Abstract
The biogenic tailoring of silver nanoparticles using plant extract is becoming an attractive approach in the current scenario. Manilkara zapota (MZ) is well known for its antibacterial, hepato-protective, anti-inflammatory, anti-tussive, anti-fungal, anti-tumour, and free radical scavenging potential. Its plants extract is a rich source of secondary metabolites. Nowadays, silver nanoparticles (AgNPs) have been advocated for a variety of biomedical applications. In present work, silver nanoparticles have been synthesized using an aqueous extract of MZ, physicochemically characterized and finally evaluated for antimicrobial effects, catalytic reduction/degradation of organic dyes and cytotoxicity. The nanosized AgNPs (~ 84 nm) were found to possess prominent antibacterial potential against gram positive and gram negative pathogens (MIC 50 μg/ml) in comparison to native plant extract. Moreover, these particles were found to be non-toxic and efficient eradicators of environmental toxicants via rapid catalytic reduction of toxic chemicals and dyes. Altogether, these results suggest promising potential of these nanoparticles that can be used as multifunctional agents for future biomedical applications.
Electronic supplementary material
The online version of this article (10.1007/s12088-020-00889-0) contains supplementary material, which is available to authorized users.
Keywords: Manilkara zapota, Phytonanotechnology, Silver nanoparticles, Catalytic reduction, Antibacterial, Antibiofilm, Antioxidant activity
Introduction
Recently, metal-based nanoparticles have been shown to possess promising potential in biomedical applications. The conversion of bulk material into nano-sized particles results in the exhibition of unique properties of matter owing to significantly increased surface area to volume ratio [1, 1 of mss]. Because of these unique properties, nanoparticles have been inculcated in the biosensor, nanocatalyst, antimicrobial, pharmaceutical, and environmental applications [2–6]. These properties have also made them active against various types of pathogens. Their mode of action has made it difficult for pathogens to develop resistance against them, which has resulted in the broadening of spectrum of antimicrobials. Further with the advent of nanobiocatalysts, which are produced by immobilizing biocatalysts (enzymes) onto functionalized nanomaterials/nanocomposites, bioprocess engineering has recorded tremendous improvements over the last few years [7–13]. The resulting conjugation has not only improved the stability and activity of the immobilized biocatalysts but also demonstrated the great potential of nanobiocatalysts in the future biotransformations.
Synthesis of nanoparticles has been the subject of immense interest to scientists/researchers worldwide. A plethora of protocols have been developed for their synthesis via chemical, physical and biological routes [14–17]. Sodium borohydride, hydrazine, ethylene glycol, trisodium citrate, etc. are some of the commonly used chemicals to produce metal/metal oxide nanoparticles, however, variable size and size distribution as well as poor stability have hampered their widespread applications. Therefore, attention has been diverted to develop clean, ecofriendly and economical physical and biological methods, which could obviate the use of toxic and hazardous chemicals. Physical methods, viz., spray pyrolysis, ball milling, thermal evaporation, pulsed laser desorption, etc., were found effective, however, these were proved to be quite tedious, expensive and required specialized equipments. Hence, biological methods were ultimately found suitable to address all the concerns of other methods [18]. Amongst them, phytonanotechnology i.e. use of plant-derived phytochemicals, is an upcoming area that deals with the manipulation of the materials within nanorange. Due to their uniform and sharp size distribution in nanometer range, silver nanoparticles (AgNPs) have been the most studied material which have shown potential as anti-cancerous, anti-inflammatory [19], anti-fungal [20], anti-bacterial [21] and anti-viral agents [22]. Water-soluble plant metabolites present in their extracts have not only acted as reducing agents but also as stabilizing agents. Therefore, the eco-friendly synthesis protocols employing extracts of the plant materials [23–26] have gained widespread interest among researchers as facile, efficient, economical and easy methods for the synthesis of particles in nano-sized range.
Herein, Manilkara zapota, a fruit majorly found in India, South East Asia, Central America, Malaysia, Thailand, Indonesia, is an evergreen tree and the fruit and leaves are the rich source of polyphenolic compounds that serve as good antioxidants and prevent the body from harmful infections [27]. Profound biological activities of its subparts have attracted its contribution for synthesizing metallic nanoparticles. Seed and leaf extract-mediated synthesis of AgNPs have exhibited various activities, viz., acaricidal, anti-fungal, anti-cancer, etc. [28–31]. In present work, we have synthesized MZ (sapodilla) aqueous leaf extract-mediated AgNPs and characterized them by physicochemical and morphological techniques (e.g. UV–VIS spectroscopy, hydrodynamic size, surface charge, FTIR, XRD, TEM, SEM, and RAMAN spectroscopy). Further, these nanoparticles were evaluated in detail for their antimicrobial competence and toxicological profile. Besides, these particles also exhibited their ability in remediation of environmental pollutants (organic dyes, chemicals, etc.).
Results and Discussion
Preparation of AgNPs and Their Characterization
Synthesis of silver nanoparticles using aqueous extract of MZ leaves was confirmed by observing a change in the color of the reaction from light yellow to dark brown as well as recording the absorption spectrum (Fig. S1). Further, these nanoparticles were characterized by various physicochemical techniques such as dynamic light scattering (DLS), transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier Transform Infrared (FTIR) and Raman spectroscopy (Figs. S2-S6). DLS results showed the average hydrodynamic diameter and surface charge of AgNPs ~ 84 nm and ~ − 18.0 mV, respectively.
Catalytic Activity
Catalytic Reduction of p-Nitrophenol to p-Aminophenol
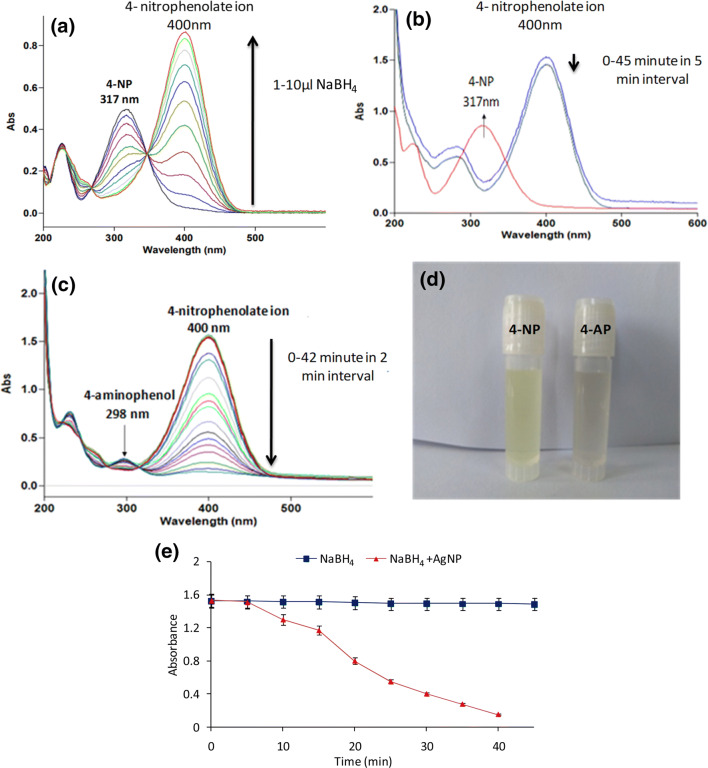
Synthesized AgNPs were evaluated for their catalytic activity by reduction of p-nitrophenol (p-NP), which is commonly found in the industrial (agriculture, paper, textiles and paints) effluents, with aqueous NaBH4 at ambient temperature to a less toxic p-aminophenol (p-AP). The conversion of p-NP into p-AP in the presence of NaBH4 is reported to be a very slow process, however, occurs rapidly under the influence of catalytic agents, overcoming the kinetic barriers by facilitating electron transfer from the donor borohydrate ion to the acceptor p-NP. Here, the reaction was also monitored via absorption spectroscopy. Amount of sodium borohydride required for the reaction was also optimised spectroscopically. In this study, absorption peak at 317 nm due to p-nitrophenol got shifted to 403 nm, which showed the conversion of p-NP into p-nitrophenolate ions with color change from almost colorless to yellow [32]. The absorption peak at 403 nm decreased marginally after 2 h of treatment with NaBH4 (100 µl; 0.1 M), while, in the presence of AgNPs, the absorption peak at 403 nm decreased very rapidly and a new peak ~ 300 nm appeared, which was due to formation of p-AP (Fig. 1). The peak due to p-nitrophenolate ions at 403 nm disappeared completely within 20 min and the color of the reaction solution changed from yellow to almost colorless further indicating the reduction of p-NP to p-AP. The results demonstrate the potential of these nanoparticles to be used as catalytic agents.
Fig. 1.
Catalytic reduction of p-NP to p-AP using NABH4 and AgNPs. a Optimization of NABH4 concentration for p-NP reduction, b reduction of p-NP in absence of AgNPs, c p-NP reduction in presence of AgNPs, d visual observation of color change from yellow (nitrophenolate) to colorless (p-AP) and e progress of reactions in the absence and presence of AgNPs
Catalytic Degradation of Organic Dyes
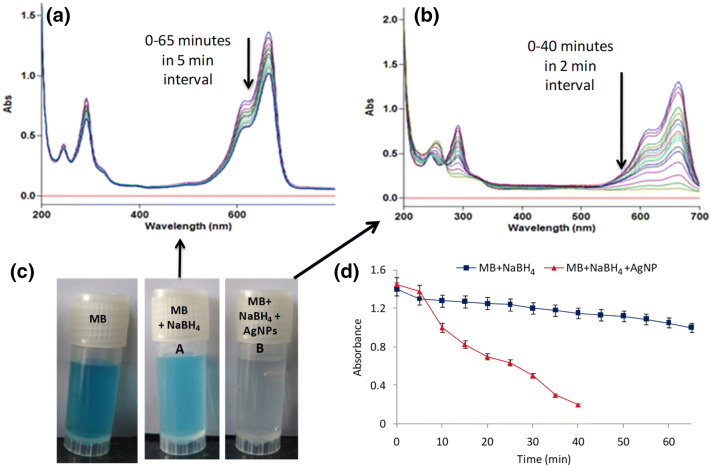
Organic dyes, methylene blue (MB) and congo red (CR), routinely used in various industries, are quite toxic which contaminate the surrounding water bodies. In this study, we have investigated the catalytic reduction of methylene blue (MB) with sodium borohydride using UV–VIS spectroscopy at different time points. Two sets of reactions were performed, viz., (a) the dye solution was treated with sodium borohydride alone, and (b) the dye solution was treated with both sodium borohydride and AgNPs. Both sets of reactions were monitored spectrophotometrically by scanning in the range of 200–700 nm. In the absence of AgNPs, the results revealed a slow progress in the reaction even after 65 min. However, in the presence of AgNPs, the reduction of MB was significantly faster and was found to be complete within 40 min with change in color from blue to colorless as shown in Fig. 2.
Fig. 2.
Catalytic degradation of methylene blue (MB) using NABH4 and AgNPs. a Degradation of MB in presence of NaBH4, b MB degradation in presence of NaBH4 and AgNPs, c visual observation of color change from blue to colorless in the presence of NaBH4 and AgNPs, and d Progress of reaction in the absence and presence of AgNPs
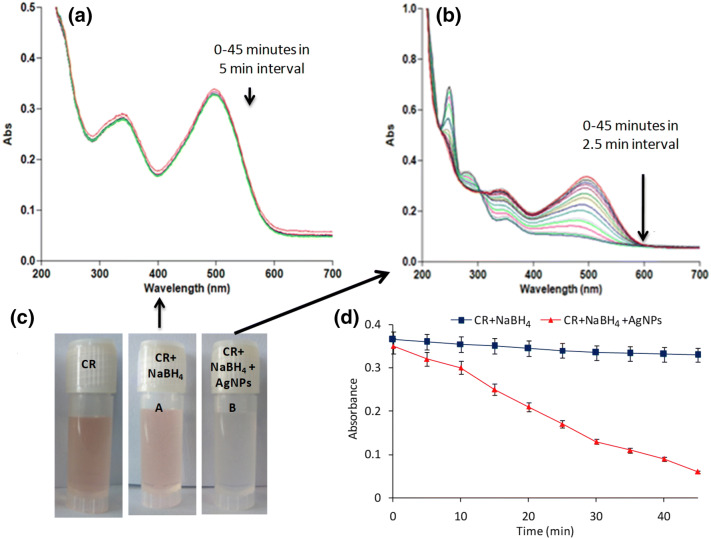
Another dye, congo red, is a well known hazardous and carcinogenic anionic dye. Its presence in aquatic ecosystem imparts harmful effects on marine life. Its degradation studies were monitored spectrophotometrically. Here too, two sets of reactions were performed, viz., in the absence and presence of AgNPs. In the absence of nanoparticles, the spectrophotometric data showed insignificant change in the absorbance values even after 45 min of treatment, whereas, in the presence of AgNPs, a fast reduction reaction was observed that occurred within 30 min with color change from red to colorless as depicted in Fig. 3.
Fig. 3.
Catalytic degradation of congo red (CR) by NABH4 and AgNPs. a Reduction of CR in presence of NaBH4, b CR degradation in the presence of AgNPs, c visual observation of color change from red to colorless in the presence of AgNPs, and d progress of reaction in the absence and presence of AgNPs
Antimicrobial Activity
Zone of Inhibition
Single disk susceptibility assay was evaluated for aqueous extract and AgNPs at different concentrations. Sample concentrations (10, 30, 50 µg/disk) were seeded on sterile disc with gentamicin sulphate as positive control and autoclaved water as negative control. Inhibited zone for silver nanoparticles was measured by zone scale (HIMEDIA) observed at maximum extent i.e. (16.66 ± 0.57 mm) at highest concentration (50 µg/disk) against gram positive instead of gram negative pathogens (14.6 ± 0.57 mm 50 µg/disk). Results of the assay revealed that the standard, gentamicin sulphate, showed smaller zone of inhibition (12.6 ± 0.57 mm) than the AgNPs in both clinical pathogenic (Staphylococcus aureus, Salmonella typhii) strains. Leaf extract did not show enough antibacterial activity compared to the standard as well as AgNPs. The bacterial inhibition by the samples among the strains might be due to the structural differences and cellular integrity. Further, internalization of cellular mechanisms for bacterial inhibition was elucidated by the minimum inhibitory count assay.
Minimum Inhibitory Count (MIC) Assay
Here, a range of concentrations was examined to determine the MIC values of both extract and AgNPs (Table S1). For determining MIC, varying concentrations of sample (5, 9, 10, 30, 50, 60, 90, 100 µg/ml) seeded with sub-bacterial culture (OD600 0.4–0.8) and LB broth were used as negative control to assure contamination during whole process. Plant mediated AgNPs inhibited bacterial cell survival for Staphylococcus aureus, Bacillus cereus, Klebseilla pneumoni at concentration of 50 µg/ml except Salmonella typhii (90 µg/ml) while extract inhibited bacterial growth by double the concentration of AgNPs i.e. 100 µg/ml for clinical pathogenic bacterial strains as mentioned in Table S1. Control group revealed prominent growth between 0.4 and 0.8 nm as well as LB broth serves as negative control. Synthesized nanoparticles reduced the free radical scavenging efficiency of ROS metabolizing enzymes i.e. flavoenzymes, catalase, SOD. Reduction in antioxidant enzymes promotes endoplasmic and mitochondrial stress which provokes chromosomal aberration, mutational changes, DNA breakage ultimately causes genotoxicities. Silver adheres on cellular membrane, produces disparate ROS species such O2−, H2O2, HOCl, hydroxyl radical and triggers hindrance in mitochondrial respiration in microbial cells, which leads to cell death. Depending on these pathways, silver ions affect oxidative signaling pathways [33].
Antibiofilm Efficacy
Congo Red Plate Agar Assay
Antibiofilm activity of AgNPs and MZ leaf extract was determined against Staphylococcus aureus and Salmonella tyhpii. MZ leaf extract showed marginal activity to inhibit biofilm formation, while AgNPs completely inhibited the formation of biofilm. The ability of AgNPs to prevent biofilm formation might be attributed to the combined effect of bioactive molecules in the leaves as well as silver nanoparticles which hindered the pathway for biofilm growth via inhibiting the formation of extracellular polymeric substances (EPS) which establish functional and structural integrity of the biofilms and make bacteria less vulnerable. In case of leaf extract alone, incomplete inhibition of the biofilm formation reflected that phenolic and other phytocompounds were solely not efficient for their prevention. Under normal conditions, a single bacterium experiences stress conditions on exposure to any drug/therapeutic molecule, loses its integrity and dies with the occlusion of internal matrix. Static biofilm growth forms the barrier for the entry of such exogenous molecules as it inflates the pathogenic effects of bacterial population residing beneath the film. The enhanced metabolic efficiencies, horizontal gene transfer, interaction between host and defenses allow these bacterial aggregates to colonize at different sites. Planktonic bacterial communities are less pathogenic than biofilm forming bacterial communities. The results of the projected study were in complete agreement with the previously reported literature [33].
Microtitre Plate Assay
In order to quantify the antibiofilm efficacy of AgNPs, microtitre plate assay was performed using AgNPs and aqueous leaf extract. The results showed that biogenic AgNPs, at concentration of 2.5 µg/ml, were found to be highly effective in the prevention of ~ 60% of biofim, which increased to ~ 90% at 30 µg/ml. On the other hand, leaf extract could prevent the formation of biofilm by ~ 45% at 2.5 µg/ml and ~ 51% at 30 µg/ml (Fig. 4). The results of this assay might be due to the fact that AgNPs acted through adherent property, reducing the chances for biofilm adherence to the surface. The combined effect of silver nanoparticles and leaf phytochemicals hindered the cellular pathway for biofilm formation. All tests were performed in replicates for reliability of results. Silver nanoparticles have tendency to adhere to the surface. The surface active metallic nanoparticles were utilized for the EPS removal which directly caused hindrance in biofilm formation [34].
Fig. 4.
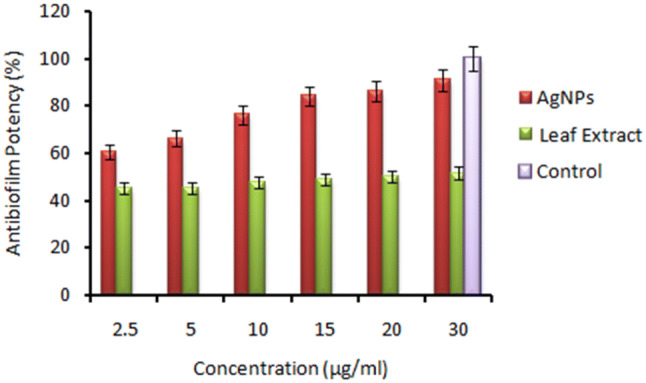
Antibiofilm biofilm activity of AgNPs and aqueous leaf extract at concentrations (2.5, 5, 10, 15, 20, 30 µg/well) against Staphylococcus aureus bacterial strain
Free Radical Scavenging Efficiency
Free radical scavenging action of AgNPs was scrutinized using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The results advocated that 10 µg/well of AgNPs and leaf extract corresponded to ~ 52% and ~ 8% of antioxidant potential, respectively. Similarly, 100 µg/well of AgNPs and leaf extract showed ~ 67% and ~ 15% antioxidant property, respectively, as represented in figure S7. The said property could be owing to the presence of polyphenols, tannins, glycosides, flavonoids, which exhibit intrinsic antioxidant potential. When these phytochemicals were bound to silver nanoparticles as stabilizers or capping agents, synergism in activity was observed. MZ leaf extract also contains these phytochemicals, however, exhibited low degree of antioxidant potential.
In Vitro Cytocompatibility
Evaluation of cytocompatibility was carried out on red blood cells (RBC) and mammalian cells, HEK293 cells. At 5× of MIC, viable RBC count was ~ 73% (Fig. S8). Hence, it was observed that the projected AgNPs were non-toxic toward RBCs. Likewise, on mammalian cells, up to 50× of MIC, these particles were found to be almost non-toxic, while at 125× of MIC, maximium of cell death was observed to be ~ 37% (Fig. S9). These observations advocated that the projected silver nanoparticles could be used in healthcare applications. The results are in agreement with the reported literature [35].
Conclusions
Use of phytonanotechnology in synthesis of silver nanoparticles has been an attraction in the field of developing nanomedicines. In this study, we have synthesized silver nanoparticles using an aqueous extract of MZ leaves and evaluated their properties. The synthesized AgNPs exhibited excellent antibacterial activity, catalytic reduction potency, radical scavenging activity, static biofilm inhibitory effects, mammalian cell compatibility behavior, etc. These results established that these particles could be used effectively as nanomedicine in future healthcare related applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
IS wishes to acknowledge Department of Science and Technology, New Delhi for awarding DST-INSPIRE to carry out this work. The authors also thank IITR-Lucknow and USIC-DU for TEM, XRD, Raman and SEM facility, respectively.
Compliance with Ethical Standards
Conflict of interest
Authors do not have any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bhawna Sharma, Email: sbhawna612@gmail.com.
Indu Singh, Email: indusingh768@gmail.com.
Somvir Bajar, Email: somvirbajar@gmail.com.
Seema Gupta, Email: seemagupta@andc.du.ac.in.
Hemant Gautam, Email: hemant@igib.res.in.
Pradeep Kumar, Email: pkumar@igib.res.in.
References
- 1.Modena MM, Rühle B, Burg TP, Wuttke S. Nanoparticle characterization: what to measure? Adv Mater. 2019;31:1901556. doi: 10.1002/adma.201901556. [DOI] [PubMed] [Google Scholar]
- 2.Bamburowicz-Klimkowska M, Poplawska M, Grudzinski IP. Nanocomposites as biomolecules delivery agents in nanomedicine. J Nanobiotechnol. 2019;17:48. doi: 10.1186/s12951-019-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 4.Patel SKS, Sandeep K, Singh M, Singh GP, Lee JK, Bhatia SK, Kalia VC. Biotechnological application of polyhydroxyalkanoates and their composites as anti-microbials agents. In: Kalia VC, editor. Biotechnological applications of polyhydroxyalkanoates. Singapore: Springer; 2019. pp. 207–225. [Google Scholar]
- 5.Otari SV, Patel SKS, Kalia VC, Kim IW, Lee JK. Antimicrobial activity of biosynthesized silver nanoparticles decorated silica nanoparticles. Ind J Microbiol. 2019;59:379–382. doi: 10.1007/s12088-019-00812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SKS, Kim JH, Kalia VC, Lee JK. Antimicrobial activity of amino-derivatized cationic polysaccharides. Ind J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SKS, Choi SH, Kang YC, Lee JK. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk–shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 8.Patel SKS, Choi SH, Kang YC, Lee JK. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Interfaces. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 9.Patel SKS, Otari SV, Li J, Kim DR, Kim SC, Cho BK, Kalia VC, Kang YC, Lee JK. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Park GD, Patel SKS, Kondaveeti S, Otari S, Anwar MZ, Kalia VC, Singh Y, Kim SC, Cho BK, Sohn JH, Kim DR, Kang YC, Lee JK. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 11.Patel SKS, Jeon MS, Gupta RK, Jeon Y, Kalia VC, Kim SC, Cho BK, Kim DR, Lee JK. Hierarchical macroporous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 12.Patel SKS, Gupta RK, Kumar V, Mardina P, Lestari R, Kalia VC, Choi MS, Lee JK. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Ind J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SKS, Choi H, Lee JK. Multimetal-based inorganic–protein hybrid system for enzyme immobilization. ACS Sustain Chem Eng. 2019;7:13633–13638. doi: 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]
- 14.Kiranmai M. Biological and non-biological synthesis of metallic nanoparticles: scope for current pharmaceutical research. Ind J Pharm Sci. 2017;79:501–512. [Google Scholar]
- 15.Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SRK, Muniyandi J, Hariharan N, Eom SH. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Coll Surf B Biointerfaces. 2009;74:328–335. doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17:1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P. Green synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol. 2018;16:84. doi: 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, Cledon M, Dalila LMA, Sarma SJ, Brar SK. Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng. 2017;2:18. doi: 10.1007/s41204-017-0029-4. [DOI] [Google Scholar]
- 19.Agarwal H, Nakara A, Shanmugam VK. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: a review. Biomed Pharmacother. 2019;109:2561–2572. doi: 10.1016/j.biopha.2018.11.116. [DOI] [PubMed] [Google Scholar]
- 20.Alananbeh K, Al-Refaee W, Al-Qodah Z. Antifungal effect of silver nanoparticles on selected fungi isolated from raw and waste water. Ind J Pharm Sci. 2017;79:559–567. doi: 10.4172/pharmaceutical-sciences.1000263. [DOI] [Google Scholar]
- 21.Slavin YN, Asnis J, Häfeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. 2017;15:65. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haggag EG, Elshamy AM, Rabeh MA, Gabr NM, Salem M, Youssif KA, Samir A, Muhsinah AB, Alsayari A, Abdelmohsen UR. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int J Nanomed. 2019;14:6217. doi: 10.2147/IJN.S214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haroon M, Zaidi A, Ahmed B, Rizvi A, Khan MS, Musarrat J. Effective inhibition of phytopathogenic microbes by eco-friendly leaf extract mediated silver nanoparticles (AgNPs) Ind J Microbiol. 2019;59:273–287. doi: 10.1007/s12088-019-00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357–1361. doi: 10.1021/la020835i. [DOI] [Google Scholar]
- 25.Chand K, Abro MI, Aftab U, Shah AH, Lakhan MN, Cao D, Mehdi G, Mohamed AMA. Green synthesis characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Adv. 2019;9:17002–17015. doi: 10.1039/C9RA01407A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KD, Kuppusamy P, Kim DH, Govindan N, Maniam GP, Choi KC. Forage crop Lolium multiflorum assisted synthesis of AgNPs and their bioactivities against poultry pathogenic bacteria in in vitro. Ind J Microbiol. 2018;58:507–514. doi: 10.1007/s12088-018-0755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulloch A, Goldson-Barnaby A, Bailey D, Gupte S. Manilkara zapota (Naseberry): medicinal properties and food applications. Int J Fruit Sci. 2020 doi: 10.1080/15538362.2019.1687071. [DOI] [Google Scholar]
- 28.Otari SV, Patil RM, Ghosh SJ, Pawar SH. Green phytosynthesis of silver nanoparticles using aqueous extract of Manilkara zapota (L.) seeds and its inhibitory action against Candida species. Mater Lett. 2014;116:367–369. doi: 10.1016/j.matlet.2013.11.066. [DOI] [Google Scholar]
- 29.Rajakumar G, Rahuman AA. Acaricidal activity of aqueous extract and synthesized silver nanoparticles from Manilkara zapota against Rhipicephalus (Boophilus) microplus. Res Vet Sci. 2012;93:303–309. doi: 10.1016/j.rvsc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Shaniba VS, Aziz AA, Jayasree PR, Kumar PRM. Manilkara zapota (L.) P. Royen leaf extract derived silver nanoparticles induce apoptosis in human colorectal carcinoma cells without affecting human lymphocytes or erythrocytes. Biol Trace Elem Res. 2019;192:160–174. doi: 10.1007/s12011-019-1653-6. [DOI] [PubMed] [Google Scholar]
- 31.Tan BL, Norhaizan ME. Manilkara zapota (L.) P. Royen leaf water extract triggered apoptosis and activated caspase-dependent pathway in HT-29 human colorectal cancer cell line. Biomed Pharmacother. 2019;110:748–757. doi: 10.1016/j.biopha.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Khoshnamvand M, Huo C, Liu J. Silver nanoparticles synthesized using Allium ampeloprasum L. leaf extract: characterization and performance in catalytic reduction of 4-nitrophenol and antioxidant activity. J Mol Struct. 2019;1175:90–96. doi: 10.1016/j.molstruc.2018.07.089. [DOI] [Google Scholar]
- 33.Shah MR, Imran M, Ullah S. Metal nanoparticles for drug delivery and diagnostic applications. Micro Nano Technol. 2019 doi: 10.1016/C2018-0-00172-3. [DOI] [Google Scholar]
- 34.Khalid HF, Tehseen B, Sarwar Y, Hussain SZ, Khan WS, Raza ZA, Bajwa SZ, Kanaras AG, Hussain I, Rehman A. Biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. J Hazard Mater. 2019;364:441–448. doi: 10.1016/j.jhazmat.2018.10.049. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava M, Hegde M, Chiruvella KK, Koroth J, Bhattacharya S, Choudhary B, Raghavan SC. Sapodilla plum (Achras sapota) induces apoptosis in cancer cell lines and inhibits tumor progression in mice. Sci Rep. 2014;4:1–9. doi: 10.1038/srep06147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.