ferulic acid (Rehman et al., 2019), some opioid derivatives (Northcutt et al., 2015), as well as anionic and cationic monosaccharide-based (Peri and Romerio, 2020), small molecule inhibitors (e.g. statins), antibodies (e.g. NI-0101 and 1A6), lipid A analog (e.g. Eritoran), and miRNAs are other TLR-4 antagonists have been explored in various experimental and clinical phases (Gao et al., 2017). Amongst other TLR-4 inhibitors, VGX-1027 has shown modulatory effects on cytokine synthesis with preventive potential on autoimmune diseases in murine models. It modulated the production of IL-1β, TNF-α and IL-10 in response to stimuli activating TLRs. In this line, a reduced activation of nuclear factor κB (NF-κB) and p38 mitogen-activated protein kinase (p38MAPK) pathways along with increased extracellular signal-regulated kinase (ERK) were also demonstrated (Stojanovic et al., 2007). From the therapeutic point of view, VGX-1027 is a safe and generally well tolerated agent with no toxicological concern towards clinical development (Lee et al., 2016). Altogether, the modulators of TLRs and TNF-α could pave the way in combating inflammatory complications of COVID-19.
4.4. JAK/STAT pathway
In addition to importance of the aforementioned receptors/mediators in the pathogenesis of COVID-19, blockade of their downstream signaling pathway (i.e., JAK/STAT) could also be of great value. The mechanism of action includes tyrosine phosphorylation of cytoplasmic domains of gp130 by JAK1 and STAT3. Several pro-inflammatory cytokines can activate JAK/STAT signaling pathways which are upregulated in COVID-19 patients (Yamaoka et al., 2018).
Based on the aforementioned mediators, JAK inhibitors are being tried to treat COVID-19 inflammatory complications. The efficacy/potential of using JAK inhibitors comes from this fact that, SARS-CoV-2 targets immune cells using cytokine receptors, and downstream JAK/STAT signaling pathway. In this line, phase III clinical trial of using ruxolitinib (NCT04120090, NCT03533790) and phase II of fedratinib (Wu and Yang, 2020) are going on in the treatment of patients with pneumonia associated COVID-19. Agents with desired selectivity for JAK/STAT pathway include peficitinib, upadacitinib, fedratanib and tofacitinib (Seif et al., 2020).
Since the JAK/STAT pathway is present in the tissues possessing Ang I receptors and immune cells, and respond to cytokine through their receptors, the idea of manipulating this pathway by JAK inhibitors as a strategy for managing COVID-19 hospitalized patients sounds good. JAK/STAT signaling pathway is the main pathway through which Ang II functions and causes vasoconstriction, chronic injuries and hypertension. Ang II exerts its cardiovascular effects by Ang I receptor, suggesting the therapeutic potential of Ang I receptor blockers (ARBs) in COVID-19 patients although contraindications should be considered when using these agents (Liu et al., 2020c). JAK/STAT pathway mediating Ang II functions is present in the cardiovascular system, proximal tubular epithelial cells of kidney, astrocytes of brainstem, hepatocytes and mesangial cells. In this way, JAK2, which is activated by Ang I receptor, activates one of the STAT family members. (i.e., STAT1, 2, 3, 4, 5A, 5B, and 6). A study showed that the use of ARBs in elderly patients (age > 65) with hypertension, caused a lower incidence of respiratory complications (Zou et al., 2020).
Some biological agents capable of blocking related upstream inflammatory pathways are being explored in the way of counteracting different inflammatory complications of COVID-19 (Seif et al., 2020). Tofacitinib, which is an orally-administered medication, blocks JAK1/2/3 and has been approved by FDA for arthritis rheumatoid (Seif et al., 2017). Besides, some cytokines like IL-2, IL-7, and IL-6 which use JAK3 as a signaling mediator, can be blocked effectively by tofacitinib (Lee et al., 2014). Until now there hasn’t been any report declaring the use of tofacitinib in the treatment of COVID-19 .
Baricitinib, as a selective JAK1/2 blocker, is used in arthritis rheumatoid. It also inhibits AAK1 (an endocytosis regulator) to serve as a potential medication of choice in treating COVID-19 patients. To reach the plasma concentration of therapeutic effect, 2–4 mg once daily dosage of baricitinib was sufficient for COVID-19 patients (Cantini et al., 2020.). Thus, blockade of this pathway in COVID-19 patients can be curative and the recent trials exploiting baricitinib in the treatment of ARDS in COVID-19 patients have shown promising results (Liu et al., 2020). AAK1 is one of the regulators of SARS-CoV-2 endocytosis into the host cell, in parallel to JAK/STAT pathway. Therefore, intervening the process of endocytosis by AAK1 inhibitors could be a way of combating COVID-19 (Cron and Chatham, 2020), as baricitinib does (Stebbing et al., 2020). Besides, combination therapy of baricitinib with antivirals like lopinavir or ritonavir and remdesivir have shown a decreased rate of viral infection with a lower incidence of uncontrolled immune responses (Pardanani et al., 2015). Other JAK1/2 inhibitors, including ruxolitinib, momelotinib, and oclacitinib have also shown potentials to be used in COVID-19 with ongoing research (Furqan et al., 2013).
Despite the advantages of their use, one of the disadvantages of JAK inhibition is the blockade of type 1 and type 2 IFNs which play remarkable beneficial roles in downregulating virus activity (Cron and Chatham, 2020; Li et al., 2020). Other JAK inhibitors adverse effects that should be concerned are generally increased risk of viral infections, lower gastrointestinal complications, anemia and leukopenia (Al-Qahtani et al., 2017).
So, more evidence is warranted to corroborate the clinical use of JAK inhibitors. As a result, JAK/STAT could be regarded as a golden target in combating COVID-19 inflammatory complications.
4.5. Cytokines and cytokine storm
Viral pneumonia caused by SARS-CoV-2 leads to ARDS, which is one of the main concerns in dealing with this novel virus. Virus-induced ARDS accompanied by hyper-inflammation, triggers the idea of using anti-cytokine therapy in the way of treating infected patients (Cameron et al., 2008.). The most common immunopathological feature of patients infected with SARS-CoV-2 is cytokine storm-mediated ARDS (Channappanavar et al., 2016). Cytokine storm is characterized by the sudden release of large amounts of pro-inflammatory factors (IFN-α, IFN-γ, IL-1β, -6, -12, -18, -33, TNF-α, TGF-β, etc.), chemokines (CCL2, 3, 5, and CXCL8, 9, 10, etc.), and poor counteracting control mechanisms of the body. This sudden release causes damages to the host organs by releasing free radicals (Huang et al., 2020), leading to multiple organ failure and severe ARDS (Gao et al., 2015). Patients with COVID-19 experience raised levels of IL-1β, IFN-γ, IP-10, MCP-1 and macrophage migration inhibitory factor, leading to the stimulation of T helper type 1 cells. The aforementioned pro-inflammatory cytokines not only are responsible for multi-organ damages but are also in a near link with psychiatric complications such as depression (Petralia et al., 2020a, 2020b) that is often observed in COVID-19 infected patients. Such studies pave the roads in providing emerging tailored therapeutic approaches in treating COVID-19. However, cytokines like IL-4 and IL-10, as anti-inflammatory cytokines secreted by T helper type 2 cells, have also been detected in COVID-19 patients. The correlation of IL-2 receptor and IL-6 levels and the severity of the disease in COVID-19 patients have been proved recently Chen et al., 2020). The levels of G-CSF, IP-10, MCP-1, MIP-1α and TNF-α are elevated/dysregulated in ICU patients in comparison with general ward patients underlying the relationships between cytokine storm and severity of the illness, mortality, and multiple organ failure (Huang et al., 2020). Higher levels of cytokines and chemokines in MERS patients were also shown to be associated with high numbers of neutrophils and monocytes in the lung tissues and peripheral blood giving rise to lung pathogenesis (Kim et al., 2016).
All of the above-mentioned consequences of the cytokine storm triggers inflammatory processes making anti-inflammatory therapeutic interventions reasonable choices to avoid disease exacerbation. It was shown that few patients who did not receive non-neutralizing antibodies against cytokine storm had persistent devastating inflammation, ARDS, and even death while other patients could survive (Zhu et al., 2020). Anti-inflammatory treatment choices could be non-steroidal anti-inflammatory drugs (NSAIDs), statins (Kruger et al., 2013), glucocorticoids, chloroquine/hydroxyl chloroquine, immunosuppressant agents, antagonists of inflammatory cytokines such as anti-IL-6 monoclonal antibodies, inhibitors of TNF-α, antagonists of IL-1, JAK and regulation of T-cell mediated responses (Yi et al., 2020). Among the candidates, selective cyclooxygenase-2 (COX-2) inhibitors, and NSAIDs showed pro-inflammatory effects in viral infections. In this regard, celecoxib in combination with oseltamivir is used in phase III clinical trial for influenza A infection (Capuano et al., 2020.). There are studies suggesting the use of anti-inflammatory medications before the initiation of cytokine storm and overwhelming inflammatory cascades in COVID-19. Thus, using corticosteroids and cytokine inhibitors such as TCZ (an IL-6 inhibitor) and anakinra (IL-1 receptor antagonist) might be appropriate options in COVID-19. Additionally, modulation of a hyper-inflammatory state can be achieved by using intravenous immune globulins (IVIG). It has been shown that IVIG administration as adjuvant treatment for COVID-19 ICU patients with pneumonia reduced the use of mechanical ventilation, hospital/ICU length of stay, and mortality of patients (Xie et al., 2020).
When patients go through storm stage, recovery will be difficult and many efforts should be made to avoid the starting of cytokine storm (Tanaka et al., 2014). In this line, etoposide by selective deletion of T cells and effectual suppression of pro-inflammatory cytokines is widely used for HLH in co-administration with cyclosporine and corticosteroids (Bergsten et al., 2017.).
Peroxisome proliferative activator receptors (PPARs) are transcription factors belonging to the ligand-activated nuclear hormone receptors family that have shown the ability to regulate inflammation (Al-Qahtani et al., 2017.). They play crucial roles in the metabolism of lipid/glucose, cell multiplication/differentiation/migration, malignancy, atherosclerosis, and vascular remodeling. Therefore, controlling inflammation by activating PPARs seems to be an option in coping with cytokine storm. There are three subtypes of PPARs, including α, β/δ, and γ (Berger and Moller, 2002). Among these subtypes, PPARγ agonists (pioglitazone and rosiglitazone) as members of thiazolidinediones (TZDs) are the best choices to be used accordingly. The main approved clinical use of TZDs are for diabetes mellitus type 2. Natural sources and foods enriched with PPAR agonists can be used as modulators of inflammatory responses (Ciavarella et al., 2020; Russell et al., 2020), with possible potentials for use against the cytokine storm in COVID-19.
As another modulator of inflammation, sphingosine-1-phosphate (S1P) which is a signaling sphingolipid, also known as lysosphingolipid, is involved in the stimulation, synthesis and release of cytokines. Since SARS-CoV-2 mainly evades the lungs epithelial/endothelial cells, S1P could be a good target in the treatment of COVID-19 through reducing cytokine responses. The drug belonging to this category is siponimod approved to be used in multiple sclerosis. Further investigations are needed to provide enough evidence for its use in SARS-CoV-2 infection (Cheng et al., 2015; Ye et al., 2020).
The main concern of using anti-inflammatory medications is putting the patient at the risk of secondary infections particularly in immuno-compromised patients causing the virus to last for a longer time in the patient's body (Ciavarella et al., 2020). Other disadvantage of using steroids, in addition to the latter, is causing nonvascular osteonecrosis which makes the prognosis poorer. High doses of steroids were not beneficial in severe respiratory compromised patients with SARS and SARS-CoV-2 but low to moderate doses and short duration of therapy were found to be helpful in COVID-19 patients (Mehta et al., 2020). In general, fighting pathogens without the involvement of inflammation as an immune system response is unimaginable. The battle starts with pathogen detection and immune cells recruitment to battlefield for the elimination of invader and repairing the left damages (Ye et al.). During cytokine storm, there are so many cytokines participating in the inflammation and using a medication with the capability of blocking an individual cytokine can’t avoid the overall devastating result of the storm. On such a basis, employing multi-target blockers looks instrumental.
So, it is important to subjugate the unleashed storm while modulating the immune system positively (Ciavarella et al., 2020).
4.6. Macrophage activation
When infection occurs with a RNA virus, TLR-7 stimulation leads to pro-inflammatory responses of mononuclear macrophages. According to the high number of mononuclear macrophages (MNP) in inflammation sites, the idea of using TLR-7 antagonists seems to be promising (Xu et al., 2020). It has been shown that alveolar macrophages in patients suffering from ARDS exert their pathological effects through agents, including proteases, reactive oxygen species (ROS), eicosanoids, phospholipids and cytokines such as IL-1, IL-6 and TNF-α (Arnaldez et al., 2020.). MNPs and pro-inflammatory molecules are more observed in severe patients in comparison to mild patients (Mehta et al., 2020). Composition of the MNPs in severe patients revealed lower counts of resident macrophages and higher numbers of pro-inflammatory monocyte-derived macrophages. Macrophages present in alveolar fluid samples showed the features of fibrosis promotion similar to macrophages involved in liver cirrhosis. This observation guided researchers to the lung pathogenesis management afforded by proper management of immune responses via macrophages (Bracaglia et al., 2017.). Immunostaining of samples taken from dead COVID-19 patients indicated that CD169+ macrophages expressed ACE2 receptor and showed the evidence of virus infection as they were positive for SARS-CoV-2 nucleoproteins. Expression of the ACE2 receptor on the macrophages may be induced by inflammatory signals through type I IFN or other receptors like CD147 that could take part in virus entry into the cell. Since now it is unclear whether the presence of the nucleoproteins in macrophages is the result of their direct infection or is due to engulfment of virus-infected cells. Prolonged activation of recruited monocytes and monocyte-derived macrophages may induce type I IFN, oxidative stress, anti-spike IgG immune complexes, as well as NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation (Kim and Nam, 2020; McGonagle et al., 2020). Virus infected macrophages expressed IL-6 and lymphocytes were severely depleted in spleen and lymph nodes. On the other hand, transcriptomic analysis confirmed the preferential activation of B lymphocytes in the lungs of patients with COVID-19 (Cavalli et al., 2020). Other organs of the COVID-19 patients also showed the existence of macrophages like CD68+ cells in kidneys that were associated with tubular damage. Other organs that may be injured throughout this disease are liver, spleen (with necrosis), lymph nodes (with focal necrosis), and heart ( Stunault et al., 2018).
Researchers should go on to comprehend the exact cause of monocyte and macrophage activation, as well as so many other factors contributing to this phenomenon. It isn’t still clearly established that cytokines are drivers of macrophage activation or viral infection/detection is the cause. It has also been found that prior infections before SARS-CoV-2 may have roles in monocytes/macrophage activation. The tissue inflammatory responses are also important. In this regard, balancing between the presence of tissue-resident macrophages and monocyte-derived macrophages is also determinant in combating COVID-19 (Abd El-Aziz and Stockand, 2020; Stunault et al., 2018).
4.7. Mammalian target of rapamycin inhibition
Preventing the dysregulation of mTOR handles the metabolism, proliferation, growth, and survival of diverse immune cells during the migration and expression of myeloid immune cells and cytokine. Administration of mTOR inhibitors seems to enhance the T-cell stimulatory activity of dendritic cells, promotes the autophagy of macrophage, blocks murine B-cell proliferation, and suppresses early B-cell production in germinal centers, also enhances the magnitude and quality of viral specific CD8+ T-cell responses to vaccination in macaques, thereby play important roles in the prevention/treatment of COVID-19 complications (Zheng et al., 2020).
On the other hand, in an in vitro study, rapamycin (a mTOR inhibitor) inhibited the replication of MERS-CoV (Kindrachuk et al., 2015). Accordingly, adjuvant treatment with mTOR inhibitors improve the outcome in ICU patients affected by H1N1 influenza virus (Wang et al., 2014). Additionally, mTOR has also been introduced as a multifunctional target in combating HIV (Nicoletti et al., 2011). In this line, rapamycin showed a powerful antiviral effect against R5 strains of HIV, in vivo (Nicoletti et al., 2009). In another study, the inhibition of mTOR suppressed multiple steps of HIV life cycle, including reverse transcription, integrase and protease (Heredia et al., 2015). In recent studies, mTOR inhibitors have been also introduced as promising candidates in combating SARS-CoV-2 (Fagone et al., 2020; Ramaiah, 2020).
Considering the role of mTOR inhibitors in limiting the proliferation of memory B cells and T-cell responses, patients treated with mTOR inhibitors are expected to a reduced early production of cross-reactive antibody, less antibody-dependent enhancement and fewer severe symptoms in COVID-19. Such promising results could draw a bright future for mTOR inhibitors in combating COVID-19.
Table 1 shows the mechanistic based use of drugs in COVID-19.
Table 1.
Selected drugs used in COVID-19 for counteracting inflammatory pathways/mediators.
| Drug | Clinical phase | Mechanism | References |
|---|---|---|---|
| Tocilizumab | phase IV for SARS-CoV-2 (ChiCTR2000029765, NCT04310228, NCT04315480, NCT04317092), Phase II for GvHD (NCT02206035, NCT04070781, NCT03434730, NCT03699631) |
human monoclonal anti-IL-6 receptor antibody | De Benedetti et al. (2012).; Kennedy et al. (2014); Xu et al. (2020) |
| Sarilumab | phase II/III study for hospitalized patients with COVID-19 | anti-IL-6 antibody | Zhang et al. (2020a) |
| Siltuximab | patients requiring ICU admission at 29 days |
anti-IL-6 antibody | Chen et al. (2016). |
| Emapalumab | approval for primary HLH | anti-IFN-γ antibody | Al-Salama (2019) |
| Anakinra | phase I for MAS (NCT02780583), phase II for MAS and sepsis (NCT03332225) | IL-1 receptor antagonist, blocking IL-1α and IL-1β |
|
| Infliximab | phase II for HLH, Phase I/II for GvHD (NCT00228839, NCT00228839, NCT00201799), Phase IV for GvHD in combination with daclizumab |
human monoclonal anti-TNF-α antibody | Henzan et al. (2006).; Neurath (2020) |
| Etanercept | Phase II/III for GvHD (NCT00726375, NCT00141739 NCT00141713, NCT00224874, ChiCTR1900024408) | competitively inhibiting TNF-α | Kitko et al. (2016) |
| Adalimumab | not available | anti-TNF-α antibody | Sanchez-Piedra et al. (2020) |
| Certulizumab | not available | anti-TNF-α antibody | Feldmann et al. (2020) |
| Golimumab | not available | anti-TNF-α antibody | Feldmann et al. (2020) |
| Ruxolitinib | phase III for HLH (NCT04120090, NCT03533790), Phase IV for GvHD (ChiCTR1900024408) | JAK inhibitor | Goker Bagca and Biray Avci (2020); Spinelli et al. (2020) |
| Fedratinib | not available | JAK inhibitor | Di Lorenzo et al. (2020) |
| Ruxolitinib | not available | JAK inhibitor | Bergsten et al. (2017). |
| Peficitinib | not available | JAK inhibitor | Bracaglia et al. (2017). |
| Upadacitinib | not available | JAK inhibitor | Luo et al. (2020) |
| Fedratanib | not available | JAK inhibitors | |
| Tofacitinib | preclinical for GvHD | JAK inhibitors | Okiyama et al. (2014) |
| Baricitinib | phase IV for SARS-CoV-2 severe pneumonia | selective JAK1/2 blocker | |
| Bevacizumab | clinical trial in China for COVID-19 (NCT04275414) | humanized monoclonal antibody targeting VEGF | Park et al. (2010); Zhang et al. (2020a),Ko et al. (2019) |
| Corticosteroids | phase II/III for SARS-CoV-2 severe pneumonia (NCT04263402, ChiCTR2000029386, ChiCTR2000029656) | dampen pro-inflammatory cytokines and possess antifibrotic property | Rochwerg et al. (2018); Zha et al. (2020)) |
| IVIG | phase II/III for SARS-CoV-2 (NCT04261426) | provides passive immunity and anti-inflammatory effects | Alijotas-Reig et al. (2020); Emmenegger et al. (2001) |
| IFNs | phase II/III for SARS-CoV-2 diagnosis during 28 days |
suppress virus replication | Zhang et al., (2020a) |
GvHD: graft versus host disease, HLH: hemophagocytic lymphohistiocytosis, IFNγ: interferon gamma, IL: interleukin, MAS: macrophage activation syndrome, TNF-α: tumor necrosis factor-alpha, VEGF: vascular endothelial growth factor, VIG: vaccinia immune globulin.
5. Conclusion
Multiple destructive signaling pathways are implicated in COVID-19 pathogenesis. Although a low level of inflammation helps toward a successful elimination of pathogens, SARS-CoV-2 utilizes overactivated inflammatory pathways leading to multiple-organ damages, physiological deterioration and eventually death. In this line, identifying the precise inflammatory pathways and therapeutic targets in COVID-19 is of high value. Growing evidence has shown the critical role of IL-6, IL-1β receptor, IFN-γ, TNF-α, TLR, JAK/STAT, RTKs and growth factor receptors in the pathogenesis of COVID-19. In the current study, the results of targeting the aforementioned inflammatory pathways in COVID-19 patients have been reviewed. Besides, we also revealed several interconnected dysregulated pathways, including PI3K/Akt/mTOR, Ras/Raf/MEK/ERK, and TRAF/TRADD/caspase as promising future targets in fighting against COVID-19.
Considering the controversial results obtained in the treatment of COVID-19, further in-depth investigations are waiting to unravel novel SARS-CoV-2 related therapeutic targets and signaling pathways to be employed for the prevention and treatment of COVID-19. Additional studies are still required to decipher more critical inflammatory pathways triggered by SARS-CoV-2.
Author contributions
Conceptualization, S.F., and H.K.; Software, S.F., drafting the manuscript, A.Y., M.Y., and S.F.; review and editing the paper: A.Y., M.Y., S.F., and H.K; revising: A.Y., S.F., and H.K.
Funding
This study was supported by the Student Research Committee (Grant no. 3010679) of Kermanshah University of Medical Sciences, Kermanshah, Iran and approved by Ethic Commettee (IR.KUMS.REC.1399.688).
Conflicts of Interest
Nothing for declaration.
References
- Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect. Genet. Evol. 2020;83:104327. doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alijotas-Reig J., Esteve-Valverde E., Belizna C., Selva-O'Callaghan A., Pardos-Gea J., Quintana A., Mekinian A., Anunciacion-Llunell A., Miró-Mur F. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102569. 102569-102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qahtani A.A., Lyroni K., Aznaourova M., Tseliou M., Al-Anazi M.R., Al-Ahdal M.N., Alkahtani S., Sourvinos G., Tsatsanis C. Middle east respiratory syndrome corona virus spike glycoprotein suppresses macrophage responses via DPP4-mediated induction of IRAK-M and PPARγ. Oncotarget. 2017;8:9053–9066. doi: 10.18632/oncotarget.14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salama Z.T. Emapalumab: first global approval. Drugs. 2019;79:99–103. doi: 10.1007/s40265-018-1046-8. [DOI] [PubMed] [Google Scholar]
- Arnaldez F.I., O’Day S.J., Drake C.G., Fox B.A., Fu B., Urba W.J., Montesarchio V., Weber J.S., Wei H., Wigginton J.M., Ascierto P.A.-O.X. The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. In. J Immunother Cancer 8 LID -. LID. 2020;000930 doi: 10.1136/jitc-2020-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Ahmad S., Irshad R., Goyal Y., Rafat S., Siddiqui N., Dev K., Husain M., Ali S., Mohan A., Syed M.A. TLRs in pulmonary diseases. Life Sci. 2019;233 doi: 10.1016/j.lfs.2019.116671. 116671-116671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., Ranieri M., Rubenfeld G., Thompson B.T., Wrigge H., Slutsky A.S., Pesenti A. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Berger J., Moller D.E. The Mechanisms of Action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bergsten E., Horne A., Aricó M., Astigarraga I., Egeler R.M., Filipovich A.H., Ishii E., Janka G., Ladisch S., Lehmberg K., McClain K.L., Minkov M., Montgomery S., Nanduri V., Rosso D., Henter J.I. Confirmed Efficacy of Etoposide and Dexamethasone in HLH Treatment: Long-Term Results of the Cooperative HLH-2004 Study. Blood. 2017;130:2728–2738. doi: 10.1182/blood-2017-06-788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector TH17 and Regulatory T Cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bracaglia C., de Graaf K., Pires Marafon D., Guilhot F., Ferlin W., Prencipe G., Caiello I., Davì S., Schulert G., Ravelli A., Grom A.A., de Min C., De Benedetti F. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76:166–172. doi: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- Bracaglia C., Prencipe G., De Benedetti F. Macrophage Activation Syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J. 2017;15 doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human Immunopathogenesis of Severe Acute Respiratory Syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancio M., Ciccocioppo R., Rocco P., Levine B., Bronte V., Bollard C.M., Weiss D., Boelens J., Hanley P.J. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies. Cytotherapy. 2020;22:474–481. doi: 10.1016/j.jcyt.2020.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canna S.W., Girard C., Malle L., de Jesus A., Romberg N., Kelsen J., Surrey L.F., Russo P., Sleight A., Schiffrin E., Gabay C., Goldbach-Mansky R., Behrens E.M. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. Allergy Clin Immunol. 2017;139:1698–1701. doi: 10.1016/j.jaci.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1792. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini, F., Niccoli, L., Matarrese, D., Nicastri, E., Stobbione, P., Goletti, D., 2020. Baricitinib Therapy in COVID-19: A Pilot Study on Safety and Clinical Impact. J Infect. 81, 318-356. LID - S0163-4453(20)30228-0 [pii] LID - 10.1016/j.jinf.2020.04.017 [doi]. [DOI] [PMC free article] [PubMed]
- Capuano A., Scavone C., Racagni G., Scaglione F. NSAIDs in patients with viral infections, including Covid-19: victims or perpetrators? Pharmacol Res. 2020;157:104849. doi: 10.1016/j.phrs.2020.104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli E., Bramanti A., Ciurleo R., Tchorbanov A.I., Giordano A., Fagone P., Belizna C., Bramanti P., Shoenfeld Y., Nicoletti F. Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: diagnostic and therapeutic perspectives. Int. J. Mol. Med. 2020;46:903–912. doi: 10.3892/ijmm.2020.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavarella, C., Motta, I., Valente, S., Pasquinelli, G.A.-O., 2020. Pharmacological (Or Synthetic) and Nutritional Agonists of PPAR-γ as Candidates for Cytokine Storm Modulation in COVID-19 Disease. Molecules. 25, 2076. LID - E2076 [pii] LID - 10.3390/molecules25092076 [doi]. [DOI] [PMC free article] [PubMed]
- Cavalli E., Petralia M.C., Basile M.S., Bramanti A., Bramanti P., Nicoletti F., Spandidos D.A., Shoenfeld Y., Fagone P. Transcriptomic analysis of COVID-19 lungs and bronchoalveolar lavage fluid samples reveals predominant B cell activation responses to infection. Int. J. Mol. Med. 2020;46:1266–1273. doi: 10.3892/ijmm.2020.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick L., Zhao S., Mysler E., Moots R.J. Review of Biosimilar Trials and Data on Etanercept in Rheumatoid Arthritis. Curr Rheumatol Rep. 2018;20:84. doi: 10.1007/s11926-018-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Liu G., Chen J., Hu A., Zhang L., Sun W., Tang W., Liu C., Zhang H., Ke C., Wu J., Chen X. Ponatinib Protects Mice from Lethal Influenza Infection by Suppressing Cytokine Storm. Front Immunol. 2019;10:1393. doi: 10.3389/fimmu.2019.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- Chen F., Teachey D.T., Pequignot E., Frey N., Porter D., Maude S.L., Grupp S.A., June C.H., Melenhorst J.J., Lacey S.F. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. doi: 10.1016/j.jim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: a Descriptive Study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Ma S., Lin D., Mei Y., Gong H., Lei L., Chen Y., Zhao Y., Hu B., Wu Y., Yu X., Zhao L., Liu H. The S1P1 receptor-selective agonist CYM-5442 reduces the severity of acute GVHD by inhibiting macrophage recruitment. Cell Mol Immunol. 2015;12:681–691. doi: 10.1038/cmi.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquette D., Bessette L., Alemao E., Haraoui B., Postema R., Raynauld J.P., Coupal L. Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry. Arthritis Res Ther. 2019;21:138. doi: 10.1186/s13075-019-1917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P., Huang X., Peiris M., Yen H.L. Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication in Vitro. Antiviral Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello F., Tortora G. EGFR Antagonists in Cancer Treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- Cron R.A.-O., Chatham W.W. The Rheumatologist’s Role in COVID-19. J Rheumatol. 2020;47:639–642. doi: 10.3899/jrheum.200334. [DOI] [PubMed] [Google Scholar]
- Davidson S., McCabe T.A.-O., Crotta S.A.-O., Gad H.H., Hessel E.M., Beinke S.A.-O., Hartmann R.A.-O.X., Wack A.A.-O. IFNλ Is a Potent Anti-influenza Therapeutic without the Inflammatory Side Effects of IFNα Treatment. EMBO Mol Med. 2016;8:1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira P.G., Termini L., Durigon E.L., Lepique A.P., Sposito A.C., Pierulivo E.M.B. Vol. 144. Med. Hypotheses; 2020. Diacerein: a potential multi-target therapeutic drug for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti F., Brunner H.I., Ruperto N., Kenwright A., Wright S., Calvo I., Cuttica R., Ravelli A., Schneider R., Woo P., Wouters C. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2385–2395. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-Approved Compound Library Identifies Four Small-Molecule Inhibitors of Middle East Respiratory Syndrome Coronavirus Replication in Cell Culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host Factors in Coronavirus Replication. Curr Top Microbiol Immunol. 2019;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: Recent Insights into Emerging Coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo G., Di Trolio R., Kozlakidis Z., Busto G., Ingenito C., Buonerba L., Ferrara C., Libroia A., Ragone G., Ioio C.d., Savastano B., Polverino M., De Falco F., Iaccarino S., Leo E. COVID 19 therapies and anti-cancer drugs: a systematic review of recent literature. Crit. Rev. Oncol. Hematol. 2020;152:102991. doi: 10.1016/j.critrevonc.2020.102991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S., Acton S. A Novel Angiotensin-Converting Enzyme-Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin. Circ Res. 2000;87:1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Du, Y.X., Chen, X.P., 2020. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 108, 242-247. LID - 10.1002/cpt.1844 [doi]. [DOI] [PubMed]
- Emmenegger U., Frey U., Reimers A., Fux C., Semela D., Cottagnoud P., Spaeth P.J., Neftel K.A. Hyperferritinemia as Indicator for Intravenous Immunoglobulin Treatment in Reactive Macrophage Activation Syndromes. Am J Hematol. 2001;68:4–10. doi: 10.1002/ajh.1141. [DOI] [PubMed] [Google Scholar]
- Fagone P., Ciurleo R., Lombardo S.D., Iacobello C., Palermo C.I., Shoenfeld Y., Bendtzen K., Bramanti P., Nicoletti F. Vol. 19. Autoimmun. Rev.; 2020. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies; p. 102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhri S., Nouri Z., Moradi S.Z., Farzaei M.H. Astaxanthin, COVID-19 and immune response: focus on oxidative stress, apoptosis and autophagy. Phytother Res. 2020 doi: 10.1002/ptr.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an Overview of Their Replication and Pathogenesis. Methods Mol Biol. 2015 doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clin. Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin‐Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M. More on COVID‐19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020;189:1060–1061. doi: 10.1111/bjh.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furqan M., Mukhi N., Lee B., Liu D. Dysregulation of JAK-STAT Pathway in Hematological Malignancies and JAK Inhibitors for Clinical Application. Biomark Res. 2013;16:5. doi: 10.1186/2050-7771-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine Phosphate Has Shown Apparent Efficacy in Treatment of COVID-19 Associated Pneumonia in Clinical Studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gao W., Xiong Y., Li Q., Yang H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front. Physiol. 2017;8:508. doi: 10.3389/fphys.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Zhou H., Wu C., Xiao Y., Ren L., Paranhos-Baccalà G., Guo L., Wang J. Antibody against Nucleocapsid Protein Predicts Susceptibility to Human Coronavirus Infection. J Infect. 2015;71:599–602. doi: 10.1016/j.jinf.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker Bagca B., Biray Avci C. S1359–6101. Cytokine Growth Factor Rev.; 2020. The Potential of JAK/STAT Pathway Inhibition by Ruxolitinib in the Treatment of COVID-19. 30158-30151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Wei Z., Wang J., Kou J., Liu W., Fu Y., Yang Z. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis. Sci. Rep. 2016;6:28370. doi: 10.1038/srep28370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzan T., Nagafuji K., Tsukamoto H., Miyamoto T., Gondo H., Imashuku S., Harada M. Success with Infliximab in Treating Refractory Hemophagocytic Lymphohistiocytosis. Am J Hematol. 2006;81:59–61. doi: 10.1002/ajh.20462. [DOI] [PubMed] [Google Scholar]
- Heredia A., Le N., Gartenhaus R.B., Sausville E., Medina-Moreno S., Zapata J.C., Davis C., Gallo R.C., Redfield R.R. Targeting of mTOR catalytic site inhibits multiple steps of the HIV-1 lifecycle and suppresses HIV-1 viremia in humanized mice. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:9412–9417. doi: 10.1073/pnas.1511144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular Cloning and Expression of an IL-6 Signal Transducer, gp130. cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S.I., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a Novel Human Interleukin (BSF-2) that Induces B Lymphocytes to Produce Immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondermarck H., Bartlett N.W., Nurcombe V. The role of growth factor receptors in viral infections: an opportunity for drug repurposing against. emerging viral diseases such as COVID-19?. FASEB Bioadv. 2020;2:296–303. doi: 10.1096/fba.2020-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilowite N.T., Prather K., Lokhnygina Y., Schanberg L.E., Elder M., Milojevic D., Verbsky J.W., Spalding S.J., Kimura Y., Imundo L.F., Punaro M.G., Sherry D.D., Tarvin S.E., Zemel L.S., Birmingham J.D., Gottlieb B.S. Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66:2570–2579. doi: 10.1002/art.38699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M., Matsunaga N., Hazeki K., Nakamura K., Takashima K., Seya T., Hazeki O., Kitazaki T., Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl) sulfamoyl] cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol. 2006;69:1288–1295. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Yang, H., Ji, W., Wu, W., Chen, S.A.-O., Zhang, W., Duan, G., 2020. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 12, 372. LID - 10.3390/v12040372 [doi] LID - 372. [DOI] [PMC free article] [PubMed]
- Jones S.A., Richards p.J., Scheller J., Rose-John S. IL-6 Transsignaling: the in Vivo Consequences. J Interferon Cytokine Res. 2005;25 doi: 10.1089/jir.2005.25.241. 241-53. [DOI] [PubMed] [Google Scholar]
- Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet Respiratory Medicine. 2020:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju M., He H., Chen S., Liu Y., Liu Y., Pan S., Zheng Y., Xuan L., Zhu D., Luo Z. Ulinastatin ameliorates LPS-induced pulmonary inflammation and injury by blocking the MAPK/NF-κB signaling pathways in rats. Med Rep. 2019;20:3347–3354. doi: 10.3892/mmr.2019.10561. [DOI] [PubMed] [Google Scholar]
- Kennedy G.A., Varelias A., Vuckovic S., Le Texier L., Gartlan K.H., Zhang P., Thomas G., Anderson L., Boyle G., Cloonan N., Leach J., Sturgeon E., Avery J., Olver S.D., Lor M., Misra A.K., Hutchins C., Morton A.J., Durrant S.T., Subramoniapillai E., Butler J.P., Curley C.I., MacDonald K.P.A., Tey S.K., Hill G.R. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15:1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- Khailany R.A., Safdar M., Ozaslan M. Genomic Characterization of a Novel SARS-CoV-2. Gene Rep. 2020;19 doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Nam J.H. Insight into the Relationship between Obesity-Induced Low-Level Chronic Inflammation and COVID-19 Infection. Int J Obes (Lond) 2020;44:1541–1542. doi: 10.1038/s41366-020-0602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U.J., Won E.J., Kee S.J., Jung S.I., Jang H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., Traynor D., Johnson R.F., Dyall J., Kuhn J.H. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E.A.-O., Choe, P.A.-O., Park, W.A.-O., Oh, H.A.-O., Kim Ej Auid- Orcid - Fau - Nam, E.Y., Nam, E.A.-O., Na, S.A.-O., Kim, M.A.-O.X., Song, K.A.-O., Bang, J.A.-O., Park, S.A.-O., Kim, H.A.-O.X., Kim, N.A.-O., Oh, M.A.-O., 2016. Clinical Progression and Cytokine Profiles of Middle East Respiratory Syndrome Coronavirus Infection. J Korean Med 31, 1717-1725. [DOI] [PMC free article] [PubMed]
- Kitko C.L., Braun T., Couriel D.R., Choi S.W., Connelly J., Hoffmann S., Goldstein S., Magenau J., Pawarode A., Reddy P., Schuler C., Yanik G.A., Ferrara J.L., Levine J.E. Combination Therapy for Graft-Versus-Host Disease Prophylaxis with Etanercept and Extracorporeal Photopheresis: Results of a Phase II Clinical Trial. Biol Blood Marrow Transplant. 2016;22:862–868. doi: 10.1016/j.bbmt.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.Y., Lee W., Kenny H.A., Dang L.H., Ellis L.M., Jonasch E., Lengyel E., Naora H. Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake. Commun Biol. 2019;2:386. doi: 10.1038/s42003-019-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger P., Bailey M., Bellomo R., Cooper D.J., Harward M., Higgins A., Howe B., Jones D., Joyce C., Kostner K., McNeil J. A Multicenter Randomized Trial of Atorvastatin Therapy in Intensive Care Patients with Severe Sepsis. Am J Respir Crit Care Med. 2013;187:743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- Ky B., Mann D.L. COVID-19 clinical trials: a primer for the cardiovascular and cardio-oncology communities. JACC (J. Am. Coll. Cardiol.): Basic to Translational Science. 2020;5:501–517. doi: 10.1016/j.jacbts.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulou V., Sergushichev A., Bambouskova M., Nair S., Vincent E.E., Loginicheva E., Cervantes-Barragan L., Ma X., Huang S.C., Griss T., Weinheimer C.J., Khader S., Randolph G.J., Pearce E.J., Jones R.G., Diwan A., Diamond M.S., Artyomov M.N. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.C., Menacherry S., Diehl M.C., Giffear M.D., White C.J., Juba R., Bagarazzi M.L., Muthumani K., Boyer J., Agarwal V. Safety, bioavailability, and pharmacokinetics of VGX‐1027—a novel oral anti‐inflammatory drug in healthy human subjects. Clinical pharmacology in drug development. 2016;5:91–101. doi: 10.1002/cpdd.193. [DOI] [PubMed] [Google Scholar]
- Lee E.B., Fleischmann R., Hall S., Hall S., Wilkinson B., Bradley J.D., Gruben D., Koncz T., Krishnaswami S., Wallenstein G.V., Zang C., Zwillich S.H., van Vollenhoven R.F. Tofacitinib versus Methotrexate in Rheumatoid Arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- Li L., Li R., Wu Z., Yang X., Zhao M., Liu J., Chen D. Therapeutic strategies for critically ill patients with COVID-19. Ann. Intensive Care. 2020;10:45. doi: 10.1186/s13613-020-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting Enzyme 2 Is a Functional Receptor for the SARS Coronavirus. Nature. 2003;426 doi: 10.1038/nature02145. 450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Huang F., Xu J., Yang P., Qin Y., Cao M., Wang Z., Li X., Zhang S., Ye L., Lv J., Wei J., Xie T., Gao H., Xu K.-F., Wang F., Liu L., Jiang C. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020 [Google Scholar]
- Locatelli F., Jordan M.B., Allen C., Cesaro S., Rizzari C., Rao A., Degar B., Garrington T.P., Sevilla J.A.-O., Putti M.C., Fagioli F., Ahlmann M., Dapena Diaz J.L., Henry M., De Benedetti F., Grom A., Lapeyre G., Jacqmin P., Ballabio M., de Min C. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N Engl J Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- Lu H. 2019. Drug Treatment Options for the 2019-new Coronavirus. [DOI] [PubMed] [Google Scholar]
- Luo W., Li Y.-X., Jiang L.-J., Chen Q., Wang T., Ye D.-W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41:531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., Wang Z., Zhao J., Jiang H., Huang J., Shi Y., Dai J., Zheng K., Li X., Yang Z. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madu I.G., Chu V.C., Lee H., Regan A.D., Bauman B.E., Whittaker G.R. Heparan Sulfate Is a Selective Attachment Factor for the Avian Coronavirus Infectious Bronchitis Virus Beaudette. Avian Dis. 2007;51:45–51. doi: 10.1637/0005-2086(2007)051[0045:HSIASA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Martens A.S., Bode J.G., Heinrich P.C., Graeve L. The Cytoplasmic Domain of the Interleukin-6 Receptor Gp80 Mediates its Basolateral Sorting in Polarized Madin-Darby Canine Kidney Cells. J Cell Sci. 2000;113:3593–3602. doi: 10.1242/jcs.113.20.3593. [DOI] [PubMed] [Google Scholar]
- Mathern D.R., Heeger P.S. Molecules Great and Small: the Complement System. Clin J Am Soc Nephrol. 2015;10 doi: 10.2215/CJN.06230614. 1636-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary E.K., Pogue J.M. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infectious Diseases 7. 2020. On Behalf of the Society of Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J.E., Mitchell H.D., Gralinski L.E., Eisfeld A.J., Josset L., Bankhead A., 3rd, Neumann G., Tilton S.C., Schäfer A., Li C., Fan S., McWeeney S., Baric R.S., Katze M.G., Waters K.M. The Effect of Inhibition of PP1 and TNFα Signaling on Pathogenesis of SARS Coronavirus. BMC Syst Biol. 2016;10:93. doi: 10.1186/s12918-016-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Preston-Hurlburt P., Janeway C.A. A Human Homologue of the Drosophila Toll Protein Signals Activation of Adaptive Immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- Michot, J.M., Albiges, L., Chaput, N., Saada, V., Pommeret, F., Griscelli, F., Balleyguier, C., Besse, B., Marabelle, A., Netzer, F., Merad, M., Robert, C., Barlesi, F., Gachot, B., Stoclin, A., 2020. Tocilizumab, an anti-IL6 Receptor Antibody, to Treat Covid-19-Related Respiratory Failure: a Case Report. Ann Oncol. 31, 961-964. LID - S0923-7534(20)36387-0 [pii] LID - 10.1016/j.annonc.2020.03.300 [doi] LID - FAU - Michot, Jean-Marie. [DOI] [PMC free article] [PubMed]
- Milewska, A., Nowak, P., Owczarek, K., Szczepanski, A., Zarebski, M., Hoang, A., Berniak, K., Wojarski, J., Zeglen, S., Baster, Z., Rajfur, Z., Pyrc, K.A.-O., 2018. Entry of Human Coronavirus NL63 into the Cell. J Virol. 92, e01933-17. LID - 10.1128/JVI.01933-17 [doi] LID - e01933-17. [DOI] [PMC free article] [PubMed]
- Morgenstern B., Michaelis M., Baer P.C., Doerr H.W., Cinatl J., Jr. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem. Biophys. Res. Commun. 2005;326:905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaca G., Negrini S., Magnani O., Penza E., Pellecchio M., Gulli R., Mandich P., Puppo F. Update upon Efficacy and Safety of Etanercept for the Treatment of Spondyloarthritis and Juvenile Idiopathic Arthritis. Mod Rheumatol. 2018;28:417–431. doi: 10.1080/14397595.2017.1366006. [DOI] [PubMed] [Google Scholar]
- Mustafa S., Balkhy H., Gabere M.N. Current Treatment Options and the Role of Peptides as Potential Therapeutic Components for Middle East Respiratory Syndrome (MERS): A Review. J Infect Public Health. 2018;11:9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narazaki M., Yasukawa K., Saito T., Ohsugi Y., Fukui H., Koishihara Y., Yancopoulos G.D., Taga T., Kishimoto T. Soluble Forms of the Interleukin-6 Signal-Transducing Receptor Component Gp130 in Human Serum Possessing a Potential to Inhibit Signals through Membrane-Anchored Gp130. Blood. 1993;82:1120–1126. [PubMed] [Google Scholar]
- Neurath M.F. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Lamenta C., Donati S., Spada M., Ranazzi A., Cacopardo B., Mangano K., Belardelli F., Perno C., Aquaro S. Inhibition of human immunodeficiency virus (HIV‐1) infection in human peripheral blood leucocytes‐SCID reconstituted mice by rapamycin. Clin. Exp. Immunol. 2009;155:28–34. doi: 10.1111/j.1365-2249.2008.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Fagone P., Meroni P., McCubrey J., Bendtzen K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov. Today. 2011;16:715–721. doi: 10.1016/j.drudis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Northcutt A., Hutchinson M., Wang X., Baratta M., Hiranita T., Cochran T., Pomrenze M., Galer E., Kopajtic T., Li C. DAT isn't all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol. Psychiatr. 2015;20:1525–1537. doi: 10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi T., Kariwa H., Yokota S.I., Iki S., Indoh T., Yokosawa N., Takashima I., Tsutsumi H., Fujii N. Cytokine Regulation in SARS Coronavirus Infection Compared to Other Respiratory Virus Infections. J Med Virol. 2006;78 doi: 10.1002/jmv.20556. 417-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiyama N., Furumoto Y., Villarroel V.A., Linton J.T., Tsai W.L., Gutermuth J., Ghoreschi K., Gadina M., O’Shea J.J., Katz S.I. Reversal of CD8 T-cell-mediated mucocutaneous graft-versus-host-like disease by the JAK inhibitor tofacitinib. J Invest Dermatol. 2014;134:992–1000. doi: 10.1038/jid.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., Zhou L., Liu J. Asymptomatic Cases in a Family Cluster with SARS-CoV-2 Infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A., Gotlib J.R., Jamieson C., Cortes J.E., Talpaz M., Stone R.M., Silverman M.H., Gilliland D.G., Shorr J., Tefferi A. Safety and Efficacy of TG101348, a Selective JAK2 Inhibitor, in myelofibrosis. JAMA Oncol. 2015;1:643–651. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.B., Oh K., Garmaa N., Seo M.W., Byoun O.J., Lee H.Y., Lee D.S. Vol. 90. -; 2010. CP-690550, a Janus kinase inhibitor, suppresses CD4+ T-cell-mediated acute graft-versus-host disease by inhibiting the interferon-γ pathway; pp. 825–835. [DOI] [PubMed] [Google Scholar]
- Peiris J.S.M., Hui K.P.Y., Yen H.L. Host Response to Influenza Virus: Protection versus Immunopathology. Curr Opin Immunol. 2010;22:475–481. doi: 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F., Romerio A. Increasing the chemical variety of small molecule-based TLR4 modulators: an overview. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01210. 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone C., Triggianese P., Bartoloni E., Cafaro G., Bonifacio A.F., Bursi R., Perricone R., Gerli R. The anti-viral facet of anti-rheumatic drugs: lessons from COVID-19. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102468. 102468-102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia M.C., Mazzon E., Fagone P., Basile M.S., Lenzo V., Quattropani M.C., Bendtzen K., Nicoletti F. Pathogenic contribution of the Macrophage migration inhibitory factor family to major depressive disorder and emerging tailored therapeutic approaches. J. Affect. Disord. 2020;263:15–24. doi: 10.1016/j.jad.2019.11.127. [DOI] [PubMed] [Google Scholar]
- Petralia M.C., Mazzon E., Fagone P., Basile M.S., Lenzo V., Quattropani M.C., Di Nuovo S., Bendtzen K., Nicoletti F. The cytokine network in the pathogenesis of major depressive disorder. Close to translation? Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102504. [DOI] [PubMed] [Google Scholar]
- Pour P.M., Fakhri S., Asgary S., Farzaei M.H., Echeverria J. The signaling pathways, and therapeutic targets of antiviral agents: focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019;10:1207. doi: 10.3389/fphar.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Cui X., Sun J., Welsh J., Natanson C., Eichacker P.Q. Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials: a meta-analysis. Care Med. 2013;41:2419–2429. doi: 10.1097/CCM.0b013e3182982add. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah M.J. Gene Reports; 2020. mTOR inhibition and p53 activation, microRNAs: the possible therapy against pandemic COVID-19; p. 100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman S.U., Ali T., Alam S.I., Ullah R., Zeb A., Lee K.W., Rutten B.P., Kim M.O. Ferulic acid rescues LPS-induced neurotoxicity via modulation of the TLR4 receptor in the mouse hippocampus. Mol. Neurobiol. 2019;56:2774–2790. doi: 10.1007/s12035-018-1280-9. [DOI] [PubMed] [Google Scholar]
- Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T., Jiang Y.Z., Xiong Y., Li Y.J., Li X.W., Li H., Fan G.H., Gu X.Y., Xiao Y., Gao H., Xu J.Y., Yang F., Wang X.M., Wu C., Chen L., Liu Y.W., Liu B., Yang J., Wang X.R., Dong J., Li L., Huang C.L., Zhao J.P., Hu Y., Cheng Z.S., Liu L.L., Qian Z.H., Qin C., Jin Q., Cao B., Wang J.W. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice T.W., Wheeler A.P., Bernard G.R., Vincent J.-L., Angus D.C., Aikawa N., Demeyer I., Sainati S., Amlot N., Cao C. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- Robinson D.R., Wu Y.M., Lin S.F. The Protein Tyrosine Kinase Family of the Human Genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- Rochwerg B., Oczkowski S.J., Siemieniuk R.A.C., Agoritsas T., Belley-Cote E., D’Aragon F., Duan E., English S., Gossack-Keenan K., Alghuroba M., Szczeklik W., Menon K., Alhazzani W., Sevransky J., Vandvik P.O., Annane D., Guyatt G. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med. 2018;46:1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruperto N., Brunner Hi, Quartier P., Constantin T., Wulffraat N., Horneff G., Brik R., McCann L., Kasapcopur O., Rutkowska-Sak L., Schneider R., Berkun Y. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012;367:2396–2406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja V., Sekhon B.S. Calixarenes and cucurbiturils: pharmaceutial and biomedical applications. Journal of Pharmaceutical Education and Research. 2013;4:16. [Google Scholar]
- Sanchez-Piedra C., Diaz-Torne C., Manero J., Pego-Reigosa J.M., Rúa-Figueroa Í., Gonzalez-Gay M.A., Gomez-Reino J., Alvaro-Gracia J.M., group B.s. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann. Rheum. Dis. 2020;79:988–990. doi: 10.1136/annrheumdis-2020-217948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., Mansouri D. JAK inhibition as a new treatment strategy for patients with COVID-19. Int Arch Allergy Immunol. 2020;181:467–475. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J., OhnesorgeFau - Rose-John NS., Rose-John S. Interleukin-6 Trans-signalling in Chronic Inflammation and Cancer. Scand J Immunol. 2006;63:321–329. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Seif F., Khoshmirsafa M., Aazami H., Mohsenzadegan M., Sedighi G., Bahar M. The Role of JAK-STAT Signaling Pathway and its Regulators in the Fate of T Helper Cells. Cell Commun Signal. 2017;15:23. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E.H., Yang I.A., Wood-Baker R., Bowman R.V., Fong K.M. Gefitinib for Advanced Non-small Cell Lung Cancer. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD006847.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020:1–3. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Krishnan V., de Bono S., Ottaviani S., Casalini G., Richardson P.J., Monteil V., Lauschke V.M., Mirazimi A., Youhanna S., Tan Y.J., Baldanti F., Sarasini A., Terres J.A.R., Nickoloff B.J., Higgs R.E., Rocha G., Byers N.L., Schlichting D.E., Nirula A., Cardoso A., Corbellino M. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic I., Cuzzocrea S., Mangano K., Mazzon E., Miljkovic D., Wang M., Donia M., Al Abed Y., Kim J., Nicoletti F. In vitro, ex vivo and in vivo immunopharmacological activities of the isoxazoline compound VGX-1027: modulation of cytokine synthesis and prevention of both organ-specific and systemic autoimmune diseases in murine models. Clin. Immunol. 2007;123:311–323. doi: 10.1016/j.clim.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Stunault M.I., Bories G., Guinamard R.R., Ivanov S. Metabolism plays a key role during macrophage activation. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/2426138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli, F.R., Conti, F., Gadina, M., 2020. HiJAKing SARS-CoV-2? the Potential Role of JAK Inhibitors in the Management of COVID-19. Sci Immunol. 5. LID - eabc5367 [pii] LID - 10.1126/sciimmunol.abc5367 [doi]. [DOI] [PubMed]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016295. a016295-a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic Implications of IL-6 Blockade for Cytokine Storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi G.M., Sota J., Vitale A., Rigante D., Emmi G., Lopalco G., Guerriero S., Orlando I., Iannone F., Frediani B., Angotti R., Messina M., Galeazzi M., Vannozzi L., Cantarini L., Fabiani C. Efficacy and Safety of Certolizumab Pegol and Golimumab in the Treatment of Non-infectious Uveitis. Clin Exp Rheumatol. 2019;37:680–683. [PubMed] [Google Scholar]
- Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., Murphy B.R., Rappuoli R., Lanzavecchia A. An Efficient Method to Make Human Monoclonal Antibodies from Memory B Cells: Potent Neutralization of SARS Coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412. doi: 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. Vol. 2002. 2020. (Characteristics of Lymphocyte Subsets and Cytokines in Peripheral Blood of 123 Hospitalized Patients with 2019 Novel Coronavirus Pneumonia (NCP)). medRxiv. [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z.A.-O., Zhong W., Xiao G. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-nCoV) in Vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-H., Chung F.-T., Lin S.-M., Huang S.-Y., Chou C.-L., Lee K.-Y., Lin T.-Y., Kuo H.-P. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit. Care Med. 2014;42:313–321. doi: 10.1097/CCM.0b013e3182a2727d. [DOI] [PubMed] [Google Scholar]
- Wu, D., Yang, X.O., 2020. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 53, 368-370. LID - S1684-1182(20)30065-7 [pii] LID - 10.1016/j.jmii.2020.03.005 [doi]. [DOI] [PMC free article] [PubMed]
- Weiss S.R., Leibowitz J.L. Coronavirus Pathogenesis. Microbiol Mol Biol Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D., Spear P.G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Cao S., Dong H., Li Q., Chen E., Zhang W., Yang L., Fu S., Wang R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka T., Kusumoto S., Ando K., Ohba M., Ohmori T. Receptor tyrosine kinase-targeted cancer therapy. Int. J. Mol. Sci. 2018;19:3491. doi: 10.3390/ijms19113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Shin W.I., Pang Y.X., Meng Y., Lai J., You C., Zhao H., Lester E., Wu T., Pang C.H. The first 75 Days of novel coronavirus (SARS-CoV-2) outbreak: recent advances, prevention, and treatment. Int. J. Environ. Res. Publ. Health. 2020;17:2323. doi: 10.3390/ijerph17072323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The Pathogenesis and Treatment of the 'Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. Cytokine storm in COVID-19 and treatment. J. Infect. 2020;80 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.-H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Y., Zhou L., Zhang S., Yan L., Gu Z., Zhang G., Zhao Y. Gadolinium polytungstate nanoclusters: a new theranostic with ultrasmall size and versatile properties for dual-modal MR/CT imaging and photothermal therapy/radiotherapy of cancer. NPG Asia Mater. 2016;8:e273. [Google Scholar]
- Zha L., Li S., Pan L., Tefsen B., Li Y., French N., Chen L., Yang G., Villanueva E.V. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med. J. Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020;S0889–1591 doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin. Immunol. 2020:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Li R., Liu S. Immunoregulation with mTOR inhibitors to prevent COVID‐19 severity: a novel intervention strategy beyond vaccines and specific antiviral medicines. J. Med. Virol. 2020 doi: 10.1002/jmv.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. 2019. A Novel Coronavirus from Patients with Pneumonia in China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Guo X., Geary K., Zhang D. Emerging therapeutic strategies for COVID-19 patients. Discoveries (Craiova) 2020;8 doi: 10.15190/d.2020.2. e105-e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziai S.A., Rezaei M., Fakhri S., Pouriran R. ACE2: its potential role and regulation in severe acute respiratory syndrome and COVID‐19. J. Cell. Physiol. 2020 doi: 10.1002/jcp.30041. [DOI] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-nCoV Infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]