Abstract
The basic leucine zipper transcription factor ATF-like 3 (BATF3) is required for the development of conventional type 1 dendritic cells that are essential for cross-presentation and CD8 T cell mediated immunity against intracellular pathogens and tumors. However, whether Batf3 intrinsically regulates CD8 T cell responses is not well studied. Here, we report a novel role for cell intrinsic Batf3 expression in regulating the establishment of circulating and resident memory T cells after foodborne Listeria monocytogenes infection of mice. Consistent with other studies, Batf3 expression by CD8 T cells was dispensable for the primary response. However, Batf3−/− T cells underwent increased apoptosis during contraction to contribute to a substantially reduced memory populations. Batf3−/− memory cells had an impaired ability to mount a robust recall response but remained functional. These findings reveal a novel cell intrinsic role of Batf3 in regulating CD8 T cell memory development.
Introduction
The basic leucine zipper transcription factor ATF-like 3 (BATF3) is a member of the AP-1 transcription factor family and required for the development of conventional type 1 dendritic cells (cDC1) (1–3). cDC1 are essential for cross-presentation of exogenous antigens to prime CD8 T cells in vivo and Batf3−/− mice that lack cDC1 have defective CD8 T cell responses to intracellular pathogens and tumors (1). Batf3-dependent cDC1 may also affect the CD8 T cell response through other functions such as providing a critical source of IL-12 during Toxoplasma gondii infection and transporting bacteria to the splenic T cell zone to establish a productive infection during systemic Listeria monocytogenes (Lm) infection (4, 5). Moreover, cDC1-mediated cross-priming has also been shown to promote skin CD8 tissue-resident memory T (TRM) cell development by inducing committed TRM cell precursors without affecting their differentiation (6).
Batf3 gene expression is low in naïve CD4 and CD8 T cells (1). However, CD4 T cells upregulate Batf3 gene expression during in vitro TH1, TH2, TH17, and TH9 cell differentiation but not Treg cell differentiation (1, 7–9). While Batf3−/− CD4 T cells show normal TH1, TH2 and TH17 cell differentiation, Batf3 induces TH9 cell differentiation by binding to the Il9 promoter through a BATF3/IRF4 complex (1, 8, 9). Batf3 inhibits Treg differentiation by repressing Foxp3 expression and Batf3−/− mice have increased Treg cells (7, 10). Batf3 is also associated with lymphomagenesis in B and T cell lymphomas. Knockdown of Batf3 expression in lymphoma cell lines resulted in reduced proliferation and enhanced apoptosis that was associated with BATF3 binding to the Myc promoter (11). Much less is known about the intrinsic role of Batf3 in CD8 T cell responses to pathogens. Batf3 expression by CD8 T cells does not appear to regulate the primary CD8 T cell response as adoptive transfer of Batf3−/− CD8 T cells into Rag2−/− mice led to a normal anti-viral CD8 T cell response 7 days after infection (1). However, whether Batf3 intrinsically regulates other phases of the CD8 T cell response has not been explored.
Consistent with previous studies, Batf3 expression by CD8 T cells was dispensable for the primary response to foodborne Lm infection. However, this study revealed the novel observation that Batf3 expression by CD8 T cells critically regulated memory T cell development. Batf3−/− CD8 T cells were largely absent from the circulation and secondary lymphoid tissues during memory homeostasis. The remaining Batf3−/− CD8 T cells isolated during memory homeostasis possessed a predominantly terminally differentiated phenotype. Moreover, Batf3−/− CD8 T cells were also unable to establish a robust TRM cell population in the intestinal epithelium. Batf3 expression by CD8 T cells played an important role in promoting the survival of CD8 T cells as Batf3−/− CD8 T cells had increased apoptosis during contraction. Furthermore, the intrinsic Batf3 expression is important for CD8 T cells to mount a robust recall response but is not required for their function. These results reveal a critical and intrinsic role of Batf3 in regulating CD8 T cell memory development and recall responses.
Materials and Methods
Mice
Female and male B6 mice (C57BL/6J and B6.SJL-Ptprca Pepcb/BoyJ) were purchased from the Jackson Laboratory and used between 8 and 12 weeks of age. OT-I Rag1−/− mice were bred in house. B6.129S(C)-Batf3tm1kmm/J (Batf3−/−) mice were purchased from the Jackson Laboratory. OT-I Rag1−/− mice were crossed with B6.129S(C)-Batf3tm1kmm/J mice to make Batf3+/− OT-I Rag1−/− heterozygous. Wild-type (WT) and Batf3−/− OT-I Rag1−/− mice used in experiments were generated by heterozygous breeding of Batf3+/− OT-I Rag1−/− mice. All mice were housed under specific-pathogen-free condition. All procedures were carried out in accordance with National Institute of Health guidelines and approved by the Stony Brook University Institutional Animal Care and Use Committee.
Bacteria and foodborne infection
Listeria monocytogenes strain 10403s carrying a mutation in the internalin A protein and expressing a truncated form of ovalbumin (InlAM rLm-OVA) was used. Prior to infection, mice were deprived of food and water for 6 hours. Foodborne infection was performed by providing ~1 cm3 piece of bread inoculated with InlAM rLm-OVA in PBS to individually housed mice. The doses for primary and recall infection were 2×109 and 2×1010 cfu, respectively.
Cell sorting
Naïve OT-I cells were obtained by sorting CD8 T cells from the spleen of OT-I Rag1−/− mice. To obtain effector OT-I cells, 1×104 naïve CD45.1+ OT-I cells were adoptively transferred into CD45.2+ recipient mice. One day later, mice were foodborne infected with 2×109 cfu InlAM rLm-OVA. 6 days post infection (dpi), effector OT-I cells were enriched from the mesenteric lymph nodes (MLN) using positive magnetic selection (Miltenyi Biotec) and sorted on a FACSARIA III cell sorter (BD Biosciences) based on the CD45 congenic marker.
Quantitative PCR
RNA was isolated using RNeasy Plus Micro Kit (QIAGEN) and cDNA was generated using iScript Advanced cDNA Synthesis Kit (Bio-Rad). Real-time RT-PCR was performed on a Bio-Rad CFX96™ using primers purchased from Bio-Rad (qMmuCID0020840 for Batf3 and qMmuCID0022816 for Hmbs).
Adoptive co-transfers
WT and Batf3−/− OT-I cells bearing different CD45 congenic markers (2×103 each) were intravenously co-transferred into naïve congenic mice one day prior to foodborne infection. Congenic allele use was based on mice availability but always performed to distinguish donors from each other and the recipient.
Flow cytometric analysis and reagents
To isolate cells for flow cytometric analysis, spleen and MLN were mashed through 70 µm cell strainers. Small intestine intraepithelial lymphocytes (IEL) were isolated as previously described (12, 13). For functional analysis, IEL were mixed with equal number of congenically different naïve splenocytes and subsequently stimulated with or without 1 µg/ml of SIINFEKL peptide at 37℃ for 5 hours in the presence of brefeldin A followed by intracellular staining of IFNγ and TNFα. Granzyme B staining was performed directly ex vivo. Reagents for flow cytometric analysis are listed in Supplemental Table 1. Data were acquired on a LSRFortessa (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Statistical Analysis
Statistical analyses were performed in Prism (GraphPad Software) using unpaired t test for Figure 1 and paired t test for all other figures. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001.
Figure 1.
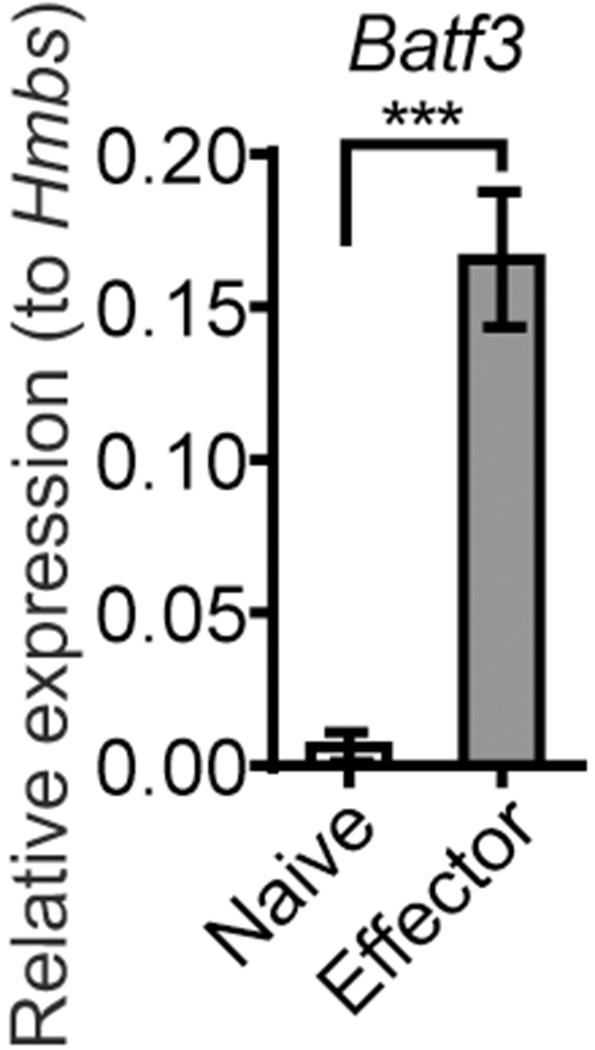
Effector CD8 T cells upregulate Batf3 gene expression. OT-I cells sorted from the spleen of naïve OT-I Rag1−/− mouse (Naïve) and from the MLN of foodborne infected mice at 6 dpi (Effector) were subjected to RNA extraction and quantitative PCR. Relative expression of Batf3 mRNA to housekeeping gene Hmbs was calculated. The data is representative of 3 independent experiments with n=4 and shown as mean ± SEM.
Results and Discussion
Batf3 expression by CD8 T cells is dispensable for the primary response but critically regulates memory development
It is well established that Batf3-dependent cDC1 play a crucial role in mounting anti-viral and anti-tumor CD8 T cell response by cross-presenting exogenous antigens to naïve CD8 T cells. However, only cursory examinations of Batf3 intrinsic effects on the CD8 T cell response have been evaluated. As such, effector OT-I cells isolated from the MLN of mice at 6 days post infection (dpi) with a mouse adapted Listeria monocytogenes expressing ovalbumin (InlAM rLm-OVA) were evaluated for Batf3 gene expression. Effector CD8 T cells rapidly upregulated Batf3 gene expression (Fig. 1), suggesting that cell intrinsic Batf3 expression may play a role in the CD8 T cell response to infection.
To determine whether cell intrinsic Batf3 expression regulates the CD8 T cell response, wild-type (WT) and Batf3−/− OT-I cells bearing different congenic markers generated from Batf3+/− OT-I Rag1−/− heterozygous breeders were co-transferred into congenically distinct B6 mice prior to foodborne infection with InlAM rLm-OVA. Donor OT-I cells were gated based on congenic markers and analyzed at different time points after infection (Fig. 2A and Supplemental Fig. 1). A comparable ratio of WT and Batf3−/− OT-I cells was observed in the blood at 6 dpi (Fig. 2B). At 9 dpi, the peak of the primary CD8 T cell response, the ratio remained comparable in the blood and spleen, while it was slightly skewed towards Batf3−/− OT-I cells in the MLN (Fig. 2B), suggesting that Batf3 does not play a critical role in CD8 T cell proliferation during the effector phase. Thus, Batf3 deficiency in CD8 T cells had little impact on the primary T cell response. At 15 dpi, the ratio of WT and Batf3−/− OT-I cells started to skew towards WT OT-I cells (Fig. 2B). Strikingly, at 30 dpi, the ratio was severely skewed towards WT OT-I cells as Batf3−/− OT-I cells were almost undetectable in the blood, spleen and MLN (Fig. 2B). Thus, CD8 T cell intrinsic Batf3 expression critically regulates the establishment of circulating memory cells.
Figure 2.
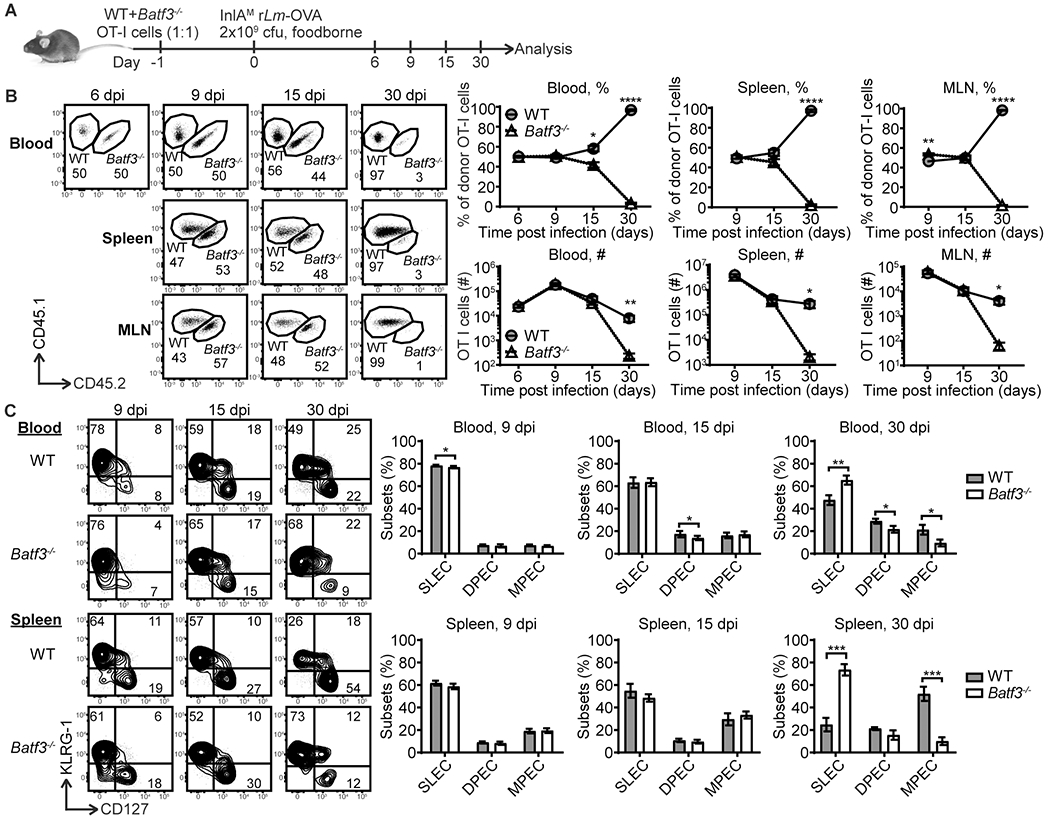
Batf3 expression by CD8 T cells is dispensable for the primary response but critically regulates memory development. (A) Experimental setup. WT and Batf3−/− OT-I cells (1:1, 2×103 cells each) bearing different congenic markers were intravenously co-transferred into naïve congenic mice one day prior to foodborne infection with InlAM rLm-OVA followed by analysis at different time points. (B) The kinetics of WT and Batf3−/− OT-I cells in the blood, spleen and MLN. (C) The kinetics of CD127 and KLRG-1 expression by WT and Batf3−/− OT-I cells in the blood and spleen. The data are pooled from 3 independent experiments for 6 and 9 dpi with n=36 for 6 dpi and n=12 for 9 dpi and 2 independent experiments for 15 and 30 dpi with n=7. The data are shown as mean ± SEM.
The memory potential of CD8 T cells was further evaluated by assessing the compositions of short-lived effector cells (SLEC; CD127− KLRG-1+), double positive effector cells (DPEC; CD127+ KLRG-1+) and memory precursor effector cells (MPEC; CD127+ KLRG-1−). SLEC rapidly undergo apoptosis during T cell contraction while MPEC have the greatest capacity to form long-lived memory cells (14). Some DPEC can lose KLRG-1 expression and also differentiate into long-live memory cells (15). At 9 dpi, WT and Batf3−/− OT-I cells had similar compositions of SLEC, DPEC and MPEC in the blood, spleen and MLN (Fig. 2C and Supplemental Fig. 2). Only subtle differences were observed. Overall, these data suggest that Batf3 expression by CD8 T cells was largely dispensable for the primary response and the differentiation of effector T cells. At 15 dpi, although the ratio started to skew towards WT OT-I cells, the composition of SLEC, DPEC and MPEC remained largely comparable between WT and Batf3−/− OT-I cells with the exception that Batf3−/− OT-I cells that had slightly less DPEC in the blood (Fig. 2C and Supplemental Fig. 2). However, by 30 dpi, Batf3−/− OT-I cells had a significantly higher proportion of SLEC phenotype cells in the blood and spleen and a significantly lower proportion of MPEC phenotype cells in the blood, spleen and MLN (Fig. 2C and Supplemental Fig. 2). Batf3−/− OT-I cells also had a significantly lower proportion of DPEC in the blood (Fig. 2C). The heavy skew towards terminally differentiated SLEC but away from the memory potential of MPEC and DPEC phenotypes between 15 and 30 dpi reflects the fate of Batf3−/− OT-I cells at 30 dpi. Thus, cell intrinsic Batf3 expression may regulate the MPEC lineage to promote memory cell development during contraction.
Batf3 expression by CD8 T cells promotes TRM cell development in the intestinal epithelium
Batf3-dependent cDC1-mediated cross-priming has been shown to promote skin CD8 TRM cell development (6). Foodborne Lm infection drives robust CD103+ TRM cell development in the intestine (16). As such, the role of cell intrinsic Batf3 expression by CD8 T cells in CD103+ TRM cell development in the intestinal epithelium was evaluated. WT and Batf3−/− OT-I cells had comparable expression of gut-homing receptors integrin α4β7 and chemokine receptor CCR9 in the blood at 6 dpi (Fig. 3A), suggesting that Batf3 expression by CD8 T cells did not regulate gut-homing receptor expression or intestinal migration capacity. At 9 dpi, the ratio of WT and Batf3−/− OT-I cells was comparable in the intestinal epithelium (IEL compartment) (Fig. 3B). Both WT and Batf3−/− OT-I cells comprised similar compositions of effector subsets (Fig. 3C). Although Batf3−/− OT-I cells had a slight disadvantage in upregulating CD69 and CD103 expression, a similar number of WT and Batf3−/− OT-I cells had differentiated into CD103+ TRM cell precursors (Fig. 3D), suggesting that Batf3 expression by CD8 T cells does not regulate the generation of intestinal CD103+ TRM cell precursors. A similar phenomenon was observed at 15 dpi (Fig. 3B–3D). However, by 30 dpi, Batf3−/− OT-I cells were significantly reduced in the IEL compartment (Fig. 3B), suggesting Batf3 deficiency in CD8 T cells impairs TRM cell development. Although Batf3−/− OT-I cells had a slightly increased ability to differentiate into MPEC and CD103+ TRM cells (Fig. 3C and 3D), the number of CD103+ TRM cells in the IEL compartment at 30 dpi was significantly lower among Batf3−/− OT-I cells (Fig. 3D). Overall, these data suggest that Batf3 expression by CD8 T cells promotes CD103+ TRM cell development in the intestinal epithelium without affecting the expression of gut-homing receptors or the generation of CD103+ TRM cell precursors. It should also be noted that TRM cells appeared less impacted by cell intrinsic Batf3 deficiency compared to their circulating counterparts, suggesting that distinct environmental signals present in the gut overcomes the defect of cell intrinsic Batf3 deficiency on CD8 T cells.
Figure 3.
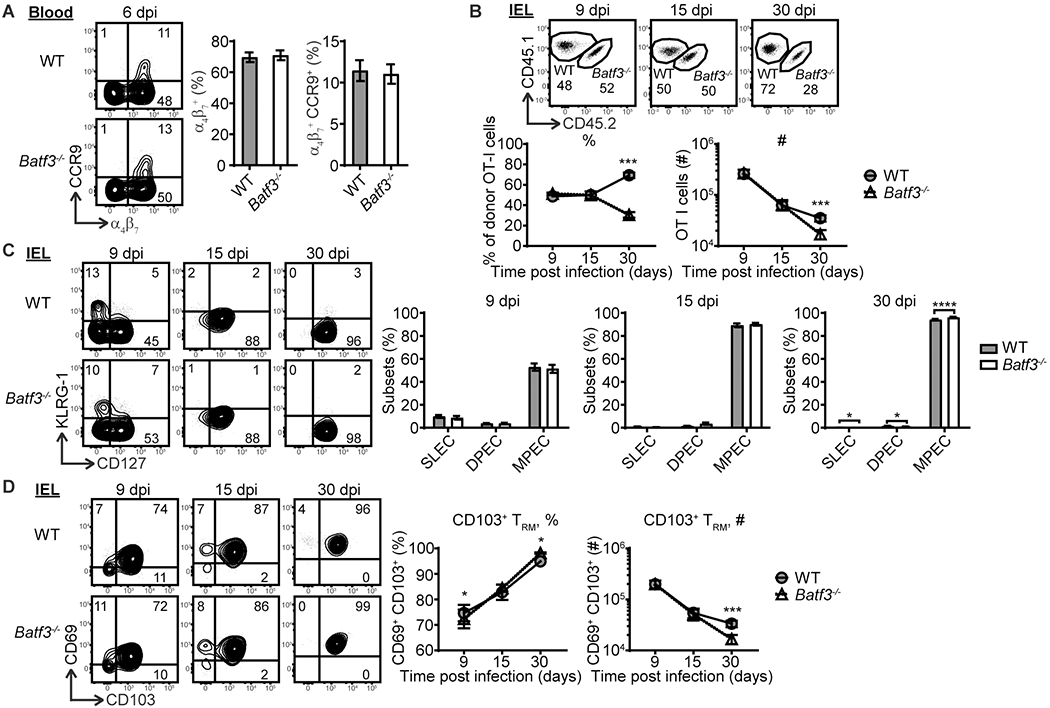
Batf3 expression by CD8 T cells promotes TRM cell development in the intestinal epithelium. (A) Integrin α4β7 and CCR9 expression by WT and Batf3−/− OT-I cells in the blood at 6 dpi. (B) The kinetics of WT and Batf3−/− OT-I cells in the IEL compartment. (C) The kinetics of CD127 and KLRG-1 expression by WT and Batf3−/− OT-I cells in the IEL compartment. (D) The kinetics of CD1103 and CD69 expression by WT and Batf3−/− OT-I cells in the IEL compartment. The data in (A) are pooled from 3 independent experiments with n=36. The data in (A) are pooled from 3 independent experiments with n=36. The The data in (B-D) are pooled from 3 independent experiments for 9 dpi with n=12 and from 2 independent experiments for 15 and 30 dpi with n=7. The data are shown as mean ± SEM.
Batf3 expression by CD8 T cells promotes CD8 T cell survival
Batf3 expression drives lymphomagenesis by promoting proliferation and survival (11). Batf3−/− OT-I cell numbers were normal during the effector phase suggesting that any potential impact on effector cell proliferation would be minimal. However, Batf3−/− OT-I cells decreased drastically between 15 and 30 dpi (Fig. 2B), suggesting an important role for Batf3 in regulating CD8 T cell survival during contraction. As such, apoptosis of WT and Batf3−/− OT-I cells was evaluated. Compared to WT OT-I cells, Batf3−/− OT-I cells had a significant increase in early apoptotic cell (Apotracker+ Live/dead−) populations at 9, 15 and 30 dpi, and the increase enlarged over time (Fig. 4). An increase in late apoptotic cells (Apotracker+ Live/dead+) also emerged in Batf3−/− OT-I cells by 30 dpi (Fig. 4). These data suggest that Batf3 expression regulates memory CD8 T cell development in part by promoting CD8 T cell survival. The survival of effector CD8 T cells during contraction is dependent on IL-7 and IL-15, which upregulates the anti-apoptotic molecule Bcl-2 to overcome the effect of TGFβ (14). As Bcl2 family members are known targets of AP-1 transcription factors (17), Batf3 may regulate memory development by promoting the anti-apoptotic machinery. The closely related molecule, BATF, can regulate Bcl-2 expression and survival of effector CD8 T cells (18). A defect in homeostatic division during memory maintenance may also contribute to loss of Batf3−/− memory T cells. This is particularly relevant for TRM cells as Batf3−/− IEL are reduced but still detectable at 30 dpi. Memory CD8 T cells undergo IL-7 and IL-15 dependent homeostatic division (19). However, homeostatic turnover is slower in intestinal TRM cells (20), which may contribute to the reduced impact of Batf3 deficiency on TRM cell populations.
Figure 4.
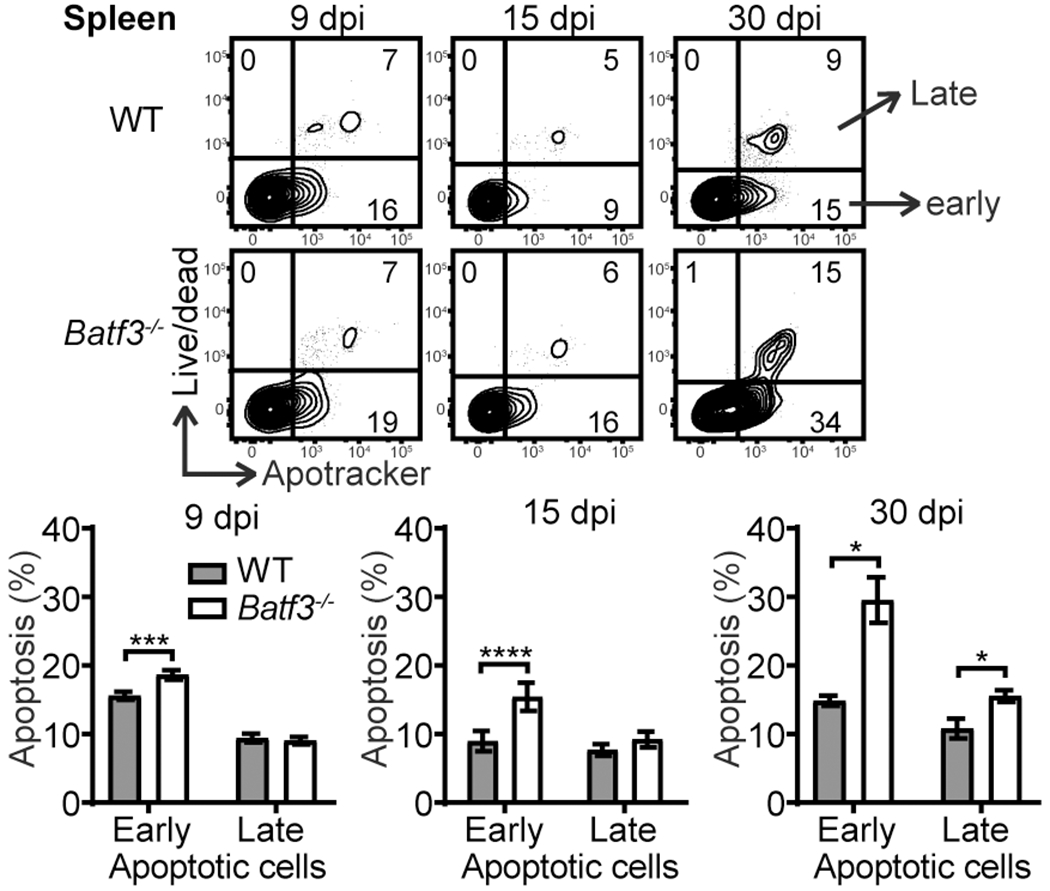
Batf3-deficient CD8 T cells undergo enhanced apoptosis. The kinetics of apoptosis analysis of WT and Batf3−/− OT-I cells in the spleen. Early apoptotic cells are defined as Apotracker+ Live/dead− and late apoptotic cells are defined as Apotracker+ Live/dead+. The data are pooled from two independent experiments with n=8 for 9 dpi and n=7 for 15 and 30 dpi and shown as mean ± SEM.
Batf3 expression by CD8 T cells is important for recall but dispensable for function.
To determine whether cell intrinsic Batf3 expression impacts recall to a secondary challenge, mice outlined in Fig. 2A were challenged at 30 dpi with a secondary infection and donor OT-I cells were analyzed at 6 days post recall infection (dpr). We were unable to detect a clear Batf3−/− OT-I cell population in the spleen and MLN (data not shown); thus, the analysis focused on donor cells from the blood and IEL compartment. While Batf3−/− OT-I cells were hardly detectable in the blood at memory and 6 dpr, their numbers increased after recall (Fig. 5A an B), suggesting that they are able to mount a recall response. However, while WT OT-I cells increased more than 20-fold after recall infection, Batf3−/− OT-I cells increased less than 10-fold (Fig. 5B), suggesting that Batf3 deficiency in CD8 T cells impaired their ability to mount a robust recall response. Furthermore, WT and Batf3−/− OT-I cells had different compositions of effector subsets at 6 dpr with Batf3−/− OT-I cells having less SLEC and more DPEC (Supplemental Fig. 3A). The analysis in the IEL compartment revealed that the ratio of WT and Batf3−/− OT-I cells was severely skewed towards WT OT-I cells (about 9:1) (Fig. 5C), which was more striking than that at memory (about 2:1) (Fig. 3B). These data suggest that Batf3-deficient OT-I cells are less competitive than their Batf3-sufficient counterparts at secondary expansion to challenge infection. The impaired ability of Batf3−/− OT-I cells to mount a robust recall response in the IEL was consistent with the lack of an emergent SLEC and CD69− population at 6 dpr (Supplemental Fig. 3B and 3C). Whether this defect is due to a lack of in situ proliferation and differentiation or a lack of newly recalled intestinal immigrants remains to be determined.
Figure 5.
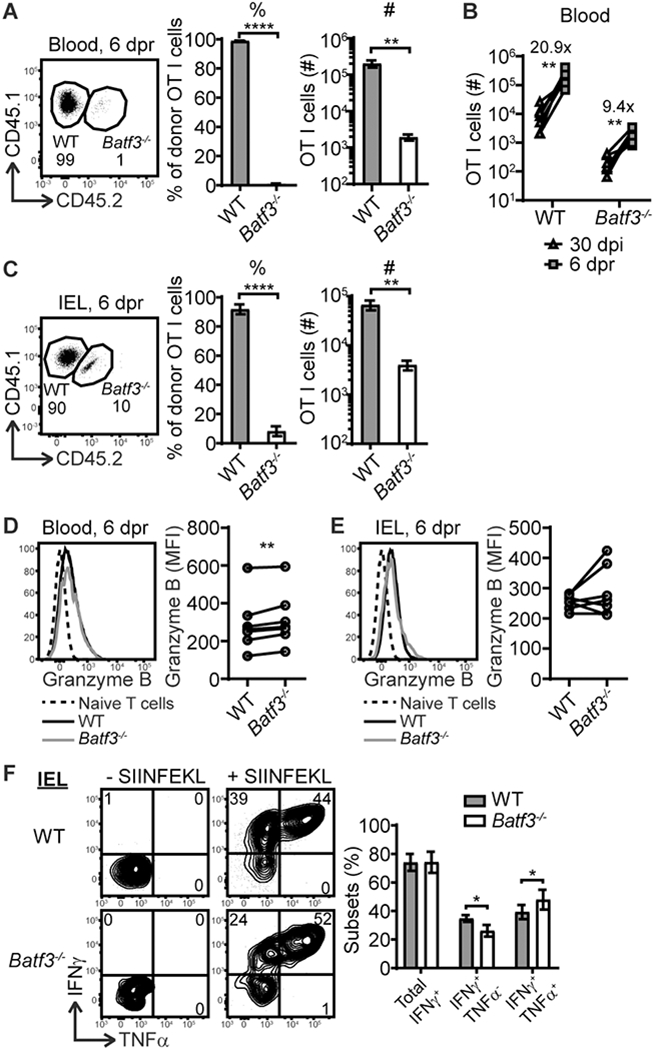
Batf3 expression by CD8 T cells is important for recall response but dispensable for their function. Mice outlined in Fig. 2A were challenged with recall infection at 30 dpi and the donor OT-I cells were analyzed at 6 dpr. (A) The percentage and number of WT and Batf3−/− OT-I cells in the blood at 6 dpr. (B) The corresponding number of WT and Batf3−/− OT-I cells in the blood at 30 dpi and 6 dpr. (C) The percentage and number of WT and Batf3−/− OT-I cells in the IEL compartment at 6 dpr. (D) The granzyme B expression by WT and Batf3−/− OT-I cells in the blood at 6 dpr. (E) The granzyme B expression by WT and Batf3−/− OT-I cells in the IEL compartment at 6 dpr. (F) The IFNγ and TNFα expression by WT and Batf3−/− OT-I cells in the IEL compartment at 6 dpr. The data are pooled from 2 independent experiments with n=7. The data in (A, C, F) are shown as mean ± SEM. In (B, D, E), each dot represents an individual animal.
Thus far, these data demonstrated that Batf3 expression by CD8 T cells critically regulated memory development and the robustness of the recall response. However, whether it regulates T cell function is unknown. The functional analysis of WT and Batf3−/− OT-I cells was performed at 6 dpr. Batf3−/− OT-I cells had a subtle but significant increase in granzyme B in the blood but not IEL (Fig. 5D and 5E). Additionally, Batf3−/− OT-I cells produced similar IFNγ, but more cells were IFNγ and TNFα double producers (Fig. 5F). Collectively, these data suggest that Batf3 deficiency in CD8 T cells did not result in functional impairment during recall.
The impact of Batf3 deficiency on the CD8 T cell response has been largely attributed to a lack of cDC1. By assessing the impact of Batf3 deficiency on the CD8 T cell response in mice with a normal compartment of DC, we uncovered a novel role for CD8 T cell intrinsic Batf3 expression in the establishment of robust circulating and resident memory T cells. In summary, intrinsic Batf3 expression by CD8 T cells was dispensable for the primary CD8 T cell response but required for the development of circulating memory T cells. In non-lymphoid tissues, cell intrinsic Batf3 expression was required for optimal TRM cell development. In the absence of Batf3, CD8 T cells underwent more apoptosis and failed to survive into memory, though other mechanisms may also contribute to the reduced memory population. Moreover, cell intrinsic Batf3 expression is dispensable for their function but is required for CD8 T cells to mount a robust recall response. Altogether, this study uncovered a novel CD8 T cell-intrinsic role of Batf3 expression in regulating memory development and recall response in part by promoting T cell survival during contraction that may be exploited for rationale vaccine design.
Supplementary Material
Key Points.
Batf3 expression by CD8 T cells critically regulates memory T cell development.
Batf3−/− CD8 T cell undergo increased apoptosis during contraction.
Batf3−/− CD8 T cells have an impaired recall ability but remain functional.
Acknowledgments
This work was supported by NIH awards K12GM102778 (Z.Q.), R01AI076457 (B.S.S.), R21AI137929 (B.S.S.), and funds provided by The Research Foundation for the State University of New York and Stony Brook University (B.S.S.).
Abbreviations:
- BATF3
basic leucine zipper transcription factor ATF-like 3
- cDC1
conventional type 1 dendritic cells
- DPEC
double positive effector cells
- dpi
days post infection
- dpr
days post recall
- IEL
intraepithelial lymphocytes
- Lm
Listeria monocytogenes
- MLN
mesenteric lymph nodes
- MPEC
memory precursor effector cells
- SLEC
short-lived effector cells
- TRM
resident memory T
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, and Murphy KM. 2008. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, and Murphy KM. 2010. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med 207: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy TL, Tussiwand R, and Murphy KM. 2013. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol 13: 499–509. [DOI] [PubMed] [Google Scholar]
- 4.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, and Murphy KM. 2011. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 35: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, Belizaire R, Aoshi T, Schreiber RD, Miller MJ, Murphy TL, Unanue ER, and Murphy KM. 2011. CD8alpha(+) dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. Immunity 35: 236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iborra S, Martinez-Lopez M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, Del Fresno C, and Sancho D. 2016. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1+ Dendritic Cells. Immunity 45: 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee W, Kim HS, Hwang SS, and Lee GR. 2017. The transcription factor Batf3 inhibits the differentiation of regulatory T cells in the periphery. Exp Mol Med 49: e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WH, Jang SW, Kim HS, Kim SH, Heo JI, Kim GE, and Lee GR. 2019. BATF3 is sufficient for the induction of Il9 expression and can compensate for BATF during Th9 cell differentiation. Exp Mol Med 51: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuda M, Hamade H, Thomas LS, Salumbides BC, Potdar AA, Wong MH, Nunnelee JS, Stamps JT, Neutzsky-Wulff AV, Barrett RJ, Wang Y, Tang J, Funari VA, Targan SR, and Michelsen KS. 2019. A role for BATF3 in TH9 differentiation and T-cell-driven mucosal pathologies. Mucosal Immunol 12: 644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Xiao X, Lan P, Li J, Dou Y, Chen W, Ishii N, Chen S, Xia B, Chen K, Taparowsky E, and Li XC. 2018. OX40 Costimulation Inhibits Foxp3 Expression and Treg Induction via BATF3-Dependent and Independent Mechanisms. Cell Rep 24: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lollies A, Hartmann S, Schneider M, Bracht T, Weiss AL, Arnolds J, Klein-Hitpass L, Sitek B, Hansmann ML, Kuppers R, and Weniger MA. 2018. An oncogenic axis of STAT-mediated BATF3 upregulation causing MYC activity in classical Hodgkin lymphoma and anaplastic large cell lymphoma. Leukemia 32: 92–101. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan BS, and Lefrancois L. 2012. Isolation of mouse lymphocytes from small intestine tissues. Curr Protoc Immunol Chapter 3: Unit3 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Z, and Sheridan BS. 2018. Isolating Lymphocytes from the Mouse Small Intestinal Immune System. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu Z, Khairallah C, and Sheridan BS. 2018. Listeria Monocytogenes: A Model Pathogen Continues to Refine Our Knowledge of the CD8 T Cell Response. Pathogens 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, Lietzenmayer M, Kroehling L, Takumi A, Kometani K, Inoue T, Kluger Y, Kaech SM, Kurosaki T, Okada T, and Flavell RA. 2018. KLRG1(+) Effector CD8(+) T Cells Lose KLRG1, Differentiate into All Memory T Cell Lineages, and Convey Enhanced Protective Immunity. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, and Lefrancois L. 2014. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 40: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eferl R, and Wagner EF. 2003. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3: 859–868. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda S, Yamazaki M, Abe M, Sakimura K, Takayanagi H, and Iwai Y. 2011. Basic leucine zipper transcription factor, ATF-like (BATF) regulates epigenetically and energetically effector CD8 T-cell differentiation via Sirt1 expression. Proc Natl Acad Sci U S A 108: 14885–14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, and Surh CD. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med 195: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, and Masopust D. 2016. IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J Immunol 196: 3920–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


