Abstract
Botulinum neurotoxins (BoNTs) and tetanus neurotoxin (TeNT) are the most potent toxins known and cause botulism and tetanus, respectively. BoNTs are also widely utilized as therapeutic toxins. They contain three functional domains responsible for receptor-binding, membrane translocation, and proteolytic cleavage of host proteins required for synaptic vesicle exocytosis. These toxins also have distinct features: BoNTs exist within a progenitor toxin complex (PTC), which protects the toxin and facilitates its absorption in the gastrointestinal tract, whereas TeNT is uniquely transported retrogradely within motor neurons. Our increasing knowledge of these toxins has allowed the development of engineered toxins for medical uses. The discovery of new BoNTs and BoNT-like proteins provides additional tools to understand the evolution of the toxins and to engineer toxin-based therapeutics. This review summarizes the progress on our understanding of BoNTs and TeNT, focusing on the PTC, receptor recognition, new BoNT-like toxins, and therapeutic toxin engineering.
Keywords: botulinum neurotoxin, tetanus neurotoxin, bacterial toxin, toxin, clostridium, protein engineering
INTRODUCTION
The concept of bacterial toxins was first proven in 1888 by Emile Roux and Alexandre Yersin, who demonstrated that bacterial culture filtration is sufficient to reproduce the disease diphtheria in animal models (1), proving that a biochemical entity released from bacteria was the major disease-causing agent. It is now well established that bacterial toxins act as guided missiles to target and disrupt specific physiological functions in animals and humans. Many of them reach the cytosol of the target cells and modify specific cellular components. These intracellular-acting toxins evolved as multidomain proteins with distinct modes of actions and diverse cellular targets. Identification and characterization of these bacterial toxins led to development of vaccines using deactivated toxins (known as toxoids), which constituted one of the major medical triumphs of the twentieth century in preventing some of the most devastating infectious diseases. In addition, a mechanistic understanding of bacterial toxins has enabled their utilization as scientific tools and therapeutic proteins, taking advantage of their evolutionarily refined ability to modulate specific cellular functions.
The most potent toxins target the nervous system and are termed neurotoxins. The focuses of this review, botulinum neurotoxins (BoNTs) and tetanus neurotoxin (TeNT), claim the top positions as the most potent toxins known (2). They are the causative agents of two distinctive diseases: botulism and tetanus, respectively. Tetanus has been a major infectious disease throughout human history. Identification of TeNT led to the development of effective vaccines, and immunization has now largely eliminated tetanus from developed countries. In contrast to tetanus, botulism is a rare disease in humans. Thus, the general population is not vaccinated against BoNTs. This lack of immunity allows BoNTs to be used as therapeutic toxins for treating a growing list of medical conditions and to be used for cosmetic purposes (3, 4). At the same time, this lack of immunity leaves society vulnerable to the potential use of BoNTs in bioterrorism attacks. Thus, BoNTs are classified as one of the most dangerous bioterrorism agents (5).
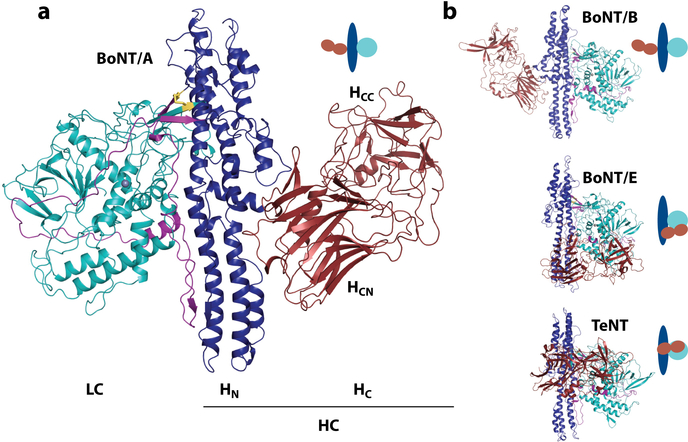
BoNTs are traditionally classified into seven serotypes (BoNT/A–BoNT/G), whereas TeNT has only a single type (6, 7). Serotyping is a classical way to distinguish biologics on the basis of whether polyclonal antisera induced by one entity can recognize/neutralize a second entity. All BoNTs and TeNT belong to the same toxin family, known as clostridial neurotoxins. These toxins share the same molecular architecture and are produced in bacteria as an ∼150-kDa protoxin with three functional domains (Figure 1a). The N-terminal domain is designated the light chain (LC) (∼50 kDa). The other two domains constitute the heavy chain (HC) (∼100 kDa). The LC is a zinc-dependent protease. The HC is the delivery vehicle, with an N-terminal domain (HN) (∼50 kDa) responsible for delivering the LC into the cytosol and a C-terminal domain (HC) (∼50 kDa) responsible for recognizing specific cell-surface receptors. The HC further contains two subdomains, including an N-terminal HCN and a C-terminal HCC. A short linker region between the LC and HC needs to be cleaved by either bacterial or host proteases to convert the protoxin into its active form. The LC and HC remain connected via an interchain disulfide bond until the LC reaches the cytosol, where the disulfide bond is reduced and the LC is released into the cytosol of neurons. The full-length structures of BoNT/A, BoNT/B, BoNT/E, and TeNT have been determined (8–11) (Figure 1b). BoNT/A and BoNT/B have a similar linear arrangement of the three domains with their HC domains isolated from the LCs, whereas the HC and LC are located on the same side of the HN in BoNT/E and TeNT, with interactions between all three domains. Additionally, a belt extends from the HN to surround the LC (Figure 1a), which potentially occupies the same cleft as the substrate proteins.
Figure 1.
(a) Structure of the botulinum neurotoxin A (BoNT/A) [Protein Data Bank (PDB) 3BTA]. The light chain (LC, catalytic domain) is shown in cyan. The heavy chain (HC) is composed of the translocation domain (HN) shown in blue with the belt in purple, and of the binding domain (HC) in red. The two subdomains of HC are indicated with the C-terminal (HCC) and N-terminal (HCN) regions. The disulfide bond linking light and heavy chains is shown as yellow sticks. The zinc ion is shown as a gray sphere. (b) The crystal structures of BoNT/B (PDB 1EPW), BoNT/E (PDB 3FFZ), and tetanus neurotoxin (TeNT) (PDB 5N0B). Domains are colored as in panel a, with a schematic representation highlighting the domain arrangements in the clostridial neurotoxins.
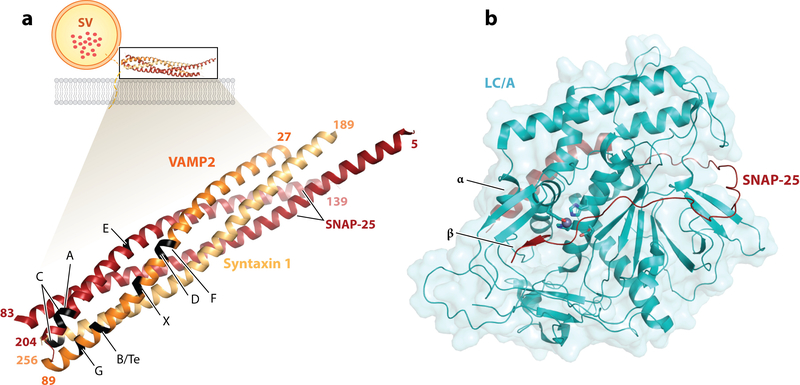
This trimodular structure underlies the mode of action for BoNTs and TeNT: They target and enter motor nerve terminals at neuromuscular junctions (NMJs) via receptor-mediated endocytosis. Acidification of the endosome triggers conformational changes of the toxins that result in transfer of the LC across endosomal membranes into the cytosol, where the LC blocks neurotransmitter release from nerve terminals by cleaving a specific set of proteins known as SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins. These proteins, including more than 60 members in mammalian cells, constitute the core complex that mediates membrane fusion events in eukaryotic cells (12–14). BoNTs and TeNT specifically cleave the set of SNARE proteins that mediate fusion of synaptic vesicle membranes to the presynaptic membrane in neurons, including the plasma membrane protein syntaxin 1, the peripheral membrane protein SNAP-25, and vesicle membrane proteins VAMP1, VAMP2, and VAMP3 (Figure 2a). The seminal discovery of SNARE proteins as the substrates for BoNTs and TeNT in the early 1990s, led by Giampietro Schiavo and Cesare Montecucco (6, 15), coincided with the groundbreaking work from James Rothman’s laboratory on the purification of the SNARE complex (16) and provided pivotal evidence for establishing the central role of the SNARE complex in mediating membrane fusion.
Figure 2.
(a) Schematic of the SNARE complex membrane fusion event and cleavage sites for BoNTs/TeNT. VAMP2 (orange), SNAP-25 (red), and syntaxin 1 (yellow) (PDB 1N7S) form a complex that mediates fusion of the vesicular membrane with the presynaptic membrane (lipid bilayer in gray) and allows neurotransmitter release (represented as red dots in the SV). The structures of the SNARE complex (PDB 1N7S) with VAMP2, SNAP-25, and syntaxin 1 are enlarged, with the cleavage site for each toxin indicated. It should be noted that the toxins cleave their substrate only when in their free form, with the complex being resistant to proteolysis. (b) Structure of the LC/A (cyan) in complex with SNAP-25 (red) (PDB 1XTG). The conserved active site residues (HExxH…E) are shown as sticks, with the zinc ion as a gray sphere. The exosites (α and β), important for substrate binding, are indicated. Abbreviations: BoNTs, botulinum neurotoxins; LC/A, light chain of botulinum neurotoxin A; PDB, Protein Data Bank; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; SV, synaptic vesicle; TeNT, tetanus neurotoxin.
Tremendous progress has been made in understanding the biology and mode of action of BoNTs and TeNT in recent years, as described in several recent excellent reviews (6, 7, 17–19). Here, we aim to provide an up-to-date discussion on our current understanding of BoNTs and TeNT.
BOTULISM AND THE BoNT PROGENITOR COMPLEX
Botulism is usually caused by food poisoning due to ingestion of BoNTs (7). These toxins are produced by a variety of anaerobic spore–forming Clostridial species categorized as Clostridium botulinum, and also include strains known as C. baratii, C. butyricum, and C. argentinense. These bacteria are ubiquitous throughout the world. There are frequent botulism outbreaks in wild animals, although the incidence of botulism among humans has become rare. Besides being a foodborne disease, botulism can also be caused by colonization of the intestine by C. botulinum. This usually occurs in infants before their normal gut microbiotas are fully developed and is known as infant botulism. C. botulinum can also colonize wounds in rare cases and causes wound botulism. The typical symptom of botulism is flaccid paralysis—the inability to contract skeletal muscles. The first sign is usually impaired vision, as muscles controlling eye movement are affected, and is followed by paralysis of facial muscles. Ultimately, patients die from respiratory failure caused by diaphragm paralysis.
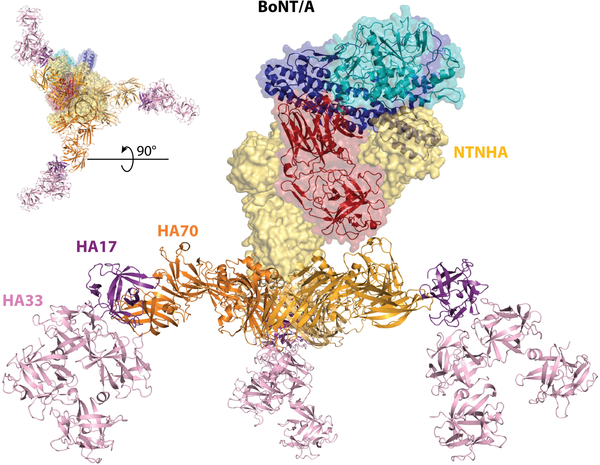
Foodborne botulism is unique in that it is caused by ingesting the toxin, rather than by live bacteria. This oral route posts a formidable challenge for a protein toxin, as it has to remain intact while passing through the gastrointestinal (GI) tract. To meet this challenge, BoNTs are produced and exist as part of a protein complex termed the progenitor toxin complex (PTC), which is encoded within a single gene cluster. Immediately preceding the bont gene is another gene encoding a protein termed NTNHA (also known as NTNH) (nontoxic nonhemagglutinin protein). BoNTs and NTNHAs form heterodimers, which constitute the minimal PTC (M-PTC). Crystal structures of the M-PTC from BoNT/A and BoNT/E have been determined (20, 21), showing that NTNHA and BoNT form a tight complex resembling interlocked hands that buries a large surface area and renders BoNTs resistant to low pH and proteases (Figure 3). BoNT–NTNHA interactions drastically change the position of the HC, and the interactions of this domain with NTNHA are critical in the formation of the complex. BoNT–NTNHA interactions are pH dependent—for instance, the M-PTC of BoNT/A is stable at pH 6.5 but disassembles at pH 7.5, providing a mechanism to release the toxin after passing the GI tract.
Figure 3.
Structure of the L-PTC/A complex, as described by Lee et al. (23), with the M-PTC complex (PDB 3V0A) made of BoNT/A (LC in cyan, HN in blue, and HC in red), the NTNHA (yellow surface), and the HA complex comprised of HA70 (orange), HA17 (purple), and HA33 (pink) (assembled from PDB 4LO4 and 4LO7). The top left corner inset shows the sample complex from below. Abbreviations: BoNT/A, botulinum neurotoxin A; HA, hemagglutinin component; HC, the receptor-binding domain; HN, translocation domain; LC, light chain; L-PTC, large progenitor toxin complex; M-PTC, minimal progenitor toxin complex; NTNHA, nontoxic nonhemagglutinin protein; PDB, Protein Data Bank.
Besides NTNHA, a BoNT gene cluster always contains one of two sets of genes: (a) an HA cluster that encodes three hemagglutinin proteins termed HA17, HA33, and HA70 on the basis of their molecular weights or (b) an OrfX cluster that encodes the proteins OrfX1, OrfX2, OrfX3, and P47. Remarkable advancements have been achieved in understanding the function and structure of the HA proteins, which form a complex that binds to the M-PTC to make the large progenitor toxin complex (L-PTC) (∼760 kDa). Negative staining electron microscopy (EM) revealed the overall domain arrangement of the native L-PTCs of BoNT/A (L-PTC/A), BoNT/B, and BoNT/D (22–24) and showed that the HA proteins form a large triskelion complex, with the NTNHA–BoNT complex bound on top of it (Figure 3). The crystal structure of the recombinant HA complex of BoNT/B revealed a triskelion-shaped structure with each arm forming a Y shape comprising two HA33s, one HA17, and one HA70 (25). The crystal structures of two subcomponents of the HA complex of BoNT/A were also determined (23). One is the central hub formed by three HA70s; the other is the extended arm comprising a part of HA70 in complex with HA17 and two HA33s (termed mini-HA). The assembled HA of BoNT/A was docked into negative stain EM density of the native L-PTC/A, revealing that the interactions between the M-PTC and the HA complex are mediated by a flexible loop in NTNHA, termed the nLoop, which binds to HA70.
Two major functions of the HA complex have been established. First, the HA complex mediates multivalent binding to cell-surface carbohydrates. The crystal structures of HA70 in complex with sialyllactose and the HA17-HA33 in complex with galactose, lactose, or LacNAc have been determined, showing that HA70 and HA33 each contain one carbohydrate binding site (23). In total, each HA complex contains nine carbohydrate binding sites. It was recently shown that carbohydrate binding mediates the enrichment of the HA of BoNT/A to glycoprotein 2 (GP2) on the microfold cells (M-cells) of the intestine (26). GP2 mediates transcytosis of microbes and particles from the intestinal lumen to the lamina propria across the M-cells as a way to expose foreign antigens to immune cells. Thus, the carbohydrate-binding capability of HA may contribute to the initial absorption of L-PTC/A through M-cells. Consistently, mice lacking M-cells or GP2 showed reduced susceptibility to oral feeding of L-PTC/A.
Second, the HA complex is capable of disrupting cell–cell junctions. Utilizing the HA complex of BoNT/B as a bait, Sugawara et al. (27) identified E-cadherin as a specific binding partner for HA. It was subsequently shown that the HA complex of BoNT/A also recognizes E-cadherin (28). E-cadherin is a member of the cadherin family that mediates cell–cell adhesions. E-cadherin is found mainly in epithelial cells and contains five tandem extracellular cadherin (EC) domains (EC1 to EC5). HA complexes of BoNT/A and BoNT/B recognize the terminal EC1-EC2 fragment. The crystal structure of a mini-HA of BoNT/A in complex with the EC1-EC2 fragment of E-cadherin has been determined (28), revealing that interactions involve binding to EC1 by HA70, binding to HA17 by both EC1 and EC2, and a small hydrophobic interface between EC1 and one of HA33. EC1 is the domain that mediates trans-dimerization between two E-cadherin molecules on neighboring cells, which mediates cell–cell adhesion. Binding of HA to EC1 blocks trans-dimerization of E-cadherin, thus disrupting cell–cell adhesions and opening up a paracellular route for absorption of BoNTs. As E-cadherin is distributed on the basolateral side of intestinal epithelial cells, it would be necessary for some L-PTC to be able to cross the intact epithelial barrier initially. Carbohydrate binding–mediated transcytosis through M-cells might contribute to this initial process.
The physiological relevance of both the carbohydrate-binding and E-cadherin interactions for the oral potency of BoNT/A was demonstrated by Lee et al. (28), who succeeded in reconstituting the complete L-PTC/A using proteins purified recombinantly in Escherichia coli. This approach allowed them to create L-PTC/A containing point mutations in HA that specifically disrupted either carbohydrate binding or E-cadherin binding. Both mutant forms of L-PTC/A showed reduced oral toxicity compared with wild-type (WT) L-PTC/A in mice.
In addition, it has been reported that the HA complexes of BoNT/C and BoNT/D can cause vacuoles in cells and induce cell death, although the physiological relevance of this observation has not yet been established (29). In contrast to HA, the function of the OrfX proteins remains unknown. In strains with the OrfX gene cluster, only the M-PTC has been isolated. It remains unclear whether OrfX proteins may form complexes with each other or with the M-PTC, although weak interactions have been observed using mass spectrometry (30). Crystal structures of recombinantly expressed OrfX2 and P47 have been determined, revealing that both proteins contain domains with a tubular lipid-binding fold (31, 32). These structural features suggest that P47 and OrfX2 might have lipid-binding capabilities, and OrfX1 and OrfX2 have been shown to bind phosphatidylinositol phosphate lipids (31).
RECOGNITION OF PRESYNAPTIC NERVE TERMINALS
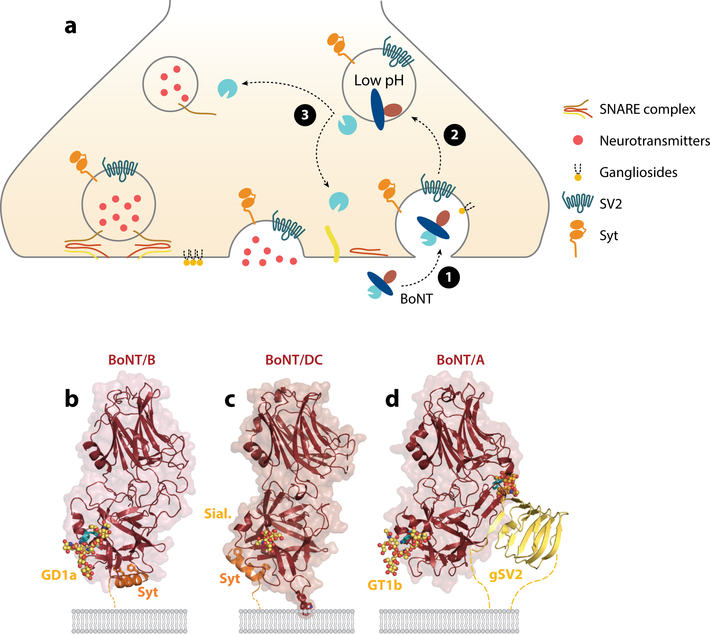
Once BoNTs enter the circulatory system, they target presynaptic motor nerve terminals. The lethal dose for mice with intraperitoneal injection of BoNTs ranges from 0.1 ng/Kg to 1 ng/Kg, making BoNTs the most potent toxins known (2). Lethality from such a low toxin concentration results from the extreme specificity with which it targets neurons. To meet these challenges, BoNTs have evolved a strategy of recognizing two distinct classes of receptors simultaneously. One set of receptors are abundantly expressed on neuronal surfaces and interact with BoNTs with relatively low affinity. The second set of receptors then firmly anchor the toxin on nerve terminals and lead to endocytosis of the toxin (Figure 4a). This double-receptor model was first proposed by Cesare Montecucco (33) in 1986 and is now well-established for the majority of BoNTs.
Figure 4.
Dual receptor binding. (a) Schematic depicting activity-facilitated binding and entry of BoNTs into neurons: dual receptor recognition of the presynaptic motoneuron, receptor-mediated endocytosis followed by pH-induced conformational change that allows translocation of LC in the cytosol, and cleavage of one of the SNARE proteins by LC. (b) BoNT/B (red) binding to Syt (orange) and ganglioside (dots) from PDB 4KBB. Conserved GBS residues are highlighted in cyan; important hydrophobic residues F47 and F54 of Syt are shown as sticks. (c) BoNT/DC (red) binding to Syt (orange) and the Sial. (dots) from the superposition of PDB 4ISR and PDB 5LR0, respectively. Residues of the hydrophobic loop involved in lipid interaction are shown as sticks (Y1251-W1252-F1253). (d) BoNT/A (red) binding to gSV2 (yellow) and ganglioside (yellow dots) from the superposition of PDB 5JLV and 2VU9, respectively. Conserved GBS residues and F953 are highlighted in cyan. Glycan linked to N559 of SV2C are shown as orange dots. The lipid bilayer is represented in gray. Dotted lines indicate the continuation of the protein receptors toward their transmembrane domain. Abbreviations: BoNT, botulinum neurotoxin; GBS, ganglioside binding site; gSV2, glycosylated synaptic vesicle glycoprotein 2; LC, light chain; PDB, Protein Data Bank; Sial., sialyl-T antigen; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; SV2, synaptic vesicle glycoprotein 2; Syt, synaptotagmin.
Ganglioside Receptors
The first set of receptors identified for BoNTs and TeNT are complex gangliosides, a class of glycosphingolipids comprising a ceramide tail and a carbohydrate headgroup with various numbers of sialic acids attached. There are more than 60 ganglioside species, and different cell types express distinct profiles of gangliosides. The carbohydrate headgroups and sialic acids of gangliosides extend from the cell surface and are utilized as attachment points for various ligands, viruses, and bacterial toxins. The complex forms of gangliosides, which contain an additional N-acetylgalactosamine (GalNAc) and galactose (Gal) in their headgroups compared with the simple form of gangliosides, are abundant on neuronal membranes. Binding of BoNTs and TeNT to complex gangliosides were reported in the 1960s and 1970s (34, 35). In recent years, the physiological relevance of complex gangliosides as receptors for BoNTs and TeNT has been established utilizing knockout (KO) mouse lines lacking different enzymes required to synthesize all or partial lines of complex gangliosides, which showed reduced susceptibility to BoNTs and TeNT (36–45).
Binding of BoNTs and TeNT to complex gangliosides has been well characterized in vitro. A ganglioside binding site (GBS) with the conserved core residues SxWY was initially mapped to the C-terminal tip of the HCC of TeNT (HCC/T) (46). This GBS was further confirmed for the HC of BoNT/A (HC/A) and BoNT/B (HC/B) and is conserved in BoNT/A, BoNT/B, BoNT/E, BoNT/F, and BoNT/G (47, 48). The crystal structures of TeNT (full-length and HC/T), BoNT/A (HC/A), BoNT/B (HC/B), and BoNT/F (HC/F) in complex with the carbohydrate headgroups of complex gangliosides have been determined (11, 46, 49–52), revealing that the GBS recognizes mainly the GalNAc-Gal moiety, together with sialic acids, thus providing a structural basis for the toxins’ selectivity toward complex gangliosides.
The SxWY motif is not conserved in BoNT/C or BoNT/D, but clear in vivo evidence shows that BoNT/C and BoNT/D require complex gangliosides as receptors (37, 44). Mutagenesis studies suggest that the position in BoNT/C and BoNT/D analogous to the conserved GBS in other BoNTs may contribute to ganglioside binding (41, 42). HC domains of BoNT/C (HC/C) and BoNT/D (HC/D) also contain an additional sialic acid–binding site, termed Sia-1 in BoNT/C. Mutations within the Sia-1 site reduce toxin binding to gangliosides, suggesting that BoNT/C and BoNT/D may contain a second ganglioside binding site at this position. TeNT also contains a sialic acid binding site (also termed the R site) at the analogous region to the Sia-1 site (46, 47, 53, 54). Mutations at this site reduced binding of TeNT to complex gangliosides, suggesting that TeNT contains two ganglioside binding sites. However, under physiological conditions, whether the second ganglioside binds to the Sia-1 site on neuronal surfaces remains to be established.
BoNT/DC is a naturally existing chimeric toxin evolutionarily related to BoNT/C and BoNT/D. Its LC and HN are almost identical to BoNT/D, whereas its HC (HC/DC) shares ∼77% identity to HC/C (55). The SxWY motif is not conserved in HC/DC. BoNT/DC is unique among all BoNTs in that its binding and entry are not affected in cultured neurons lacking complex gangliosides, and the toxicity of BoNT/DC in mice lacking complex gangliosides is similar to that in WT mice (45). The crystal structure of HC/DC in complex with the terminal components of a complex ganglioside headgroup revealed that HC/DC recognizes carbohydrates at a site analogous to the conserved GBS of other BoNTs (Figure 4c), but HC/DC only recognizes the sialic acid moiety and does not interact with the carbohydrate backbone. Therefore, BoNT/DC is able to utilize a wide range of sialic acid–containing surface molecules as receptors. As gangliosides are the major sialic acid–containing molecules, accounting for ∼65% of the total sialic acids in neuronal plasma membranes, neurons lacking gangliosides still showed greatly reduced levels of binding and entry of BoNT/DC.
Protein Receptors: Synaptotagmin I and II
Nishiki et al. (56, 57) first identified two homologous synaptic vesicle membrane proteins, synaptotagmin I and II (Syt-I and Syt-II), as BoNT/B binding partners in the 1990s. Later studies established that Syt-I and Syt-II are functional protein receptors that mediate binding and entry of BoNT/B into cells (58). Subsequently, BoNT/G and BoNT/DC were also reported to utilize Syt-I and Syt-II as functional receptors (38, 59, 60). Syt-I and Syt-II are single-spanning transmembrane proteins, with a C-terminal cytoplasmic domain and a short N-terminal tail inside the vesicle lumen (Figure 4a). Syt-I/Syt-II are key synaptic vesicle membrane proteins, and their cytoplasmic domain functions as the Ca2+ sensors for triggering synaptic vesicle exocytosis (61). Using Syt-I/Syt-II as receptors suggests an entry pathway for BoNTs (Figure 4a): These proteins are usually hidden inside neurons within synaptic vesicles. When neurons are active and releasing neurotransmitters, the luminal tail of Syt-I/Syt-II becomes transiently exposed on cell surfaces and available for toxin binding. Neurons actively recycle Syt-I/Syt-II to regenerate synaptic vesicles, which provides an efficient access route for the toxin. This activity-facilitated entry pathway enhances neuronal specificity and enables the toxins to preferentially attack active neurons.
Defining Syt-I and Syt-II as the functional receptors for BoNT/B, BoNT/DC, and BoNT/G was straightforward, as purified recombinant Syt-I and Syt-II luminal fragments directly and specifically bind to all three toxins in vitro (57–60). Neurons lacking Syt-I/Syt-II become resistant to the above toxins, and expression of Syt-I/Syt-II restored binding and entry of these toxins (38, 60). The binding sites for BoNT/B, BoNT/G, and BoNT/DC have been mapped to the same short sequence adjacent to the transmembrane domain within the luminal tail of Syt-I/Syt-II. The minor residue differences between Syt-I and Syt-II within this binding region account for their differences in binding affinity toward BoNTs, with Syt-II showing higher binding affinity than Syt-I (57, 62, 63). Syt-II was expressed at all motor nerve terminals, whereas Syt-I was only coexpressed in ∼40% of motor nerve terminals (64). Thus, Syt-II likely acts as the major toxin receptor at NMJs. The physiological relevance of Syt-I and Syt-II as toxin receptors has been further demonstrated for BoNT/B and BoNT/DC using competition assays: Recombinant Syt-II fragments containing the toxin binding site reduced toxicity of BoNT/B and BoNT/DC in vivo in mice (58, 60).
The crystal structure of BoNT/B in complex with Syt-II was determined in 2006 in two parallel studies (62, 65). The structure revealed that the Syt-II fragment binds to a hydrophobic groove in the HCC of BoNT/B (Figure 4b). The binding is mainly mediated by hydrophobic interactions, particularly through the key residues F47 and F54 in Syt-II. Multiple other interactions influence the binding specificity and affinity. F47 and F54 are highly conserved in Syt-II across a wide range of vertebrate species, but human and chimpanzee Syt-II contains an L residue at the F54 position. This single residue difference severely reduces binding of BoNT/B, BoNT/G, and BoNT/DC to human Syt-II compared with mouse/rat Syt-II, which might explain why humans are less sensitive to BoNT/B compared with BoNT/A (60, 66).
The Syt binding site in HC/B is located close to, but separated from, the GBS. The HC of BoNT/G (HC/G) shares ∼50% sequence identity with HC/B, and the crystal structure of HC/G showed that the Syt binding site is largely conserved (67, 68). In contrast, HC/DC shares a rather low (∼33%) sequence identity with HC/B. The crystal structures of HC/DC in complex with human Syt-I and Syt-II peptides have been determined (69) (Figure 4c). Syt-I and Syt-II are recognized in a similar manner, with Syt-I (37–48) and Syt-II (43–54) both forming an amphipathic helix docking into the hydrophobic pocket at the C-terminus of HC/DC. Although the key Syt residues involved in binding are the same, BoNT/DC presents a distinct Syt binding site, located perpendicular to the Syt binding site in BoNT/B. Interestingly, the C terminus of the Syt-II peptides appears at a similar location when bound to either BoNT/B or BoNT/DC, with the rest of Syt-II peptide extending perpendicularly. Because the C terminus is next to the transmembrane domain of Syt-II, both toxins would be anchored at a similar position relative to the membrane.
The relationship between ganglioside binding and Syt binding was further clarified by the first ternary crystal structure of an HC/B–Syt–ganglioside complex (50) (Figure 4b). The structure demonstrated that the GBS and Syt binding site are isolated from each other, with no significant conformational changes upon simultaneous binding to the two receptors. Thus, the GBS and Syt binding site provide two independent anchoring points in the double-receptor model.
Protein Receptors: Synaptic Vesicle Glycoprotein 2
As hijacking the synaptic vesicle recycling process appears to be a preferred entry pathway, searching for receptors for BoNTs was carried out by systematically screening synaptic vesicle membrane proteins. These studies led to the discovery that BoNT/A utilizes another synaptic vesicle membrane protein, SV2 (synaptic vesicle glycoprotein 2), as its receptor (70, 71). This discovery is consistent with the finding that internalized BoNT/A is largely localized within synaptic vesicles (72).
SV2 includes three isoforms (SV2A, SV2B, and SV2C) in mammals. SV2A is selectively expressed in a subpopulation of motor nerve terminals of slow muscle fibers, whereas SV2B and SV2C are broadly expressed in motor nerve terminals (73). SV2 contains 12 transmembrane helices. Its N terminus and C terminus are on the cytosolic side. BoNT/A recognizes the fourth luminal domain of SV2 (SV2-L4) and can utilize all three homologs as its receptors (Figure 4d). The evidence for SV2 as a BoNT/A receptor is considerable. First, BoNT/A binds directly and specifically to SV2C-L4 recombinantly expressed in E. coli and also showed weak binding to SV2A-L4 and SV2B-L4 (70, 71). Second, binding and entry of BoNT/A into neurons lacking all SV2s are blocked and can be rescued by expression of full-length SV2A/B/C, or a chimeric receptor containing the L4 of SV2A/B/C (40, 70). On neuronal surfaces, the three SV2 isoforms are similarly capable of mediating binding and entry of BoNT/A. Finally, BoNT/A showed a reduced binding to the NMJs of SV2B KO mice and a lower toxicity on SV2B KO mice in vivo compared with WT mice (70).
The crystal structure of HC/A in complex with human SV2C-L4 expressed in E. coli was determined (74). The structure revealed that SV2C-L4 forms a quadrilateral β-helix with overlapping β-strands (Figure 4d), a fold similar to pentapeptide repeat proteins. This type of architecture is prone to forming higher-order aggregates, similar to the formation of amyloid fibrils. HC/A appears to utilize this weakness in this fold and stacks its own β-strands onto the exposed β-strands of SV2C-L4. The interactions are mainly through backbone-to-backbone hydrogen bonds, thus largely depending on recognizing the overall β-strand conformation, rather than specific residues. This binding model is in sharp contrast to the BoNT–Syt-I/Syt-II interactions, which include extensive side-chain interactions that ensure high binding specificity. Another major difference is that the Syt binding site is located within the HCC, whereas the SV2 binding site involves residues from both HCN and HCC. The HC/A structure in the HC/A–SV2C–L4 complex is similar to apo-HC/A, suggesting that SV2C binding does not cause any significant conformation change of HC/A.
The promiscuous nature of backbone-to-backbone interactions is difficult to reconcile with the highly specific receptor recognition of BoNT/A and raises the question of whether additional interactions with SV2 are involved. SV2-L4 contains three N-linked glycosylation sites conserved across SV2A, SV2B, and SV2C. By expressing glycosylation-deficient mutant forms of SV2A in SV2-null neurons, it was shown that the first two of the three glycosylation sites did not affect binding and entry of BoNT/A. However, removing the third glycosylation site (N573 in SV2A), which is located within the SV2–BoNT/A binding interface, diminished the efficacy of BoNT/A entry into neurons, suggesting that glycosylation at this site is critical for high-affinity binding of BoNT/A to SV2 (40, 75, 76).
To understand the role of the N-linked glycan in BoNT/A binding, Yao et al. (75) determined the structure of HC/A in complex with a glycosylated human SV2C-L4 (Figure 4d). The protein–protein interface is largely the same as the structure of nonglycosylated SV2C-L4 with HC/A, but a complex glycan was clearly observed extending from the third glycosylation site (N559 in SV2C) into a pocket of HC/A. The two innermost GlcNAc, the third mannose and a fucose, are well defined and are recognized through a network of hydrogen bonds and van der Waals interactions. These interactions nearly double the binding interface between HC/A and SV2C-L4 from 557 Å2 to 925 Å2. Consistently, binding of HC/A to glycosylated SV2C-L4 showed a significantly slower dissociation rate compared with its binding to nonglycosylated SV2C-L4 (75, 76). The physiological relevance of glycan binding was further established as a mutant BoNT/A containing F953R showed no detectable toxicity at mouse diaphragm motor nerve terminals (75). Interestingly, this glycan binding site overlaps with the epitope region for a well-established BoNT/A-neutralizing human monoclonal antibody (77). The structure of N-linked glycosylation is highly heterogeneous, but the base of all N-linked glycans is conserved across species, starting with two GlcNAc and a mannose. In essence, BoNT/A utilizes conserved carbohydrate moieties to expand the binding site and enhance the specificity and avidity of the BoNT/A–SV2 interactions. This composite binding model, which combines a backbone-to-backbone–mediated conformation-dependent recognition with an invariable posttranslational modification moiety, has the advantage of tolerating a high degree of residue variations in host species as it does not depend on any specific side-chain interactions.
BoNT/E was also found to utilize SV2 as its functional receptor, as binding and entry of BoNT/E into neurons lacking all SV2s are blocked, and it can be rescued by expression of full-length SV2A or SV2B (but not SV2C) or by a chimeric receptor containing the L4 of SV2A or SV2B (40). Furthermore, BoNT/E showed lower potency on ex vivo preparation of diaphragm tissues from SV2B KO mice and lower toxicity in SV2B KO mice in vivo. Unlike BoNT/A, which can utilize all three SV2s, BoNT/E can use only SV2A or SV2B, but not SV2C, at least not in cultured hippocampal/cortical neurons (40). Binding of BoNT/E appears to be more dependent on the N-linked glycan at the third glycosylation site of SV2 than does BoNT/A, as mutating the N573 site in SV2A completely abolished its function to mediate binding and entry of BoNT/E. Consistently, BoNT/E showed no detectable binding to nonglycosylated SV2-L4. Direct binding of HC/E to glycosylated SV2A-L4 has been shown, and mutagenesis approaches suggest that the SV2 binding site involves the interface region between HCN/E and HCC/E (78). However, the exact interacting interface remains to be established.
BoNT/D has also been shown to utilize all three SV2s as its functional receptors on cultured hippocampal/cortical neurons (44). However, direct binding of BoNT/D to SV2 has yet to be demonstrated, and the in vivo relevance of SV2 for BoNT/D remains to be validated. Interestingly, the binding mechanism of BoNT/D appears to be distinct from BoNT/A and BoNT/E. Mutating any one of the three conserved glycosylation sites in SV2A showed no effect on its ability to mediate binding and entry of BoNT/D (44). Furthermore, chimeric receptors containing SV2-L4 could not mediate binding and entry of BoNT/D, suggesting that isolated SV2-L4 is not sufficient to mediate BoNT/D binding (44).
TeNT was also shown to utilize SV2 as its functional receptors in cultured hippocampal/cortical neurons (79). Furthermore, TeNT showed reduced toxicity in SV2B KO mice in vivo, suggesting that SV2B contributes to TeNT entry at NMJs in vivo. However, direct interactions of TeNT with any SV2s remain to be established. Similar to BoNT/D, mutating any one of the three conserved N-linked glycosylation sites does not affect binding and entry of TeNT mediated by SV2A, and SV2-L4 in chimeric receptors cannot mediate binding and entry of TeNT. Besides SV2, it has been reported that TeNT may interact with extracellular matrix proteins of the nidogen family (80). Two peptides derived from two nidogen family members can reduce binding of TeNT to neurons and reduce toxicity of TeNT in vivo in mice. Binding of HC/T to NMJs was reduced in the nidogen-2 KO mice, and TeNT showed a slightly reduced toxicity in nidogen-2 KO mice. The molecular details of TeNT–nidogen interactions and the physiological role of this interaction remain to be further elucidated.
Whether BoNT/F and BoNT/C have their own protein receptors is not clear. BoNT/F has been reported to bind glycosylated SV2s (41, 81). However, entry of BoNT/F into SV2 KO hippocampal/cortical neurons is at levels similar to entry into WT neurons, suggesting that SV2 is not an essential receptor for BoNT/F (44, 79). It has been reported that BoNT/A may utilize fibroblast growth factor receptor 3 as another receptor (82), but its role remains to be confirmed.
The Lipid Binding Loop
Besides gangliosides and the protein receptors, the lipid membrane itself could contribute to the overall affinity of the toxins for cell surfaces. Chai et al. (62) first proposed that an exposed loop of BoNT/B, located between the GBS and Syt binding site, is in an ideal position to interact with lipid membranes through hydrophobic interactions (Figure 4b). All BoNTs contain such a loop, although they differ significantly. For instance, the loops in HC/C and HC/DC are long and structurally similar to the loop in HC/B, whereas the loop in HC/A is significantly shorter, which may prevent its interaction with membranes. It has been shown that point mutations at the tip of this loop reduced binding of HC/C, HC/D, and HC/DC to gangliosides; thus, this loop was initially proposed as a potential ganglioside binding loop (43, 83–86). However, this loop could contribute to ganglioside binding by interacting with the ceramide lipid anchors of gangliosides nonspecifically. Indeed, analyzing soluble ganglioside carbohydrate headgroups revealed that the toxin has far lower affinity for the isolated carbohydrate moiety than expected (52), suggesting that the lipid membrane itself could contribute to the apparent affinity of the toxins for gangliosides. This question was directly addressed experimentally in a recent study, which showed that HC/DC is capable of binding to ganglioside-free liposomes in liposome flotation assays, and point mutations at the tip of the HC/DC loop abolished this ganglioside-independent binding to liposomes, demonstrating that this loop binds to lipids in a ganglioside-independent manner (45) (Figure 4c). A recent study also examined the role of this loop using nanodiscs with both gangliosides and Syt embedded within a membrane environment (87). Although direct binding of HC/B, HC/DC, and HC/G to immobilized nanodiscs containing lipid alone was not detectable under these assay conditions, they found that deletions at the tip of the loop reduced binding of these HC domains to nanodiscs containing gangliosides alone, Syt-II alone, or a combination of gangliosides plus Syt-II. Furthermore, point mutations and deletion of residues at the tip of this loop reduced the toxicity of BoNT/B, BoNT/DC, and BoNT/G (87), demonstrating a critical role of this loop in the potency of these toxins.
RETROGRADE TRANSPORT OF TeNT
The sequence and structure of the TeNT is highly similar to BoNTs. However, the disease tetanus, caused by TeNT, is drastically different from botulism and is characterized by periodic hypercontraction of skeletal muscles (termed spastic paralysis). TeNT is produced by Clostridium tetani, in which the toxin is encoded on a plasmid. There are no other toxin-associated proteins, which is consistent with the fact that TeNT does not need to pass through the GI tract. Instead, TeNT is produced in situ and directly enters the circulatory system when C. tetani spores contaminate deep wounds. TeNT and BoNTs both target and enter peripheral motor nerve terminals, but their distinct destination determines their symptoms (88): Whereas the LC of BoNTs is released into the cytosol of motor neurons, the majority of TeNT is transported retrogradely along the motor neuron axon to the soma (Figure 5). TeNT is then released from motor neurons and reenters connecting inhibitory neurons, where the LC/T (LC of TeNT) is finally released into the cytosol and blocks neurotransmitter release. Losing inhibitory input leads to hyperactivity of motor neurons, resulting in spastic paralysis.
Figure 5.
Retrograde transport. Schematic representation of the intracellular pathway followed by the clostridial neurotoxins. BoNTs mainly act at the NMJ (orange arrows), whereas TeNT undergoes retrograde transport along the axon (black arrows) and transcytosis to reach the inhibitory interneurons of the CNS (blue arrows). Abbreviations: BoNTs, botulinum neurotoxins; CNS, central nervous system; NMJ, neuromuscular junction; TeNT, tetanus neurotoxin.
The potential retrograde traffic routes for TeNT have been thoroughly discussed in recent reviews (88, 89). The molecular basis for sorting TeNT into retrograde transport pathways remains a mystery. It had been previously assumed that HC/T (HC of TeNT)–receptor interactions govern TeNT’s retrograde sorting. However, this view has been challenged by recent studies. Wang et al. (90) generated a series of chimeric toxins by switching different domains of BoNTs with their counterparts in TeNT. Surprisingly, chimeric toxins containing either HC/T or even the entire HC of TeNT both induced flaccid paralysis in mice, suggesting that the HC of TeNT is not sufficient to mediate efficient retrograde transport (Figure 5). Conversely, replacing HC/T with HC/A also resulted in a chimeric toxin that induced flaccid paralysis. These data suggest that HC/T is required but not sufficient to mediate efficient retrograde transport of TeNT. Other parts of TeNT including its LC are required for efficient sorting. These results are consistent with the observation that TeNT showed a low degree of colocalization with HC/T in cells and neurons and a much higher retrograde transport efficacy than HC/T (91–93). The crystal structure of TeNT was recently determined, revealing a unique domain arrangement in a closed conformation (Figure 1b) and a pH-mediated domain rearrangement between closed and open conformations (11). The exact contribution of such domain rearrangements to retrograde sorting of TeNT remains to be examined.
Interestingly, the distinction between TeNT and BoNTs in terms of retrograde sorting is not absolute. It has been shown that TeNT induces flaccid paralysis at relatively high toxin concentrations (94), suggesting that it can escape from endosomes within motor nerve terminals above a certain dose threshold. Conversely, retrograde transport of BoNT/A has been suggested by the earlier observation that radioactivity was detected in the ventral horn of the spinal cord when radiolabeled BoNT/A was injected in the rat gastrocnemius muscle (95). Recent studies showed that BoNT/A injected in the superior colliculus caused cleavage of SNAP-25 within rat retina, and retrograde transport of BoNT/A occurs in both hippocampal neurons and motor neurons (96, 97). Utilizing microfluidic devices that separate the axonal terminal versus soma of neurons, Restani et al. (98) showed that fluorescence-labeled BoNT/A and BoNT/E were both retrogradely transported in cultured motor neurons, and Bomba-Warczak et al. (99) showed that BoNT/A, BoNT/D, and TeNT added into the axonal chamber resulted in cleavage of SNARE proteins in second-order neurons that do not extend any axons into the axonal chamber. Adding neutralizing antibodies blocked cleavage of SNARE proteins in second-order neurons, suggesting that these toxins were released from the first-order neurons into media before they reenter the second-order neurons. Bomba-Warczak et al. (99) also utilized SV2A/B KO neurons and found that the retrograde transport process of these toxins was not affected, suggesting that the retrograde sorting of these toxins is independent of their entry receptor SV2. How TeNT and BoNTs are sorted into the retrograde transport pathways and the molecular determinants for this sorting process remain to be elucidated.
TRANSLOCATION
Membranes form a formidable barrier for any toxins acting in the cytosol. The HN of BoNTs and TeNT facilitates the translocation of their LCs across endosomal membranes. Presumably, low pH induces conformational changes in the HN, leading to its interactions with membranes and eventual translocation of the LCs. Once exposed in the cytosol, the disulfide bond connecting the HN and the LC is reduced, which is facilitated by the NADPH-thioredoxin reductase-thioredoxin system in the cytosol (100, 101).
The molecular details of the membrane translocation process remain to be established. This represents a major question not only for BoNTs and TeNT but also for similar bacterial toxins that are produced as single polypeptides, such as diphtheria toxin and Clostridium difficile toxins. Our current knowledge on translocation of the clostridial neurotoxins has been thoroughly discussed in several recent reviews (17, 102), with two major models proposed. One model suggests that HN forms a protein transduction channel, which allows unfolded LCs to cross the membrane. Consistently, planar lipid bilayer studies and a patch clamp approach revealed that an ion-conducting channel is formed by the HN (103–105). In this model, formation of an HN channel is a prerequisite for LC translocation, and the LCs do not contact the lipid membrane. An alternative model proposes that the LCs and HN are induced into a molten globule state in the presence of both low pH and negatively charged lipid membranes. The molten globule state is a partially unfolded state that exposes hydrophobic regions of the protein, enabling interactions with the hydrophobic core of lipid membranes. In this model, the LC is part of the protein complex exposed to lipids. This model is supported by earlier studies showing that both the HC and the LC of BoNTs and TeNT are labeled by photoactive lipid labeling in model membranes (106, 107). An ion-conducting channel might still be formed by the HN in this model during or after translocation of LCs. Elucidating the molecular details of the translocation process remains a challenging task and would require further development of reconstitution models and structural approaches.
THE LIGHT CHAIN AND SNARE PROTEIN CLEAVAGE
The LCs of BoNTs and TeNT act as zinc-dependent proteases in the cytosol of neurons. BoNT/A, BoNT/C, and BoNT/E cleave SNAP-25. BoNT/B, BoNT/D, BoNT/F, BoNT/G, and TeNT cleave VAMP1, VAMP2, and VAMP3 (Figure 2a). In addition, BoNT/C also cleaves syntaxin 1. Cleavage of any of the three SNARE proteins is sufficient to block formation of SNARE complexes, thus preventing synaptic vesicle exocytosis. Blocking synaptic transmission appears to be the only physiologically relevant function of BoNTs and TeNT. In addition, high concentrations of BoNT/C and BoNT/E can induce death of cultured neurons (108, 109). This is because syntaxin 1 and SNAP-25 are both required for additional membrane fusion events, including essential plasma membrane recycling processes in neurons, independent of their roles in mediating synaptic vesicle exocytosis (109). This neuronal cytotoxicity occurs only when the concentrations of BoNT/C and BoNT/E reach a level that can cleave the majority of syntaxin 1 and SNAP-25 in neurons, which is far higher than the lethal dose of these BoNTs.
Although the LCs share only approximately 30% sequence identity across all serotypes, this domain presents a highly conserved fold, belonging to the M27 family of metalloproteases in the MEROPS database (110). The core structure of the LC remains largely unchanged, whether as a single domain or as part of the full-length toxin. An open catalytic pocket with surrounding negative surface potential generally provides access to the LC active sites. The strictly conserved HExxH…E motif presents a typical tetrahedral architecture around the Zn2+ ion that involves the His/Glu/His triad and a fourth, water-mediated coordination to the second glutamic acid. This water molecule delivers the nucleophile base required for proteolysis. In addition, R362 and Y365 of BoNT/A were demonstrated to be involved in stabilization of the reaction’s transition state and are also conserved across all clostridial neurotoxins (111).
LCs are proteases with strong specificity for neuronal SNARE proteins. This specificity is the result of a complex mechanism of toxin–substrate interaction that involves multiple recognition sites (112). This binding mechanism was illustrated by the X-ray crystal structure of LC/A in complex with SNAP-25, which defined two exosites (α- and β-) on LC/A that interact with SNAP-25 (113) (Figure 2b). The structures of LC/F bound to VAMP-derived peptide inhibitors also showed key exosites (114). Variation in the composition and localization of the LC exosites seems to govern which SNARE proteins can be cleaved as well as the position of their cleavage sites.
Residue changes in SNARE proteins render some vertebrate species resistant, or less susceptible, to cleavage by BoNTs. For instance, among homologous VAMP1, VAMP2, and VAMP3, rat VAMP1 contains a residue change at the BoNT/B and TeNT cleavage site, and rats are resistant to both toxins. Human VAMP1 contains a change from mouse VAMP1 at residue 48, with residue I in humans and residue M in mice. This location is critical for binding of LC/D, and the presence of an isoleucine reduces the cleavage efficacy of LC/D and renders humans less sensitive to BoNT/D (115, 116). A survey of 17 major primate species revealed frequent residue changes in VAMP1 at position 48, with either residue M or I (116). Although these M/I changes could be random and neutral events, their effect on the sensitivity to BoNT/D suggests this toxin could have exerted selective evolutionary pressure.
Intriguingly, LC/A has the extraordinary ability to maintain its activity within neurons for several months (117, 118). This is a key pharmacological property and underlies its success as a therapeutic toxin. Two mechanisms have been proposed. First, LC/A interacts with the cytoskeleton component septin, which might sequester LC/A within stable cytoskeleton structures underneath the plasma membrane (119). Consistently, motifs at the C terminus of LC/A have been shown to be essential for both binding to septin and its long half-life in vivo (119–121). The details of the LC/A–septin interactions remain to be established at the structural level. Second, it has been shown that the C terminal part of LC/A is able to recruit the deubiquitinating enzyme VCIP135/VCPIP1, thus actively inhibiting its degradation by the proteasome (122). In contrast, LC/E is rapidly degraded in neurons, potentially via its association with TRAF2, a RING finger protein that promotes the ubiquitin/proteasome pathway (123). The paralysis caused by BoNT/E consistently showed a much shorter duration than that caused by BoNT/A (124, 125).
NEW SEROTYPES, SUBTYPES, AND BoNT-LIKE TOXINS
BoNTs are traditionally classified on the basis of their distinct serological properties. Recent progress in sequencing toxin genes has begun to reveal a growing number of subtypes within the same serotype, defined by significant levels of protein sequence differences (2.6–31.6%) (126, 127) (Figure 6). They are designated with an Arabic number (e.g., BoNT/A1–A8) and can vary significantly in their activity; for example, BoNT/F5 cleaves VAMP1 at a novel site, distinct from the canonical site for BoNT/F (128).
Figure 6.
A phylogenetic split network covering the clostridial neurotoxins and selected homologs. The diagram illustrates their potential evolutionary relationships, as well as conflicts arising from chimerisms, based on their protein sequences (133). The sequences were clustered with UCLUST to 98% identity. Abbreviations: BoNT, botulinum neurotoxin; TeNT, tetanus neurotoxin.
In 2013, a strain isolated from an infant botulism patient expressed both BoNT/B and a novel BoNT that cannot be effectively neutralized by standard antisera assays (129, 130). The novel BoNT was initially designated serotype H. However, genomic sequencing revealed that this BoNT/H could also be considered a chimeric toxin (BoNT/FA), with its LC similar to the LC of BoNT/F5 and its HC similar to the HC of BoNT/A1 (131). Its toxicity can be neutralized by antibodies against the HC of BoNT/A1, although higher concentrations of antibodies are required than the standard assays (131).
Discovering and defining new bacterial toxins are traditionally disease-centric processes. However, rapidly accumulating microbial genomic information has begun to reveal novel toxin genes that were not previously directly linked to diseases. In 2015, the genome of C. botulinum strain 111, which was originally isolated from an infant botulism patient, was sequenced. This strain is known to express BoNT/B2 encoded on a plasmid, and the toxicity of its culture can be neutralized by antisera against BoNT/B (132). Surprisingly, a novel BoNT gene cluster exists in its genome, encoding a typical BoNT (denoted BoNT/X), an NTNHA, and the OrfX proteins (133). The LC of BoNT/X (LC/X) cleaves VAMP1/2/3 at a site distinct from the known cleavage sites for all other BoNTs. Interestingly, LC/X is also capable of cleaving other VAMP family members, including VAMP4, VAMP5, and Ykt6, although the physiological relevance of cleaving these noncanonical substrates remains to be examined. BoNT/X is practically not toxic to mice. This is likely because its HC does not recognize mouse/rat neurons effectively. Linking the LC-HN of BoNT/X with HC/A resulted in a chimeric toxin with much higher toxicity than when linked to HC/X (133).
Following the discovery of BoNT/X, another BoNT-like toxin was recently identified in the genome of Enterococcus faecium (134). The strain was isolated from cow feces. Genomic sequencing revealed a complete BoNT-like gene cluster located on a conjugative plasmid, containing a BoNT-like gene, an NTNHA-like gene, and OrfX-like genes. This represents the first BoNT-like gene cluster found outside the Clostridium genus. This toxin is denoted as BoNT/En [also known as eBoNT/J (135)]. BoNT/En and BoNT/X are on the same emerging branch in the family tree and share significant (37%) sequence identity (Figure 6). Functional characterization revealed that LC/En cleaves VAMP1/2/3 at a novel site (134). Interestingly, LC/En is also capable of cleaving SNAP-25 in neurons, although its cleavage of recombinant SNAP-25 in vitro is not efficient. The cleavage site is located on the N-terminal half of SNAP-25, which is distinct from all known BoNT cleavage sites (134). Similar to BoNT/X, HC/En appears unable to recognize mouse/rat neurons, as BoNT/En is not effective in cultured neurons or in mice, whereas linking the LC-HN of BoNT/En with HC/A generates a potent toxin capable of targeting neurons and inducing paralysis in mice (134).
A distantly related BoNT-like toxin gene was also discovered by bioinformatical analysis in the genome of bacterium Weissella oryzae, designated BoNT/Wo (136, 137). This potential toxin showed only ∼14–16% sequence identity with other BoNTs, whereas the normal range for members of the BoNT family is >28%. It lacks the conserved interchain disulfide bond, and no typical bont gene cluster is associated with BoNT/Wo, although a neighboring gene showed a low degree of similarity to ntnh. BoNT/Wo was reported to cleave rat VAMP2 at a unique tryptophan-tryptophan site (137). However, this cleavage has yet to be confirmed in neurons, and the physiological relevance of this activity toward rat VAMP2 remains to be determined. BoNT/Wo likely represents a distant relative of BoNTs. BoNT-like toxin domains have also been discovered in other species and metagenomic data (138). It is possible that these BoNT-like toxins do not target humans or animals but rather other organisms that have attracted little attention in the past. For instance, insects represent one of the largest biomasses in nature; whether these emerging toxins target insects remains an intriguing question (139).
TOXIN ENGINEERING
Clinical use of BoNTs is a great example of turning natural toxins into useful therapeutics. It began with collaborations between Dr. Allen Scott, an ophthalmologist who was seeking nonsurgical methods to weaken overactive muscles, and Dr. Edward Schantz, a microbiologist who studies BoNTs (3, 4). Experiments were carried out first on monkeys and later in humans, showing that BoNT/A is safe and effective in weakening muscles. BoNT/A is now widely used for treating a growing list of medical conditions, including muscle spasms, chronic pain, and overactive bladder, as well as for cosmetic purposes. BoNT/B is also approved for medical uses. Two key pharmacological properties make BoNTs ideal therapeutic proteins. First, because only a minute amount of toxin is needed to attenuate the activity of target neurons, patients usually do not generate neutralizing antibodies even after repeated injections over many years. Second, because the effects of BoNT/A last more than 3–6 months in humans, a single injection is sufficient to maintain the therapeutic effect for months.
With the ever-expanding medical uses of BoNTs, further improvements of the pharmacological properties of BoNTs, such as higher efficacy, lower immunogenicity, longer duration, and faster onset time, are highly desired. For instance, enhancing the efficacy of BoNTs would reduce the amount of toxins needed for injection, thus decreasing the risk of generating neutralizing antibodies and adverse diffusion of toxins. Furthermore, longer duration of therapeutic effects is particularly beneficial for medical applications that require invasive procedures such as the treatment of overactive bladder.
Improvement of toxin activity can be made through point mutations in HC, HN, and LC of BoNTs. Mutagenesis studies have been carried out on both HC/A and HC/B to enhance their binding to gangliosides and protein receptors, with the rationale that enhancing the receptor-binding step would increase the efficacy of the toxin to target and enter human neurons. Crystal structures of toxin-receptor complexes provide a solid knowledge basis for structure-based mutagenesis approaches. A recent successful example addressed the reduced binding of WT BoNT/B to human Syt-II, which contributes to the observed lower potency of BoNT/B in patients. Tao et al. (140) carried out a mutagenesis screen in HC/B and identified mutations that enhance binding of BoNT/B to human Syt-II. The engineered toxin showed approximately 11-fold higher efficacy in blocking neurotransmission compared with WT BoNT/B on cultured neurons expressing human Syt-II. Another example is that mutations in the GBS of BoNT/A that enhance its interactions with gangliosides also increased the potency of BoNT/A (US Patent 9234011B2) (141). Enhancing LC activity has also been explored. For instance, a single mutation (S201P) significantly increased the catalytic activity of LC/B on VAMP in vitro (142). However, full-length BoNT/B containing this mutation did not present any advantage over WT BoNT/B in multiple cell-based assays or in vivo (143). These results suggest that the rate-limiting step in BoNT efficacy resides in the initial neuronal recognition rather than the later intracellular activity. The LC/A has been modified to enhance its stability or alter its half-life in neurons (121, 144). The LC of BoNT/C (LC/C) has been modified to maintain cleavage of syntaxin 1, but SNAP-25 cleavage is diminished, which serves as a useful scientific tool to demonstrate the role of syntaxin 1 for synaptic transmission and a potential therapeutic toxin targeting syntaxin 1 (145, 146). Modification of the translocation process is challenging, as its molecular mechanism remains unknown. Pirazzini et al. (147) mutated conserved negatively charged residues in the LC and HN of BoNT/B on the basis of the rationale that protonation of these negatively charged residues is required for the interaction of the toxin with the negatively charged membranes. They identified a triple mutant that showed enhanced activity on hemi-diaphragm models as well as faster onset time, potentially due to enhanced translocation efficacy.
The modular structure of BoNT and the variations in activity between serotypes have led to the development of chimeric toxins displaying improved pharmacological properties. For example, two different studies generated chimeric toxins consisting of the HC/B with the LC-HN of BoNT/A (90, 148). These recombinant toxins displayed enhanced potency and duration of action in mice compared with WT BoNT/A, possibly due to increased binding and entry into neurons, as neurons express higher levels of Syt-I/Syt-II than SV2s (149).
Besides improving the pharmacological properties of BoNTs, another goal of toxin engineering is to expand their use to additional cell types, such as nonneuronal cells and sensory neurons. Because the secretion events in these cells may not utilize the same set of SNARE proteins as synaptic vesicle exocytosis, alteration/expansion of the substrate selectivity of BoNTs might be required. Several attempts have been made to engineer BoNT to cleave SNAP-23, which is involved in a number of secretion processes in nonneuronal cells (12). Sikorra et al. (150) recently developed a yeast screen–based system to identify the key binding pocket mutations to modify LC/A to cleave SNAP-23. A single point mutation (K224D) was previously identified in LC/E that enables efficient cleavage of both SNAP-25 and SNAP-23 (151). This mutant LC/E was capable of degrading SNAP-23 in cultured human epithelial cells and inhibiting mucin and interleukin-8 secretion. To target nonneuronal cells effectively, the receptor-binding properties of BoNTs need to be altered as well. This can be achieved by replacing the HC with a targeting agent that binds specifically to the intended cell types. One example of this approach includes fusing the LC-HN of BoNT/D to the growth hormone–releasing hormone (152). The resulting molecule is effective in inhibiting growth hormone secretion from rat pituitary glands for treating pituitary gland–related diseases.
The ability of BoNTs to target motor neurons and deliver their LCs into the cytosol effectively also raised interest in utilizing inactive toxins as delivery tools to transport therapeutics into motor neurons. For instance, various cargo proteins fused to the N terminus of BoNT/D can be delivered into neurons (153), and inactive BoNT/C has been developed as a vehicle to deliver therapeutics into the cytosol of neurons (154). If BoNTs are to be used as delivery tools, their toxicity has to be abolished, which is usually achieved by mutating key catalytic residues in LCs. However, at high doses, the inactive toxins still induce flaccid paralysis in mice (154). The reason for this residual toxicity remains to be elucidated.
Although clostridial neurotoxins are already the most potent toxins known, our increasing understanding of their mechanisms of action has allowed the development of molecules with increased potency or altered activity and specificity to meet specific medical needs. The discovery of new toxin serotypes and BoNT-like proteins with analogous functions will provide valuable additional tools to engineer toxins with novel pharmacological properties.
ACKNOWLEDGMENTS
We thank Dr. Daniel Lundin for help with the phylogenetic network; Markel Martinez, Jonathan Davies, and members of the Dong laboratory for discussions; and Dr. Sicai Zhang for help with figures. This study was partially supported by National Institutes of Health (NIH) grants (R01NS080833, R01AI132387, R01AI39087, R21NS106159) to M.D. and by the Swedish Research Council (2014-5667), the Wenner-Gren Foundation, and the Swedish Cancer Society (to P.S.). M.D. acknowledges support from the NIH-funded Harvard Digestive Disease Center (P30DK034854) and Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center (P30HD18655). M.D. holds the Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund. Owing to space limitations as well as the narrow focus of this review, discussion and citation of much significant work had to be excluded. We apologize for those omissions.
Footnotes
DISCLOSURE STATEMENT
P.S. and M.D. have patents and patent applications regarding BoNTs. P.S., G.M., and M.D. have ongoing research collaborations with Ipsen.
LITERATURE CITED
- 1.Alouf JE. 2006. A 116-year story of bacterial protein toxins (1888–2004): from “diphtheritic poison” to molecular toxinology In The Comprehensive Sourcebook of Bacterial Protein Toxins, ed. Alouf J, Popoff M, pp. 3–21. Burlington, MA: Academic; 3rd ed. [Google Scholar]
- 2.Gill DM. 1982. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46: 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schantz EJ, Johnson EA. 1992. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol. Rev. 56: 80–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson EA. 1999. Clostridial toxins as therapeutic agents: benefits of nature’s most toxic proteins. Annu. Rev. Microbiol. 53: 551–75 [DOI] [PubMed] [Google Scholar]
- 5.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, et al. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285: 1059–70 [DOI] [PubMed] [Google Scholar]
- 6.Schiavo G, Matteoli M, Montecucco C. 2000. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80: 717–66 [DOI] [PubMed] [Google Scholar]
- 7.Rossetto O, Pirazzini M, Montecucco C. 2014. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 12: 535–49 [DOI] [PubMed] [Google Scholar]
- 8.Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. 1998. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat. Struct. Biol. 5: 898–902 [DOI] [PubMed] [Google Scholar]
- 9.Swaminathan S, Eswaramoorthy S. 2000. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat. Struct. Biol. 7: 693–9 [DOI] [PubMed] [Google Scholar]
- 10.Kumaran D, Eswaramoorthy S, Furey W, Navaza J, Sax M, Swaminathan S. 2009. Domain organization in Clostridium botulinum neurotoxin type E is unique: its implication in faster translocation. J. Mol. Biol. 386: 233–45 [DOI] [PubMed] [Google Scholar]
- 11.Masuyer G, Conrad J, Stenmark P. 2017. The structure of the tetanus toxin reveals pH-mediated domain dynamics. EMBO Rep. 18: 1306–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahn R, Scheller RH. 2006. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7: 631–43 [DOI] [PubMed] [Google Scholar]
- 13.Sudhof TC, Rothman JE. 2009. Membrane fusion: grappling with SNARE and SM proteins. Science 323: 474–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q, Zhou P, Wang AL, Wu D, Zhao M, et al. 2017. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 548: 420–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, et al. 1992. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359: 832–35 [DOI] [PubMed] [Google Scholar]
- 16.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, et al. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature 362: 318–24 [DOI] [PubMed] [Google Scholar]
- 17.Montal M 2010. Botulinum neurotoxin: a marvel of protein design. Annu. Rev. Biochem. 79: 591–617 [DOI] [PubMed] [Google Scholar]
- 18.Rummel A 2015. The long journey of botulinum neurotoxins into the synapse. Toxicon 107: 9–24 [DOI] [PubMed] [Google Scholar]
- 19.Pirazzini M, Rossetto O, Eleopra R, Montecucco C. 2017. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol. Rev. 69: 200–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu S, Rumpel S, Zhou J, Strotmeier J, Bigalke H, et al. 2012. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science 335: 977–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eswaramoorthy S, Sun J, Li H, Singh BR, Swaminathan S. 2015. Molecular assembly of Clostridium botulinum progenitor M complex of type E. Sci. Rep. 5: 17795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benefield DA, Dessain SK, Shine N, Ohi MD, Lacy DB. 2013. Molecular assembly of botulinum neurotoxin progenitor complexes. PNAS 110: 5630–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Gu S, Jin L, Le TT, Cheng LW, et al. 2013. Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLOS Pathog. 9: e1003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa K, Watanabe T, Suzuki T, Yamano A, Oikawa T, et al. 2007. A novel subunit structure of Clostridium botulinum serotype D toxin complex with three extended arms. J. Biol. Chem. 282: 24777–83 [DOI] [PubMed] [Google Scholar]
- 25.Amatsu S, Sugawara Y, Matsumura T, Kitadokoro K, Fujinaga Y. 2013. Crystal structure of Clostridium botulinum whole hemagglutinin reveals a huge triskelion-shaped molecular complex. J. Biol. Chem. 288: 35617–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumura T, Sugawara Y, Yutani M, Amatsu S, Yagita H, et al. 2015. Botulinum toxin A complex exploits intestinal M cells to enter the host and exert neurotoxicity. Nat. Commun. 6: 6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugawara Y, Matsumura T, Takegahara Y, Jin Y, Tsukasaki Y, et al. 2010. Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J. Cell Biol. 189: 691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K, Zhong X, Gu S, Kruel AM, Dorner MB, et al. 2014. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science 344: 1405–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyashita S, Sagane Y, Suzuki T, Matsumoto T, Niwa K, Watanabe T. 2016. “Non-toxic” proteins of the botulinum toxin complex exert in-vivo toxicity. Sci. Rep. 6: 31043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalb SR, Baudys J, Smith TJ, Smith LA, Barr JR. 2017. Characterization of hemagglutinin negative botulinum progenitor toxins. Toxins 9: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafsson R, Berntsson RP, Martinez-Carranza M, El Tekle G, Odegrip R, et al. 2017. Crystal structures of OrfX2 and P47 from a botulinum neurotoxin OrfX-type gene cluster. FEBS Lett. 591: 3781–92 [DOI] [PubMed] [Google Scholar]
- 32.Lam KH, Qi R, Liu S, Kroh A, Yao G, et al. 2018. The hypothetical protein P47 of Clostridium botulinum E1 strain Beluga has a structural topology similar to bactericidal/permeability-increasing protein. Toxicon 147: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montecucco C 1986. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 11: 314–17 [Google Scholar]
- 34.Van Heyningen WE, Miller PA. 1961. The fixation of tetanus toxin by ganglioside. J. Gen. Microbiol. 24: 107–19 [DOI] [PubMed] [Google Scholar]
- 35.Simpson LL, Rapport MM. 1971. Ganglioside inactivation of botulinum toxin. J. Neurochem. 18: 1341–43 [DOI] [PubMed] [Google Scholar]
- 36.Kitamura M, Takamiya K, Aizawa S, Furukawa K. 1999. Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim. Biophys. Acta 1441: 1–3 [DOI] [PubMed] [Google Scholar]
- 37.Tsukamoto K, Kohda T, Mukamoto M, Takeuchi K, Ihara H, et al. 2005. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid: novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 280: 35164–71 [DOI] [PubMed] [Google Scholar]
- 38.Dong M, Tepp WH, Liu H, Johnson EA, Chapman ER. 2007. Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J. Cell Biol. 179: 1511–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bullens RW, O’Hanlon GM, Wagner E, Molenaar PC, Furukawa K, et al. 2002. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J. Neurosci. 22: 6876–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. 2008. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol. Biol. Cell 19: 5226–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rummel A, Hafner K, Mahrhold S, Darashchonak N, Holt M, et al. 2009. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J. Neurochem. 110: 1942–54 [DOI] [PubMed] [Google Scholar]
- 42.Strotmeier J, Lee K, Volker AK, Mahrhold S, Zong Y, et al. 2010. Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 431: 207–16 [DOI] [PubMed] [Google Scholar]
- 43.Strotmeier J, Gu S, Jutzi S, Mahrhold S, Zhou J, et al. 2011. The biological activity of botulinum neurotoxin type C is dependent upon novel types of ganglioside binding sites. Mol. Microbiol. 81: 143–56 [DOI] [PubMed] [Google Scholar]
- 44.Peng L, Tepp WH, Johnson EA, Dong M. 2011. Botulinum neurotoxin D uses synaptic vesicle protein SV2 and gangliosides as receptors. PLOS Pathog. 7: e1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Berntsson RPA, Tepp WH, Tao L, Johnson EA, et al. 2017. Structural basis for the unique ganglioside and cell membrane recognition mechanism of botulinum neurotoxin DC. Nat. Commun. 8: 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fotinou C, Emsley P, Black I, Ando H, Ishida H, et al. 2001. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J. Biol. Chem. 276: 32274–81 [DOI] [PubMed] [Google Scholar]
- 47.Rummel A, Bade S, Alves J, Bigalke H, Binz T. 2003. Two carbohydrate binding sites in the H(CC)-domain of tetanus neurotoxin are required for toxicity. J. Mol. Biol. 326: 835–47 [DOI] [PubMed] [Google Scholar]
- 48.Rummel A, Mahrhold S, Bigalke H, Binz T. 2004. The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol. Microbiol. 51: 631–43 [DOI] [PubMed] [Google Scholar]
- 49.Stenmark P, Dupuy J, Imamura A, Kiso M, Stevens RC. 2008. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLOS Pathog. 4: e1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berntsson RP, Peng L, Dong M, Stenmark P. 2013. Structure of dual receptor binding to botulinum neurotoxin B. Nat. Commun. 4: 2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benson MA, Fu Z, Kim JJ, Baldwin MR. 2011. Unique ganglioside recognition strategies for clostridial neurotoxins. J. Biol. Chem. 286: 34015–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamark C, Berntsson RP, Masuyer G, Henriksson LM, Gustafsson R, et al. 2017. Glycans confer specificity to the recognition of ganglioside receptors by botulinum neurotoxin A. J. Am. Chem. Soc. 139: 218–30 [DOI] [PubMed] [Google Scholar]
- 53.Jayaraman S, Eswaramoorthy S, Kumaran D, Swaminathan S. 2005. Common binding site for disialyllactose and tri-peptide in C-fragment of tetanus neurotoxin. Proteins 61: 288–95 [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Fu Z, Kim JJ, Barbieri JT, Baldwin MR. 2009. Gangliosides as high affinity receptors for tetanus neurotoxin. J. Biol. Chem. 284: 26569–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moriishi K, Koura M, Abe N, Fujii N, Fujinaga Y, et al. 1996. Mosaic structures of neurotoxins produced from Clostridium botulinum types C and D organisms. Biochim. Biophys. Acta 1307: 123–26 [DOI] [PubMed] [Google Scholar]
- 56.Nishiki T, Kamata Y, Nemoto Y, Omori A, Ito T, et al. 1994. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J. Biol. Chem. 269: 10498–503 [PubMed] [Google Scholar]
- 57.Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, et al. 1996. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 378: 253–57 [DOI] [PubMed] [Google Scholar]
- 58.Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER. 2003. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 162: 1293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rummel A, Karnath T, Henke T, Bigalke H, Binz T. 2004. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J. Biol. Chem. 279: 30865–70 [DOI] [PubMed] [Google Scholar]
- 60.Peng L, Berntsson RP, Tepp WH, Pitkin RM, Johnson EA, et al. 2012. Botulinum neurotoxin D-C uses synaptotagmin I and II as receptors, and human synaptotagmin II is not an effective receptor for type B, D-C and G toxins. J. Cell Sci. 125: 3233–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapman ER. 2002. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 3: 498–508 [DOI] [PubMed] [Google Scholar]
- 62.Chai Q, Arndt JW, Dong M, Tepp WH, Johnson EA, et al. 2006. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature 444: 1096–100 [DOI] [PubMed] [Google Scholar]
- 63.Rummel A, Eichner T, Weil T, Karnath T, Gutcaits A, et al. 2007. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. PNAS 104: 359–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, et al. 2006. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J. Neurosci. 26: 13493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin R, Rummel A, Binz T, Brunger AT. 2006. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature 444: 1092–95 [DOI] [PubMed] [Google Scholar]
- 66.Strotmeier J, Willjes G, Binz T, Rummel A. 2012. Human synaptotagmin-II is not a high affinity receptor for botulinum neurotoxin B and G: increased therapeutic dosage and immunogenicity. FEBS Lett. 586: 310–13 [DOI] [PubMed] [Google Scholar]
- 67.Stenmark P, Dong M, Dupuy J, Chapman ER, Stevens RC. 2010. Crystal structure of the botulinum neurotoxin type G binding domain: insight into cell surface binding. J. Mol. Biol. 397: 1287–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willjes G, Mahrhold S, Strotmeier J, Eichner T, Rummel A, Binz T. 2013. Botulinum neurotoxin G binds synaptotagmin-II in a mode similar to that of serotype B: tyrosine 1186 and lysine 1191 cause its lower affinity. Biochemistry 52: 3930–38 [DOI] [PubMed] [Google Scholar]
- 69.Berntsson RP, Peng L, Svensson LM, Dong M, Stenmark P. 2013. Crystal structures of botulinum neurotoxin DC in complex with its protein receptors synaptotagmin I and II. Structure 21: 1602–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, et al. 2006. SV2 is the protein receptor for botulinum neurotoxin A. Science 312: 592–96 [DOI] [PubMed] [Google Scholar]
- 71.Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. 2006. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 580: 2011–14 [DOI] [PubMed] [Google Scholar]
- 72.Colasante C, Rossetto O, Morbiato L, Pirazzini M, Molgo J, Montecucco C. 2013. Botulinum neurotoxin type A is internalized and translocated from small synaptic vesicles at the neuromuscular junction. Mol. Neurobiol. 48: 120–27 [DOI] [PubMed] [Google Scholar]
- 73.Chakkalakal JV, Nishimune H, Ruas JL, Spiegelman BM, Sanes JR. 2010. Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development 137: 3489–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benoit RM, Frey D, Hilbert M, Kevenaar JT, Wieser MM, et al. 2014. Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A. Nature 505: 108–11 [DOI] [PubMed] [Google Scholar]
- 75.Yao G, Zhang S, Mahrhold S, Lam KH, Stern D, et al. 2016. N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat. Struct. Mol. Biol. 23: 656–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahrhold S, Bergstrom T, Stern D, Dorner BG, Astot C, Rummel A. 2016. Only the complex N559-glycan in the synaptic vesicle glycoprotein 2C mediates high affinity binding to botulinum neurotoxin serotype A1. Biochem. J. 473: 2645–54 [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Rodriguez C, Levy R, Arndt JW, Forsyth CM, Razai A, et al. 2007. Molecular evolution of antibody cross-reactivity for two subtypes of type A botulinum neurotoxin. Nat. Biotechnol. 25: 107–16 [DOI] [PubMed] [Google Scholar]
- 78.Mahrhold S, Strotmeier J, Garcia-Rodriguez C, Lou J, Marks JD, et al. 2013. Identification of the SV2 protein receptor-binding site of botulinum neurotoxin type E. Biochem. J. 453: 37–47 [DOI] [PubMed] [Google Scholar]
- 79.Yeh FL, Dong M, Yao J, Tepp WH, Lin G, et al. 2010. SV2 mediates entry of tetanus neurotoxin into central neurons. PLOS Pathog. 6: e1001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bercsenyi K, Schmieg N, Bryson JB, Wallace M, Caccin P, et al. 2014. Tetanus toxin entry. Nidogens are therapeutic targets for the prevention of tetanus. Science 346: 1118–23 [DOI] [PubMed] [Google Scholar]
- 81.Fu Z, Chen C, Barbieri JT, Kim JJ, Baldwin MR. 2009. Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F. Biochemistry 48: 5631–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacky BP, Garay PE, Dupuy J, Nelson JB, Cai B, et al. 2013. Identification of fibroblast growth factor receptor 3 (FGFR3) as a protein receptor for botulinum neurotoxin serotype A (BoNT/A). PLOS Pathog. 9: e1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strotmeier J, Lee K, Volker AK, Mahrhold S, Zong Y, et al. 2010. Botulinum neurotoxin serotype D attacks neurons via two carbohydrate binding sites in a ganglioside-dependent manner. Biochem. J. 431: 207–16 [DOI] [PubMed] [Google Scholar]
- 84.Karalewitz AP, Fu Z, Baldwin MR, Kim JJ, Barbieri JT. 2012. Botulinum neurotoxin serotype C associates with dual ganglioside receptors to facilitate cell entry. J. Biol. Chem. 287: 40806–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kroken AR, Karalewitz AP, Fu Z, Kim JJ, Barbieri JT. 2011. Novel ganglioside-mediated entry of botulinum neurotoxin serotype D into neurons. J. Biol. Chem. 286: 26828–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kroken AR, Karalewitz AP, Fu Z, Baldwin MR, Kim JJ, Barbieri JT. 2011. Unique ganglioside binding by botulinum neurotoxins C and D-SA. FEBS J. 278: 4486–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stern D, Weisemann J, Le Blanc A, von Berg L, Mahrhold S, et al. 2018. A lipid-binding loop of botulinum neurotoxin serotypes B, DC and G is an essential feature to confer their exquisite potency. PLOS Pathog. 14: e1007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Surana S, Tosolini AP, Meyer IFG, Fellows AD, Novoselov SS, Schiavo G. 2018. The travel diaries of tetanus and botulinum neurotoxins. Toxicon 147: 58–67 [DOI] [PubMed] [Google Scholar]
- 89.Bercsenyi K, Giribaldi F, Schiavo G. 2013. The elusive compass of clostridial neurotoxins: deciding when and where to go? Curr. Top. Microbiol. Immunol. 364: 91–113 [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Zurawski TH, Meng J, Lawrence GW, Aoki KR, et al. 2012. Novel chimeras of botulinum and tetanus neurotoxins yield insights into their distinct sites of neuroparalysis. FASEB J. 26: 5035–48 [DOI] [PubMed] [Google Scholar]
- 91.Ovsepian SV, Bodeker M, O’Leary VB, Lawrence GW, Dolly JO. 2015. Internalization and retrograde axonal trafficking of tetanus toxin in motor neurons and trans-synaptic propagation at central synapses exceed those of its C-terminal-binding fragments. Brain Struct. Funct. 220: 1825–38 [DOI] [PubMed] [Google Scholar]
- 92.Blum FC, Przedpelski A, Tepp WH, Johnson EA, Barbieri JT. 2014. Entry of a recombinant, full-length, atoxic tetanus neurotoxin into Neuro-2a cells. Infect. Immun. 82: 873–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blum FC, Tepp WH, Johnson EA, Barbieri JT. 2014. Multiple domains of tetanus toxin direct entry into primary neurons. Traffic 15: 1057–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuda M, Sugimoto N, Ozutsumi K, Hirai T. 1982. Acute botulinum-like intoxication by tetanus neurotoxin in mice. Biochem. Biophys. Res. Commun. 104: 799–805 [DOI] [PubMed] [Google Scholar]
- 95.Wiegand H, Erdmann G, Wellhoner HH. 1976. 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn-Schmiedeberg’s Arch. Pharmacol. 292: 161–65 [DOI] [PubMed] [Google Scholar]
- 96.Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. 2008. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 28: 3689–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Restani L, Novelli E, Bottari D, Leone P, Barone I, et al. 2012. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic 13: 1083–89 [DOI] [PubMed] [Google Scholar]
- 98.Restani L, Giribaldi F, Manich M, Bercsenyi K, Menendez G, et al. 2012. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLOS Pathog. 8: e1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bomba-Warczak E, Vevea JD, Brittain JM, Figueroa-Bernier A, Tepp WH, et al. 2016. Interneuronal transfer and distal action of tetanus toxin and botulinum neurotoxins A and D in central neurons. Cell Rep. 16: 1974–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pirazzini M, Tehran DA, Zanetti G, Lista F, Binz T, et al. 2015. The thioredoxin reductase–Thioredoxin redox system cleaves the interchain disulphide bond of botulinum neurotoxins on the cytosolic surface of synaptic vesicles. Toxicon 107: 32–36 [DOI] [PubMed] [Google Scholar]
- 101.Pirazzini M, Azarnia Tehran D, Zanetti G, Megighian A, Scorzeto M, et al. 2014. Thioredoxin and its reductase are present on synaptic vesicles, and their inhibition prevents the paralysis induced by botulinum neurotoxins. Cell Rep. 8: 1870–78 [DOI] [PubMed] [Google Scholar]
- 102.Pirazzini M, Tehran DA, Leka O, Zanetti G, Rossetto O, Montecucco C. 2016. On the translocation of botulinum and tetanus neurotoxins across the membrane of acidic intracellular compartments. Biochim. Biophys. Acta 1858: 467–74 [DOI] [PubMed] [Google Scholar]
- 103.Fischer A, Montal M. 2007. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. PNAS 104: 10447–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koriazova LK, Montal M. 2003. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 10: 13–18 [DOI] [PubMed] [Google Scholar]
- 105.Fischer A, Montal M. 2007. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J. Biol. Chem. 282: 29604–11 [DOI] [PubMed] [Google Scholar]
- 106.Montecucco C, Schiavo G, Brunner J, Duflot E, Boquet P, Roa M. 1986. Tetanus toxin is labeled with photoactivatable phospholipids at low pH. Biochemistry 25: 919–24 [DOI] [PubMed] [Google Scholar]
- 107.Montecucco C, Schiavo G, Dasgupta BR. 1989. Effect of pH on the interaction of botulinum neurotoxins A, B and E with liposomes. Biochem. J. 259: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berliocchi L, Fava E, Leist M, Horvat V, Dinsdale D, et al. 2005. Botulinum neurotoxin C initiates two different programs for neurite degeneration and neuronal apoptosis. J. Cell Biol. 168: 607–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng L, Liu H, Ruan H, Tepp WH, Stoothoff WH, et al. 2013. Cytotoxicity of botulinum neurotoxins reveals a direct role of syntaxin 1 and SNAP-25 in neuron survival. Nat. Commun. 4: 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rawlings ND, Barrett AJ, Bateman A. 2010. MEROPS: the peptidase database. Nucleic Acids Res. 38: D227–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Binz T, Bade S, Rummel A, Kollewe A, Alves J. 2002. Arg362 and Tyr365 of the botulinum neurotoxin type a light chain are involved in transition state stabilization. Biochemistry 41: 1717–23 [DOI] [PubMed] [Google Scholar]
- 112.Rossetto O, Schiavo G, Montecucco C, Poulain B, Deloye F, et al. 1994. SNARE motif and neurotoxins. Nature 372: 415–16 [DOI] [PubMed] [Google Scholar]
- 113.Breidenbach MA, Brunger AT. 2004. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature 432: 925–29 [DOI] [PubMed] [Google Scholar]
- 114.Agarwal R, Schmidt JJ, Stafford RG, Swaminathan S. 2009. Mode of VAMP substrate recognition and inhibition of Clostridium botulinum neurotoxin F. Nat. Struct. Mol. Biol. 16: 789–94 [DOI] [PubMed] [Google Scholar]
- 115.Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, et al. 1994. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J. Biol. Chem. 269: 12764–72 [PubMed] [Google Scholar]
- 116.Peng L, Adler M, Demogines A, Borrell A, Liu H, et al. 2014. Widespread sequence variations in VAMP1 across vertebrates suggest a potential selective pressure from botulinum neurotoxins. PLOS Pathog. 10: e1004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whitemarsh RC, Tepp WH, Johnson EA, Pellett S. 2014. Persistence of botulinum neurotoxin A subtypes 1–5 in primary rat spinal cord cells. PLOS ONE 9: e90252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keller JE, Neale EA, Oyler G, Adler M. 1999. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 456: 137–42 [DOI] [PubMed] [Google Scholar]
- 119.Vagin O, Tokhtaeva E, Garay PE, Souda P, Bassilian S, et al. 2014. Recruitment of septin cytoskeletal proteins by botulinum toxin A protease determines its remarkable stability. J. Cell Sci. 127: 3294–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang J, Zurawski TH, Meng J, Lawrence G, Olango WM, et al. 2011. A dileucine in the protease of botulinum toxin A underlies its long-lived neuroparalysis: transfer of longevity to a novel potential therapeutic. J. Biol. Chem. 286: 6375–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scheps D, Lopez de la Paz M, Jurk M, Hofmann F, Frevert J. 2017. Design of modified botulinum neurotoxin A1 variants with a shorter persistence of paralysis and duration of action. Toxicon 139: 101–8 [DOI] [PubMed] [Google Scholar]
- 122.Tsai YC, Kotiya A, Kiris E, Yang M, Bavari S, et al. 2017. Deubiquitinating enzyme VCIP135 dictates the duration of botulinum neurotoxin type A intoxication. PNAS 114: E5158–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsai YC, Maditz R, Kuo CL, Fishman PS, Shoemaker CB, et al. 2010. Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. PNAS 107: 16554–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, Meng J, Lawrence GW, Zurawski TH, Sasse A, et al. 2008. Novel chimeras of botulinum neurotoxins A and E unveil contributions from the binding, translocation, and protease domains to their functional characteristics. J. Biol. Chem. 283: 16993–7002 [DOI] [PubMed] [Google Scholar]
- 125.Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, et al. 2003. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem. 278: 1363–71 [DOI] [PubMed] [Google Scholar]
- 126.Hill KK, Smith TJ, Helma CH, Ticknor LO, Foley BT, et al. 2007. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J. Bacteriol. 189: 818–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hill KK, Xie G, Foley BT, Smith TJ. 2015. Genetic diversity within the botulinum neurotoxin-producing bacteria and their neurotoxins. Toxicon 107: 2–8 [DOI] [PubMed] [Google Scholar]
- 128.Kalb SR, Baudys J, Webb RP, Wright P, Smith TJ, et al. 2012. Discovery of a novel enzymatic cleavage site for botulinum neurotoxin F5. FEBS Lett. 586: 109–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barash JR, Arnon SS. 2014. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins. J. Infect. Dis. 209: 183–91 [DOI] [PubMed] [Google Scholar]
- 130.Dover N, Barash JR, Hill KK, Xie G, Arnon SS. 2014. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 209: 192–202 [DOI] [PubMed] [Google Scholar]
- 131.Maslanka SE, Luquez C, Dykes JK, Tepp WH, Pier CL, et al. 2016. A novel botulinum neurotoxin, previously reported as serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes A and F and is neutralized with serotype A antitoxin. J. Infect. Dis. 213: 379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kozaki S, Kamata Y, Nishiki T, Kakinuma H, Maruyama H, et al. 1998. Characterization of Clostridium botulinum type B neurotoxin associated with infant botulism in Japan. Infect. Immun. 66: 4811–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang S, Masuyer G, Zhang J, Shen Y, Lundin D, et al. 2017. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 8: 14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang S, Lebreton F, Mansfield MJ, Miyashita SI, Zhang J, et al. 2018. Identification of a botulinum neurotoxin-like toxin in a commensal strain of Enterococcus faecium. Cell Host Microbe 23: 169–76.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brunt J, Carter AT, Stringer SC, Peck MW. 2018. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 592: 310–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mansfield MJ, Adams JB, Doxey AC. 2015. Botulinum neurotoxin homologs in non-Clostridium species. FEBS Lett. 589: 342–48 [DOI] [PubMed] [Google Scholar]
- 137.Zornetta I, Azarnia Tehran D, Arrigoni G, Anniballi F, Bano L, et al. 2016. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 6: 30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Doxey AC, Mansfield MJ, Montecucco C. 2018. Discovery of novel bacterial toxins by genomics and computational biology. Toxicon 147: 2–12 [DOI] [PubMed] [Google Scholar]
- 139.Montecucco C, Rasotto MB. 2015. On botulinum neurotoxin variability. mBio 6: e02131–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tao L, Peng L, Berntsson RP, Liu SM, Park S, et al. 2017. Engineered botulinum neurotoxin B with improved efficacy for targeting human receptors. Nat. Commun. 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burns JR, Lambert GS, Baldwin MR. 2017. Insights into the mechanisms by which clostridial neurotoxins discriminate between gangliosides. Biochemistry 56: 2571–83 [DOI] [PubMed] [Google Scholar]
- 142.Guo J, Pan X, Zhao Y, Chen S. 2013. Engineering clostridia neurotoxins with elevated catalytic activity. Toxicon 74: 158–66 [DOI] [PubMed] [Google Scholar]
- 143.Elliott M, Maignel J, Liu SM, Favre-Guilmard C, Mir I, et al. 2017. Augmentation of VAMP-catalytic activity of botulinum neurotoxin serotype B does not result in increased potency in physiological systems. PLOS ONE 12: e0185628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lopez de la Paz M, Scheps D, Jurk M, Hofmann F, Frevert J. 2018. Rational design of botulinum neurotoxin A1 mutants with improved oxidative stability. Toxicon 147: 54–57 [DOI] [PubMed] [Google Scholar]
- 145.Wang D, Zhang Z, Dong M, Sun S, Chapman ER, Jackson MB. 2011. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry 50: 2711–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zanetti G, Sikorra S, Rummel A, Krez N, Duregotti E, et al. 2017. Botulinum neurotoxin C mutants reveal different effects of syntaxin or SNAP-25 proteolysis on neuromuscular transmission. PLOS Pathog. 13: e1006567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pirazzini M, Henke T, Rossetto O, Mahrhold S, Krez N, et al. 2013. Neutralisation of specific surface carboxylates speeds up translocation of botulinum neurotoxin type B enzymatic domain. FEBS Lett. 587: 3831–36 [DOI] [PubMed] [Google Scholar]
- 148.Rummel A, Mahrhold S, Bigalke H, Binz T. 2011. Exchange of the HCC domain mediating double receptor recognition improves the pharmacodynamic properties of botulinum neurotoxin. FEBS J. 278: 4506–15 [DOI] [PubMed] [Google Scholar]
- 149.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, et al. 2006. Molecular anatomy of a trafficking organelle. Cell 127: 831–46 [DOI] [PubMed] [Google Scholar]
- 150.Sikorra S, Litschko C, Muller C, Thiel N, Galli T, et al. 2016. Identification and characterization of botulinum neurotoxin A substrate binding pockets and their re-engineering for human SNAP-23. J. Mol. Biol. 428: 372–84 [DOI] [PubMed] [Google Scholar]
- 151.Chen S, Barbieri JT. 2009. Engineering botulinum neurotoxin to extend therapeutic intervention. PNAS 106: 9180–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Somm E, Bonnet N, Martinez A, Marks PM, Cadd VA, et al. 2012. A botulinum toxin-derived targeted secretion inhibitor downregulates the GH/IGF1 axis. J. Clin. Investig. 122: 3295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bade S, Rummel A, Reisinger C, Karnath T, Ahnert-Hilger G, et al. 2004. Botulinum neurotoxin type D enables cytosolic delivery of enzymatically active cargo proteins to neurones via unfolded translocation intermediates. J. Neurochem. 91: 1461–72 [DOI] [PubMed] [Google Scholar]
- 154.Vazquez-Cintron EJ, Beske PH, Tenezaca L, Tran BQ, Oyler JM, et al. 2017. Engineering botulinum neurotoxin C1 as a molecular vehicle for intra-neuronal drug delivery. Sci. Rep. 7: 42923. [DOI] [PMC free article] [PubMed] [Google Scholar]