Abstract
The search for promising yeasts that surpass the fermentative capacity of commercial strains, such as Saccharomyces cerevisiae CAT-1, is of great importance for industrial ethanol processes in the world. Two yeasts, Pichia kudriavzevii BB2 and Saccharomyces cerevisiae BB9, were evaluated in comparison to the industrial yeast S. cerevisiae CAT-1. The objective was to evaluate the performance profile of the three studied strains in terms of growth, substrate consumption, and metabolite formation, aiming to determine their behaviour in different media and pH conditions. The results showed that under cultivation conditions simulating the medium used in the industrial process (must at 22° Brix at pH 3.0) the highest ethanol productivity was 0.41 g L−1 h−1 for S. cerevisiae CAT-1, compared to 0.11 g L−1 h−1 and 0.16 g L−1 h−1 for P. kudriavzevii and S. cerevisiae BB2, respectively. S. cerevisiae CAT-1 produced three times more ethanol in must at pH 3.0 (28.30 g L−1) and in mineral medium at pH 3.0 (29.17 g L−1) and 5.0 (30.70 g L−1) when compared to the value obtained in sugarcane must pH 3.0 (9.89 g L−1). It was concluded that S. cerevisiae CAT-1 was not limited by the variation in pH in the mineral medium due to its nutritional composition, guaranteeing better performance of the yeast even in the presence of stressors. Only S. cerevisiae CAT-1 expressed he constitutive invertase enzyme, which is responsible for hydrolysing the sucrose contained in the must.
Electronic supplementary material
The online version of this article (10.1007/s12088-020-00891-6) contains supplementary material, which is available to authorized users.
Keywords: Sugar consumption, Must, Mineral medium, Invertase, Process condition
Introduction
There are two types of energy: non-renewable and renewable. The major non-renewable energy resources are coal, hydrocarbons (petroleum and natural gases), and nuclear processes. On the other hand, renewable energy cannot be depleted, or it can be replenished within a human’s lifetime. Bioenergy, a type of renewable energy, is produced from biological material (including plants, animals, and their by-products) called biomass. Bioenergy can be utilised to generate heat, electricity, and fuels. Thus, it replaces petrochemical fuels and reduces greenhouse gas emissions [1–5]. Considering the environmental benefits of renewable sources of energy, several countries are mandating the share of bioenergy in their national energy matrix. Brazil is recognised worldwide as one of the leaders in the production of sugarcane (Saccharum officinarum), being responsible for 1/3 of the world production [6, 7], and it was the second largest producer of ethanol in 2016, after the U.S. [6]. The production of first-generation ethanol from sugarcane is of great interest, mainly due to its high yields and low costs [7]. Despite that, there are many initiatives for the production of second-generation ethanol that will be necessary with increasing world demand [8, 9]. However, the use of lignocellulosic biomass of sugarcane rather than molasses requires an improvement in pre-treatment technologies, which is not yet economically competitive because of the high cost of the enzymes [7, 9].
The yeast Saccharomyces cerevisiae is a dominant species in the production of ethanol [9, 10] due to its ability to break down sucrose present in sugarcane and its resistance to the selective conditions of the process [10]. It is estimated that S. cerevisiae converts 56% of sucrose into ethanol during the fermentation process [10]. Nevertheless, the stresses present in the alcoholic fermentation may cause drastic damage to cellular organelles and membranes, which leads to growth inhibition or cell death. In addition, many strains have a high tolerance to ethanol and other inhibitors (aldehydes, esters, alcohol, higher alcohol, sulphur compounds, phenols, biomass, methanol, and furfuraldehyde) formed during fermentation, and they have the ability to ferment quickly [5]. Thus, it is necessary to acquire or induce different cellular mechanisms of stress adaptation, e.g., stress protein induction, leading to favourable changes in the membrane structure [11] to keep and/or improve fermentation efficiency.
Therefore, the aim of the present work was to evaluate two fermentative yeast strains, Pichia kudriavzevii BB2 and S. cerevisiae BB9, which were recently isolated in the Brazilian Midwest Region [12], and the industrial yeast S. cerevisiae CAT-1 under conditions similar to those found in industrial processes in order to determine their behaviours in different media and pH conditions.
Materials and Methods
The following two strategies were employed in the present study: (1) cultivations were carried out under simulated industrial media conditions with the yeast strains P. kudriavzevii BB2, S. cerevisiae BB2, and S. cerevisiae CAT-1, and (2) additional experiments were conducted with the yeast isolate that presented the best fermentative performance (Fig. S1).
Yeast Isolates, Maintenance, and Activation
Pichia kudriavzevii BB2 and S. cerevisiae BB9 were previously isolated from the Barralcool S/A Alcohol Plant, Barra do Bugres, MT, Brazil [13]. These isolates are deposited in the Brazilian Midwest Yeast Collection (RECOL). S. cerevisiae CAT-1 was kindly provided by the São Fernando Sugar and Alcohol Plant (USFAA), Dourados, MS, Brazil.
The isolates were preserved in test tubes with 5 mL of “Yeast Extract Pepton Dextrose Agar” (YEPD: yeast extract, 10 g L−1; peptone, 20 g L−1; dextrose, 20 g L−1; agar 15 g L−1) autoclaved at 121 °C for 15 min and added with 20% glycerol (cryoprotectant). The tubes were sealed and stored in an ultra-freezer at – 80 °C and were peeled when necessary.
Cell Activation and Multiplication
The yeasts were reactivated in sterile YEPD agar for 24 h and then transferred to a new agar. Then, the cells were incubated in a 30 °C oven for another 24 h. For cell multiplication, one slope of each selected isolate was transferred to an Erlenmeyer flask (250 mL) containing 200 mL of sterile YEPD broth (yeast extract, 10 g L−1; peptone, 20 g L−1; glucose, 20 g L−1) and incubated for 16–18 h on a rotating orbital shaker (200 rpm, 30 °C). After that, the media were centrifuged (1,500 g per 5 min) in a centrifuge tube (Quimis) and the cells were transferred to the must. Inocula were standardised with an initial cell concentration of 2 × 106 CFU mL−1 [14], equivalent to a concentration of 9 g yeast L−1, which is close to that utilised in the sugar ethanol plants, where fermentations starts with 10–20 g yeast L−1 [15].
Preparation of Must
The must was prepared from concentrated sugarcane juice broth in the pre-crystallisation phase from an industrial concentrator. This creamy looking broth, called “magma,” is composed of two distinct fractions: crystallised sugars (sucrose, glucose, and fructose) and molasses, which is basically composed of the uncrystallised fraction of the sugars. The magma was dissolved to 22°Brix and present pH value of 5.4.
The Brix and pH values were chosen to represent more severe conditions than those found in the industry at the beginning of the process when pH is corrected to 2.0 to 3.0, depending on the level of must bacterial contamination. So the experiments were conducted with two conditions: at pH 3.0 (simulating stressful condition) and at pH 5.0 (close to ideal) (Fig. S2).
Fermentation of Must and Sampling
The fermentation assays simulating the industrial condition were performed with 22°Brix must at pH 5.0. The cultivations with the must were carried out in 500 mL Erlenmeyer flasks containing 300 mL of must, capped with silicone stoppers to avoid air intake. The flasks were incubated in an oven at 30 °C, and the assays were performed in duplicate.
Every 1 h, the flasks were opened for sample collection, with partial aeration of the flask and exit of CO2. Samples were taken in the first 10 h, with a volume of 5 mL for each sampling, totalling 50 mL. The remaining media was collected after 24 h of cultivation. The collected samples were centrifuged (1500 × g, 5 min), and the supernatants were stored in a freezer at – 18 °C prior to the analyses.
Mineral Medium (Verduyn Media)
The mineral medium was prepared according to Nascimento and Fonseca [16]. The carbon source was prepared in order to obtain the same concentrations present in the must at 22° Brix (sucrose 122 g L−1; glucose 51 g L−1; and fructose 38 g L−1). Solutions containing the three sugars were autoclaved separately and then mixed with all other components after reaching room temperature.
Sucrose Hydrolysis Assay
Samples of the must at 22°Brix were collected, treated in duplicate under five different conditions, and analysed using UPLC (ultra performance liquid chromatography) in order to evaluate sucrose hydrolysis and the concentration of sucrose, glucose, and fructose in musts before starting the culture. The treatments were the following: natural must (NM, pH 5.4), sterilised must (SM, pH 5.4), and must acidified to pH 3.0 (SAM). H2SO4 was used to adjust the sample to the correct pH.
Quantification of Extracellular Metabolites and Determination of Biomass
The collected samples were centrifuged in a refrigerated microcentrifuge (NT805, Brazil) (5 min, 17609 × g, 5 °C). The supernatant was used to determine the amounts of residual substrate, ethanol, glycerol, and organic acids in an Agilent 1290 UPLC system equipped with a Rezex ROA-Organic Acid H + (8%) HPLC column (Phenomenex). The mobile phase was trifluoroacetic acid (TFA, 5 mM), with a flow rate of 0.6 mL min−1 at 55 °C for ethanol glycerol and organic acids or 0.3 mL min−1 at 25 °C for residual substrate. The injected volume was 20 µL. These compounds were detected using an Agilent 1260 refractive index detector (RID) coupled to a data acquisition module [17].
The biomass pellet obtained after sample centrifugation was dried in an oven (105 °C) until constant weight. The dried cell mass (g L−1) was obtained by the quotient of the difference of weighing by the volume of centrifuged medium. The biomass concentration (X) was also indirectly determined via Optical Density (OD) measurements performed with a spectrophotometer (Biospectro sp-220) at 600 nm. For this purpose, the absorbance values were converted into mass values using a linear relationship (OD units per gram of dry cell mass) determined for each experiment. The ethanol productivity (Peth) was defined as the difference between the final and initial product concentration divided by time [16].
Quantification of Invertase Activity in Biomass
Enzymatic assays were performed in order to quantify the production of the invertase enzyme and to verify the nature of the invertase, i.e., if it was inducible or constitutive [18]. For this, cells were grown in mineral medium containing three different sources of carbon: sucrose (122 g L−1), glucose (51 g L−1), and a mixture of sucrose (122 g L−1), glucose (51 g L−1), and fructose (38 g L−1) at an initial pH of 5.0 to obtain the same quantity of sugarcane juice at 22 oBrix. The samples were collected at 0, 2, 4, 8, 10, 24, and 48 h.
The reaction mixture was composed of 0.9 mL of 0.1 M sodium acetate buffer (pH 4.5) containing 1% sucrose and 0.1 mL of cell suspension, maintained for 10 min at 50 °C (adapted from work by Barbosa et al. [18]). The reducing sugar that was released was quantified at 540 μm by the DNS (3,5-dinitrosalicylic acid) classic method, which is described elsewhere [19]. The enzymatic activity was expressed as the amount of enzyme producing 1 μmol of product per minute of reaction (U). All experiments were performed in triplicate, and the data are presented as the mean values ± standard deviations. The statistical significance was analysed by measuring the variance (ANOVA, α = 0.05) [20].
Carbon Balance
The carbon balance was determined by estimating the concentration of sugar and biomass input compared to the outputs of residual sugars, biomass, CO2, and ethanol at 24 h and 48 h in terms of C-mol C-mol substrate−1 [21]. The CO2 was theoretically estimated by difference, considering despicable the cellular maintenance rate and the formation of other metabolites.
Results and Discussion
Fermentation in Must at pH 3.0
The sugar consumption and ethanol production curves are shown for S. cerevisiae CAT-1 (Fig. 1a), P. kudriavzevii BB2 (Fig. 1b), and S. cerevisiae BB9 (Fig. 1c) in 22°Brix must (pH 3.0) following 24 h of cultivation. These musts initially demonstrated a sucrose concentration of 46.2 g L−1, wich decreased to 8.2 g L−1 within 2 h (Fig. 1) indicating that the sucrose was broken down during acidification with H2SO4, generating more glucose and fructose. Within 2 h of the beginning of cultivation, the sucrose concentration decreased to 8.1 g L−1 in the medium with S. cerevisiae CAT-1, releasing 19.0 g L−1 h−1 of the substrate. In parallel, the glucose concentration increased from 83.8 to 94.9 g L−1 (5.5 g L−1 h−1 of productivity) and the fructose concentration increased from 91.6 to 109.1 g L−1 (8.8 g L−1 h−1 of productivity) (Fig. 1a). The kinetic profile reveals that the rate of sucrose inversion was higher than the rate of consumption by S. cerevisiae CAT-1 due to the accumulation of the substrate. In addition, it was verified that all sucrose hydrolysis occurred before consuption of all free glucose in the medium, which also probably indicates continuous synthesis of invertase [22], i.e., independently of the carbon source present in the medium, free sucrose is hydrolysed (Fig. 1a). The yeast S. cerevisiae presents two routes for sucrose utilisation: extracellular hydrolysis predominantly by the action of the extracellular periplasmic invertase [18], encoded by the SUC2 gene that encodes for the invertase enzyme [11], releasing glucose and fructose, which are captured by facilitated diffusion through transporters encoded by members of the HXT gene family [23]; and another route involving the active transport of sucrose with subsequent intracellular hydrolysis of sugar [24]. After the depletion of sucrose (9 h of culture), the consumption of glucose and fructose increased slightly, with glucose being slightly preferred over fructose (Fig. 1a). Glucose transport from sucrose hydrolysis occurs using a low affinity system while the free glucose concentration is high, repressing the consumption of other sugars. Since the glucose concentration reaches lower rates, the high affinity system relieves repression over transport of other sugars, allowing the consumption of both partially repressed sugars [17]. It is believed that S. cerevisiae CAT-1 as an industrial yeast presents dominant and resistant physiological aspects, which guarantee its permanence in stressful environments due to its superiority and adaptation compared to other yeasts [25]. The complete sequencing of the genome showed a greater number of genes related to the metabolism of vitamins B1 and B6, which increase competitiveness and guarantee their predominance in relation to wild and laboratory yeasts [26]. Thus, fermentation with S. cerevisiae CAT-1 presents advantages for the industrial process, e.g., reduced foam formation, flocculation, and consumption of inputs. An acidic pH is important for controlling bacterial contamination in the industrial process. Although, in plants, a reduction in pH to 2.0–3.0 through the use of H2SO4 has been adopted for a few hours to control bacterial contamination, this can cause physiological disturbances in yeast, causing a decrease in cell viability [27]. The continuous exposure of the acid to cells in cultures for up to 48 h at pH 3.0 probably caused damage to S. cerevisiae CAT-1 cells, especially because the carbon sources remained in the media (Fig. 1a). The longer the time and the lower the pH, the more severe the impact on yeast metabolism.
Fig. 1.
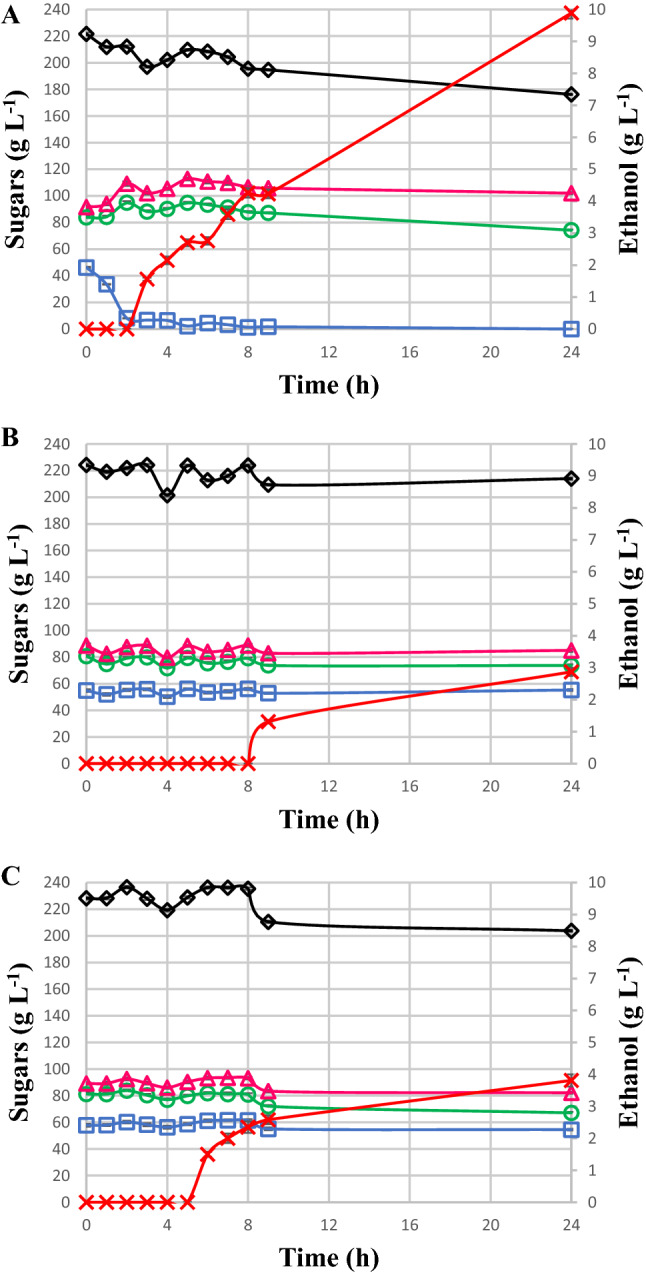
Kinectics of sugar consumption and ethanol production for a S. cerevisiae CAT-1, b P. kudriavzevii BB2 and c S. cerevisiae BB9 in must (pH 3.0) during 24 h of cultivation. Sucrose (open square), glucose (open circle), fructose (open triangle), sum of sugars (open diamond) and ethanol (X). Results are expressed in terms of averages and standard deviations of triplicates (p < 0.05)
Ethanol production occurred after 3 h, reaching 9.9 g ethanol L−1 after 24 h of cultivation (Fig. 1a). It is believed that the stressful situation caused physiological changes, e.g., reduction in viability, sugar consumption, and ethanol production rate. Della-Bianca et al. [27] reported that the low tolerance to growth at pH 3.0 for S. cerevisiae PE-2 is possible due to the synergism between an acidic pH and some unknown factor, significantly affecting ethanol production. Analyses carried out elsewhere showed that S. cerevisiae strains have a locus of the SUC2 gene in their genome, leading to the postulation that it is an adaptation to sucrose-rich broths [25]. Despite the presence of a single copy of SUC2 [28], the strong regulation of S. cerevisiae shows invertase activity, providing efficient consumption of the substrates containing a mixture of sucrose, glucose, and fructose [25]. The hydrolysis profile of sucrose confirms the superiority of S. cerevisiae CAT-1 in acidic must cultures in relation to the other two yeasts evaluated (Fig. 1). This was evident by the decreasing profile of the sucrose curve, even in the presence of high concentrations of glucose in the medium, which could repress invertase production and its activity. S. cerevisiae CAT-1 is a strain selected from the selective pressure of the industrial process. It predominates in fermentation vessels, presenting characteristics essential for its survival under conditions of cellular stress [16]. These characteristics may explain why S. cerevisiae CAT-1 had higher invertase production, even under stressful conditions, e.g., acidic pH of 3.0 and high osmotic pressure at 22°Brix. However, P. kudriavzevii BB2 (Fig. 1b) and S. cerevisiae BB9 (Fig. 1c) did not present differentiated sucrose hydrolysis that could be perceptible from the obtained results. The sucrose profiles of the tested yeasts remained constant, probably not showing the same invertase expression as the yeast S. cerevisiae CAT-1 unless hydrolysis of the available sucrose occurred. The high concentration of sugars in the must at 22°Brix was possibly stressful to the cells, leading to a decrease in viability and, consequently, a reduction in substrate consumption. S. cerevisiae CAT-1 consumed 45 g L−1 of the substrate within 24 h of cultivation, i.e., 20% of the total available substrate (Fig. 1a).
Saccharomyces cerevisiae CAT-1 was highlighted in relation to the other two yeasts during growth in must (pH 3.0) due to its higher ethanol productivity (Peth) of 0.5 g−1 h−1 compared to 0.2 and 0.3 g ethanol g−1 h−1 for P kudriavzevii BB2 and S. cerevisiae BB9, respectively, at 9 h (Table S1). The kinetic profile of P. kudriavzevii BB2 was a virtually constant consumption profile of the three carbon sources present in the must (Fig. 1b), with a reduction of only 10.2 g substrate L−1 during 24 h of culture. Ethanol production started after 9 h and at 24 h 2.9 g L−1 had been produced (Fig. 1b). Similarly, it was not possible to verify any changes in the profile of sucrose consumption by the yeast S. cerevisiae BB9. After 24 h, a total of 18.2 g substrate L−1 had been consumed. S. cerevisiae BB9 produced ethanol during 6 h of culture, reaching 3.8 g ethanol L−1 after 24 h (Fig. 1c). It was reported [29] that S. cerevisiae CAT-1 presented better efficiency in ethanol production, with higher sugar consumption and higher ethanol production when compared to another yeasts in sugarcane juice-based must. The obtained results allowed us to conclude that the yeasts presented different consumption profiles and yields when cultivated with must. Under stressful conditions (pH 3.0, 22°Brix), the industrial strain S. cerevisiae CAT-1 presented greater ability to hydrolyse the available sucrose in the medium (Fig. 1a), even though P. kudriavzevii BB2 also presented good sucrose fermenting capacity, as reported elsewhere [13]. In addition, it showed higher sugar consumption and better ethanol production in relation to P. kudriavzevii BB2 (Fig. 1b) and S. cerevisiae BB9 (Fig. 1c). A laboratory yeast strain was reported to produce less ethanol than an industrial strain in molasses-based media with a high concentration of sugars [30, 31]. The low yield of the laboratory yeast in molasses was attributed to the limitation of sucrose hydrolysis due to non-activation of invertase enzyme and because laboratory strains are more sensitive to certain inhibitory factors in molasses.
Sucrose Hydrolysis During Pre-treatment
From the results obtained with the must (Fig. 1a), it was verified that the initial concentration of sucrose in the acidified must (22°Brix, pH 3.0) was lower than that found for glucose and fructose. However, a much higher concentration of sucrose was expected in relation to the other sugars. It is well known that sucrose corresponds to approximately 60% of the total sugars in magma, while glucose and fructose represent 40% [32]. This led us to investigate must concentrations under different pre-treatment conditions (Table 1).
Table 1.
Effect of pH and temperature on sucrose hydrolysis in must
| Medium | Sugar concentration (g L−1) and relative percentage (%) | |||
|---|---|---|---|---|
| Sucrose | Glucose | Fructose | Total sugar | |
| NM | 126.5 ± 0.46 (59.8)a | 46.0 ± 0.68 (21.7)a | 39.1 ± 0.32 (18.5)a | 211.7 ± 1.47a |
| SM | 130.8 ± 0.95 (61.5)a | 44.6 ± 0.76 (21.0)a | 37.1 ± 0.45 (17.5)a | 213.3 ± 0.64a |
| AM | 125.0 ± 0.65 (58.6)a | 48.3 ± 0.96 (22.7)a | 39.9 ± 0.88 (18.7)a | 213.2 ± 2.49a |
| SAM | 34.5 ± 0.65 (16.1)b | 87.1 ± 1.49 (40.7)b | 92.4 ± 0.45 (43.2)b | 214.0 ± 1.29a |
NM natural must, SM sterilised must, AM acidified must (pH 3.0), SAM sterilised and acidified must (pH 3.0). The values in parenthesis refer to the relative sugar percentage (%) of the initial concentration for each treatment
*Different letters in the same column indicate a significant difference (p < 0.05) according to the Tukey test
The natural must presented a pH of 5.4. H2SO4 was added to reduce the pH to 3.0 before sterilisation. It was observed that acidification and sterilisation alone were not enough to statistically (P > 0.05) alter the composition of the sugars in the must. However, acidification, together with the heat and pressure of the autoclave during sterilisation, triggered the breakdown of sucrose to 16.1% of the total sugars in magma, releasing glucose and fructose, which, in turn, had their concentrations increased to 40.7% and 43.2%, respectively (Table 1). As the industrial must is not sterilised [15], no change in the sugar profile occurred.
Considering the strains studied, S. cerevisiae CAT-1 presented visible sucrose hydrolysis in the must (Fig. 1a). We sought to understand the mechanism adopted by this strain to explain this physiological behaviour. The yeast showed intense breakdown of the sucrose before consuming the free glucose in the medium. To understand this metabolic option adopted by S. cerevisiae CAT-1, new trials were carried out. Thus, must at pH 5.0 was utilised to compare the fermentative capacity at different pH values. Moreover, two other assays using the mineral medium with the same carbon concentrations of the must at 22°Brix at pH 3.0 and pH 5.0 were tested. The mineral medium was prepared in order to reproduce the sugar concentrations of the must. These media of known composition were used to evaluate whether the high sugar concentration of the must could influence the sucrose preference or whether another compound present in the must would inhibit S. cerevisiae CAT-1.
Cultivation of Saccharomyces cerevisiae CAT-1 with Must at pH 5.0 and Mineral Medium at pH 3.0 and 5.0
HCl (1 M) was used to correct the pH of must to 5.0, and the sucrose concentration obtained was 108.70 g L−1. At this time, hydrolysis was found to have occurred at a lower rate compared to the other pre-treatments (Table 1) because a weaker acid was utilised and, obviously, a lower amount of acid was needed to reduce the pH, which resulted in lower hydrolysis during sterilisation.
In this study, there was also a decrease in the sucrose concentration in the must at pH 5.0. After only 2 h of cultivation, this concentration was reduced to 73.8 g L−1, and after 4 h its concentration was reduced to 52.8 g L−1, i.e., 48.5% of the total content was hydrolysed, probably due to the presence of the invertase enzymes. On the other hand, there was an increase in the glucose content of 35.2 g L−1 and an increase in the fructose content of 33.9 g L−1 in 4 h, indicating that the rate of consumption of these sugars by yeast was lower than the breakage [16]. After 24 h of culture, the residual amount of sucrose is zero and, glucose presented an apparent preferential consumption in relation to fructose, that present concentration of 50.8 g L−1 and 93.7 g L−1 respectively (Fig. 2a). In the must at pH 5.0, the ethanol production started after 4 h, reaching 21.8 g ethanol L−1.
Fig. 2.
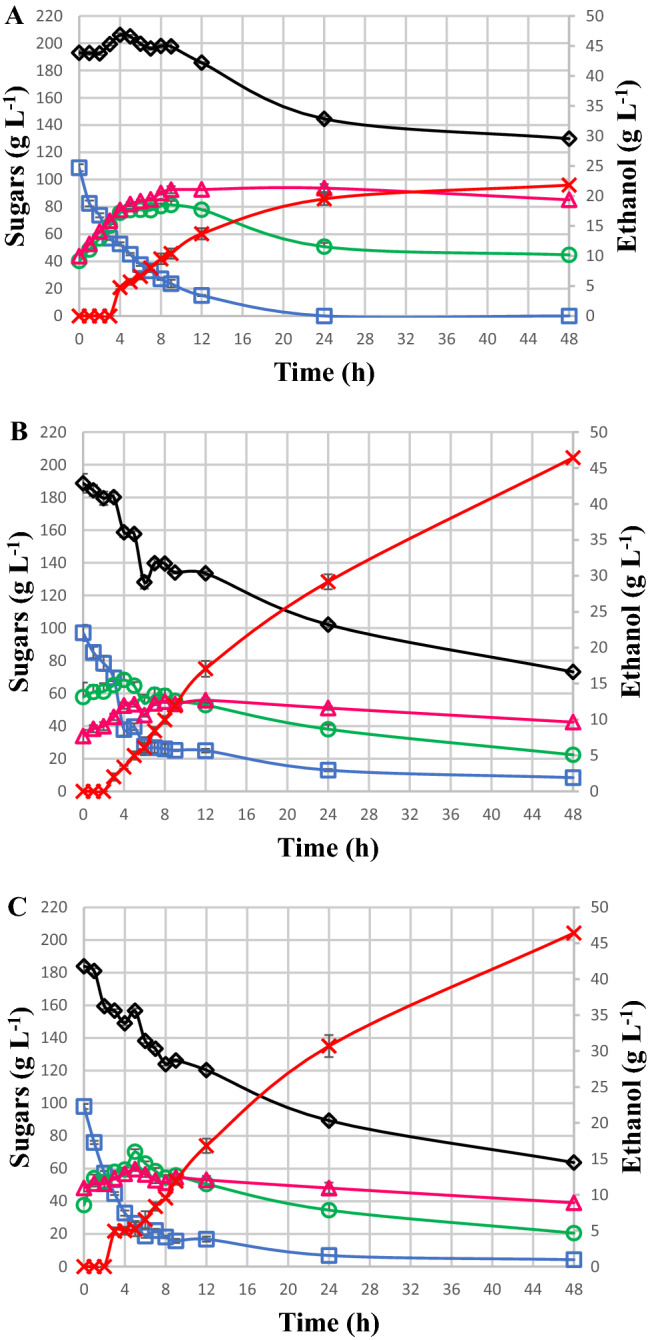
Kinetics of sugar consumption and ethanol production for S. cerevisiae CAT-1 in must (pH 5.0—a) and mineral medium (pH 3.0—b and pH 5.0—c) during 48 h of cultivation. Sucrose (open square), glucose (open circle), fructose (open triangle), sum of sugars (open diamond) and ethanol (X). Results are expressed in terms of averages and standard deviations of triplicates (p < 0.05)
When cultivated in mineral media at pH 5.0, the ethanol production started after 3 h, reaching 46.4 g ethanol L−1 after 48 h, which is very similar to the mineral media at pH 3.0. The final ethanol production in both types of mineral media was two times higher than the ethanol production in must at pH 5.0. The highest ethanol productivity was observed from 12 to 24 h, producing 1.2 g of ethanol L−1 h−1 (Fig. 2a). The increase in fructose up to 12 h of culture in mineral media at pH 3.0 and 5.0 resulted from the breakdown of sucrose. When the glucose concentration was reduced to 13.0 L−1 h−1 at pH 3.0 after 24 h and to 16.8 g L−1 at pH 5.0 after 12 h, the fructose was finally consumed, indicating a preference for glucose, as reported in the literature [17].
Biomass Production
The catabolism of the substrates by S. cerevisiae can occur via aerobic and anaerobic routes. It is verified that in yeasts, even in the presence of oxygen, the anaerobic pathway can also be utilised [33]. The low biomass accumulated indicates that the metabolism was practically fully fermentative. The fermentation process causes an increase in energy consumption for the maintenance and deviation of carbon to the formation of metabolites, such as ethanol. Fermentation can be triggered by the inhibition of respiration due a high concentration of glucose. Glucose represses the expression of genes that encode Krebs Cycle enzymes, respiratory chain enzymes, and mitochondrial structures and induce the expression of genes involved in fermentative metabolism [34]. The cultivations presented significant changes in biomass production. It was verified that the mineral medium presented higher production in a shorter culture time in relation to the must medium (Fig. S3). The growth in mineral medium at pH 5.0 produced higher biomass content (2 g L−1) after 9 h of culture. In the same period, the growth in must at pH 5.0 produced only 0.90 g L−1 (Fig. 2b). It is believed that the defined composition of the mineral medium favors the development of yeasts due to the higher amount of nutrients. Moreover, the amount of nitrogen is limited in musts, and only ammonium may be considered the nitrogen source for the observed effect on fermentation. Ammonium and nitrate can be found up to 250 mg L−1. However, S. cerevisiae is incapable of assimilating nitrate, and it has very little capacity to assimilate traces of proteins, peptides, and peptide-like compound that can be present in the medium [35]. Nitrogenous compounds are important for the success and conclusion of industrial fermentation processes. They are involved in the metabolism and growth of yeasts, affecting the correct evolution of fermentation and ensuring quality in ethanol production [36]. On the other hand, it is presumed that a decrease in the viability of the yeasts in the mineral media at pH 3.0 and 5.0 after 9 h may have been caused by the production and accumulation of ethanol.
In its composition, mineral medium contains nitrogenous components, potassium, magnesium, and vitamins such as biotin and thiamine [16]. An already described phenomenon is that yeasts do not grow under thiamine limitation [32]. It is believed that these components are of extreme nutritional importance for the development of yeasts, favouring the production of biomass. The must at pH 5.0 had a higher adaptation phase and lower values of biomass in relation to the mineral medium. During 12 h of cultivation, 0.9 g biomass L−1 was produced in the must, while the cultures with mineral medium reached the same value within 6 h. The cultivation with must showed a decrease in biomass production after 24 h of cultivation. This was possibly caused by the high concentration of ethanol and other metabolites in the medium, acting as a stressing factor on the yeasts (Fig. 2c). However, in the mineral medium, biomass production was reduced after 9 h, which corresponded to the period of higher ethanol production (Fig. 2b, c), indicating a probable loss of yeast viability, possibly due to the increased ethanol concentrations.
Ethanol stress inhibits the amino acid and glucose transport systems, leading to the dissolution of the proton gradient concentration and a loss of nutrition in the cell, thereby inhibiting yeast metabolic activity and reducing cellular viability [37, 38]. Beyond affecting the composition of the yeast membrane, ethanol affects the physiology of the yeast, inhibiting its growth and causing enzymatic inactivation, which leads to decreased cellular viability. Media with low nutrient concentrations have limited growth and low biomass yields in the substrate [17]. Recycling cells during the industrial process causes an increase in dead cells that serve as a food source, enriching the fermentative medium by releasing amino acids, vitamins, and minerals.
Carbon Balance
The carbon balance demonstrates the preferred metabolic routes for each pH and media condition. The carbon balance showed a direct relactionship between residual sugars and pH when compared to the mineral media. At a lower pH, there was more residual sugar. When comparing the mineral media (richer in nutrients) with must, it is clear that the must does not have the nutrients necessary for a higher consumption of the sugars, indicated by the higher residual sugar content, probably due to its poor composition in nitrogen sources, which directly interferes in the metabolism [17, 35, 36] (Table S2). The same can be observed in the production of ethanol in must that reaches half of the production of the mineral media at both pH values 3.0 and 5.0.
The CO2 produced was present in the same proportion of ethanol, indicating that the metabolism was strictly fermentative since the stoichiometric C-molar relationship between CO2 and ethanol was the same in this pathway [15]. In accordance, the biomass formation was not relevant in terms of carbon consumption. In fact, the variation in cell concentration was very small for the conditions studied (Table S2). This demonstrates that under anaerobic conditions there is no considerable cell growth, which is in accordance with what is observed in fuel alcohol industries.
Determination of Invertase Activity in Cells
After cultures with S. cerevisiae CAT-1 in mineral medium and must at pH 3.0 and 5.0, it was verified that independent of the condition the yeast presented an expressive capacity to hydrolyse the sucrose, presumably due to the expression of invertase enzymes by the yeast, as reported elsewhere [39].
From these results, S. cerevisiae CAT-1 was cultured again in mineral medium (pH 5.0) in order to evaluate the presence of the invertase enzyme and its nature. The three sources of carbon present in the natural must were sucrose (122 g L−1), glucose (51 g L−1), and a mixture of sucrose (122 g L−1), glucose (51 g L−1), and fructose (38 g L−1).
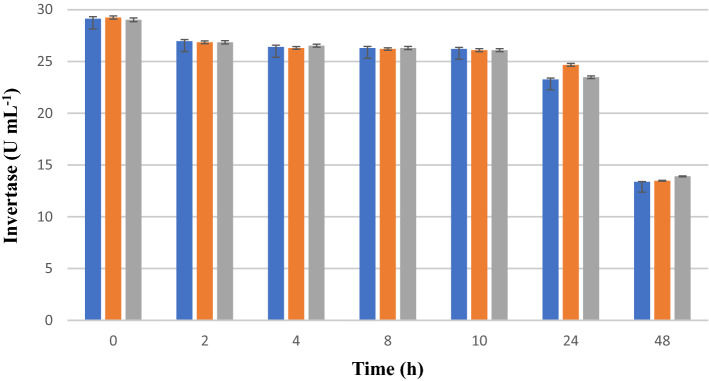
Saccharomyces cerevisiae CAT-1 produced the same dosage of invertase in the experiments with the three carbon sources tested. Thus, the assays demonstrated that invertase is a constitutive enzyme (Fig. 3). Constitutive enzymes are produced continuously by microorganisms, whether a filamentous or a yeast fungus, and regardless of the presence of a substrate they are produced in constant quantities without taking into account the physiological requirement or the concentration of the substrate. They are synthesised continuously because their role in the maintenance of cellular or structural processes are indispensable [40].
Fig. 3.
Invertase activity (U mL−1) of S. cerevisiae CAT-1 cultivated in mineral medium (pH 5.0) during 48 h of cultivation. In blue: sucrose (122 g L−1), glucose (51 g L−1), and fructose (38 g L−1); in orange: sucrose (122 g L−1); and in gray: glucose (51 g L−1). Results are expressed in terms of averages and standard deviations of triplicates (p < 0.05)
These results on the presence of the enzyme and its constitutive nature explain the breakdown of sucrose, independently of the presence of free glucose in the medium during the experiments. Therefore, whatever the available sugar is, S. cerevisiae CAT-1 will produce enzymes capable of metabolising the breakage of the sucrose present in the medium, releasing monosaccharides (glucose and fructose). This characteristic can be one of the reasons for the superiority of this industrial yeast in alcohol plants, where sucrose is rapidly hydrolysed to glucose and fructose, accelerating the fermentation process for ethanol production.
Beyond the high invertase activity, S. cerevisiae also presents several SUC loci in the genome when exposed to media of cultures where sucrose is the main sugar to be fermented (e.g., must). The SUC2 gene is present in all yeasts of the genus Saccharomyces [25]. Another mechanism was described for the entry of sucrose into the cell, mediated by AGT1 permeas [39]. Sequencing of S. cerevisiae CAT-1 showed the presence of both SUC2 and AGT1 genes [41].
This rapid hydrolysis of the sucrose provoked by the enzyme invertase releases a large amount of glucose and fructose in the medium and may favour the development of other yeasts that do not have invertase, including contaminating strains. However, studies have shown that due to stressful conditions in the industrial process, S. cerevisiae CAT-1 is still the most widely used yeast and is best suited for ethanol production in a large scale. This increase in free monosaccharides in the medium can cause alterations in the gene expression of the cells of other S. cerevisiae strains due to changes in the osmotic equilibrium of the medium caused by the high concentrations of glucose and fructose released [28].
Saccharomyces cerevisiae CAT-1 initially produced 29 U mL−1 invertase in the three different substrate conditions (Fig. 3). During the 24 h of culture, the enzymatic production remained constant. After 48 h of cultivation, the production was reduced to 13 U mL−1 for the three carbon sources (Fig. 3). This may occur due to the inhibition of the metabolism of yeast or due to the presence of other compounds, e.g., the presence of ethanol in the medium, which denatures the protein.
However, what makes S. cerevisiae CAT-1 well suited to the rigorous plant conditions and guarantees its dominance and persistence in relation to other yeasts is not fully known [5].
Conclusion
Pichia kudriavzevii BB2 and S. cerevisiae BB9 did not show industrial potential for ethanol production since they did not perform well under the conditions studied. S. cerevisiae CAT-1 had better fermentative performance compared to the other yeasts tested. It presented better production of ethanol and physiological characteristics that ensured good performance against stressful aspects. S. cerevisiae CAT-1 showed low ethanol production at pH 3.0 due to an altered composition of sugar and the presence of stressful factors in the must, but the mineral media present good fermentative adaptation with the mineral media at pH 3.0 due to the rich composition of the mineral medium, which provided greater resistance to yeast during cultivation. Still S. cerevisiae CAT-1 presented better results at pH 5.0, demonstrating that the pH stress increase in the nutrient-poor natural environment. The rate of invertase production was the same for the three carbon sources tested during cultivation of S. cerevisiae CAT-1. The results showed that regardless of the nature of the sugar or concentration, the yeast produced the same amounts of invertase, which allowed us to conclude that invertase is a constitutive enzyme in S. cerevisiae CAT-1. Other studies carried out under the process conditions with S. cerevisiae CAT-1 will be important to determining its behaviour when compared to bench studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the Brazilian research funding agencies: Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) for their financial support.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel SKS, Kumar V, Mardina P, Li J, Lestari R, Kalia VC, Lee JK. Methanol production from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Biores Technol. 2018;263:25–32. doi: 10.1016/j.biortech.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Patel SKS, Gupta RK, Otari SV, Gao H, Lee J, Zhang L. Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J. 2019;302:1–8. doi: 10.1002/biot.201800468. [DOI] [PubMed] [Google Scholar]
- 3.Patel SKS, Jeon MS, Gupta RK, Jeon Y, Kalia VC, Kim SC, Lee J-K. Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 4.Patel SKS, Ray S, Prakash J, Wee JH, Kim S-Y, Lee J-K, Kalia VC. Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol. 2019 doi: 10.1007/s12088-018-00777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Della-Bianca BE, Basso TO, Stambuk BU, Basso LC, Gombert AK. What do we know about the yeast strains from the Brazilian fuel ethanol industry? Appl Microbiol Biotechnol. 2013;97:979–991. doi: 10.1007/s00253-012-4631-x. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho AL, Antunes CH, Freire F. Economic-energy-environment analysis of prospective sugarcane bioethanol production in Brazil. Appl Energy. 2016;181:514–526. doi: 10.1016/j.apenergy.2016.07.122. [DOI] [Google Scholar]
- 7.Raza G, Ali K, Hassan MA, Ashraf M, Kahan MT, Kahan IA. Sugarcane as a Bioenergy Source. In: Kahan MT, Kahan IA, editors. Sugarcane biofuels: status, potential, and prospects of the sweet crop to fuel the world. Switzerland: Springer; 2019. pp. 3–19. [Google Scholar]
- 8.Bechara R, Gomez A, Saint-Antonin V, Schweitzer J-M, Maréchal F, Ensinas A. Review of design works for the conversion of sugarcane to first and second-generation ethanol and electricity. Renew Sust Energy Rev. 2018;91:152–164. doi: 10.1016/j.rser.2018.02.020. [DOI] [Google Scholar]
- 9.Carvalho DJ, Moretti RR, Colodette JL, Bizzo WA. Assessment of the self-sustained energy generation of an integrated first and second generation ethanol production from sugarcane through the characterization of the hydrolysis process residues. Energy Convers Manag. 2020;203:112267. doi: 10.1016/j.enconman.2019.112267. [DOI] [Google Scholar]
- 10.Wang L, Xin-qing Zhao, Xue C, Feng-Wu Bai. Impact of osmotic stress and ethanol inhibition in yeast cells on process oscillation associated with continuous very-high-gravity ethanol fermentation. Biotechnol Biofuels. 2013;6:133. doi: 10.1186/1754-6834-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dangi AK, Dubey KK, Shukla P. Strategies to improve Saccharomyces cerevisiae: technological advancements and evolutionary engineering. Indian J Microbiol. 2017;57:378–386. doi: 10.1007/s12088-017-0679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo JZ, Nascimento VM, Stefanello I, Silva CAA, Gonçalves FA, Perdomo IC, Vilela DM, Simionatto S, Pereira RM, Paz MF, Leite RSR, Gelinski JMLN, Fonseca GG. Biochemical evaluation, molecular characterization and identification of novel yeast strains isolated from Brazilian savannah fruits, chicken litter and a sugar and alcohol mill with biotechnological potential for biofuel and food industries. Biocatal Agric Biotechnol. 2018;16:390–399. doi: 10.1016/j.bcab.2018.09.006. [DOI] [Google Scholar]
- 13.Silva RO, Batistote M, Cereda MP. Wild strains of fermenting yeast isolated of sugar cane juice from an alcohol distillery from Mato Grosso, Brazil. J Biotechnol Biodivers. 2011;2:22–27. doi: 10.1590/s1516-89132013000200001. [DOI] [Google Scholar]
- 14.Araque E, Parra C, Rodriguez M, Freer J, Baeza J. Selection ofthermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production. Enzyme Microbial Technol. 2008;43:120–123. doi: 10.1016/j.enzmictec.2008.02.007. [DOI] [Google Scholar]
- 15.Lima UA, Aquarone E, Borzani W, Schmidell W. Biotecnologia Industrial: processos Fermentativos e Enzimáticos. São Paulo: Blucher; 2001. [Google Scholar]
- 16.Nascimento VM, Fonseca GG. Effects of the carbon source and the interaction between carbon sources on the physiology of the industrial Saccharomyces cerevisiae CAT-1. Prep Biochem Biotechnol. 2019;11:1–8. doi: 10.1080/10826068.2019.1703192. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca GG, Carvalho NMB, Gombert AK. Growth of the yeast Kluyveromyces marxianus CBS 6556 on different sugar combinations as sole carbon and energy source. App Microbiol Biotechnol. 2013;97:5055–5067. doi: 10.1007/s00253-013-4748-6. [DOI] [PubMed] [Google Scholar]
- 18.Barbosa PMG, Morais TP, Silva CAA, Santos FRS, Garcia NFL, Fonseca GG, Leite RSR, Paz MF. Biochemical characterization and evaluation of invertases produced from Saccharomyces cerevisiae CAT-1 and Rhodotorula mucilaginosa for the production of fructooligosaccharides. Prep Biochem Biotechnol. 2018;48:506–513. doi: 10.1080/10826068.2018.1466155. [DOI] [PubMed] [Google Scholar]
- 19.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 20.Patel SKS, Shanmugam R, Kalia VC, Lee J-K. Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour Technol. 2020 doi: 10.1016/j.biortech.2020.123022. [DOI] [PubMed] [Google Scholar]
- 21.Stephanopoulos GN, Aristidou AA, Nielsen J. Metabolic engineering principles and methodologies. San Diego: Academic Press; 1998. [Google Scholar]
- 22.Sankaran S, Qureshi MAA, Subbaiah S, Kavitha M. Isolation and optimization of constitutively synthesized invertase, from Saccharomyces cerevisiae mutant type strain. Int J Appl Nat Sci. 2017 doi: 10.13140/rg.2.2.17876.35207. [DOI] [Google Scholar]
- 23.Lin Z, Li W-H. Expansion of hexose transporter genes was associated withthe evolution of aerobic fermentation in yeasts. Mol Biol Evol. 2010;28:131–142. doi: 10.1093/molbev/msq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marques WL, Mans R, Marella ER, Cordeiro RL, van den Broek M, Daran J-MG, Pronk JT, Gombert AK, van Maris AJA. Elimination of sucrose transport and hydrolysis in Saccharomyces cerevisiae: a platform strain for engineering sucrose metabolism. FEMS Yeast Res. 2017;9:1–17. doi: 10.1093/femsyr/fox006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stambuk BU, Dunn B, Alves SL, Jr, Duval EH, Sherlock G. Industrial fuel ethanol yeasts contain adaptive copy number changes in genes involved in vitamin B1 and B6 biosynthesis. Gen Res. 2009;19:2271–2278. doi: 10.1101/gr.094276.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogowska A, Pomastowski P, Złoch M, Railean-Plugaru V, Król A, Rafńska K, Szultka-Młyńska M, Buszewski B. The infuence of diferent pH on the electrophoretic behaviour of Saccharomyces cerevisiae modifed by calcium ions. Sci Rep. 2018;8:7261. doi: 10.1038/s41598-018-25024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della-Bianca BE, Hulster E, Pronk JT, van Maris AJA, Gombert AK. Physiology of the fuel ethanol strain Saccharomyces cerevisiae PE-2 at low pH indicates a context-dependent performance relevant for industrial applications. FEMS Yeast Res. 2014;14:1196–1205. doi: 10.1111/1567-1364.12217. [DOI] [PubMed] [Google Scholar]
- 28.Marques WL, Raghavendran V, Stambuk BU, Gombert AK. Sucrose and Saccharomyces cerevisiae: a relationship most sweet. FEMS Yeast Res. 2016;16:1–16. doi: 10.1093/femsyr/fov107. [DOI] [PubMed] [Google Scholar]
- 29.Batistote M, Cardoso CAL, Ramos DD, Ernandes JR. Desempenho de leveduras obtidas em indústrias de Mato Grosso do Sul na produção de etanol em mosto a base de cana de açúcar. Ciênc Nat. 2010;32:83–95. doi: 10.5902/2179460x9487. [DOI] [Google Scholar]
- 30.Wu R, Chen D, Cao S, Lu Z, Huang J, Lu Q, Chen Y, Chen X, Guan N, Weia Y, Huang R. Enhanced ethanol production from sugarcane molasses by industrially engineered Saccharomyces cerevisiae via replacement of the PHO4 gene. RSC Adv. 2020;10:2267–2276. doi: 10.1039/c9ra08673k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes ML, Paulillo SCL, Godoy A, Cherubin RA, Lorenzi MS, Giometti FHC, Bernardino CD, Neto HBA, Amorim HV. Ethanol production in Brazil: a bridge between science and industry. Braz J Microbiol. 2016;47:64–76. doi: 10.1016/j.bjm.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonseca GG, Gombert AK, Heinzle E, Wittmann C. Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 2007;7:422–435. doi: 10.1111/j.1567-1364.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 33.Imura M, Nitta K, Iwakiri R, Matsuda F, Shimizu H, Fukusaki E. Comparison of metabolic profiles of yeasts based on the difference of the Crabtree positive and negative. J Biosci Bioeng. 2020;129:52–58. doi: 10.1016/j.jbiosc.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Traven A, Wong JMS, Xu D, Sopta M, Ingles CJ. Interorganellar communication: altered nuclear gene expression profiles in a yeast mitochondrial DNA mutant. J Biol Chem. 2000;276:4020–4027. doi: 10.1074/jbc.m006807200. [DOI] [PubMed] [Google Scholar]
- 35.Souza RB, Menezes JAS, Souza RFR, Dutra ED, Morais MA. Mineral composition of the sugarcane juice and its influence on the ethanol fermentation. Appl Biochem Biotechnol. 2015;175:209–222. doi: 10.1007/s12010-014-1258-7. [DOI] [PubMed] [Google Scholar]
- 36.Roca-Mesa H, Sendra S, Mas A, Beltran G, Torija M-J. Nitrogen preferences during alcoholic fermentation of different Non-Saccharomyces yeasts of oenological interest. Microorganisms. 2020;8:157. doi: 10.3390/microorganisms8020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Wu D, Lin Y, Wang X, Kong H, Tanaka S. Substrate and product inhibition on yeast performance in ethanol fermentation. Energy Fuels. 2015;29:1019–1027. doi: 10.1021/ef502349v. [DOI] [Google Scholar]
- 38.Basso TO, Kok S, Dario M, Espírito-Santo JCA, Muller G, Schlong PS, Silva CP, Tonso A, Daran J, Gombert AK, Maris AJAV, Pronk JT, Stambuk BU. Engineering topology and kinetics of sucrose metabolism in Saccharomyces cerevisiae for improved ethanol yield. Metabol Eng. 2011;13:694–703. doi: 10.1016/j.ymben.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Marques WL, Mans R, Marella ER, Cordeiro RL, Van Den Broek M, Daran JG, Pronk JT, Gombert AK, Van Maris JA. Elimination of sucrose transport and hydrolysis in Saccharomyces cerevisiae: a platform strain for engineering sucrose metabolism. FEMS Yeast Res. 2017;17:1–11. doi: 10.1093/femsyr/fox006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li B, Liu H, Zhang Y, Kang T, Zhang L, Tong J, Xiao L, Zhang H. Constitutive expression of cell wall invertase genes increases grain yield and starch content in maize. Plant Biotechnol J. 2013;11:1080–1091. doi: 10.1111/pbi.12102. [DOI] [PubMed] [Google Scholar]
- 41.Babrzadeh F, Jalili R, Wang C, Shokralla S, Pierce S, Robinson-Mosher A, Nyren P, Shafer RW, Basso LC, Amorim HV, Oliveira AJ, Davis RW, Ronaghi M, Gharizadeh B, Stambuk BU. Whole-genome sequencing of the efficient industrial fuel-ethanol fermentative Saccharomyces cerevisiae strain CAT-1. Mol Gen Genom. 2012;287:485–494. doi: 10.1007/s00438-012-0695-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.