Abstract
Coronavirus disease 2019 (COVID-19) appears to be associated with increased arterial and venous thromboembolic disease. These presumed abnormalities in hemostasis have been associated with filter clotting during continuous renal replacement therapy (CRRT). We aimed to characterize the burden of CRRT filter clotting in COVID-19 infection and to describe a CRRT anticoagulation protocol that used anti-factor Xa levels for systemic heparin dosing. Multi-center study of consecutive patients with COVID-19 receiving CRRT. Primary outcome was CRRT filter loss. Sixty-five patients were analyzed, including 17 using an anti-factor Xa protocol to guide systemic heparin dosing. Fifty-four out of 65 patients (83%) lost at least one filter. Median first filter survival time was 6.5 [2.5, 33.5] h. There was no difference in first or second filter loss between the anti-Xa protocol and standard of care anticoagulation groups, however fewer patients lost their third filter in the protocolized group (55% vs. 93%) resulting in a longer median third filter survival time (24 [15.1, 54.2] vs. 17.3 [9.5, 35.1] h, p = 0.04). The rate of CRRT filter loss is high in COVID-19 infection. An anticoagulation protocol using systemic unfractionated heparin, dosed by anti-factor Xa levels is reasonable approach to anticoagulation in this population.
Electronic supplementary material
The online version of this article (10.1007/s11239-020-02301-6) contains supplementary material, which is available to authorized users.
Keywords: Continuous venovenous hemofiltration, CRRT, CVVH, Acute kidney injury, End stage renal disease, Hemodialysis, Hemofiltration, Coronavirus, SARS, SARS-CoV2, Hypercoagulability, Thrombosis
Highlights
We reviewed our first 65 patients who received continuous renal replacement therapy with COVID-19 infection to measure rates of filter loss.
83% of patients lost at least one filter with a median filter life of only 6.5 hours.
Initiation of systemic heparin, dosed by anti-factor Xa levels, resulted in improved long term filter life.
Background
Coronavirus disease 2019 (COVID-19) frequently leads to critical illness and up to 20% of these patients require renal replacement therapy [1, 2]. Continuous renal replacement therapy (CRRT) has emerged as the preferred renal replacement modality in the critically ill in severe COVID-19 infection [3]. COVID-19 appears to lead to coagulation abnormalities including thrombocytopenia, prolonged prothrombin time (PT)/partial thromboplastin time (PTT), and increased fibrin degradation products [4–11]. Clotting of the CRRT filter is a major limitation to care, as it leads to inefficient dialysis, causes blood loss, and depletes limited resources (CRRT filters) [12, 13]. These risks can be mitigated via administration of systemic anticoagulation [14]. However, systemic unfractionated heparin (UFH) is associated with increased bleeding events in CRRT [14]. The goal of this study was to characterize the burden of CRRT filter clotting in COVID-19 infection and to describe a CRRT anticoagulation pilot protocol that used anti-factor Xa levels given the unreliability of PTT levels in patients with COVID-19.
Methods
Setting and patient population
This study was conducted at three hospitals in the Mass General Brigham healthcare system. Consecutive patients with COVID-19 infection diagnosed via nasopharyngeal swab/polymerase chain reaction testing admitted between March 16, 2020 and April 27, 2020 and required CRRT were included. CRRT was performed as continuous venovenous hemofiltration (CVVH) using the NxStage One machine (Lawrence, MA, USA) with pre-filter replacement fluid and CAR-500/505 filter sets. CVVH prescriptions were at the discretion of treating clinicians.
Data collection and outcome
All data were extracted retrospectively from the electronic medical record. The primary outcome (filter clotting) was defined as CRRT circuit failure due to thrombosis requiring unplanned time off the machine to replace the filter, not attributed to venous access malfunction, either with or without blood loss. This study was approved by the Institutional Review Board of Partners Healthcare and abided by the Declaration of Helsinki. The need for informed consent was waived.
Pilot anticoagulation protocol
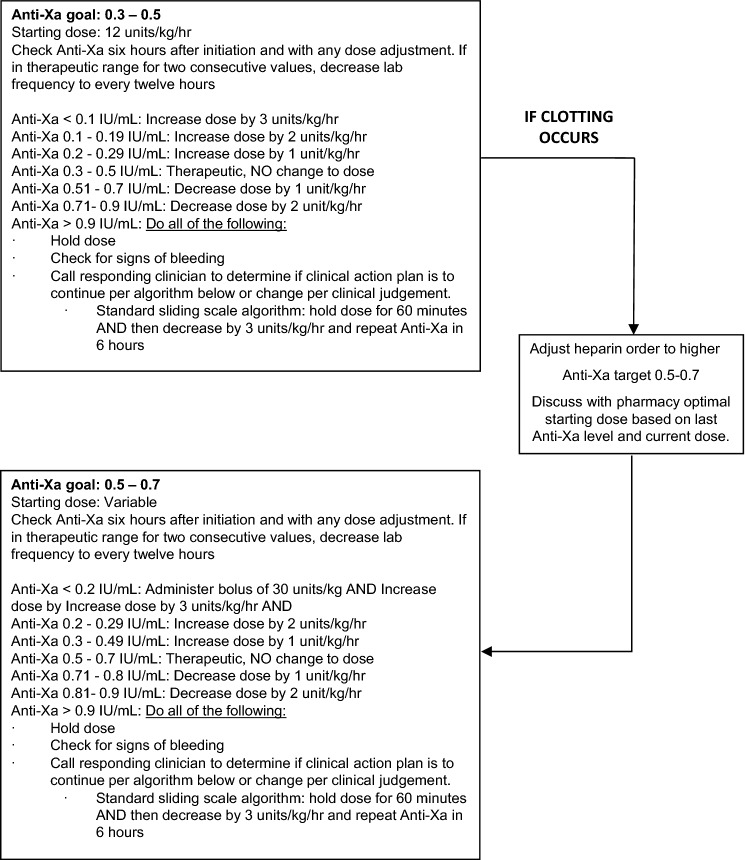
A COVID-specific CVVH anticoagulation protocol was piloted starting April 13, 2020. This protocol dosed systemic UFH by anti-factor Xa levels [15] due to the unreliability of PTT levels in patients with critical illness and COVID-19. The protocol was developed as a quality improvement measure during the COVID-19 pandemic in response to high rates of filter clotting (Fig. 1).
Fig. 1.
COVID-19 heparin sliding scale doing schedule for continuous renal replacement therapy using anti-factor Xa levels.
Outcomes and statistical analysis
The primary outcome was time to filter loss. We also measured “Severe Clotting,” defined as experiencing > 2 filter losses in 48 h or a filter loss < 8 h into CVVH. Secondary outcomes included inpatient mortality, renal recovery off dialysis by discharge, and adverse events (major bleeding [16] and heparin-induced thrombocytopenia [HIT]). Non-parametric testing was performed. Two-tailed p values < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (Cary, North Carolina).
Results
Patient demographics
Sixty-five consecutive patients treated with CVVH were analyzed (Supplement 1). Median Sequential Organ Failure Assessment (SOFA) score was 10 [8, 12] for all patients. Fifty-seven out of 65 patients (88%) initiated CVVH for AKI, whereas 8/65 patients (12%) had end stage renal disease. At the time of CVVH initiation, 64/65 patients (98%) were mechanically ventilated, 22/65 patients (34%) required prone ventilation, and 59/65 patients (91%) were on intravenous vasopressors. Patients spent a median of 6 [2, 13] days on CVVH. Bicarbonate replacement fluid was used in 54/65 patients (83%).
Filter outcomes
Fifty-four out of 65 patients (83%) lost at least one filter (median first filter survival time of 6.5 [2.5, 33.5] h). Severe filter clotting was observed in 29/65 patients (44%). Patients with Severe Clotting had a shorter first filter life (4.5 [2, 6] vs. 31 [10, 59] h, p < 0.001) and lost more filters in the first 48 h of CVVH (2.4 ± 1.27 vs. 0.56 ± 0.69 filters, p < 0.001). Few variables were associated Severe Clotting: lower serum potassium (4.3 [4.0, 4.8] vs. 4.7 [4.4, 5.4] mEq/L, p = 0.04), lower aspartate aminotransferase (55.5 [32.5, 100] vs. 100 [45, 170] U/L, p = 0.03), and higher C-reactive protein (229 [148, 292] vs. 141 [99.6, 216.9], p = 0.01. The use of citrate replacement fluid was not associated with either severe clotting or increased filter life. Systemic UFH use was associated with longer filter survival time compared with no systemic UFH use (31 [5.5, 59] vs. 7.5 [3.5, 31] h, p = 0.03).
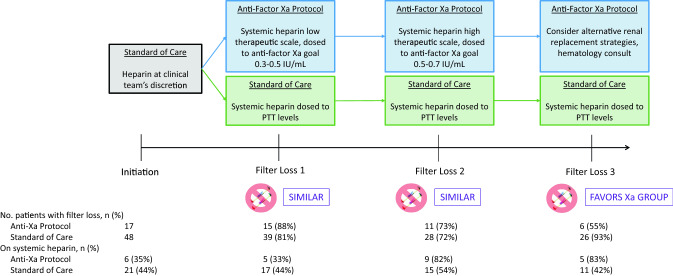
Seventeen patients received protocolized anticoagulation dosed by anti-factor Xa levels, whereas 48 were treated with standard of care anticoagulation dosed by PTT level (Fig. 2). There were no major differences between groups in age, sex, race, ethnicity, body mass index, or baseline medications. There was no difference between groups in percentage who lost their first filter (88% vs. 81%), or second filter (73% vs. 72%). However, fewer patients lost their third filter in the protocolized group (55% vs. 93%) resulting in a longer median third filter survival time (24 [15.1, 54.2] vs. 17.3 [9.5, 35.1] h, p = 0.04). Of note, more patients were treated with systemic UFH over time in the protocolized group than in the standard of care group (Fig. 2).
Fig. 2.
Study design and systemic heparin use while on continuous renal replacement therapy. Among total patients at risk, the percent displayed under Filter Loss 1, 2, and 3 represents the number who lost a filter divided by the total number who entered that period at risk
Other clinical outcomes
At the time of final analysis, 3/65 patients (5%) were still admitted to the hospital, 30/65 patients (46%) died while inpatient and 32/65 patients (49%) survived to discharge. Twenty out of 22 (91%) non-ESRD patients recovered off renal replacement therapy by discharge. Major bleeding occurred in 9/65 patients (14%). Two patients (3%) developed heparin-induced thrombocytopenia.
Discussion
We report CRRT filter clotting in COVID-19 infection and demonstrated that severe filter clotting is an important clinical problem that affects 44% of this population. Our median filter life of 6.5 h was considerably less than what is reported in the literature (~ 20–40 h), suggesting that these patients are more prone to clotting than those typically enrolled in ICU clinical trials [14]. Based on early descriptive studies of COVID-19, this was not unexpected, as several studies have noted signs of intense inflammation and coagulopathy, including elevated d-dimer, fibrinogen, and inflammatory markers [6, 9].
After piloting an anticoagulation protocol with a standard algorithm, we observed favorable results using systemic UFH dosed by anti-factor Xa levels. This study serves as proof of concept that a graduated anticoagulation plan may be a reasonable approach in this population, but requires a larger cohort and/or comparative studies to validate the optimal intensity of anticoagulation in this population.
It is important to acknowledge the unique clinical circumstances surrounding this study period that influenced our design and results. Even now, there are no evidence-based guidelines around CRRT in COVID-19 infection, which emphasizes the importance of this work, as we prepare for persistent rise of COVID-19 cases across the country. Interventions that can improve clinical and prevent wasting of potentially limited resources in a disaster setting to prevent the need for rationing care of patients with kidney disease [17]. While there remains uncertainty about the overall risk and benefit of systemic anticoagulation in COVID-19, we have demonstrated that anticoagulation targeting anti-factor Xa levels has potential to decrease filter clotting.
Our study has several limitations. Because our anticoagulation protocol was derived out of clinical necessity during a pandemic, we were not able to draw comparisons across patient populations, rather evaluating the within-patient changes in time to filter loss to determine its effectiveness. The only renal replacement performed in this study was CVVH, and care should be taken when extrapolating these results to other CRRT modalities. Our study was not powered to detect differences in outcomes such as mortality or bleeding events. Despite these limitations, we believe we have provided important observations as to the use of CRRT in this population.
In conclusion, the rate of CRRT filter loss is high in COVID-19 infection. An anticoagulation protocol using systemic UFH, dosed by anti-factor Xa levels is reasonable approach to anticoagulation in this population. These results require confirmation in prospective clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 133.9 kb)
Acknowledgements
The authors would like to acknowledge the clinical and support staff in the intensive care units at Massachusetts General Hospital, Brigham and Women’s Hospital, and Newton Wellesley Hospital for their commitment and professionalism in providing care during this exceptional time.
Abbreviations
- COVID-19
Coronavirus disease 2019
- CRRT
Continuous renal replacement therapy
- UFH
Unfractionated heparin
- ICU
Intensive care unit
- AKI
Acute kidney injury
- CVVH
Continuous venovenous hemofiltration
Author contributions
PE, RR, RJR, NL, DJRS, NTR, ASA designed the study. PE, SZ, SK, SP, OK, FRM, PGC, WM participated in data collection and analysis. PE, RR, RJR, NL, MES, ALL, EPR, KAH, CCH developed and enacted clinical protocols. ASA drafted the manuscript. All authors read and approved the final manuscript.
Funding
ASA is supported by American Heart Association Award 18CDA34110131. KAH is supported by the National Heart, Lung and Blood Institute (RO1-AI138999-01, UO1-HL123022-06). FRM is supported by NIH grants K23DK102511, R03DK122240, and U01DK096189. CH reports research funding from Astra-Zeneca. RR reports institutional research funding from BMS, Janssen. PGC is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K08DK123400). ASA reports advisory board fees from Mallinckrodt Pharmaceuticals. DJRS reports consulting fees from HealthReveal and Blackstone Life Sciences. RR reports institutional research support from BMS, Janssen and advisory/consulting fees from BMS, Janssen, Portola, and Dova. PGC reports advisory/consulting fees from Reata and Alexion.
Compliance with ethical standards
Conflict of interest
All authors declare they have no conflict of interest
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolwani A. Continuous renal-replacement therapy for acute kidney injury. N Engl J Med. 2012;367:2505–2514. doi: 10.1056/NEJMct1206045. [DOI] [PubMed] [Google Scholar]
- 4.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:154. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R. Continuous is not continuous: the incidence and impact of circuit “down-time” on uraemic control during continuous veno-venous haemofiltration. Intensive Care Med. 2003;29:575–578. doi: 10.1007/s00134-003-1672-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Ni H, Lu B. Variables associated with circuit life span in critically ill patients undergoing continuous renal replacement therapy: a prospective observational study. ASAIO J. 2012;58:46–50. doi: 10.1097/MAT.0b013e31823fdf20. [DOI] [PubMed] [Google Scholar]
- 14.Bai M, Zhou M, He L, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med. 2015;41:2098–2110. doi: 10.1007/s00134-015-4099-0. [DOI] [PubMed] [Google Scholar]
- 15.Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012;32:546–558. doi: 10.1002/j.1875-9114.2011.01049.x. [DOI] [PubMed] [Google Scholar]
- 16.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 17.Division of Nephrology CUVCoP Disaster response to the COVID-19 pandemic for patients with kidney disease in New York City. J Am Soc Nephrol. 2020;31:1371–1379. doi: 10.1681/ASN.2020040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 133.9 kb)