Abstract
The recognition of a prodromal period preceding the onset of frank psychosis dates back to its first descriptions. Despite insights gained from a prospective approach to the study of the Clinical High Risk syndrome for psychosis (CHR-P), a prospectively-based understanding of the duration of the psychosis prodrome and the factors that may influence is not well-established. Here we analyze data from the second North American Prodrome Longitudinal Study (NAPLS-2) to characterize prodrome duration in those who converted to psychosis. Of the 764 participants identified as being at CHR-P, 94 converted to psychosis and 92 of these had recorded estimates of prodrome onset. Estimates of prodrome duration were derived from CHR-P syndrome onset and conversion dates from the Structured Interview for Psychosis-risk Syndromes. Results identified a mean prodrome duration of 21.6 months. Neither CHR-P sub-syndrome nor medication exposure was found to significantly influence prodrome duration in this sample. These results provide the most precise estimate of prodrome duration to date, although results are limited to prodromes identified by ascertainment as being at CHR-P. Our findings also suggest a rule of thirds with regard to prodrome duration in those followed for two years: one third of CHR-P patients who convert will do so by 1 year after CHR-P syndrome onset, another third 1–2 years after onset, and the final third more than 2 years after onset.
Introduction
Early intervention in psychosis can improve outcomes: those treated early attempt suicide less frequently (Melle et al. 2006), require fewer hospitalizations (Srihari et al. 2015; Kane et al. 2016), and have higher quality of life (Marshall et al. 2005). Early intervention during the prodromal phase may be particularly important; indeed, while duration of untreated psychosis (DUP) correlates with a host of long-term outcomes (Penttila et al. 2014), efforts to reduce DUP at the time of the first psychotic episode have met with mixed results (Oliver et al. 2018). In contrast, intervention during the prodromal phase of the illness has been associated a DUP among those who nevertheless convert than among entering care at the point of first episode (Valmaggia et al. 2015). Moreover, Rosengard and colleagues (Rosengard et al. 2018) showed that those with a period consistent with a state of attenuated psychotic symptoms exhibited poorer functioning and more severe symptoms compared with those who did not, consistent with the importance of the prodrome to clinically-relevant outcomes.
Not only is the prodrome itself of importance to early intervention, but its duration is also. Clinicians designing programs hoping to prevent psychosis among patients showing prospectively-identified prodromal symptoms would benefit from knowing the expected duration of the prodrome in order to determine the length of the program. Similarly, researchers designing studies investigating prediction or prevention of psychosis also need to plan follow-up duration. Despite its clinical importance, however, a basic understanding of the typical duration of the psychosis prodrome has thus far largely escaped even partly prospective characterization.
We are aware of only one such previous partly prospective sample, in which 110 nonpsychotic patients prospectively identified as experiencing subjective disturbances of thought, speech, memory, perception, and action termed “basic symptoms” were ascertained prospectively in five German university psychiatry departments between 1987 and 1991 and followed up an average of 10 years later (Klosterkotter et al. 2001; Schultze-Lutter et al. 2007). The more recent publication (Schultze-Lutter et al. 2007) reported on prodrome duration among the 79 converters. The onset of the prodrome was defined as the time of the first basic symptom, and onset of frank psychosis was defined as the time when any Present State Examination criterion for psychotic symptoms was first noticed. The mean time interval between these two points was 67 months. This duration of the prodrome, like that from the present report, derived only partly from a prospective method in that the portion of the time between prodrome onset and case ascertainment was determined retrospectively. A similar earlier sample ascertained in three German cities from 1970 to 1988 and followed up for an average of 8 years did not report on the duration of the prodrome (Gross 1997; Klosterkötter et al. 1997). No study has reported partly-prospective prodrome duration estimates where prodrome onset has been based on the Clinical High Risk Syndrome for psychosis (CHR-P) (Yung and McGorry 1996; Miller et al. 1999) that has been frequently investigated in recent years (Woods et al. 2019).
Fully retrospective estimates of prodrome duration are listed in Table 1, showing 23 papers dating back as far as 1938 and including the seminal ABC (Age Beginning Course) study of Häfner and colleagues (Hafner et al. 1993). Table 1 includes summaries of the samples and definitions of prodrome onset and offset. An additional seven studies were excluded from Table 1 since they used a treatment-related definition of prodrome offset, such as first hospitalization for psychosis or first antipsychotic treatment, that conceptually overlaps with duration of untreated psychosis (McGlashan 1984; Hafner et al. 1989; Riecher et al. 1991; Haas and Sweeney 1992; Ho et al. 2000; Singh et al. 2005; Raballo et al. 2014). Four studies included in Table 1 did not report duration as a mean (Cameron 1938; Conrad 1958; Varsamis and Adamson 1971; Bechdolf et al. 2002), and in one study 6 of 82 subjects were excluded from the mean as outliers (Lappin et al. 2007).
Table 1.
Descriptions of prodrome duration from fully-retrospective literature.
| Citation | Sample | Method | Onset Definition | Offset Definition | Duration, months |
|---|---|---|---|---|---|
| (Cameron 1938) | 100 1st admission schizophrenia | U | Nonspecific sxa | Specific sxb | Weeks to years |
| Specific sxb | Hospitalization | Days to months to years | |||
| (Conrad 1958)c | nr | U | Onset of ‘trema’ | ‘becoming manifest’ | Very brief to several years |
| (Chapman 1966) | 40 recent onset schizophrenia | Sd | 1st noticeable illness | nr | mean 11, range 1 to 33 |
| (Gross 1969)e | 290 cases | nr | 1st sign | 1st contact | mean 42 |
| (Varsamis and Adamson 1971) | 44 1st admission schizophrenia | U | 1st somatic or psychological sx | Manifest or overt psychosis | median 30, range 0.03 to 84 |
| (Huber et al. 1980; Gross 1997) | 502 hospitalized for schizophrenia | U | 1st noncharacteristic continuous sxf | 1st psychotic manifestation | mean 38.4 |
| 1st noncharacteristic discontinuous sxf | mean > 120 months | ||||
| (Rabiner et al. 1986) | 64 1st episode psychosis | U | 1st noticeable sx | 1st study interview | mean 14.5 |
| (Loebel et al. 1992) | 70 1st episode schizophrenia | U | 1st noticeable sx | 1st psychotic sx | 22.7±36.2 |
| (Beiser et al. 1993) | 1411st episode schizophrenia | Sg | 1st noticeable sx | 1st prominent psychotic sx | 26.1±34.4 |
| (Hafner et al. 1993) | 267 1st admission schizophrenia | Sh | 1st psychiatric sx | onset psychosis | mean 32.4 |
| 1st positive sx | mean 30 | ||||
| (McGorry et al. 1996) | 130 1st episode psychosis | Si | operationally defined | operationally defined | 15.0±26.9 |
| (Häfner 2000) | 232 1st episode schizophrenia | Sh | 1st neg or nonspec sx | onset psychosis | mean 60 |
| (Bechdolf et al. 2002) | 27 relapsing paranoid schizophrenia in full remission | Sk | 1st basic symptom | Not specified | median 3.8 |
| (Gourzis et al. 2002) | 100 hospitalized with recent onset schizophrenia | U | 1st noticeable sx | 1st active phase sx | 15.1±11.6 |
| (Malla et al. 2002) | 88 1st episode nonaffective psychosis | U | 1st psychiatric sx | 1st psychotic sx | 28.6±46.2 |
| (Harrigan et al. 2003) | 565 1st episode psychosis | Si | 1st deviation from premorbid personality | 1st sustained psychotic sx | 12.1±19.8 |
| (Keshavan et al. 2003) | 104 1st episode psychosis | U | 1st contiguous attenuated positive or negative sx | 1st onset of psychotic sx | mean 20.6 |
| (Lappin et al. 2007) | 82 1st episode psychosis | U | 1st fundamental change in functioning | 1st week of psychotic sx | 4.8±8.3 (6 outliers > 39 excluded) |
| (Shioiri et al. 2007) | 219 hospitalized for schizophrenia | U | 1st psychiatric sx | onset schizophrenia | mean 37.2 |
| (Cotton et al. 2009) | 661 1st episode psychosis | U | not defined | first sustained positive symptom | 13.0±18.7 |
| (Renwick et al. 2015) | 320 1st episode psychosis | Sm | 1st noticeable signs | onset of psychosis | 17.6±32.0, median 4.0 IQR 0–21 |
| (Schultze-Lutter et al. 2015) | 126 1st episode psychosis | Sn | 1st unspecific symptom | 1st psychotic sx | mean 100.5 |
| 1st attenuated positive or cognitive or perceptive basic sx | 53.3±71.8 | ||||
| (Shah et al. 2017) | 238 1st episode psychosis | So | 1st sx contiguous with FEP | 1st psychotic sx | mean 23.2 |
| 1st attenuated positive sx | mean 39.2 |
Abbreviations: sx--symptom or symptoms. U--unstructured interview or unclear, S--structured interview, A-administrative database
Nonspecific symptoms included negative, anxiety, and depressive symptoms.
Specific symptoms were mostly positive symptoms and delusional mood.
Findings as related in English by (Varsamis and Adamson 1971) and (Fish 1961).
interviews were apparently structured but no instrument was named
Original in German, results summarized in (Häfner 2000)
Characteristic schizophrenic deficiency symptoms were mostly negative symptoms.
record review with symptom checklist.
IRAOS structured interview.
Royal Park Multidiagnostic Instrument for Psychosis.
Comprehensive Assessment of Symptoms and History.
ERIraos-CL and “sequence of symptoms assessment”
Nottingham Onset Schedule
Onset Questionnaire
Early Recognition Instrument based on the Instrument for the Retrospective Assessment of the Onset of Schizophrenia
Circumstances of Onset and Relapse Schedule
Methods varied across the remaining 18 studies. Most determined prodrome duration cases ascertained during the early course after onset, but three studies ascertained more chronic cases (Gross 1969; Huber et al. 1980; Gross 1997; Shioiri et al. 2007). The majority used structured interview methods to determine prodrome onset and offset, but several did not or did not report this methodological detail (Gross 1969; Huber et al. 1980; Rabiner et al. 1986; Loebel et al. 1992; Gross 1997; Gourzis et al. 2002; Malla et al. 2002; Shioiri et al. 2007; Cotton et al. 2009). Prodrome onset definitions in Table 1 mainly identified onset as the first appearance of any kind of behavioral symptom or change from the usual state, without regard to whether the prodrome remitted at any point between onset and the appearance of frank psychosis (14 studies reporting the full distribution, median mean duration 27.3 months, minimum 11 months, maximum 100.5 months, IQR 15.7 to 38.1 months). Two of these fourteen studies (Hafner et al. 1993; Schultze-Lutter et al. 2015) provided alternative definitions of prodrome onset as the first appearance of a symptom characteristic of schizophrenia (positive, negative, or basic) but not sufficiently severe to qualify for frank psychosis. The mean duration by this definition as expected was shorter than the duration when starting from the first nonspecific symptom in both studies. Two additional studies in Table 1 required that onset be defined by specific symptoms that were contiguous with the later first-episode psychosis (Keshavan et al. 2003; Shah et al. 2017), in one case as compared to the first lifetime appearance and yielding a shorter duration than the lifetime onset definition (Shah et al. 2017). In two studies prodrome onset definitions were not stated in detail (McGorry et al. 1996; Cotton et al. 2009). The onset of psychosis ending the prodrome was retrospectively determined in all studies in Table 1 and in most cases was not based on specifically-defined criteria.
Here we present data on prodrome duration derived from the second North American Prodrome Longitudinal Study (NAPLS) cohort (Addington et al. 2012). While descriptive in nature, this analysis provides partly-prospectively and heretofore unreported data on prodrome duration where prodrome onset has been based on the CHR-P syndrome and the onset of psychosis was prospectively determined using reliable structured methods. Additionally, we investigate whether different sub-types of CHR-P may present with different prodrome durations. In particular, those with the Brief Limited Intermittent Psychotic Symptoms (BLIPS) sub-type (Fusar-Poli et al. 2016a; Fusar-Poli et al. 2017a) may be hypothesized to have shorter prodromes.
Methods
Participants and Procedures
A total of 764 participants meeting Clinical High Risk syndrome for Psychosis (CHR-P (Fusar-Poli 2017a)) criteria and 280 healthy control participants were recruited for participation in the second phase of the North American Prodrome Longitudinal Study (NAPLS-2). Total sample characteristics have been described elsewhere (Addington et al. 2015). Of the 764 CHR-P participants, 94 transitioned to psychosis during the two-year follow-up period (mean +/− SD: 9.306 ± 9.422 months), and 92 of these had estimates for prodromal syndrome onset date. These 92 converters are the subject of the present analyses.
All procedures and consent forms were approved by each individual site’s Institutional Review Boards for human subject research. Criteria for Psychosis-risk Syndromes (COPS) from the Structured Interview for Psychosis-risk Syndromes and Scale of Psychosis-risk Symptoms (SIPS/SOPS) (Miller et al. 1999; Miller et al. 2002) were applied for inclusion in the CHR-P group (McGlashan et al. 2010). The COPS is based upon the SIPS and has three possible prodromal syndromes: attenuated positive symptom syndrome (APSS), requiring the presence of at least one attenuated positive psychotic symptom of insufficient severity to meet diagnostic criteria for a psychotic disorder; genetic risk and deterioration (GRD), requiring a combination of both functional decline (≥ 30% drop in Global Assessment of Function score in the past month, compared to one year prior) and genetic risk (defined as having either schizotypal personality disorder or a first-degree relative with a schizophrenia-spectrum disorder); and brief intermittent psychotic syndrome (BIPS), requiring the presence of any one or more threshold psychotic symptoms that are too brief to meet diagnostic criteria for psychosis. The interrater reliability of these COPS diagnoses has been established in more than 20 published samples (median kappa 0.89) (Woods et al. 2019), and COPS diagnoses were further confirmed in the present study by presentation of each case at a weekly consensus diagnostic conference call. Conversion to psychosis was identified when participants met Presence of Psychotic Symptoms (POPS) criteria from the SIPS (Miller et al. 2003) and was also confirmed by consensus diagnostic conference. Conversion diagnoses were established using the Structured Clinical Interview for DSM-IV-TR axis I disorders (SCID) (First et al. 2002).
Onset of prodromal symptoms was determined during the SIPS interview as supplemented by caregivers and treatment records. For those qualifying for more than one CHR-P sub-syndrome, the earliest CHR-P syndrome onset date was used. Onset dates, along with all information gathered during SIPS interviews were checked for accuracy and discussed by weekly phone call with representatives from all sites, including all those involved in the interview in question whenever possible. All interviewers were certified to perform the SIPS, including determination of onset of CHR-P and psychotic symptom dates.
The majority of participants qualified as APSS (n = 88), although GRD (n = 15) and BIPS (n = 9) were also represented. There was significant overlap between the diagnoses, with 18 participants meeting criteria for more than one, and 2 meeting criteria for all three.
Regarding conversion diagnoses, each participant who converted was assessed by SCID interview at conversion, a year later, or at both times. If two SCID interviews were performed, the earlier of the two available assessments was used for purposes of diagnostic classification in this study. Conversion diagnoses (determined by SCID, see below) included primary psychosis-spectrum disorders (total n = 74; Psychotic Disorder NOS, n = 35; Schizophrenia, n = 20; Schizophreniform Disorder, n = 8; Schizoaffective Disorder, n = 7; Delusional Disorder, n = 3; Brief Psychotic Disorder, n = 1) and primary mood disorders (total n = 8; Bipolar I Disorder, n = 6; Major Depressive Disorder, n = 2). A subset of the 92 participants (n = 10) were not able to be assessed for conversion diagnosis. Other relevant sample characteristics are included in Table 2.
Table 2.
Demographics and clinical data of participants.
| Mean ± SD | N | |
|---|---|---|
| Age at CHR-P Onset | 17.60 ± 3.63 | 92 |
| Female, No. (%) | 35 (38.04) | 92 |
| APS, No. (%) | 88 (95.65) | 92 |
| BIPS, No. (%) | 9 (9.78) | 92 |
| GRD, No. (%) | 15 (16.30) | 92 |
| SPD diagnosis, No. (%) | 10 (10.87) | 92 |
| SOPS Positive Score | 13.62 ± 3.65 | 91 |
| SOPS Negative Score | 12.44 ± 6.37 | 91 |
| SOPS Disorganization Score | 6.38 ± 3.77 | 91 |
| SOPS General Score | 9.75 ± 4.27 | 91 |
| Calculated Baseline Risk of Conversion | 26.20% ± 12.65 | 92 |
| Decline in GAF in the Past Year | 13.98 ± 12.56 | 92 |
| Decline in GFR in the Past Year | 1.54 ± 1.79 | 92 |
| Decline in GFS in the Past Year | 0.99 ± 1.15 | 92 |
SOPS = Scale Of Psychosis-risk Symptoms; GFR Change = change in Global Role Function score in the year prior to baseline assessment; GFS Change = change in Global Social Function score in the year prior to baseline; GAF Change = change in Global Assessment of Functioning score in the year prior to baseline assessment; Baseline BIPS = presence of Brief Intermittent Psychosis Syndrome at baseline assessment; Baseline APS = presence of Attenuated Psychotic Symptom Syndrome at baseline assessment; Baseline GRD = presence of Genetic Risk and Deterioration Syndrome at baseline assessment; Baseline SPD = presence of Schizotypal Personality Disorder at baseline assessment; Calculated Risk = subject-wise calculated risk using the NAPLS Risk Calculator; Average Tanner Stage = self-reported average of pubic and penile/breast development at baseline assessment.
Data Analysis
All data for the 92 converters of interest were compiled and analyzed. Prodrome duration was taken to be the length of time from retrospectively-identified syndrome onset at baseline assessment to date of conversion. Psychosis-risk syndrome onset date was defined retrospectively at initial SIPS evaluation as being the date at which the participant first exhibited symptoms qualifying him or her for a CHR-P diagnosis. Similarly, conversion date was defined as being the date at which the participant first exhibited a worsening or emergence of symptoms qualifying him or her for a psychosis diagnosis by POPS criteria.
All data were analyzed via MATLAB (https://www.mathworks.com/products/matlab.html).
Exploratory Analysis: Effects of Medication Exposure
Retrospective records of lifetime exposure to medications were obtained for each participant up to the time of ascertainment and prospectively after enrollment in NAPLS-2. Lifetime exposure to antipsychotics, antidepressants, benzodiazepines, mood stabilizers, and stimulants were calculated at the following time points: 1) at time of reported attenuated symptom onset; 2) at time of ascertainment; and 3) at time of conversion. Relationships between previous medication exposure and 1) time to attenuated symptom onset, 2) duration of time from attenuated symptom onset to ascertainment, and 3) duration of time between ascertainment and conversion were analyzed via multiple regression analysis.
Results
Group Demographics and Clinical Data
Demographics and baseline clinical data are summarized in Table 1.
Length of Prodrome
Across all 92 observations, a mean (± SD) of 21.59 (±18.90) months elapsed between estimated CHR-P syndrome onset date and conversion to psychosis by COPS criteria (including 12.29 ± 17.43 months from onset to presentation and 9.31± 0.98 months from presentation to conversion). Median duration was 16.03 months.
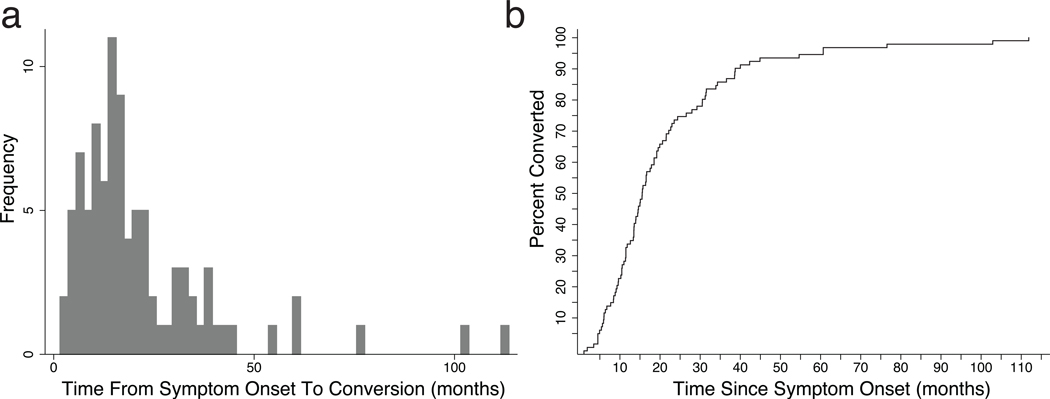
The overall distribution (Fig. 1a) exhibited a left skew (2.58) and high kurtosis (11.25), reflecting the natural floor at t = 0 and a larger tail than is seen in normal distributions, driven by two long prodromal durations greater than 100 months. Examination of the data in the form of a Nelson-Aalen cumulative distribution plot (Fig. 1b) reveals that 95% of all enrollees who would eventually convert did so by 61.02 months after estimated prodrome onset.
Figure 1. Prodrome Duration.
a. Histogram of prodrome duration in months when binned into 40 bins of equal duration. b. Nelson-Aalen cumulative probability plot for same data. CHR-P syndrome = Clinical High Risk for Psychosis syndrome.
Relationship to Medication Exposure
Analysis of the relationship between: 1) lifetime medication exposure prior to attenuated symptom onset and time to attenuated symptom onset; 2) lifetime medication exposure prior to ascertainment and duration of time from attenuated symptom onset to ascertainment; and 3) lifetime medication exposure prior to conversion and duration of time between ascertainment and conversion were analyzed via multiple regression analysis. None of these models reached statistical significance (all p>0.10).
Prodrome Duration by CHR Sub-Syndrome
As illustrated in Figure S1, Nelson-Aalen plots diverge based upon membership in CHR-P sub-groups, whether plotted inclusive of those with overlapping sub-group diagnoses (Fig. S1a) or without (Fig. S1b). Mean prodrome duration for participants qualifying for BIPS was lower (mean ± SD: 12.70 ± 5.61 months) than for those who did not (22.56 ± 19.59 months), although this difference did not reach statistical significance (t = −1.50; p = 0.138). Because of the significant overlap between the diagnoses, categorical sub-syndrome diagnoses were entered as predictors of prodrome duration in a multivariate regression model. None of the predictors or the overall model reached statistical significance (RMSE = 18.8; R2 = 0.039; F91,88 = 1.2; p = 0.316).
Prodrome Duration by NAPLS-2 Site
Figure S2 presents prodrome duration in months plotted as a function of NAPLS-2 Consortium site. Total duration differed by site (F1,7 = 2.15; p = 0.0472), with the longest duration at site 5 (University of North Carolina, mean 33.96 months) and the shortest duration at site 2 (Emory University, mean 11.63 months). There was substantial overlap in prodrome durations even between these two sites (Figure S2), and inspection of the distributions suggests that the longer duration at site 5 was influenced by two outliers (Grubbs 1950) at this site. Repeat analysis revealed that the effect of site was no longer statistically significant when these outliers were omitted (F1,7 = 1.6; p = 0.1482). Time to conversion after ascertainment did not differ by site (F1,7 = 0.84; p = 0.55) and prodrome duration at time of ascertainment differed at only a trend level (F1,7 = 1.88; p = 0.083).
Discussion
Prospective Characterization of Prodrome Duration
We used converters to psychosis from the NAPLS-2 cohort to provide the first estimate of psychosis prodrome duration derived in part from prospectively-gathered data and based on CHR-P definitions of prodrome onset and offset. Given the standardization of measurement that prospective follow-up affords, we have been able to minimize the error associated with one component of prodrome duration: time from CHR-P ascertainment to conversion. Moreover, the component of prodrome duration that we assessed retrospectively, time from CHR-P syndrome onset to CHR-P ascertainment, was determined closer to the time of prodrome onset than in previous retrospective studies. Additionally, defining prodrome duration in the context of such a cohort has allowed us to identify factors that could affect this duration and to determine whether it influences final diagnostic outcome.
The mean duration of prodrome in the present study (22 months) was shorter than that (67 months) in the only other study that used a similar, partly prospective design (Klosterkotter et al. 2001; Schultze-Lutter et al. 2007). As noted in the Limitations, part of this difference could be due to the relatively short two-year prospective follow-up interval in the current study, which may have led to the exclusion of some patients with longer prodromes. Another difference is that the previous study identified the onset of the prodrome as the first basic symptom, whereas ours identified the onset of the prodrome based on CHR-P criteria, which largely depends on the first attenuated positive symptom. Basic symptoms are thought to begin earlier in the course of the disorder than attenuated positive symptoms (Bechdolf et al. 2012), in agreement with the long prodrome duration (mean 53.3 months) in one retrospective study in Table 1 that included the first basic symptom as part of the prodrome onset definition (Schultze-Lutter et al. 2015). Consistent with this interpretation, our mean 22 months duration was shorter than the 27-month median of means from retrospective studies in Table 1 that identified prodrome onset by such terms as “first noticeable illness,” “first sign,” “first noncharacteristic symptom,” “first psychiatric symptom,” etc. Our mean prodrome duration is also somewhat shorter, however, than that in two retrospective studies in Table 1 identifying prodromal onset by positive symptoms: mean 30 months (Hafner et al. 1993) and mean 39.2 months (Shah et al. 2017) but similar to the mean 20.6 months in a study that identified prodrome onset by the first contiguous attenuated positive or negative symptom (Keshavan et al. 2003). Our prodrome onset definition did not require that the qualifying symptoms be contiguous with later psychosis.
The frequency data highlighted in Figure 1 are similar to previous descriptions of the distribution of prodrome length, with left skew and a larger-than-expected tail, reflective of the presence of a smattering of extraordinarily long prodrome durations (Häfner et al. 1995; Moller and Husby 2000). Extending these findings using the cumulative distribution plot in Figure 1b, we may propose a rule of thirds: one third of CHR-P patients who convert will do so by one year after CHR-P syndrome onset, another third between one and two years after onset, and the final third greater than two years after onset. Currently available data are unable to aid in discerning whether individuals with extraordinarily long prodrome durations may represent a sub-population that possesses protective factors that delay conversion; alternatively, these individuals may have more of an insidious onset that is characterized by prominent negative symptoms or by cognitive impairment that may affect the accuracy of recall about symptom onset. Future studies may be better suited to explore this possibility.
Medication Exposure and Prodrome Duration
The possibility that medications—especially antipsychotics—may help to delay or even prevent conversion to psychosis among those at risk has been proposed by many, although the cumulative evidence for the efficacy of antipsychotics in achieving delay or prevention is sparse (Stafford et al. 2013). The efficacy of antidepressant medications in determining outcomes is presently difficult to ascertain, although recent evidence may support the use of these medications in CHR as a means of treating comorbid symptoms with the hope of also affecting illness trajectory (Fusar-Poli et al. 2015). Our results revealed no effect of medication exposure at any point in the lifetime of patients on time to symptom onset, time to presentation, or on time to conversion, consistent with the preponderance of existing evidence, although we must emphasize that evaluation of treatment effects in observational data is confounded by selection biases.
Limitations
Limitations of this work include the fact that retrospective dating of prodrome onset played a role in the current analysis, even if the contribution of this time was smaller compared to past wholly retrospective analyses. It may also be true that the two years allowed for participant follow-up placed an artificial cap on prodrome duration. Another limitation is that our sample contains only subjects who presented for evaluation in CHR-P research clinics, and for participation in the NAPLS-2 study in particular. This means that generalization may be an issue. Adding to this is the fact that only a very small proportion of those who will eventually experience a first episode of psychosis are initially recognized and evaluated by CHR-P research clinics (Fusar-Poli 2017b; Fusar-Poli et al. 2017b), and that only two-thirds of first episode cases appear to experience a prodromal period at all (Shah et al. 2017). Conceivably, prodromes could be shorter or longer among subjects who do not respond to CHR-P recruitment efforts. Greater precision on both these points will require employment of large-scale epidemiological sampling with frequent longitudinal follow-up, a challenging endeavor. In addition, the sample was limited to subjects initially ascertained as at CHR-P by the SIPS. The SIPS definition of CHR-P places emphasis on the magnitude of recent change, and thus prodromes with slower change, which could also be longer prodromes, may have been excluded. Similarly, the CHR-P definition relies largely on the presence of positive symptoms; future studies might also measure onset of mood, anxiety or other psychiatric symptoms to broaden our understanding of the prodromal course of psychosis. We were also limited by a relatively small sample size, especially with regard to some data points. Lastly, an additional limitation of the present study concerns the interrater reliability of our prodrome onset determination. Although several studies looking retrospectively from the first episode have reported that the determination of prodrome onset was reliable (Beiser et al. 1993; Hafner et al. 1993; Gourzis et al. 2002; Malla et al. 2002), and although the interrater reliability of the SIPS has been reported to be excellent in more than 20 studies of diagnostic and symptom severity agreement (Woods et al. 2019), we are not aware of studies investigating the reliability of the prodrome onset determination using the SIPS. This is another area of needed additional research. Despite these several limitations, however, the current findings provide perhaps the most precise estimate of prodrome duration available at the current time. Epidemiologic samples with fully-prospective longitudinal follow-up would provide even greater precision without the sampling bias attendant to the sampling methods employed in this study (Fusar-Poli et al. 2016b).
Supplementary Material
Nelson-Aalen cumulative probability plots for all participants (including those who overlap in their sub-group membership) (a) and only participants who belong to one distinct CHR-P sub-group (b).
Prodrome duration in months plotted as a function of NAPLS-2 Consortium site. Duration differed by site (F1,7 = 2.15; p = 0.0472).
References
- Addington J, Cadenhead KS, Cornblatt BA, et al. (2012) North American Prodrome Longitudinal Study (NAPLS 2): Overview and recruitment. Schizophrenia Research 142:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Liu L, Buchy L, et al. (2015) North American Prodrome Longitudinal Study (NAPLS 2): The Prodromal Symptoms. J Nerv Ment Dis 203:328–335 doi: 10.1097/NMD.0000000000000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechdolf A, Schultze-Lutter F, Klosterkötter J (2002) Self-experienced vulnerability, prodromal symptoms and coping strategies preceding schizophrenic and depressive relapses. European Psychiatry 17:384–393 [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Wagner M, Ruhrmann S, et al. (2012) Preventing progression to first-episode psychosis in early initial prodromal states. British Journal of Psychiatry 200:22–29 doi: 10.1192/bjp.bp.109.066357 [DOI] [PubMed] [Google Scholar]
- Beiser M, Erickson D, Fleming JA, Iacono WG (1993) Establishing the onset of psychotic illness. Am J Psychiatry 150:1349–1354 [DOI] [PubMed] [Google Scholar]
- Cameron DE (1938) Early schizophrenia. American Journal of Psychiatry 95:567–578 [Google Scholar]
- Chapman J (1966) The early symptoms of schizophrenia. Br J Psychiatry 112:225–251 [DOI] [PubMed] [Google Scholar]
- Conrad K (1958) Die beginnende Schizoprenie. Georg Thieme Verlag, Stuttgart, Germany [Google Scholar]
- Cotton SM, Lambert M, Schimmelmann BG, Foley DL, Morley KI, McGorry PD, Conus P (2009) Gender differences in premorbid, entry, treatment, and outcome characteristics in a treated epidemiological sample of 661 patients with first episode psychosis. Schizophrenia Research 114:17–24 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. In. SCID-I/P [Google Scholar]
- Fish F (1961) A neurophysiological theory of schizophrenia. Journal of Mental Science 107:828–838 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P (2017a) The Clinical High-Risk State for Psychosis (CHR-P), Version II. Schizophr Bull 43:44–47 doi: 10.1093/schbul/sbw158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P (2017b) Extending the benefits of indicated prevention to improve outcomes of first-episode psychosis. JAMA Psychiatry 74:667–668 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Bonoldi I, et al. (2016a) Prognosis of Brief Psychotic Episodes: A Meta-analysis. JAMA Psychiatry 73:211–220 doi: 10.1001/jamapsychiatry.2015.2313 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, De Micheli A, et al. (2017a) Diagnostic and Prognostic Significance of Brief Limited Intermittent Psychotic Symptoms (BLIPS) in Individuals at Ultra High Risk. Schizophr Bull 43:48–56 doi: 10.1093/schbul/sbw151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Frascarelli M, Valmaggia L, et al. (2015) Antidepressant, antipsychotic and psychological interventions in subjects at high clinical risk for psychosis: OASIS 6-year naturalistic study. Psychological Medicine 45:1327–1339 doi: 10.1017/S003329171400244X [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Rutigliano G, Stahl D, Davies C, Bonoldi I, Reilly T, McGuire P (2017b) Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry 74:493–500 doi: 10.1001/jamapsychiatry.2017.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Schultze-Lutter F, Cappucciati M, et al. (2016b) The Dark Side of the Moon: Meta-analytical Impact of Recruitment Strategies on Risk Enrichment in the Clinical High Risk State for Psychosis. Schizophr Bull 42:732–743 doi: 10.1093/schbul/sbv162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourzis P, Katrivanou A, Beratis S (2002) Symptomatology of the initial prodomal phase in schizophrenia. Schizophrenia Bulletin 28:415–429 [DOI] [PubMed] [Google Scholar]
- Gross G (1969) Prodrome und vorpostensyndrome schizophrener erkrankungen In: Huber G (ed) Schizophrenie und Zyklothymie. Ergebnisse und Probleme. Thieme, Stuttgart, pp 177–187 [Google Scholar]
- Gross G (1997) The onset of schizophrenia. Schizophrenia Research 28:187–198 [DOI] [PubMed] [Google Scholar]
- Grubbs FE (1950) Sample Criteria for Testing Outlying Observations. Annals of Mathematical Statistics 21:27–58 doi: DOI 10.1214/aoms/1177729885 [DOI] [Google Scholar]
- Haas GL, Sweeney JA (1992) Premorbid and onset features of first-episode schizophrenia. Schizophrenia Bulletin 18:373–386 doi: 10.1093/schbul/18.3.373 [DOI] [PubMed] [Google Scholar]
- Häfner H (2000) Onset and early course as determinants of the further course of schizophrenia. Acta Psychiatrica Scandinavica, Supplement 102:44–48 [PubMed] [Google Scholar]
- Häfner H, Maurer K, Löffler W, Bustamante S, Van der Heiden W, Riecher-Rössler A, Nowotny B (1995) Onset and early course of schizophrenia In: Search for the Causes of Schizophrenia. Springer, pp 43–66 [Google Scholar]
- Hafner H, Maurer K, Loffler W, Riecher-Rossler A (1993) The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry 162:80–86 [DOI] [PubMed] [Google Scholar]
- Hafner H, Riecher A, Maurer K, Loffler W, Munk-Jorgensen P, Stromgren E (1989) How does gender influence age at first hospitalization for schizophrenia? A transnational case register study. Psychol Med 19:903–918 [DOI] [PubMed] [Google Scholar]
- Harrigan SM, McGorry PD, Krstev H (2003) Does treatment delay in first-episode psychosis really matter? Psychological Medicine 33:97–110 [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Flaum M, Nopoulos P, Miller D (2000) Untreated initial psychosis: its relation to quality of life and symptom remission in first-episode schizophrenia. American Journal of Psychiatry 157:808–815 [DOI] [PubMed] [Google Scholar]
- Huber G, Gross G, Schuttler R, Linz M (1980) Longitudinal studies of schizophrenic patients. Schizophr Bull 6:592–605 [DOI] [PubMed] [Google Scholar]
- Kane JM, Robinson DG, Schooler NR, et al. (2016) Comprehensive Versus Usual Community Care for First-Episode Psychosis: 2-Year Outcomes From the NIMH RAISE Early Treatment Program. Am J Psychiatry 173:362–372 doi: 10.1176/appi.ajp.2015.15050632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Haas G, Miewald J, Montrose DM, Reddy R, Schooler NR, Sweeney JA (2003) Prolonged untreated illness duration from prodromal onset predicts outcome in first episode psychoses. Schizophrenia Bulletin 29:757–769 [DOI] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F (2001) Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry 58:158–164 [DOI] [PubMed] [Google Scholar]
- Klosterkötter J, Schultze-Lutter F, Gross G, Huber G, Steinmeyer EM (1997) Early self-experienced neuropsychological deficits and subsequent schizophrenic diseases: An 8-year average follow-up prospective study. Acta Psychiatrica Scandinavica 95:396–404 [DOI] [PubMed] [Google Scholar]
- Lappin JM, Dazzan P, Morgan K, et al. (2007) Duration of prodromal phase and severity of volumetric abnormalities in first-episode psychosis. Br J Psychiatry Suppl 51:s123–127 [DOI] [PubMed] [Google Scholar]
- Loebel AD, Lieberman JA, Alvir JM, Mayerhoff DI, Geisler SH, Szymanski SR (1992) Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry 149:1183–1188 [DOI] [PubMed] [Google Scholar]
- Malla AK, Norman RM, Manchanda R, et al. (2002) One year outcome in first episode psychosis: influence of DUP and other predictors. Schizophrenia Research. 54:231–242 [DOI] [PubMed] [Google Scholar]
- Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T (2005) Association between duration of untreated psychosis and in cohorts of first-episode outcome patients - A systematic review. Archives of General Psychiatry 62:975–983 doi: DOI 10.1001/archpsyc.62.9.975 [DOI] [PubMed] [Google Scholar]
- McGlashan TH (1984) The Chestnut Lodge Follow-up Study: II. Long-term Outcome of Schizophrenia and the Affective Disorders. Archives of General Psychiatry 41:586–601 [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Walsh B, Woods S (2010) The psychosis-risk syndrome : handbook for diagnosis and follow-up. Oxford University Press, New York [Google Scholar]
- McGorry PD, Edwards J, Mihalopoulos C, Harrigan SM, Jackson HJ (1996) EPPIC: an evolving system of early detection and optimal management. Schizophr Bull 22:305–326 [DOI] [PubMed] [Google Scholar]
- Melle I, Johannesen JO, Friis S, et al. (2006) Early detection of the first episode of schizophrenia and suicidal behavior. Am J Psychiatry 163:800–804 doi: 10.1176/ajp.2006.163.5.800 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, et al. (2003) Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 29:703–715 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW (2002) Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 159:863–865 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, et al. (1999) Symptom assessment in schizophrenic prodromal states. Psychiatr Q 70:273–287 [DOI] [PubMed] [Google Scholar]
- Moller P, Husby R (2000) The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull 26:217–232 [DOI] [PubMed] [Google Scholar]
- Oliver D, Davies C, Crossland G, Lim S, Gifford G, McGuire P, Fusar-Poli P (2018) Can We Reduce the Duration of Untreated Psychosis? A Systematic Review and Meta-Analysis of Controlled Interventional Studies. Schizophr Bull. 44:1362–1372. doi: 1310.1093/schbul/sbx1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttila M, Jaaskelainen E, Hirvonen N, Isohanni M, Miettunen J (2014) Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. British Journal of Psychiatry 205:88–94 doi: 10.1192/bjp.bp.113.127753 [DOI] [PubMed] [Google Scholar]
- Raballo A, Meneghelli A, Cocchi A, et al. (2014) Shades of vulnerability: Latent structures of clinical caseness in prodromal and early phases of schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 264:155–169 [DOI] [PubMed] [Google Scholar]
- Rabiner CJ, Wegner JT, Kane JM (1986) OUTCOME STUDY OF 1ST-EPISODE PSYCHOSIS .1. RELAPSE RATES AFTER 1 YEAR. American Journal of Psychiatry 143:1155–1158 [DOI] [PubMed] [Google Scholar]
- Renwick L, Lyne J, Donoghue BO, et al. (2015) Prodromal symptoms and remission following first episode psychosis. Schizophrenia Research 168:30–36 [DOI] [PubMed] [Google Scholar]
- Riecher A, Maurer K, Löffler W, et al. (1991) Sex differences in age at onset and course of schizophrenic disorders: A contribution to the understanding of the disease? In: Häfner H, Gattaz WF (eds) Search for the Causes of Schizophrenia. Springer, Berlin, pp 14–33 [Google Scholar]
- Rosengard RJ, Malla A, Mustafa S, et al. (2018) Association of Pre-onset Subthreshold Psychotic Symptoms With Longitudinal Outcomes During Treatment of a First Episode of Psychosis. JAMA Psychiatry doi: 10.1001/jamapsychiatry.2018.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Lutter F, Rahman J, Ruhrmann S, Michel C, Schimmelmann BG, Maier W, Klosterkötter J (2015) Duration of unspecific prodromal and clinical high risk states, and early help-seeking in first-admission psychosis patients. Social Psychiatry and Psychiatric Epidemiology 50:1831–1841 doi: 10.1007/s00127-015-1093-3 [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F, Ruhrmann S, Hoyer C, Klosterkötter J, Leweke FM (2007) The initial prodrome of schizophrenia: different duration, different underlying deficits? Comprehensive Psychiatry 48:479–488 [DOI] [PubMed] [Google Scholar]
- Shah JL, Crawford A, Mustafa SS, Iyer SN, Joober R, Malla AK (2017) Is the Clinical High-Risk State a Valid Concept? Retrospective Examination in a First-Episode Psychosis Sample. Psychiatr Serv 68:1046–1052 doi: 10.1176/appi.ps.201600304 [DOI] [PubMed] [Google Scholar]
- Shioiri T, Shinada K, Kuwabara H, Someya T (2007) Early prodromal symptoms and diagnoses before first psychotic episode in 219 inpatients with schizophrenia. Psychiatry and Clinical Neurosciences 61:348–354 [DOI] [PubMed] [Google Scholar]
- Singh SP, Cooper JE, Fisher HL, et al. (2005) Determining the chronology and components of psychosis onset: The Nottingham Onset Schedule (NOS). Schizophrenia Research 80:117–130 [DOI] [PubMed] [Google Scholar]
- Srihari VH, Tek C, Kucukgoncu S, et al. (2015) First-Episode Services for Psychotic Disorders in the U.S. Public Sector: A Pragmatic Randomized Controlled Trial. Psychiatr Serv 66:705–712 doi: 10.1176/appi.ps.201400236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T (2013) Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ 346:f185 doi: 10.1136/bmj.f185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmaggia LR, Byrne M, Day F, et al. (2015) Duration of untreated psychosis and need for admission in patients who engage with mental health services in the prodromal phase. Br J Psychiatry 207:130–134 doi: 10.1192/bjp.bp.114.150623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsamis J, Adamson JD (1971) Early schizophrenia. Can Psychiatr Assoc J 16:487–497 [DOI] [PubMed] [Google Scholar]
- Woods SW, Walsh BC, Powers III AR, McGlashan TH (2019) Reliability, validity, epidemiology, and cultural variation of the Structured Interview for Psychosis-risk Syndromes (SIPS) and the Scale Of Psychosis-risk Symptoms (SOPS) In: Li H, Shapiro DI, Seidman LJ (eds) Handbook of Attenuated Psychosis Syndrome Across Cultures: International Perspectives on Early Identification and Intervention. Springer, New York, pp 85–113 [Google Scholar]
- Yung AR, McGorry PD (1996) The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull 22:353–370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nelson-Aalen cumulative probability plots for all participants (including those who overlap in their sub-group membership) (a) and only participants who belong to one distinct CHR-P sub-group (b).
Prodrome duration in months plotted as a function of NAPLS-2 Consortium site. Duration differed by site (F1,7 = 2.15; p = 0.0472).