Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) is the second most common malignancy of primary liver cancer. Among the several pathological types of ICC, only five cases of the clear cell type have been reported, including the one presented below. Here we report a unique case of clear cell type ICC following laparoscopic hepatectomy.
Case presentation
A 67-year-old woman had a history of hepatitis B virus. Computed tomography revealed a ring-like enhanced mass 35 mm in diameter at segment 7 in the early phase. The enhancement was prolonged to the late phase through the portal phase, while the shape was irregular. Ethoxybenzy magnetic resonance imaging revealed that the tumor had a low signal intensity on T1-weighted imaging and a high signal intensity on T2-weighted imaging. Diffusion-weighted images identified that the tumor had remarkably high signal intensity. Tumor enhancement was not detected throughout the tumor in the hepatocyte phase. Upon ICC diagnosis, a laparoscopic S7 subsegmentectomy was performed. The patient’s postoperative course was uneventful. An immunohistochemical examination revealed that the cells tested positive for cytokeratin 7 (CK7), CK19, and CD56 and negative for CK20, CD10, α-fetoprotein, thyroid transcription factor-1. At 2 years after surgery, the patient remains alive without recurrence.
Conclusions
Here we presented a case of clear cell ICC that was treated by laparoscopic hepatectomy. Immunological analysis, especially by CD56 and several CK markers, is helpful for diagnosing this disease.
Keywords: Clear cell type, Intrahepatic cholangiocarcinoma, Laparoscopic hepatectomy
Background
Intrahepatic cholangiocarcinoma (ICC) is the second most common malignancy of primary liver cancer and has a dismal prognosis. Previous reports demonstrated a 5-year overall survival rate of around 40% even after curative surgery [1, 2]. The establishment of a systemic perioperative chemotherapy regimen is currently underway. Recently, the efficacy of neoadjuvant chemotherapy for locally advanced ICC was reported [3]. ICC has several types, including clear cell. Cases of clear cell ICC are limited, as only five have been reported including the one presented here; thus, its imaging characteristics and prognosis remain unclear. Here we report a case of clear cell ICC following laparoscopic hepatectomy.
Case presentation
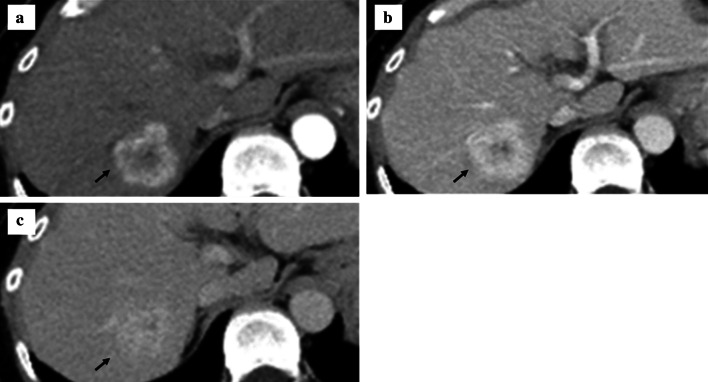
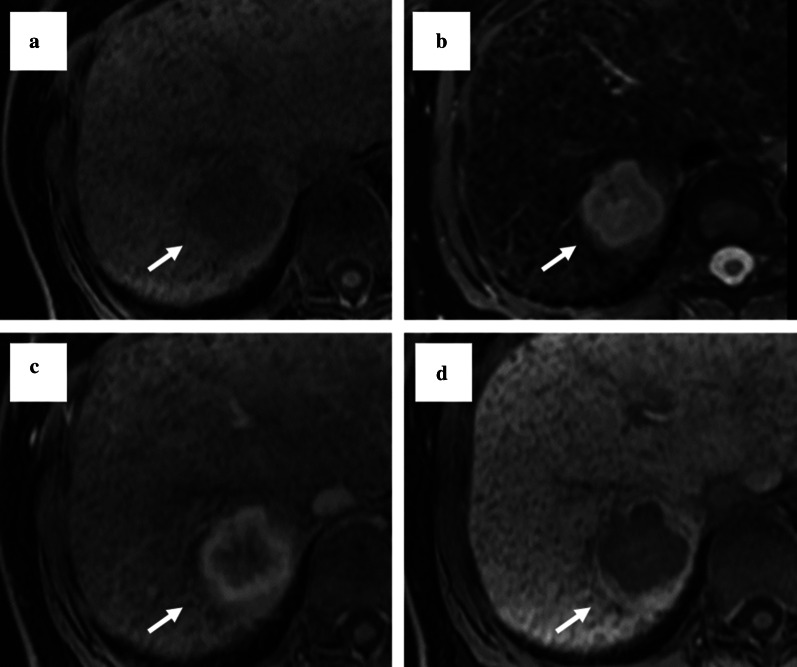
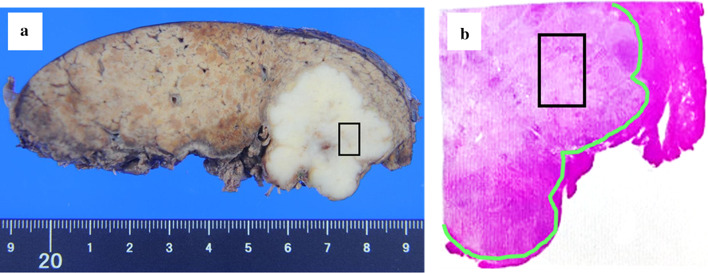
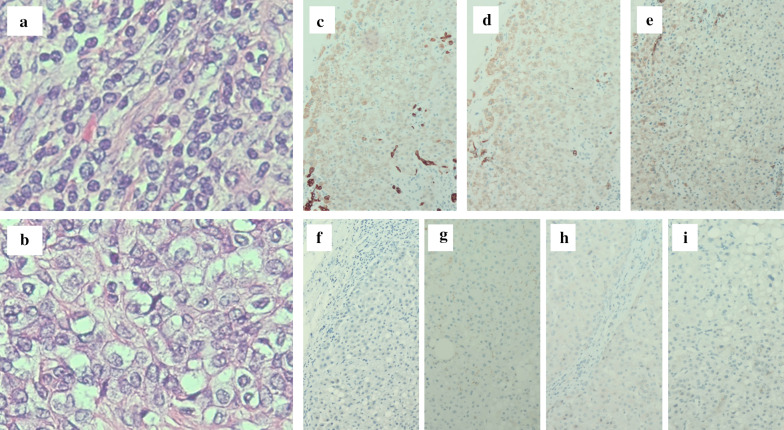
A 67-year-old woman was admitted to our hospital with the chief complaint of high fever. She had a past medical history of hepatitis B virus. Laboratory data showed Child–Pugh liver disease grade A and liver damage grade A. Levels of tumor makers such as carcinoembryonic antigen, carbohydrate antigen 19-9, α-fetoprotein (AFP), and protein induced by vitamin K absence or antagonist-II were within the normal ranges. Dynamic abdominal computed tomography represented a ring-like enhanced mass 35 mm in diameter at segment 7 at the early phase (Fig. 1a). The enhancement was prolonged to the late phase through the portal phase, while the shape was irregular (Fig. 1b, c). Dynamic magnetic resonance imaging revealed that the tumor had a low signal intensity upon T1 weighting (Fig. 2a) and a high signal intensity upon T2 weighting (Fig. 2b). Diffusion-weighted images identified that the tumor had remarkably high signal intensity. The tumors have ring-like enhancement at the early phase (Fig. 2c) that was not detected throughout the tumor at the hepatocyte phase (Fig. 2d). Radiological findings suggested that the tumor was ICC or combined hepatocellular carcinoma (HCC)–cholangiocellular carcinoma for which a laparoscopic S7 subsegmentectomy was performed. On operative finding, the surface of the liver was slightly irregular due to the hepatitis B viral infection. Under intraoperative ultrasonography guidance, the tumor was closed to the right hepatic vein without direct invasion and a laparoscopic S7 subsegmentectomy was completed. The operative time was 528 min, and the intraoperative blood loss was 500 mL. On macroscopic examination, the tumor was solid and whitish with irregular margins (Fig. 3a). The tumor was 35 × 32 × 30 mm in dimension. Microscopically, most of the tumor cells had an enlarged nucleus–cytoplasmic ratio, and they proliferated into funicular or small alveolar structures with stromal tissue consisted of hyaline collagen fiber. The differentiation was poor adenocarcinoma with abundant clear cytoplasm (Fig. 4a, b). An immunohistochemical examination revealed that the cells tested positive for cytokeratin 7 (CK7), CK19, and CD56 (Fig. 4c–i) but negative for CK20, CD10, AFP, and thyroid transcription factor-1 (Fig. 4f–i). Based on these findings, the tumor was diagnosed as a clear cell ICC, T1aN0M0, stage IA according to the American Joint Committee on Cancer/Union for International Cancer Control staging classification 8th edition.
Fig. 1.
Findings of dynamic abdominal computed tomography. a The arterial phase showing a ring-like enhanced mass at segment 7 measuring 35 mm in diameter shaped like an irregular arrow. b Portal phase. c The enhancement was prolonged to the late phase
Fig. 2.
Findings of magnetic resonance imaging (MRI). a The tumor shows a low signal intensity on a T1-weighted image. b The tumor shows a high signal intensity on a T2-weighted image. c At the early phase on ethoxybenzyl magnetic resonance imaging (EOB-MRI), the tumor shows ring-like enhancement. d The enhancement is not detected throughout the tumor in the hepatocyte phase on EOB-MRI
Fig. 3.
Imaging. a Gross appearance of the cut surface showing a solid whitish mass measuring 35 mm × 32 mm × 30 mm with irregular margins. In the area surrounded by the black line on a and b, clear cells are abundantly clear
Fig. 4.
Histopathological findings. a, b The tumor consists of poorly differentiated adenocarcinoma tissue (a) and clear cells with atypical nuclei (b) (hematoxylin and eosin stain); c CK7 expression is positive; d CK19 expression is positive; e CD56 expression is positive; f CK20 expression is negative; g CD10 expression is negative; h α-fetoprotein expression is negative; i thyroid transcription factor-1 expression is negative
On the 7th postoperative day, she was discharged without complications. Although no adjuvant chemotherapy was administered, her postoperative course was uneventful without a recurrence at 2 year after surgery.
Conclusions
Histopathologically, ICC develops as various tissues form, such as well-differentiated adenocarcinoma, adenosquamous carcinoma, mucinous carcinoma, mucoepidermoid carcinoma, squamous carcinoma, sarcomatoid carcinoma, and signet-ring cell carcinoma [4, 6]. Among them, the clear cell type is particularly rare. Due to its rarity, its prognosis and radiological findings remain unclear. Nevertheless, radiological characteristic might be sustained intra-tumor staining in the late phases of dynamic CT. Clear cell carcinoma is generally thought to be derived from the ovary and kidney. Some cancers show a clear cell type, such as ICC, HCC, gallbladder carcinoma, and metastasis of lung or thyroid cancer [4, 6–8]. The incidence of clear cell gallbladder carcinoma is reportedly around 1% [7].
Immunohistochemical analysis would play an important role in distinguishing between clear cell HCC and other types. Positivity for CK7 and the absence of CK20 staining are considered features of cholangiocarcinoma [4, 5]. It has been reported that CD56 positivity relates to the clear cell change [3, 9], while ICC shows negativity for vimentin and CD56 [3, 10]. Glycogen, mucin, and lipids also cause the clear cell change [7, 11, 12]; in this case, glycogen reactivity was shown (Fig. 4). We could distinguish clear cell ICC from clear cell HCC in terms of differences in the reactivities of HepPar-l, AFP, and Hepatocyto [4, 9, 11–14]. Renal cell carcinoma is also a well-known clear cell tumor, and its immunohistochemical features include negativity for vimentin, CD10, and S-100 protein [4, 8–11, 15].
Table 1 shows the cases of clear cell type ICC reported to date. Curative surgery was performed in most cases. Among them, three patients survived without recurrence after receiving curative surgery. Based on this observation, clear cell ICC might have a better prognosis than ordinary ICC. Therefore, aggressive surgical resection of this disease is recommended. To the best of our knowledge, this is the first report of a clear cell ICC case undergoing laparoscopic hepatectomy. Laparoscopic hepatectomy is increasingly used to treat primary liver tumors with reportedly fewer postoperative complications. Ban et al. proposed that degree of difficulty of laparoscopic hepatectomy is related to the maximal tumor diameter, tumor location, and liver function. In this case, regardless of a relatively high difficulty score, laparoscopic hepatectomy was achieved [16]. In the present case, no adjuvant chemotherapy was administered and the patient’s postoperative course was uneventful without recurrence for 12 months. Clear cell ICC is a rare malignancy with no effective perioperative chemotherapy; therefore, careful follow-up is necessary to improve postoperative prognosis.
Table 1.
Reported cases of clear cell type intrahepatic cholangiocarcinoma
| Author | Year | Age/sex | Size(cm) | Treatment | Adjuvant chemotherapy | Immunohistochemical analysis | Prognosis |
|---|---|---|---|---|---|---|---|
| Tihan T, et al. | 1998 | 72/M | 15 | Surgery | Not described | N/A | Alive at 30 m |
| Logani S, et al. | 1998 | 64/F | 12 | Surgery | Not described | N/A | Not described |
| Falta EM, et al. | 1999 | 50/M | 1.5 | Surgery | Not described | N/A | Not described |
| Fernandes SR, et al. | 2017 | 51/F | 9.5 | Surgery | + | CK7(+), CK20(−) | Alive at 2 yr |
| Our case | 2020 | 67/F | 3.5 | Surgery | − | CK7(+), CK19, CD56(−), CK20, CD10, TTF1 | Alive at 2 yr |
In conclusion, here we presented a case of clear cell ICC following laparoscopic hepatectomy. Immunological analysis, especially by CD56 and several CK markers, is helpful for diagnosing this disease.
Acknowledgements
Not applicable.
Abbreviations
- AFP
α-Fetoprotein
- CK
Cytokeratin
- EOB-MRI
Ethoxybenzyl magnetic resonance imaging
- HCC
Hepatocellular carcinoma
- ICC
Intrahepatic cholangiocarcinoma
- MRI
Magnetic resonance imaging
- PAS
Periodic acid–Schiff
Authors’ contributions
TA and TY conceived the presented idea and developed the theory and performed the computations. TM, TY, AO, TK, SY, MN, HO and TN encouraged to investigate a specific aspect and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
Funding
There is none of the funding body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics approval and consent to participate
All procedures used in this research were approved by the Ethical Committee of our institution.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ercolani G, Vetrone G, Grazi G, Gian L, Aramaki O, Cescon M, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252:107–114. doi: 10.1097/SLA.0b013e3181e462e6. [DOI] [PubMed] [Google Scholar]
- 2.Abe T, Amano H, Shimamoto F, Hattori M, Kuroda S, Kobayashi T, et al. Prognostic evaluation of mucin-5AC expression in intrahepatic cholangiocarcinoma, mass-forming type, following hepatectomy. Eur J Surg Oncol. 2015;41:1515–1521. doi: 10.1016/j.ejso.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 4.Toriyama E, Nanashima A, Hayashi H, Abe K, Kinoshita N, Yuge S, et al. A case of intrahepatic clear cell cholangiocarcinoma. World J Gastroenterol. 2010;16:2571–2576. doi: 10.3748/wjg.v16.i20.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes SR, Baldaia C, Marques HP, Tortosa F, Ramalho F. Intrahepatic clear cell cholangiocarcinoma—an uncommon histologic subtype: case report and literature review. Rev Esp Enferm Dig. 2017;109:382–385. doi: 10.17235/reed.2017.4239/2016. [DOI] [PubMed] [Google Scholar]
- 6.Buettner S, van Vugt JLA, Izermans JN, Koerkamp B. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther. 2017;10:1131–1142. doi: 10.2147/OTT.S93629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardaman C, Albores-Saavedra J. Clear cell carcinoma of gallbladder and extrahepatic bile ducts. Am J Surg Pathol. 1995;19:91–99. doi: 10.1097/00000478-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa M, Torii T, Toshimitsu Y, Kamizasa N, Suzuki T, Shinozuka N, et al. α-Fetoprotein-producing clear cell carcinoma of the extrahepatic bile ducts. J Clin Gastroenterol. 2006;40:555–557. doi: 10.1097/00004836-200607000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Haas S, Gütgemann I, Wolff M, Fischer HP. Intrahepatic clear cell cholangiocarcinoma: immunohistochemical aspects in a very rare type of cholangiocarcinoma. Am J Surg Pathol. 2007;31:902–906. doi: 10.1097/PAS.0b013e31802c0c8a. [DOI] [PubMed] [Google Scholar]
- 10.Nakanuma Y, Sasaki M, Ikeda H, Sato Y, Zen Y, Kosaka K, et al. Pathology of peripheral intrahepatic cholangiocarcinoma with reference to tumorigenesis. Hepatol Res. 2008;38:325–334. doi: 10.1111/j.1872-034X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 11.Tihan T, Blumgart L, Klimstra DS. Clear cell papillary carcinoma of the liver: an unusual variant of peripheral cholangiocarcinoma. Hum Pathol. 1998;29:196–200. doi: 10.1016/S0046-8177(98)90235-0. [DOI] [PubMed] [Google Scholar]
- 12.Albores-Saavedra J, Hoang MP, Murakata LA, Sinkre P, Yaziji H. Atypical bile duct adenoma, clear cell type: a previously undescribed tumor of the liver. Am J Surg Pathol. 2001;25:956–960. doi: 10.1097/00000478-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Falta EM, Rubin AD, Harris JA. Peripheral clear cell cholangiocarcinoma: a rare histologic variant. Am Surg. 1999;65:592–595. [PubMed] [Google Scholar]
- 14.Adamek HE, Spiethoff A, Kaufmann V, Jakobs R, Riemann JF. Primary clear cell carcinoma of noncirrhotic liver: immunohistochemical discrimination of hepatocellular and cholangiocellular origin. Dig Dis Sci. 1998;43:33–38. doi: 10.1023/A:1018859617522. [DOI] [PubMed] [Google Scholar]
- 15.Logani S, Adsay V. Clear cell cholangiocarcinoma of the liver is a morphologically distinctive entity. Hum Pathol. 1998;29:1548–1549. doi: 10.1016/S0046-8177(98)90031-4. [DOI] [PubMed] [Google Scholar]
- 16.Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745–753. doi: 10.1002/jhbp.166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.