Abstract
In the treatment of cancer, chemotherapy plays an important role though the efficacy of anti-cancer drug administered orally is limited, due to their poor solubility in physiological medium, inability to cross biological membrane, high Para-glycoprotein (P-gp) mediated drug efflux, and pre-systemic metabolism. These all factors cumulatively reduce drug exposure at the target site leading to multidrug resistance (MDR). Lipid based carriers systems has been explored to overcome solubility and permeability related issues of anti-cancer drugs. The lipid based formulations have also been reported to circumvent the effect of P-gp and CYP3A4. Further long chain triglycerides (LCT) has shown their ability to access Lymphatic route over Medium Chain Triglycerides, as the former has been extensively used for targeting anti-cancer drugs at proliferating cells through lymphatic route. Therefore this review tries to reflect the usefulness of lipid based drug carriers systems (viz. liposome, solid lipid nanoparticle, nano-lipid carriers, self-emulsifying, lipidic pro-drugs) in targeting lymphatic system and overcoming issues related to solubility and permeability of anti-cancer drugs. Moreover, we have also tried to reflect how critically lipid based carriers are important in maximizing therapeutic safety and efficacy of anti-cancer drugs.
Keywords: Lipid based carriers, Lymphatic system, Metastasis, P-gp, CYP3A4
Introduction
Cancer can be regarded as a multiplex of disease states generated with an outcome of prolonged injuries at tissue and cellular level through interactions with cancer causing agents known as the carcinogens. The administration of tobacco (people consuming tobacco products, or are in the vicinity of tobacco smoke), irradiation with ultraviolet light (ultraviolet-A produces genetic damage and suppression of immune system whereas ultraviolet-B has penetrability to the epidermis an evident factor for skin melanoma) and various viral infections which may integrate by incorporating their genetic material into the host’s DNA leading to mutation (hepatitis B virus- liver carcinoma, human papillomavirus- causative agent for cervical carcinoma, human herpesvirus 8- significantly affecting the integumentary system), epigenetic changes (alteration in gene expression without any significant change in DNA sequence namely DNA-methylation and histone modifications) and global transcriptome changes (through inflammatory pathways) these all factors increases the probability of converting healthy cells into cancerous cells.1 Cancer cells proliferate in the human body by gaining access into the blood vasculature and lymphatic streams, producing secondary tumors through metastasis.Strategically the treatment of patient suffering from cancer is undertaken through removal of cancerous or suspected tissues employing surgical intervention which is normally a first line approach accompanied by targeted radiation therapy to kill cancerous cell and reduce the size of the tumor, and finally systemically delivering the anti-cancer and immunomodulatory agents by chemotherapy and immunotherapy. A conventional chemotherapy strategy delivers anti-cancer drugs not only to cancer cells but also have access to normal healthy cells thereby producing plethora of side effects. Therefore, several attempts have been made in this regard to target anti-cancer agents at tumor sites by researchers in the last forty years through newer delivery approaches, among them lipid based drug delivery systems have gained sufficient interest, as these systems can address the complexity of anti-cancer drug delivery safely and effectively to localized tumors as well as in the vicinity of metastatic sites.2 This review is an attempt to address the role of lipid based drug carriers in lymphatic route drug targeting (an alternative voyage for delivering anti-cancer agents to metastatic cancer cells), avoiding hepatic first pass metabolism (cytochrome P-450), circumventing the effect of p-glycoprotein pump (as drug effluxer), we have also compared other nanoparticulate delivery system (polymeric and metallic) with lipid based nano-carriers in terms of formulation characteristics and their clinical interpretations.
Challenges and opportunities in targeting anticancer drugs
On a cumulative note while addressing the severities of cancer, tumor metastasis accounts for largest percentage of adversities and mortalities. Cancer cells gains access to lymphatics by invading the broad intercellular pores just adjacent to endothelial cells together with irregular basement membrane.3,4 The cancer cell after gaining access to lymphatics uses it as a reservoir site, which results in further advancement of carcinoma distant to primary site of malignancy.5-7
Role of lymphatic route
The lymphatic system is a nexus that comprises of lymph nodes, lymphatic vessels, spleen, thymus, Peyer’s patches and tonsils which function in coordination with the circulatory system and a circulating fluid called lymph. Lymph serves the purpose of transporting lymphocytes and antigen presenting cells into the blood and bones via lymph nodes. Further, the lymphatic system serves the purpose of maintaining the homeostasis by regulating the balance of water in body through transporting cells of the immune system back to the nodes of lymph and making delivery of extracellular fluid again in the central circulation.8,9 The potential aspects of Lymphatic drug transport for immunomodulatory agents and anti-cancer drugs using lipid based drug carrier systems have been explored by the researchers, as the lymphatic system serves as a pathway for lymphocytes particularly T and B and tumor metastasis.10-17 As the structure of lymphatic capillaries is extensively porous owing to their single layer of non-fenestrated endothelial cells which in turn allows the access of large particles.18
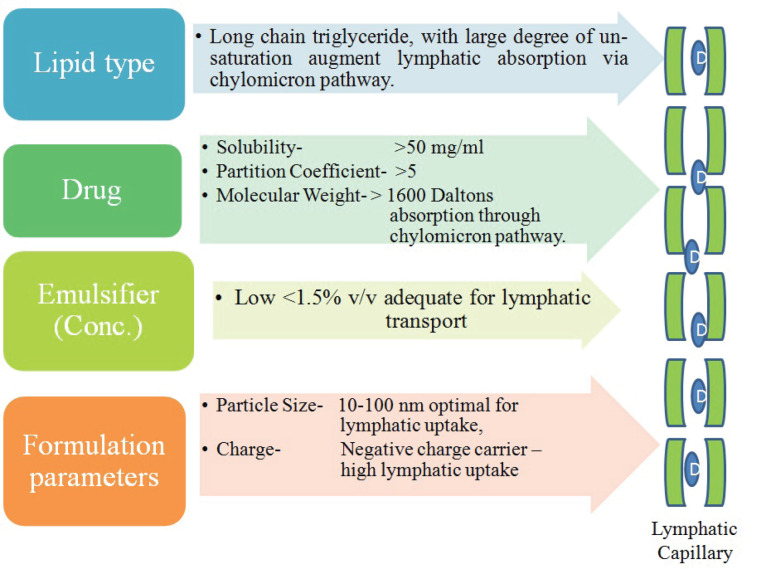
Among the other factors like lipid composition, effective potential on the oil globules, molecular weight, dose, and particle size, the drug in-vivo behavior is more closely related with the particle size. As nano size particles persist larger surface area as compared to macro sized, therefore overall interaction with the biological membranes is enhanced providing better absorption into the lymphatic system, therefore it has been advocated that particles below 200 nm are effectively targeted into the lymphatic route. Charge on the formulations also governs the passage through luminal membrane having negative potential as a whole owing to glycosaminoglycans presence, therefore it is advocated that formulation those are neutral or bearing negative charge can be effectively transported into the lymphatic system as they can easily avoid electrostatic interaction compared with formulations having positive charge that persist stronger affinity towards the luminal membrane hence get retained.19 Key factors responsible for effective lymphatic route targeting are represented in Figure 1.
Figure 1.
Schematic representation of key factors for lymphatic drug targeting by lipid based delivery systems (D= Drug reaching to lymphatic circulation).
Lymphatic transport of lipid products
The membrane-bound transporter proteins play a pivotal role for the transport of lipid digested products either by active or passive transport. The lipid transportation is advocated by enterocyte’s apical membrane post-parandially, as this is the time when lipid concentration in the lumen is substantially high which in turn promote passive transportation.20-22 The mechanistic approach at microvillus for fatty acid uptake may be related with sodium concentration directly or indirectly together with inclusion of fatty acid binding proteins and transporters.23-25 The intracellular transformation of lipid subsequent to enterocyte’s absorption is governed by its chain length. Lipids with short and medium carbon chain length (i.e. having C<12) generally spread beyond the enterocytes into the capillaries for gaining access to portal vein, whereas lipid having long-chain (i.e. C≥12) gain access to mesenteric lymph after migrating from the site of absorption with subsequent re-esterification and fabrication by the endoplasmic reticulum into the lipoproteins of the intestine (chylomicron and very low density lipoproteins), here chylomicron has direct relationship with lipid load.26-29 The magnitude of lipid access to lymphatic transport is not merely related to lipid chain length but also to its preference towards lymph (i.e. log P>5).30,31 Vahouny & Treadwell32 concluded from their experiments that co-administration of long chain triglycerides (LCT) increased lymphatic transport of cholesterol.Nankervis et al33 demonstrated the effectiveness of linoleic acid and cottonseed oil (Long chain lipids) for enhancing the lymphatic absorption of retinoids over Miglyol 812, (medium-chain lipid) the latter though were less sensitive to oxidation and have comparatively higher solvent capacity.34-38
Oral administration has always been attempted largely as compared with other routes considering potential benefits of patient’s compliance and cost effectiveness. But oral anti-cancer drug access to systemic circulation and at the target site suffers from multitude of factors like poor aqueous solubility, extensive pre-systemic metabolism, efflux transporter activity, and these all factors collectively accounts for resistance against anti-cancer drugs.
Pre-systemic cytochrome based drug metabolism and P-glycoprotein mediated drug efflux
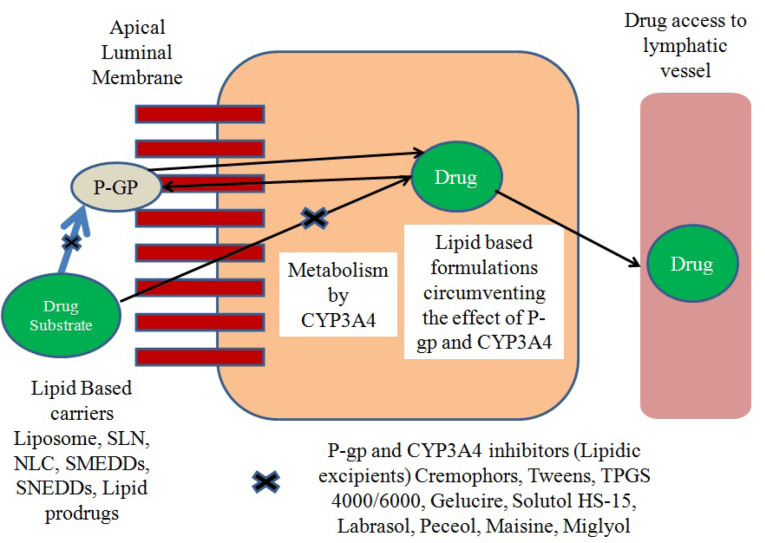
P-glycoprotein pump (multi-drug effluxer) and Cytochrome P450 (drug metabolizer) perhaps work concomitantly as a protective barrier and are the most evident factors for reduced bioavailability of BCS Class II and IV drugs.39-42 The first pass effect on drug metabolism is a summation of enzymatic metabolism at brush border (alkaline phosphatase, isomaltase and sucrase and peptidases) of the lumen and at the intracellular level by microsomal enzymes on the endoplasmic reticulum in the cytoplasm.43 Cytochrome P450 particularly CYP 3A4, are the major contributors in phase I metabolism and accounts for oxidation of many anti-cancer agents, thereby reducing the bioavailability of drugs with ester moiety like capecitabine.44,45 The fraction of drug absorbed from the gastrointestinal tract reaches to liver (metabolizing house) through entero-hepatic circulation and undergoes biotransformation. This is known as first pass hepatic metabolism and it is the major contributor for lower oral bioavailability of many anti-cancer agents like Tamoxifen substrate for CYP3A4.46,47 The tandem situation becomes more critical when a drug is a substrate for both CYP3A4 and p-glycoproteins (p-gp).48 After the discovery of P-gp transporters (from the family of ATP-binding cassette transporters) expressed at carcinogenic cells, blood-brain barrier as major obstructing and excretory tissues by Juliano and Ling in the late 1970s, further studies started in establishing the role of P-gp as anti-cancer effluxer. Anti-cancer agents which are substrate to P-gp have compromised effectiveness owing to their altered pharmacokinetics. This condition is a major contributor in multidrug resistance (MDR). Furthermore, as the drug is effluxed out by p-gp, and is now again in the vicinity of CYP3A4 to show its metabolizing affect, the drug’s bioavailability gets synergistic reduction.49-54 A number of strategies to tackle the poor bioavailability due to synergistic or lone effect for drug which is substrate to either CYP3A4 or P-gp or both have been investigated. The concomitant use of P-gp inhibitors, surfactants and polymer with anti-cancer agents in the formulations are some of the most commonly explored approaches. Sandhu et al55 prepared SNEDDs of paclitaxel (PTX) with co-administration of curcumin. Sesame oil having high polyunsaturated fatty acids (PUFA) content was used as an oil phase, whereas Labrasol and sodium deoxycholate were used as surfactant and co-surfactant respectively. The In situ intestinal perfusion study exhibited sufficient increase in permeability and absorption characteristics by PTX-Cu-SNEDDs as compared to PTX-SNEDDs and PTX suspension. Therefore we have tried to highlight the most widely used class of agents (lipid vehicles and surfactants) in lipid based drug carriers which can tackle the synergistic effect of CYP3A4 and P-gp as shown as in Figure 2.56
Figure 2.
Schematic representation of role of lipid excipients in circumventing the effect of P-glycoprotein pump and Cytochrome 3A4 mediated drug efflux and metabolism.
Lipid based carriers for anti-cancer delivery
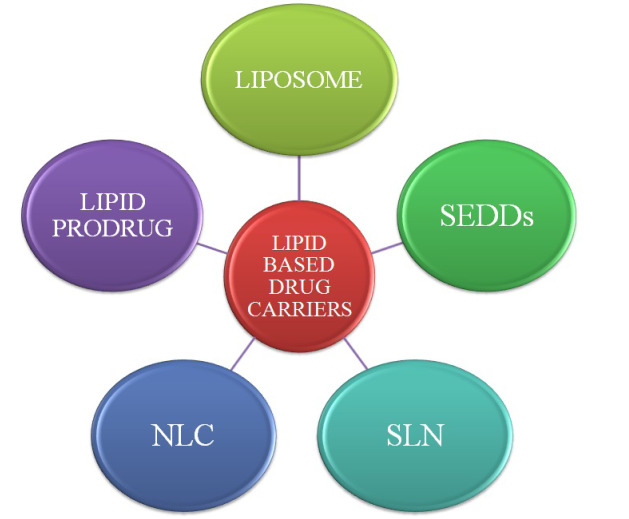
Lipid based formulations have attained popularity as targeted, safe and effective delivery strategy for anti-cancer agents by overcoming the drawbacks of conventional drug delivery system during last five decades. Lipid based carriers system employed for anti-cancer drug delivery has been represented underneath in Figure 3.
Figure 3.
Schematic diagram of lipid based drug carriers.
Liposome
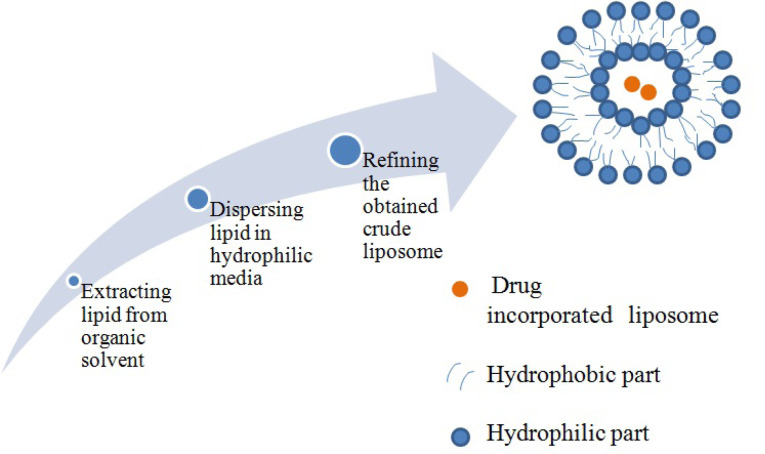
Liposome vesicle is bilayered, spherical in shape and usually composed of monomers with polar head and a non polar tail. The lipophilic head groups are oriented inside the bilayer. Liposomes were the first structured lipid based nanoparticle and possess unique characteristics to encapsulate both hydrophobic (at nonpolar lipid bilayer) and hydrophilic drug (at polar core). Their peculiar quality to permeate through the enterocytes and enhanced stability for drugs has gained sufficient interest for effective lymphatic drug targeting.57 Liposome preparation involves some generalized sequence of events as depicted in Figure 4.58
Figure 4.
Generalized method for liposome preparation.
Ling et al59 reported that cefotaxime (having poor bioavailability and high water solubility) in liposomal formulation have enhanced bioavailability from 2.3 to 2.7 times compared to drug solution and physical mixture respectively, moreover the concentration of liposomal formulation was also substantially more in lymph thereby suggesting possible utility of liposome in targeting lymphatic vessels and addressing bioavailability related issues. Recent studies suggested that merely encapsulating the drug into liposomes may sometimes cannot provide sufficient lymphatic targeting, owing to other properties like the particle size, which governs the passage through bio-membranes.60-62 In an another study reported by Hashida et al63 liposomal formulation of carboxyfluorescein were inefficient in significantly permeating the mucosa of the intestine, but could enter intestinal mucosa when it was co-administered with lipid-surfactant mixed micelles as the latter could interact with intestinal luminal cell membrane, advocating the role of lipid based formulation with concomitant use of surfactants. Ye et al64 explored the potential of synthetic borneol as permeation enhancer in lymphatic targeting of 7-ethyl-10-hydroxycamptothecin incorporated nanoliposome (SN-38-Lips) and increase in lymph node uptake when administered through subcutaneous route. It was evident from their results that nanoliposomes when administered with 2mg/ml of Borneol exhibited enhanced lymphatic node retention and uptake.
Liposome with coating material for enhanced performance
Liposomes with surface modifications have also been developed for combating issues of drug resistance, enzymatic metabolism and membrane permeability. Li et al65 have developed the formulation of polyethylene glycol (PEG)-coated liposomes of recombinant human epidermal growth factor. The area under the curve (AUC) enhanced form 1.7 to 2.5 folds which might have been an outcome of enhanced resistance towards enzymatic degradation and altered permeability.66 Iwanaga et al67 In an another study while examining the effect of coating on peptide drug (insulin as model drug) reported that coating the liposomal surface with polyethylene glycol, sugar portion of mucin increases the gastro-intestinal stability. Further, the coated liposomes were able to manage longer hypoglycemic effect over the uncoated liposomes. Moribe et al68 were able to increase the encapsulation of nystatin into liposome with distearoyl-N-(monomethoxy poly (ethylene glycol) succinyl) phosphatidylethanolamine (DSPE-PEG). The usefulness of liposome based products as delivery vehicle for anti-cancer drugs has been reflected by recent research reports as summarized in Table 1.
Table 1. Anti-cancer drugs benefitted by liposome .
| Drug | Lipid Component | Interpretation | Special feature | Ref. |
| Raloxifene hydrochloride | DSPC: Cholesterol, diethylene triamine penta acetic acid | Raloxifene loaded liposomal formulation provided effective uterine targeting as compared to non-liposomal drug. | Gamma scintigraphy studies depicted selective uptake of radiolabeled RLH. | 69 |
| Raloxifene |
Dipalmitoyl phosph-atidylcholine, dioctyl phosphatidyl choline and calcium chloride. Dimethyl-β-cyclodextrin and sodium taurocholate |
Raloxifene dimethyl-β-CD cochleate formulations were found to be successful in reducing breast tumors. Further matrix metalloproteinase-2 (MMP-2) enzyme was also found to be inhibited. | MCF-7 cell lines were used to evaluate antitumor activity. | 70 |
| Adriamycin |
EPC/Chol 55:45 Chloroform, isopropanol, Water. |
Adriamycin loaded liposome on local administration effectively inhibiting the proliferating cells by inducing apoptosis thereby reducing lymph node metastasis. | Highest apoptotic index of 21.73%. | 71 |
| Doxorubicin |
Phosphatidylcholine, and cholesterol |
Doxorubicin-loaded liposomes offered enhanced permeability and retention effect. | Passive targeting at cancer cells. | 72 |
Solid lipid nanoparticle (SLN)
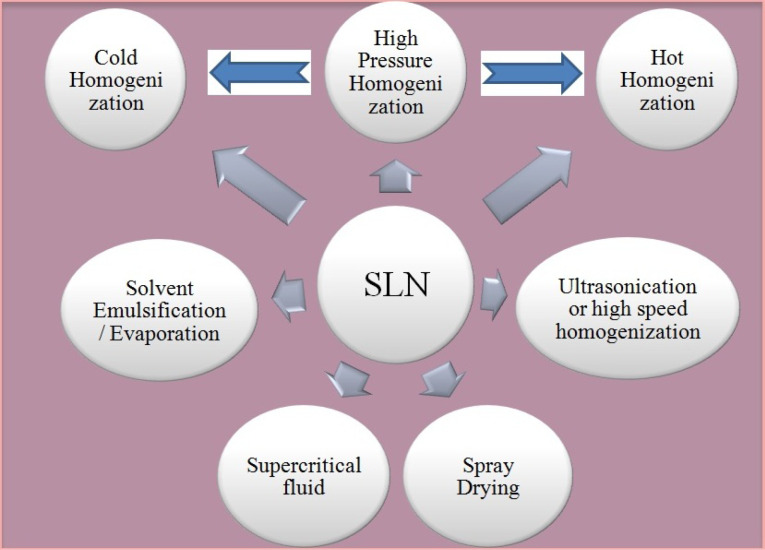
SLN are lipid based drug carries which utilizes certain physiological lipids like mixtures of mono-, di-, or triglycerides along with surfactants (poloxamer and tweens). Moreover SLN are solid at room and physiological temperature. These unique characteristics offers reduced systemic toxicity and enhanced physical stability. Effective drug targeting (particularly to lymphatics) and increased drug encapsulation are additional benefits of SLN.73-75 The exclusion of biotoxic solvents during the manufacturing of SLN by high pressure homogenization (HPH) supports exclusive reduced biological toxicity.76,77 Most commonly explored technologies for SLN preparation has been depicted in Figure 5 and comparison in Table 2.
Figure 5.
SLN preparation technologies.
Table 2. Comparison of technologies employed for SLN/NLC preparation78-82 .
| Lipid Nanoparticle Technology | Methodology | Advantage | Disadvantage |
| High pressure Homogenization |
Hot Homogenization Drug incorporated lipid melt dispersed in surfactant rich hot aqueous phase, followed by homogenization under high pressure. |
• Bulk manufacturing • High stability and loading of drugs. |
• Not recommended for thermolabile drugs. • Drug may enter in aqueous medium under homogenization |
|
Cold Homogenization Drug dissolved in melted lipid, swiftly cooled under liquid nitrogen/dry ice, followed by milling. Then milled powder disperse in surfactant rich aqueous solution, followed by homogenization under high pressure. |
• No drug partition in aqueous phase. • Thermolabile drugs can also be incorporated |
• Surfactant involved may cause irritation/sensitization. | |
| Solvent emulsification/evaporation | Lipid dissolved in organic solvent immiscible with water, followed by formation of an emulsion stabilized by surfactant. Solvent removal by solvent removal under reduced pressure. | • No thermal stress. |
• Organic solvent used for dissolving lipid may possess toxicity. • Varied particle size of nanoparticles. |
| Super critical fluid technology | Supercritical fluids like carbon dioxide have been used for solvent extraction through o/w emulsions. | • Carbon-dioxide can serve as an alternative to other toxic organic solvents. |
• Majority of organic solvents utilized are hazardous. • Large quantity of surfactant concentration required. |
| Ultrasonification | Lipid phase is dispersed surfactant rich aqueous phase, followed by homogenization under high shear/ultrasonication | • Simple manufacturing process. |
• Polydispersed nanoparticles. • High concentration of surfactant. |
| Spray drying | SLN can be prepared from aqueous dispersion. |
• Cost effective and substitute to lyophilization. |
• Particles aggregation. • Lipids with melting point above 70° C can only be used |
In comparison with other techniques, HPH can efficiently be explored for large-scale manufacturing of SLN. Homogenization can be done under hot and cold conditions. Both the process essentially involves dispersion/dissolution of drug into the lipid melt before HPH. Thereafter fluid is allowed to pass under high pressure (upto 200 bars) from a narrow passage in homogenizer. Dolatabadi et al83 employed simple homogenization technique to develop Alendronate SLN for inhalational use. The developed SLN formulation had particle size below 100 nm and low polydispersibility index 0.25. Furthermore the developed formulation exhibited better systemic bioavailability.Bakhtiary et al84 also employed hot homogenization technique to prepare Erlotinib SLN as dry powder inhalation product. Erlotinib SLN exhibited particle with spherical shape and size below 100 nm. As a model for non-small cell lung cancer human alveolar adenocarcinoma epithelial A549 cell lines were used for cytotoxic investigation, and the developed formulation exhibited higher anticancer activity.
Role of SLNs in increasing anti-cancer drug absorption and permeability and cancer treatment
Eskandani et al85 successfully incorporated Shikonin-Act into SLN through hot homogenization method for enhancement in anti-proliferative activity of Shikonin-Act. Shikonin-Act loaded SLN exhibited better therapeutic potential against intact Shikonin in MTT (cytotoxicity) and comet assay (genotoxicity).In another study form Eskandani et al86 reported significant improvement in antiproliferative action of galbanic acid (GBA) by incorporating GBA into SLN. Hot homogenization method was used for preparation of SLN; the entrapment efficiency of developed SLN was more than 98%. GBA-SLN inhibited growth rate of A549 cells, moreover the cytotoxic effect of GBA-SLN was more prominent after 48 hours. Therefore result of their study depicted sustained and enhanced antiproliferative action form GBA-SLN.Findings from Hamishehkar et al87 were also in the agreement that sclareol (poorly water soluble) incorporated SLN was able to exhibit sustained (after 48 hours) and improved antiproliferative activity in A549 cell line.
Reports from the study conducted by Reddy et al88 on formulation of radiolabelled (99mTc) etoposide loaded tripalmitin (ETPL) solid lipid nanoparticle depicted that among the three route explored for administration; subcutaneous, intraperitoneal and intravenous the tumor uptake specificity was highest after 24 hours in subcutaneous followed by intraperitoneal and intravenous administration which was 8-folds and 59-folds respectively, their finding suggested the specificity of ETPL in targeting lymphatic route associated metastasis.
Few recent research findings depicting the potential of SLN in enhancing anti-cancer drug efficacy are given in Table 3.
Table 3. List of anti-cancer drugs formulated as SLNs .
| Drug Name | Lipid Components | Interpretation | Special Feature | Ref. |
| Docetaxel | Trimyristin | Strong antitumor activity was observed from DCX-SLN, as compared to solubilized mixture of DCX in Tween 80/ethanol in cell culture and pre-established tumor in mouse model. | Decreased accumulation in vital organs depicted safety of the formulation. | 89 |
| Paclitaxel |
Compritol 888 ATO, Precirol ATO5 |
Developed SLN of PTX could significantly enhanced in-vitro cancer cell death than Taxol, against MXT-B2 murine breast cancer cell line. | Drug entrapment efficiency was above 97% | 90 |
| Paclitaxel | Stearic acid, lecithin and poloxamer 188 | SLN entrapped PTX showed enhanced cytotoxicity against PTX alone in cultured hepatocellular carcinoma cells. | PTX formulation exhibited sustains release with Higuchi kinetics | 91 |
| Paclitaxel | Folate-(PEG)-phosphatidyl-ethanolamine | Paclitaxel-loaded solid-liquid lipid nanoparticle showed enhanced rate of tumor inhibition on S180 tumor-bearing mice compared to PTX alone. | Sustained in-vitro release property | 92 |
| Paclitaxel | Glyceryl palmitostearate nanoparticle | Anti-proliferative activity was intact for PTX against B16F10 cell lines in chemosensitive assay. | Mean particle size of the formulation was (172-253 nm) | 93 |
| Paclitaxel | PEG and folate modified monostearin SLN | Cellular uptake and cytotoxicity of PTX was enhanced through folate and PEG modified SLN. | Octadecylamine–fluorescein isothiocyanate was used to formulate fluorescent SLN. | 94 |
| Methotrexate |
Sodium taurodeoxy-cholate, soya lecithin and stearic acid |
The survival time of Ehrlich ascites carcinoma suffering mice was increased owing to better anti-cancer activity of methotrexate-SLN. | MTX-SLN was reported to have average particle size of 270 nm with 51.3% of drug loading. | 95 |
| Vinorelbine bitartrate | PEG2000-stearic acid | PEG modification of SLN inhibited phagocytosis of VB-pSLNs by RAW264.7 cells as well as increased cellular uptake by MCF-7 and A549 cell lines. | Octadecylamine–fluorescein isothiocyanate was used as florescent marker in cellular uptake studies. | 96 |
| Camptothecin | Poloxamer 188, stearic acid, soya lecithin | The mean residence time and area AUC of camptothecin loaded SLN was higher as compared to camptothecin solution. | Camptothecin-SLN has 196.8 mean particle size with -69.3 mV of zeta potential. | 97 |
|
Tamoxifen citrate |
Glycerol behenate, sodium tauroglyco-cholate | Tamoxifen citrate incorporated solid lipid nanoparticle exhibited 3 fold and 3.5 fold increase in t (1/2) and mean residence time respectively. | Smaller mean diameter particle was obtained with homogenization at 15 000 psi for 3 cycles of SLN. | 98 |
| 5-Flurouracil | Dynasan, soyalecithin, polyvinyl alcohol* | 5-flurouracil (5-FU) incorporated SLN could sustain delivery up to 48 hours. | Mean particle size of 402.5 nm ± 34.5 with PDI of 0.005 | 99 |
* As stabilizer
Nano-structured lipid carriers (NLCs)
The SLN suffered from multitude of drawbacks particularly of insufficient drug loading capacity and leaching of drug during storage (alteration to β type); thereby lipid-based nano-carriers were fabricated to address the shortcomings of SLN, using both solid and liquid characteristics of lipids.100 This peculiar characteristic offers better drug entrapment during product shelf life, which in turn increases drug solubilizing potential of NLCs system. Studies have also been conducted for exploring the potential of SLN for targeted and sustained delivery of drugs. Moreover judiciously formulated NLCs system (tailoring the composition and formulation processing parameters) can be used for effective intestinal absorption of drugs which can synergistically improves drug bioavailability.101 Therefore NLCs are categorized as second generation lipid based nano-drug carriers.102-106 Dolatabadi et al107 successfully prepared Ketotifen incorporated NLCs with enhanced systemic exposure and reduced cellular toxicity. Hot homogenization and ultrasonication method was used for NLCs preparation. Ketotifen loaded NLC has particle size below 100 nm with high encapsulation efficiency of 70%.
NLCs and their role in increasing anti cancer drug absorption and permeability
Delivery of drug substances with poor bioavailability owing to their low aqueous solubility, permeability and high metabolic activity has been a herculious task for the formulation scientist since long. Majority of problems can be addressed by incorporating them into NLCs such as fenofibrate, isoliquiritigenin, baicalin.108-110 Metabolism of CYP3A4 substrate drug can be prevented by using surfactant (polysorbates, cremophors, and poloxamer) which are inhibitor of the CYP3A4 activity. Moreover conversion of drug physical state from crystalline to amorphous enhances dissolution velocity and increases the uptake of drug by lymphatic system. Making an overall enhancement in drug absorption and bioavailability.111-114 The entrapment of drug into the lipid matrix provides stealth effect to photolabile and also to drug prone to hydrolytic degradation. Tailoring the globular size of NLCs in the range from 120 to 200 nm reduces the uptake by RES, thereby providing effective enhancement in oral bioavailability.115,116 Ding et al117 reported the usefulness of NLCs in co-delivery of PTX and indocyanine green (ICG). The developed formulation have dual characteristic of chemo as well as photodynamic therapy. ICG was reported to enhance the drug release by laser irritation, further increased intracellular uptake and cytotoxicity was obtained by concomitant use of PTX and ICG.
Limited lymphatic uptake of NLCs
Drug incorporated NLCs which are tailor made convert into mixed micelles on interaction with bile salts in the GIT, making them capable of gaining access into lymphatic system thereby circumventing the metabolic activity of liver. The limited lymphatic access also serves the purpose of dose frequency reduction, enhanced concentration of anti-cancer drug at localized and metastatic tumor cells. These factors advocate effective management in cancer chemotherapy and associated side effects.118 Anti-cancer drugs benefitted by NLCs are shown in Table 4.
Table 4. List of anti-cancer drugs formulated as NLCs .
| Drug | Lipid vehicle | Interpretation and remark | Ref. |
| Docetaxel |
Soy lecithin, glyceryl monostearate, and fatty acids |
Prepared DTX-NLC (murine melanoma treatment) accounts for lower toxicity in therapeutics dose and selective cytotoxicity to A549 cells through apoptosis. | 119 |
| Docetaxel oleate |
Poloxamer F68, oleic acid, and Glyceryl monostearate |
Prepared prodrug NLC of Docetaxel exhibited improved membrane permeability in in-situ single pass intestinal perfusion and 4.04 fold increase in bioavailability | 120 |
| Biochanin A | Glycerol monostearate, medium chain triglyceride, soya lecithin, and Tween 80* | Biochanin loaded NLC exhibited higher AUC values with prolonged in-vitro residence time | 121 |
| Tamoxifen citrate | Poloxamer 188, glyceryl monostearate, Labrafil WL 2609 BL, Tween 20*, and Tween 80* | Similar cytotoxic activity was observed both on human (MCF-7) and mice (4T1) breast cancer cell lines form Tamoxifen and Tamoxifen-NLC | 122 |
| Tamoxifen citrate | Glyceryl monostearate, Labrafil WL 2609 BL,Tween20*, Tween 80*, Poloxamer 188* | Tamoxifen loaded NLC exhibited 2.71 and 7.10 fold increment in bioavailability and T1/2 respectively, also measurable amount was detected in mesenteric lymph node. | 123 |
| Etoposide |
Glyceryl monostearate, soybean oil, soya lecithin |
Etoposide loaded-DSPE-NLC was highly cytotoxic to human epithelial-like lung cell carcinoma cells, and even relative bioavailability was 3.5 fold to Etoposide-suspension | 124 |
*Emulsifier.
Self-Emulsifying Drug Delivery Systems (SEDDs)
SEDDs are complex mixture of Lipid, Surfactant and Co-surfactant the later may or may not be used. Lipid Based drug delivery system have emerged from simplistic oily solutions; they can be either self-micro emulsifying drug delivery system (SMEDDs) or self-nanoemulsifying drug delivery systems (SNEDDs). Whether SEDDs, SMEDDs or SNEDDs they have unique characteristic to self-emulsify into oil-in-water (O/W) emulsion in-vivo on subsequent dilution and agitation by the physiological system.125-128 SEDDs formulations have proven their utility in addressing the issue of bioavailability for BCS Class II and IV candidates and essentially comprises of lipid globule size in the range of 200 nm-5 mm.129-131 When the globular size of the inner phase ranges from 100-200 nm they are termed as SMEDDs and makes transparent micro-emulsions on dilution. Whereas, those self emulsifying lipid based formulations having lipid globular size below 100 nm are categorized as SNEDDs.132,133
A plethora of research article have been published which clearly exhibits the usefulness of SMEDDs and SNEDDs for lymphatic route targeting. As the drug is in pre-solubilized state into the lipid component it presents drug in ready to be absorbed condition, it also circumvents the effect of metabolic enzymes and drug effluxer, therefore contributes to overall enhancement in drug bioavailability.134,135 The faith of drug substance incorporated into lipid based drug delivery system in-vivo is dependent on the factors like physiochemical properties of drug, nature of lipid used (SCT, MCT or LCT). Therefore, judicious selection of surfactant and co-surfactant blend and its ratio is required as it is a guiding process in drug absorption into the luminal lymphatic pathway and effective drug concentration to tumor cells.136-138 The type of oils used for the formulation of SMEDDs is given in Table 5. Anti-cancer drugs benefitted by SEDDs and SMEDDs are given in Table 6.
Table 5. List of lipids explored in formulation of SMEDDs34 .
| Type of Lipid | Description |
| Long chain triglycerides | Sunflower oil, Sesame oil, Soybean oil, arachis oil, Rice bran oil, castor oil, cottonseed oil, maize (corn) oil, canola oil, hydrolyzed corn oil, triolein olive oil, safflower oil, palm oil, peanut oil, |
| Medium chain triglycerides and related esters | Crodamol GTCC®), Caprylic/capric triglycerides (Akomed E, Akomed R, Miglyol 810, and Captex 355, Captex 200, Triacetin Neobee M5®, fractionated coconut oil (Miglyol 810 and 812 ), Labrafac CC, Captex 300, |
|
Medium chain mono and di-glycerides Long-chain mono glycerides Propylene glycol (PG) fatty acid esters |
Mono and diglycerides of capric/caprylic acid. (Capmul MCM and Imwitor), Glycerylmonooleate (Capmul GMO, Peceol), glycerylmonolinoleate (Maisine-CC/Maisine 35-1) PG Diester of caprylic/capric acid (Labrafac PG), PG monocaprylic ester (Sefsol-218), PG monolaurate (Lauroglycol FCC, Lauroglycol90, Capmul PG-12), PG dicaprylate (Miglyol 840) |
Table 6. List of anti-cancer agents benefited by SEDDs/SMEDDs .
| Drug | Oil | Emulsifier/Co-Emulsifier | Outcome | Ref. |
| Docetaxel | Glyceryl tricaprylate | Cremophor RH 40, diethylene glycol monoethyl ether; HPMC for promoting super saturation | Docetaxel loaded SEDDs formulation exhibited 8.77 folds higher AUC | 139 |
|
Doxorubicin and LYP-1 (anticancer peptide) |
Maisine/Peceol | PEG 300, TPGS, Gelucire 44/14, Labrasol, Tween 80 propylene glycol | Co-administration of doxorubicin with LYP-1 through SMEDDs formulation showed reduction in tumor growth and metastasis | 140 |
| Etoposide | PC-complexed; octyl and decyl mono-glyceride | Cremophor EL and PEG 400 | 60-fold increase in bioavailability | 141 |
| 9-Nitrocamptothecin (9-NC) | Ethyl oleate | Tween-80/ Cremophor EL and PEG-400/ethanol | SMEDDs based formulation produced better tumor reduction as compared to oral suspension | 142 |
| Exemestane | Capryol 90 | Cremophor ELP and Transcutol P and | Exemestane loaded formulation exhibited 2.8-fold higher oral bioavailability against un emulsified formulation. | 143 |
| Raloxifene | Mono and di glyceride of capric/ caprylic acid Capmul MCM C8 | Akrysol K-140, PEG-200 | In-vitro intestinal permeability studies exhibited enhanced permeability as compared to drug suspension. | 144 |
| Mitotane | Capryol 90 | Tween 80 and Cremophor EL | The optimized mitotane formulation exhibited better permeability from intestinal barrier against mitotane solution (14.85 ± 0.8 vs. 3.03 ± 0.2 μM/cm 2 ), together with 3.4-fold higher bioavailability | 145 |
| Etoposide | Medium chain triglyceride | Polyoxyethylene sorbitan monooleate-20, diethylene glycol monoethyl ether, propylene glycol monolaurate type-I | The % drug release was 1.6 and 1.4 folds for optimized formulation in simulated gastric fluid and simulated intestinal fluid | 146 |
| Curcumin | Ethyl oleate, | OP: Cremophor, (PEG 400), isopropyl myristate, Cremophor RH40R, | The Curcumin loaded SMEDDS absorption was 3.86 times or 12.73 times compared with a Curcumin suspension. | 147 |
| Curcumin | Castor oil | Tween 80 and ethanol | Curcumin solubility in SEDDs was 1.93 mg/mL | 148 |
| Curcumin | Ethyl oleate |
Transcutol P Cremophor RH 40 |
SMEDDs based formulation was targeted for colonic delivery through pulsatile capsule. | 149 |
| Curcumin | Lauroglycol | Labrasol and Transcutol HP | The Cmax and AUC for solid SEDDs was 4.6 and 7.6- folds higher | 150 |
| Curcumin (Type IV lipid based formulation) | Gelucire 44/14 | Labrasol, Vitamin E TPGS and PEG 400 | Lipid based oral formulation exhibited enhanced Cmax and AUC0-35.8-fold increase in the oral bioavailability as compared to control | 151 |
| Curcumin | Isopropyl myristate | Cremophor RH 40 and ethanol | Curcumin SMEDDs relative bioavailability was 1213% as compared to Curcumin oral suspension | 152 |
| Paclitaxel | Vitamin E | DOC-Na, TPGS, Propylene glycol, Cremophor RH 40 | On use of P-gp inhibitor (Cyclosporine-A) oral absorption of Paclitaxel was enhanced | 153 |
| Paclitaxel | Glyceryl dioleate |
Cremophor EL, PEG 400; HPMC as super saturation Promoter |
5-fold higher bioavailability | 154 |
| Paclitaxel | DL-alpha tocopherol | TPGS, tyloxapol, DOC-Na | 5 fold increase in Paclitaxel loading as compared to i.v. formulation with less toxicity. | 155 |
SNEDDs for hydrophobic drugs
As the absorption of drug substance (hydrophobic) is the limiting factor owing to crystalline structure of the molecule, the entire dose of drug in SNEDDs exists in dissolved state in the lipid concentrate, therefore overall enhancement in absorption of the drug substance takes place. This finally promotes partitioning through the enterocytes and into the systemic circulation.156-160
Furthermore the lipid based formulations (emulsified in the stomach) or lipid digestion products after reaching to the enteric system (duodenum) instigate bile juice secretion (bile salts and biliary lipids) stored in the gall bladder. The composition of bile juice is such that it supports further processing of lipid based formulations as they act as a medium for the solubilization of hydrophobic fatty acids, mono and di-glyceride which are incorporated in series of colloidal structure at intestinal lumen. The hydrophilic part of the micelles orients into the aqueous phase whereas hydrophobic chain forms the central part.161-163
The overall solubilizing potential of the intestinal lumen is enhanced by the formation of mixed micellar phase, through lipid based formulations reducing precipitation of hydrophobic drugs. Their solubilizing potential is related with the ability to utilize enormous interfacial surface where the hydrophobic drug partitions.164,165
SNEDDs as remedy for CYP3A4, P-gp substrate drugs
The mechanistic approach behind enhanced oral bioavailability of SNEDDs based formulation as hypothesized by many researchers might be an outcome of enhanced permeability by trans-cellular route, as the formulation components associates with trans-cellular membrane makes it more fluidic which in turn provide massive passive permeation of formulation components.166,167 The other theories have originated from the functionality of lipid based excipients; as they have ability to inhibit the efflux transporters making conformational changes to the efflux causing pumps (p-gp) thereby enhancing drug concentration at the target site.168,169 Conventional theory advocated the dominant role of liver in pre-systemic metabolism of drugs whereas the newer finding suggests that intestinal lumen is a major obstacle for drug substance permeability. This hypothesis was generalized on experimental findings which reflected high level of concentration of proteins associated with drug metabolizing enzymes in liver rather than small intestine.170 Though CYP3A associated drug metabolizing enzymes were largely expressed at the developed villus tip of enterocytes in the small intestine.171 The CYP3A sub-family of enzymes is considered to have a major role in Phase I metabolism in human beings. In fact, the CYP3A sub-family is responsible for the oxidative metabolism of ~50% of currently marketed drugs.172 Recent research done by several researchers has shown that more than 70% of the enzymes associated with CYP3A4 prevails in small intestine, whereas its prevalence in hepatic was comparatively low to 30%, which somewhat have demystified the dominant role of liver in first pass effect.173
The SNEDDs formulation serves the purpose of delivering hydrophobic drugs by presenting the drug in pre-solubilized state in nano sized globules which are thermodynamically stable with enhanced absorptive potential.The overall bioavailability is also enhanced owing to reduced pre-systemic metabolism by CYP3A4 and reduced or negligible P-gp mediated drug efflux.174 The role of CYP3A4 and P-gp was discussed when addressing low bioavailability of cyclosporine by Wu et al175 a BCS Class II compound and P-gp, CYP3A4 substrate. The SNEDDs formulation exhibited better permeability through membranes when administered in solubilized state with corn oil as lipid vehicle, but reduced bioavailability was observed owing to higher metabolism by CYP3A4 and also as it was highly effluxed out from the cells. These suggestions were in agreement that merely solubilizing the drug in lipid do not suffices the condition of poor bioavailability as the incorporation of vehicles that can inhibit the effect of P-gp and CYP3A4 is also equally important. Akhtar et al176 developed SNEDDs of etoposide (a p-gp substrate), and reported higher permeability coefficient in apical to basolateral direction from optimized SNEDDs formulation across Caco-2 monolayers as 2.6 and 11-fold in comparison to marketed formulation and lone drug solution respectively. Enhanced permeability and bioavailability has been attributed to Cremophor RH 40 (as surfactant) ability to inhibit the P-gp efflux activity in the gut and ability of Transcutol P (as co-surfactant) to alter membrane permeability. The SNEDDs has been explored for maximizing the therapeutic potential of anti-cancer drugs and results of their study are listed in Table 7.
Table 7. List of anti-cancer agents benefitted by SNEDDs .
| Drug | Lipid Components | Interpretation | Ref. |
| Etoposide |
Oleoylmacrogol-6-glycerides, Gelucire 44/14, Peceol, Transcutol** P, Plurol Oleique and Labrasol* |
The formulation showed better transportation of etoposide across the Caco-2 cell lines and higher cytotoxicity than the Etosid® and free drug against A549 human epithelial lung carcinoma cell lines | 176 |
| Docetaxel | Capryol 90, Labrasol*, and Transcutol HP** | 17% higher oral bioavailability for D-SNEDDs as compared to 2.6 % for Docetaxel solution, with high anti-tumor efficacy and reduced toxicity. | 177 |
|
Tamoxifen citrate |
Maisine 35-1, Capryol 90, Cremophor RH40*, propylene glycol** | Higher drug release from SNEDDs as compared to Tamoxifen citrate suspension. | 178 |
| Docetaxel | Maisine 35-1and Capmul MCM with Tween 80* and Transcutol-HP** | Self Nano Emulsifying Lipidic Nano-micelles System (SNELS) Formulation enriched with PUFA (Long Chain Glyceride) exhibited better dissolution, enhanced p-gp inhibition and reduced pre-systemic metabolism as depicted from in vivo pharmacokinetic studies, better permeability and absorption was also reflected from ex-vivo permeation andin situ perfusion studies. Moreover LCG based formulation were highly targeted to lymphatic system as compared to MCG (medium-chain fatty acid glyceride) | 179 |
*Emulsifier, **Co-emulsifier.
Lipid prodrug
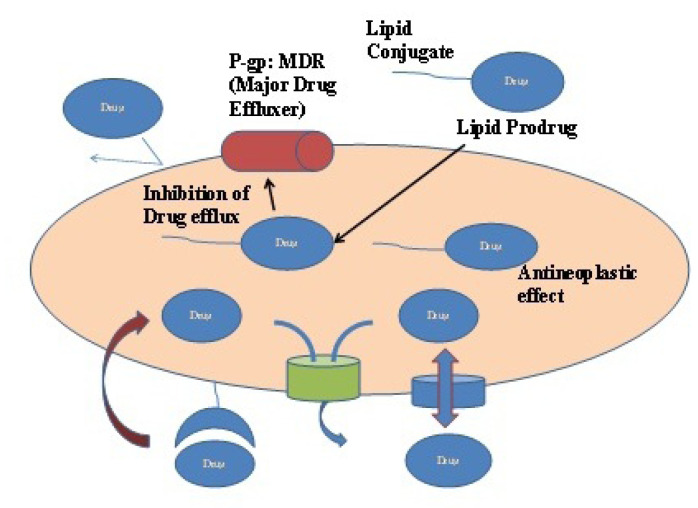
A plethora of lipid carriers have been investigated for the development of lipid prodrug which are generally a unique mixture of glyceride, fatty acids, and phospholipids. The drug is linked either at the carboxylate group or at the ω-position of the carbon chain at its terminal part.180 A Glyceride conjugation is an outcome of attaching carboxylate group of drug moiety through ester linkage, whereas the drug can also be linked through phosphate group or at the glycerol backbone. The enhanced permeability exhibited by drugs attached to the glycerol backbone is attributed to their ability to permeate apical membrane of the intestinal lumen and the blood brain through natural absorption pathway of phospholipids.181 Drug-lipid conjugations can reduce total quantity of drug being effluxes out from the cells by P-gp or MDR transporters which in turn will increase permeation to cancerous cells and prolonging the retention of anti-cancer agents Figure 6.182 Numerous lipid-taxane conjugates, with alterations at 2 or 7-OH position of series of second generation taxoids (Paclitaxel, Docetaxel SB-T-1103, SB-T-1104, SB-T-1213, SB-T-1214, SB-T-1216, and SB-T-1217) have also been investigated.183
Figure 6.
Lipid prodrug can effectively reduce drug efflux from P-gp and MDR.
Targeted lipid nano-carriers for cancer therapeutics
Targeting anti-cancer drugs to proliferating cells through lipid based delivery systems have proven benefits in selectively targeting the cancerous cells, avoidance of exposure to healthy cells, hydrophobic drug delivery and prolonged exposure over conventional chemotherapy strategies which have dose dependent side effects due to their non-selective bio-distribution.184 Targeted delivery of anti-cancer drugs can be achieved either by active or passive targeting. The active targeting is achieved by attaching lipid based nano-carriers with tumor specific binding ligand. These specific bindings results in selective accumulation at target site. Whereas, passive targeting approach delivers the drug cargo passively to proliferating cells utilizing the rapid vascularization of hyper-permeable cells. The nano-particulate size (10-100 nm) and its surface properties (hydrophilic) are decisive in passive targeting and assembly within tumors.185-187 Jain et al188 successfully investigated tumor targeting specificity of Ferritin integrated 5-flurouracil SLN. Fr-SLNs showed 7.7-folds more binding affinity with MDA-MB-468 breast cancer cell lines in contrast to non Ferritin integrated SLN of 5-FU under in-vitro studies. Further under therapeutic investigation in MDA-MB-468 cancer induced mice, formulation with Fr-SLNs exhibited significant reduction in tumor growth as compared with plain 5-FU and non-Ferritin integrated SLN of 5-FU.Yang et al189 explored target specificity to CD44 overexpressing tumors with PTX incorporated SLN having hyaluronic acid-coating. Melt extrusion technique was employed for preparing cationic PTX-NLC, which were further coated with hyaluronic acid. Tumor specificity, pharmacokinetics and biodistribution were investigated in B16-bearing Kunming mice. It was concluded from their research that Hyaluronic acid coated SLN of PTX exhibited tumor targeting efficiency of 14.46 %, which was around 1.4 fold higher against Taxol® and drug circulation was also enhanced from 3h to 6h. Liu et al190 successfully explored synergistic anticancer action against cervical cancer from engineered SLN with trans-activating transcriptional activator (TAT) for co-delivery of PTX coupled with α-tocopherol succinate-cisplatin prodrug (TOS-CDDP) (TAT PTX/TOS-CDDP SLNs). The in-vitro and in-vivo studies were in agreement that developed formulation showed specific antitumor action on HeLa cells and cervical cancer suppression with reduced toxicity in vivo. Furthermore to get an insight into the characteristics of different nano-particles (polymeric, metallic) in comparison with lipid based nano-carriers for cancer therapeutics we have summarized recent research trends in the form of a comprehensive Table 8.
Table 8. Comprehensive table showing different characteristics of nano-particles with special emphasis on lipid based drug carriers .
| Nano-carrier types | Drug | Composition | Cancer Type/ Cell lines investigated | Formulation Characteristics | Outcome | Ref. |
| SNEDDs | Sunitinib malate (SM) |
BLauroglycol-90 (oil), Triton- X100 (surfactant) and Transcutol-P (cosurfactant) |
HT-29 colon cells | PS* 42.3 nm, PDI** (0.174), ZP*** value (-36.4mV) | Optimized SNEDDs formulation was about two times more efficacious in comparison to plain SM | 191 |
| SNEDDs | Etoposide | GT and PGMCE (1:1) Cremophor RH 40, Transcutol P | For cytotoxicity A549 human epithelial lung carcinoma cell lines, and Caco-2 cell monolayers for transportation | PS* 36.01 nm, ZP*** (-27.1mV) | AUC was increased to 7.9 fold compared to etoposide drug suspension | 176 |
| SLN | Galbanic acid | glyceryl palmitostearate (Precirol ATO 5), Poloxamer 407 | A549 cells | PS* 92nm, ZP*** value( -23.39mV), Entrapment efficiency- >98 % | Sustained and enhanced antiproliferative action form GBA-SLN | 86 |
| SLN | Paclitaxel | E.wax and Brij 78 | U-118 and HCT-15 cell lines, brain uptake in situ rat brain perfusion model | PS* <100 nm, with high drug loading. | Significant modulation of p-gp at Blood brain barrier by Brij 78 | 192 |
| Targeted-NLC | Docetaxel | 1,2-Distearoyl-sn-glycero- 3-phosphoethanolamine-N- [amino(polyethylene glycol)-2000] | Murine model bearing B16 | PS*168nm, Encapsulation efficiency >95% | Increased accumulation of drug in both tumor and tumor vasculature, therefore successful targeting to VEGFR-2 | 193 |
| SMEDDs | Curcumin | Semi-synthetic oleic acid derived bicephalous heterolipid, E1E, Solutol HS-15, Transcutol HP | Human cervix cancer cell line HeLa | PS* 22.39 nm, PDI** 0.243, Curcumin loading efficiency 70.52 | 26 fold increment in absorption | 194 |
| SMEDDs | Dutasteride | CapryolTM 90, Cremophor® EL, Transcutol® HP | Pharmacokinetic studies in Male Sprague–Dawley rats | PS*35.3nm | 10.5 and 13.2 fold increment in AUC 0→24h and Cmax respectively | 195 |
| Amorphous polymeric NPs | Cisplatin | P(3HV-co-4HB)-b-mPEG amphiphilic block copolymer | MTT assay using DU145 cell line | PS* 155 nm, PDI** 0.154, ZP*** value (-18mV) | The polymeric nanoparticles of cisplatin exhibited sustained drug delivery | 196 |
| PLGA-based nanoparticles | Paclitaxel | PEGylated PLGA-based nanoparticles | MTT assay on Human Cervix Carcinoma cells (HeLa) | PS* 112 nm, PDI** 0.18, ZP*** (-0.556 mv) | The developed nanoparticle exhibited significant anti-proliferative activity compared to Taxol on HeLa cell lines. | 197 |
| Carbon nanotubes | Doxorubicin | Anti body P-gp functionalized hydrophilic single walled nanotubes | Multidrug resistant leukemia (K562R) cells | Effective loading and targeted delivery of doxorubicin | Significant anti-proliferative activity in K562R leukemia cells with 2.4 times higher cytotoxicity | 198 |
| Cyclodextrin based Nanoparticles | Docetaxel | Poly(e-caprolactone) (PCL) and poly(ethylene glycol)-block-poly(e-caprolactone) (mePEG-PCL) nano particles with hydroxypropyl-β-cyclodextrin (CD) coating | MCF-7 human breast adenocarcinoma cell lines | PS* 60-132 nm, ZP*** (-22 and -37mV) , Encapsulation efficiency ranges from 46 and 73% | hydroxypropyl-β-cyclodextrin (CD) and PCL based nanoparticles exhibited significant antiproliferative activity against MCF-7 human breast adenocarcinoma cell lines | 199 |
| Superparamagnetic iron oxide nanoparticles (SPIONs) | Doxorubicin | polyamidoamine (rPAA) with poly(ethylene glycol)(PEG)/dodecyl amine graft | Xenograft MDA-MB-231 breast tumor in mice | PS* ˜150nm | rPAA@SPION effectively inhibited tumor growth in xenograft MDA-MB-231 breast tumor induced mice | 200 |
| Gold nanoparticle | Oxaliplatin | Functionalized with a thiolated poly(ethylene glycol) (PEG) monolayer capped with a carboxylate group | A549 lung epithelial cancer cell line and colon cancer cell lines HCT116,HCT15, HT29, and RKO | PS* 30-40 nm, | Functionalized NP exhibited 6 fold increments in cytotoxicity in A549 lung epithelial cancer cell line and 5.6-fold more cytotoxic in colon cancer. | 201 |
*PS = Particle Size, **PDI = Polydispersibility index, ***ZP = Zeta Potential, VEGFR-2 = Vascular endothelial growth factor receptors, UGT = UDP-glucuronosyltransferase
Conclusion and future prospects
Lipid based formulation have emerged as a panacea for anti-cancer drug therapeutics. Lipid component as delivery vehicle for hydrophobic drugs have been successfully utilized for enhancing their oral bioavailability. Moreover, lipid component like Peceol, Maisine 35-1 and Gelucire 44/14 have proved their usefulness in lymphatic system targeting and effective P-gp and CYP3A4 modulatory effect. Further, as per our discussion in this article the judicious selection of lipid components is advocated as selecting long chain triglyceride over short and medium chain triglycerides when lymphatic drug targeting is desired. Therefore, lipid based formulation can be employed for lymphatic voyage of anti-cancer drugs with additional benefits of P-gp and CYP3A4 modulation for drugs having poor hydrophilicity, low intestinal permeation and high CYP3A4 mediated metabolic activity.
Ethical Issues
Not applicable
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Acknowledgments
The manuscript was not financed from any source.
References
- 1. Lieu CH, William WN, Lippman SM. Cancer chemoprevention. In: Garrett-Mayer E, ed. Principles of Anticancer Drug Development. New York, NY: Springer; 2011. p. 463-81. 10.1007/978-1-4419-7358-0_16 [DOI]
- 2.Xie Y, Bagby TR, Cohen MS, Forrest ML. Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv. 2009;6(8):785–92. doi: 10.1517/17425240903085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011;71(4):1214–8. doi: 10.1158/0008-5472.can-10-3277. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto K, Sawai K, Minato H, Yada H, Shirasu M, Sakakura C. et al. Number and anatomical extent of lymph node metastases in gastric cancer: analysis using intra-lymph node injection of activated carbon particles (CH40) Jpn J Clin Oncol. 1999;29(2):74–7. doi: 10.1093/jjco/29.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Brodt P. Characterization of two highly metastatic variants of Lewis lung carcinoma with different organ specificities. Cancer Res. 1986;46(5):2442–8. [PubMed] [Google Scholar]
- 6.Ward PM, Weiss L. Metachronous seeding of lymph node metastases in rats bearing the MT-100-TC mammary carcinoma: the effect of elective lymph node dissection. Breast Cancer Res Treat. 1989;14(3):315–20. doi: 10.1007/bf01806303. [DOI] [PubMed] [Google Scholar]
- 7.Crile G Jr, Isbister W, Deodhar SD. Demonstration that large metastases in lymph nodes disseminate cancer cells to blood and lungs. Cancer. 1971;28(3):657. doi: 10.1002/1097-0142(197109)28:3<657::aid-cncr2820280319>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106(5):920–31. doi: 10.1161/circresaha.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296(6):E1183–94. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty S, Shukla D, Mishra B, Singh S. Lipid--an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73(1):1–15. doi: 10.1016/j.ejpb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, Patel HR. The metastatic cascade in prostate cancer. Surg Oncol. 2006;15(3):117–28. doi: 10.1016/j.suronc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Muranishi S. [Lymphatic delivery of drugs and its application to cancer chemotherapy (author’s transl)] Yakugaku Zasshi. 1980;100(7):687–98. [PubMed] [Google Scholar]
- 13.Garzon-Aburbeh A, Poupaert JH, Claesen M, Dumont P, Atassi G. 1,3-dipalmitoylglycerol ester of chlorambucil as a lymphotropic, orally administrable antineoplastic agent. J Med Chem. 1983;26(8):1200–3. doi: 10.1021/jm00362a021. [DOI] [PubMed] [Google Scholar]
- 14.McCarter MD, Clarke JH, Harken AH. Lymphangiogenesis is pivotal to the trials of a successful cancer metastasis. Surgery. 2004;135(2):121–4. doi: 10.1016/s0039-6060(03)00342-8. [DOI] [PubMed] [Google Scholar]
- 15.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 16.Sleeman JP. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res. 2000;157:55–81. doi: 10.1007/978-3-642-57151-0_6. [DOI] [PubMed] [Google Scholar]
- 17.Sleeman JP. The relationship between tumors and the lymphatics: what more is there to know? Lymphology. 2006;39(2):62–8. [PubMed] [Google Scholar]
- 18. Charman WN, Stella VJ, O’Driscoll C. Anatomy and physiology of the lymphatics. In: Charman WN, Stella VJ, eds. Lymphatic Transport of Drugs. Boca Raton: Routledge; 2019. p. 1-30.
- 19.Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1-2):143–56. doi: 10.1016/s0169-409x(01)00154-5. [DOI] [PubMed] [Google Scholar]
- 20.Thomson AB, Schoeller C, Keelan M, Smith L, Clandinin MT. Lipid absorption: passing through the unstirred layers, brush-border membrane, and beyond. Can J Physiol Pharmacol. 1993;71(8):531–55. doi: 10.1139/y93-078. [DOI] [PubMed] [Google Scholar]
- 21.Schoeller C, Keelan M, Mulvey G, Stremmel W, Thomson AB. Role of a brush border membrane fatty acid binding protein in oleic acid uptake into rat and rabbit jejunal brush border membrane. Clin Invest Med. 1995;18(5):380–8. [PubMed] [Google Scholar]
- 22.Schoeller C, Keelan M, Mulvey G, Stremmel W, Thomson AB. Oleic acid uptake into rat and rabbit jejunal brush border membrane. Biochim Biophys Acta. 1995;1236(1):51–64. doi: 10.1016/0005-2736(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 23.Stremmel W, Lotz G, Strohmeyer G, Berk PD. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J Clin Invest. 1985;75(3):1068–76. doi: 10.1172/jci111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stremmel W. Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J Clin Invest. 1988;82(6):2001–10. doi: 10.1172/jci113820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirier H, Degrace P, Niot I, Bernard A, Besnard P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine Comparison with fatty acid-binding proteins (FABP) Eur J Biochem. 1996;238(2):368–73. doi: 10.1111/j.1432-1033.1996.0368z.x. [DOI] [PubMed] [Google Scholar]
- 26.Bloom B, Chaikoff IL, Reinhardt Reinhardt. Intestinal lymph as pathway for transport of absorbed fatty acids of different chain lengths. Am J Physiol. 1951;166(2):451–5. doi: 10.1152/ajplegacy.1951.166.2.451. [DOI] [PubMed] [Google Scholar]
- 27.Chaikoff IL, Bloom B, Stevens BP, Reinhardt WO, Dauben WG. Pentadecanoic acid-5-C 14; its absorption and lymphatic transport. J Biol Chem. 1951;190(2):431–5. [PubMed] [Google Scholar]
- 28.Bloom B, Chaikoff IL, Reinhardt WO, Dauben WG. Participation of phospholipides in lymphatic transport of absorbed fatty acids. J Biol Chem. 1951;189(1):261–7. [PubMed] [Google Scholar]
- 29.Kiyasu JY, Bloom B, Chaikoff IL. The portal transport of absorbed fatty acids. J Biol Chem. 1952;199(1):415–9. [PubMed] [Google Scholar]
- 30.Porter CJ, Charman WN. Intestinal lymphatic drug transport: an update. Adv Drug Deliv Rev. 2001;50(1-2):61–80. doi: 10.1016/s0169-409x(01)00151-x. [DOI] [PubMed] [Google Scholar]
- 31.Prajapati HN, Patel DP, Patel NG, Dalrymple DM, Serajuddin ATM. Effect of difference in fatty acid chain lengths of medium-chain lipids on lipid-surfactant-water phase diagrams and drug solubility. J Excip Food Chem. 2011;2(3):73–88. [Google Scholar]
- 32.Vahouny GV, Treadwell CR. Comparative effects of dietary fatty acids and triglycerides on lymph lipids in the rat. Am J Physiol. 1959;196(4):881–3. doi: 10.1152/ajplegacy.1959.196.4.881. [DOI] [PubMed] [Google Scholar]
- 33.Nankervis R, Davis SS, Day NH, Shaw PN. Intestinal lymphatic transport of three retinoids in the rat after oral administration: effect of lipophilicity and lipid vehicle. Int J Pharm. 1996;130(1):57–64. doi: 10.1016/0378-5173(95)04265-2. [DOI] [Google Scholar]
- 34.Pouton CW, Porter CJ. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60(6):625–37. doi: 10.1016/j.addr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Yáñez JA, Wang SW, Knemeyer IW, Wirth MA, Alton KB. Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev. 2011;63(10-11):923–42. doi: 10.1016/j.addr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurram AK, Deshpande PB, Kar SS, Nayak UY, Udupa N, Reddy MS. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J Pharm Sci. 2015;77(3):249–57. doi: 10.4103/0250-474x.159596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter CJH, Charman WN. Uptake of drugs into the intestinal lymphatics after oral administration. Adv Drug Deliv Rev. 1997;25(1):71–89. doi: 10.1016/s0169-409x(96)00492-9. [DOI] [Google Scholar]
- 38.Hiroshi Y, Shozo M, Chiharu K, Hitoshi S. Bifunctional delivery system for selective transfer of bleomycin into lymphatics via enteral route. Int J Pharm. 1981;8(4):291–302. doi: 10.1016/0378-5173(81)90069-7. [DOI] [Google Scholar]
- 39.Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid tissues I Orally administered biodegradable microspheres target the peyer’s patches. J Control Release. 1990;11(1-3):205–14. doi: 10.1016/0168-3659(90)90133-e. [DOI] [Google Scholar]
- 40.Hawley AE, Davis SS, Illum L. Targeting of colloids to lymph nodes: influence of lymphatic physiology and colloidal characteristics. Adv Drug Deliv Rev. 1995;17(1):129–48. doi: 10.1016/0169-409x(95)00045-9. [DOI] [Google Scholar]
- 41.Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer’s patches. Am J Physiol. 1998;275(1):G130–7. doi: 10.1152/ajpgi.1998.275.1.G130. [DOI] [PubMed] [Google Scholar]
- 42.Wells JM, Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol. 2008;6(5):349–62. doi: 10.1038/nrmicro1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terwogt JM, Schellens JH, Huinink WW, Beijnen JH. Clinical pharmacology of anticancer agents in relation to formulations and administration routes. Cancer Treat Rev. 1999;25(2):83–101. doi: 10.1053/ctrv.1998.0107. [DOI] [PubMed] [Google Scholar]
- 44.Hunter J, Hirst BH. Intestinal secretion of drugs The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv Drug Deliv Rev. 1997;25(2-3):129–57. doi: 10.1016/s0169-409x(97)00497-3. [DOI] [Google Scholar]
- 45.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27(1):17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Wu CY, Benet LZ, Hebert MF, Gupta SK, Rowland M, Gomez DY. et al. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: studies with cyclosporine. Clin Pharmacol Ther. 1995;58(5):492–7. doi: 10.1016/0009-9236(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 47. Rubino JT. Encyclopedia of pharmaceutical science and technology. In: Swarbrick J, Boylan JC, eds. Encyclopedia of Pharmaceutical Science and Technology. Vol.3. New York: Marcel Dekker, Inc; 1988. p. 375-98.
- 48.Barthe L, Woodley J, Houin G. Gastrointestinal absorption of drugs: methods and studies. Fundam Clin Pharmacol. 1999;13(2):154–68. doi: 10.1111/j.1472-8206.1999.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 49.Thummel KE, Kunze KL, Shen DD. Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv Drug Deliv Rev. 1997;27(2-3):99–127. doi: 10.1016/s0169-409x(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 50.Tabata T, Katoh M, Tokudome S, Nakajima M, Yokoi T. Identification of the cytosolic carboxylesterase catalyzing the 5’-deoxy-5-fluorocytidine formation from capecitabine in human liver. Drug Metab Dispos. 2004;32(10):1103–10. doi: 10.1124/dmd.104.000554. [DOI] [PubMed] [Google Scholar]
- 51. Gonzalez FJ. Drug metabolism. In: Brunton LL, Hilal-Dandan R, Knollmann BC, ed. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill Companies; 2006. p. 71-92.
- 52.Shin SC, Choi JS, Li X. Enhanced bioavailability of tamoxifen after oral administration of tamoxifen with quercetin in rats. Int J Pharm. 2006;313(1-2):144–9. doi: 10.1016/j.ijpharm.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 53.Mouly S, Paine MF. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res. 2003;20(10):1595–9. doi: 10.1023/a:1026183200740. [DOI] [PubMed] [Google Scholar]
- 54.Martin-Facklam M, Burhenne J, Ding R, Fricker R, Mikus G, Walter-Sack I. et al. Dose-dependent increase of saquinavir bioavailability by the pharmaceutic aid cremophor EL. Br J Clin Pharmacol. 2002;53(6):576–81. doi: 10.1046/j.1365-2125.2002.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandhu PS, Beg S, Mehta F, Singh B, Trivedi P. Novel dietary lipid-based self-nanoemulsifying drug delivery systems of paclitaxel with p-gp inhibitor: implications on cytotoxicity and biopharmaceutical performance. Expert Opin Drug Deliv. 2015;12(11):1809–22. doi: 10.1517/17425247.2015.1060219. [DOI] [PubMed] [Google Scholar]
- 56.Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16(4-5):237–46. doi: 10.1016/s0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 57.Mountfield RJ, Senepin S, Schleimer M, Walter I, Bittner B. Potential inhibitory effects of formulation ingredients on intestinal cytochrome P450. Int J Pharm. 2000;211(1-2):89–92. doi: 10.1016/s0378-5173(00)00586-x. [DOI] [PubMed] [Google Scholar]
- 58.Riaz M. Liposomes preparation methods. Pak J Pharm Sci. 1996;9(1):65–77. [PubMed] [Google Scholar]
- 59.Ling SS, Magosso E, Khan NA, Yuen KH, Barker SA. Enhanced oral bioavailability and intestinal lymphatic transport of a hydrophilic drug using liposomes. Drug Dev Ind Pharm. 2006;32(3):335–45. doi: 10.1080/03639040500519102. [DOI] [PubMed] [Google Scholar]
- 60.El Maghraby GM, Williams AC, Barry BW. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int J Pharm. 2004;276(1-2):143–61. doi: 10.1016/j.ijpharm.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 61.Godin B, Touitou E. Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J Control Release. 2004;94(2-3):365–79. doi: 10.1016/j.jconrel.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Fang JY, Hwang TL, Huang YL, Fang CL. Enhancement of the transdermal delivery of catechins by liposomes incorporating anionic surfactants and ethanol. Int J Pharm. 2006;310(1-2):131–8. doi: 10.1016/j.ijpharm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Hashida N, Murakami M, Yoshikawa H, Takada K, Muranishi S. Intestinal absorption of carboxyfluorescein entrapped in liposomes in comparison with its administration with lipid-surfactant mixed micelles. J Pharmacobiodyn. 1984;7(3):195–203. doi: 10.1248/bpb1978.7.195. [DOI] [PubMed] [Google Scholar]
- 64.Ye T, Wu Y, Shang L, Deng X, Wang S. Improved lymphatic targeting: effect and mechanism of synthetic borneol on lymph node uptake of 7-ethyl-10-hydroxycamptothecin nanoliposomes following subcutaneous administration. Drug Deliv. 2018;25(1):1461–71. doi: 10.1080/10717544.2018.1482973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, Song JH, Park JS, Han K. Polyethylene glycol-coated liposomes for oral delivery of recombinant human epidermal growth factor. Int J Pharm. 2003;258(1-2):11–9. doi: 10.1016/s0378-5173(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 66.Iwanaga K, Ono S, Narioka K, Morimoto K, Kakemi M, Yamashita S. et al. Oral delivery of insulin by using surface coating liposomes: improvement of stability of insulin in GI tract. Int J Pharm. 1997;157(1):73–80. doi: 10.1016/s0378-5173(97)00237-8. [DOI] [Google Scholar]
- 67.Iwanaga K, Ono S, Narioka K, Kakemi M, Morimoto K, Yamashita S. et al. Application of surface-coated liposomes for oral delivery of peptide: effects of coating the liposome’s surface on the GI transit of insulin. J Pharm Sci. 1999;88(2):248–52. doi: 10.1021/js980235x. [DOI] [PubMed] [Google Scholar]
- 68.Moribe K, Maruyama K, Iwatsuru M. Encapsulation characteristics of nystatin in liposomes: effects of cholesterol and polyethylene glycol derivatives. Int J Pharm. 1999;188(2):193–202. doi: 10.1016/s0378-5173(99)00222-7. [DOI] [PubMed] [Google Scholar]
- 69.Patel A, Tyagi A, Sharma RK, Thakkar H. A gamma scintigraphy study to investigate uterine targeting efficiency of raloxifene-loaded liposomes administered intravaginally in New Zealand white female rabbits. Drug Deliv. 2016;23(9):3330–8. doi: 10.1080/10717544.2016.1177137. [DOI] [PubMed] [Google Scholar]
- 70.Ağardan NB, Değim Z, Yılmaz Ş, Altıntaş L, Topal T. The effectiveness of raloxifene-loaded liposomes and cochleates in breast cancer therapy. AAPS PharmSciTech. 2016;17(4):968–77. doi: 10.1208/s12249-015-0429-3. [DOI] [PubMed] [Google Scholar]
- 71.Ling R, Li Y, Yao Q, Chen T, Zhu D, Jun Y. et al. Lymphatic chemotherapy induces apoptosis in lymph node metastases in a rabbit breast carcinoma model. J Drug Target. 2005;13(2):137–42. doi: 10.1080/10611860400027725. [DOI] [PubMed] [Google Scholar]
- 72.Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 73.Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev. 2011;63(10-11):901–8. doi: 10.1016/j.addr.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 75.Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2-3):165–96. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 76.Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56(9):1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Yuan H, Chen J, Du YZ, Hu FQ, Zeng S, Zhao HL. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids Surf B Biointerfaces. 2007;58(2):157–64. doi: 10.1016/j.colsurfb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–77. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Yoon G, Park JW, Yoon IS. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. J Pharm Investig. 2013;43(5):353–62. doi: 10.1007/s40005-013-0087-y. [DOI] [Google Scholar]
- 80.ALHaj NA, Abdullah R, Ibrahim S, Bustamam A. Tamoxifen drug loading solid lipid nanoparticles prepared by hot high pressure homogenization techniques. Am J Pharmacol Toxicol. 2008;3(3):219–24. doi: 10.3844/ajptsp.2008.219.224. [DOI] [Google Scholar]
- 81.Parhi R, Suresh P. Preparation and characterization of solid lipid nanoparticles-a review. Curr Drug Discov Technol. 2012;9(1):2–16. doi: 10.2174/157016312799304552. [DOI] [PubMed] [Google Scholar]
- 82.Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349–58. doi: 10.4103/0250-474x.57282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ezzati Nazhad Dolatabadi J, Hamishehkar H, Valizadeh H. Development of dry powder inhaler formulation loaded with alendronate solid lipid nanoparticles: solid-state characterization and aerosol dispersion performance. Drug Dev Ind Pharm. 2015;41(9):1431–7. doi: 10.3109/03639045.2014.956111. [DOI] [PubMed] [Google Scholar]
- 84.Bakhtiary Z, Barar J, Aghanejad A, Saei AA, Nemati E, Ezzati Nazhad Dolatabadi J. et al. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev Ind Pharm. 2017;43(8):1244–53. doi: 10.1080/03639045.2017.1310223. [DOI] [PubMed] [Google Scholar]
- 85.Eskandani M, Nazemiyeh H. Self-reporter shikonin-Act-loaded solid lipid nanoparticle: formulation, physicochemical characterization and geno/cytotoxicity evaluation. Eur J Pharm Sci. 2014;59:49–57. doi: 10.1016/j.ejps.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Eskandani M, Barar J, Ezzati Nazhad Dolatabadi J, Hamishehkar H, Nazemiyeh H. Formulation, characterization, and geno/cytotoxicity studies of galbanic acid-loaded solid lipid nanoparticles. Pharm Biol. 2015;53(10):1525–38. doi: 10.3109/13880209.2014.991836. [DOI] [PubMed] [Google Scholar]
- 87.Hamishehkar H, Bahadori MB, Vandghanooni S, Eskandani M, Nakhlband A, Eskandani M. Preparation, characterization and anti-proliferative effects of sclareol-loaded solid lipid nanoparticles on A549 human lung epithelial cancer cells. J Drug Deliv Sci Technol. 2018;45:272–80. doi: 10.1016/j.jddst.2018.02.017. [DOI] [Google Scholar]
- 88.Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RS. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release. 2005;105(3):185–98. doi: 10.1016/j.jconrel.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 89.Naguib YW, Rodriguez BL, Li X, Hursting SD, Williams RO 3rd, Cui Z. Solid lipid nanoparticle formulations of docetaxel prepared with high melting point triglycerides: in vitro and in vivo evaluation. Mol Pharm. 2014;11(4):1239–49. doi: 10.1021/mp4006968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Videira MA, Arranja AG, Gouveia LF. Experimental design towards an optimal lipid nanosystem: a new opportunity for paclitaxel-based therapeutics. Eur J Pharm Sci. 2013;49(2):302–10. doi: 10.1016/j.ejps.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Pandita D, Ahuja A, Velpandian T, Lather V, Dutta T, Khar RK. Characterization and in vitro assessment of paclitaxel loaded lipid nanoparticles formulated using modified solvent injection technique. Pharmazie. 2009;64(5):301–10. doi: 10.1691/ph.2009.8338. [DOI] [PubMed] [Google Scholar]
- 92.Wu L, Tang C, Yin C. Folate-mediated solid-liquid lipid nanoparticles for paclitaxel-coated poly(ethylene glycol) Drug Dev Ind Pharm. 2010;36(4):439–48. doi: 10.3109/03639040903244472. [DOI] [PubMed] [Google Scholar]
- 93.Shenoy VS, Gude RP, Ramchandra Murthy RS. Paclitaxel-loaded glyceryl palmitostearate nanoparticles: in vitro release and cytotoxic activity. J Drug Target. 2009;17(4):304–10. doi: 10.1080/10611860902737938. [DOI] [PubMed] [Google Scholar]
- 94.Yuan H, Miao J, Du YZ, You J, Hu FQ, Zeng S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int J Pharm. 2008;348(1-2):137–45. doi: 10.1016/j.ijpharm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 95.Ruckmani K, Sivakumar M, Ganeshkumar PA. Methotrexate loaded solid lipid nanoparticles (SLN) for effective treatment of carcinoma. J Nanosci Nanotechnol. 2006;6(9-10):2991–5. doi: 10.1166/jnn.2006.457. [DOI] [PubMed] [Google Scholar]
- 96.Wan F, You J, Sun Y, Zhang XG, Cui FD, Du YZ. et al. Studies on PEG-modified SLNs loading vinorelbine bitartrate (I): preparation and evaluation in vitro. Int J Pharm. 2008;359(1-2):104–10. doi: 10.1016/j.ijpharm.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 97.Yang S, Zhu J, Lu Y, Liang B, Yang C. Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharm Res. 1999;16(5):751–7. doi: 10.1023/a:1018888927852. [DOI] [PubMed] [Google Scholar]
- 98.Reddy LH, Vivek K, Bakshi N, Murthy RS. Tamoxifen citrate loaded solid lipid nanoparticles (SLN): preparation, characterization, in vitro drug release, and pharmacokinetic evaluation. Pharm Dev Technol. 2006;11(2):167–77. doi: 10.1080/10837450600561265. [DOI] [PubMed] [Google Scholar]
- 99.Yassin AE, Anwer MK, Mowafy HA, El-Bagory IM, Bayomi MA, Alsarra IA. Optimization of 5-flurouracil solid-lipid nanoparticles: a preliminary study to treat colon cancer. Int J Med Sci. 2010;7(6):398–408. doi: 10.7150/ijms.7.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54 Suppl 1:S131–55. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 101.Saupe A, Wissing SA, Lenk A, Schmidt C, Müller RH. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) -- structural investigations on two different carrier systems. Biomed Mater Eng. 2005;15(5):393–402. [PubMed] [Google Scholar]
- 102.Jenning V, Thünemann AF, Gohla SH. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199(2):167–77. doi: 10.1016/s0378-5173(00)00378-1. [DOI] [PubMed] [Google Scholar]
- 103.Luan J, Zheng F, Yang X, Yu A, Zhai G. Nanostructured lipid carriers for oral delivery of baicalin: in vitro and in vivo evaluation. Colloids Surf A Physicochem Eng Asp. 2015;466:154–9. doi: 10.1016/j.colsurfa.2014.11.015. [DOI] [Google Scholar]
- 104.Zhang X, Qiao H, Zhang T, Shi Y, Ni J. Enhancement of gastrointestinal absorption of isoliquiritigenin by nanostructured lipid carrier. Adv Powder Technol. 2014;25(3):1060–8. doi: 10.1016/j.apt.2014.02.012. [DOI] [Google Scholar]
- 105.Tran TH, Ramasamy T, Truong DH, Choi HG, Yong CS, Kim JO. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS PharmSciTech. 2014;15(6):1509–15. doi: 10.1208/s12249-014-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Severino P, Andreani T, Macedo AS, Fangueiro JF, Santana MH, Silva AM. et al. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Deliv. 2012;2012:750891. doi: 10.1155/2012/750891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ezzati Nazhad Dolatabadi J, Azami A, Mohammadi A, Hamishehkar H, Panahi-Azar V, Rahbar Saadat Y. et al. Formulation, characterization and cytotoxicity evaluation of ketotifen-loaded nanostructured lipid carriers. J Drug Deliv Sci Technol. 2018;46:268–73. doi: 10.1016/j.jddst.2018.05.017. [DOI] [Google Scholar]
- 108.Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415(1-2):232–43. doi: 10.1016/j.ijpharm.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 109.Suresh G, Manjunath K, Venkateswarlu V, Satyanarayana V. Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS PharmSciTech. 2007;8(1):24. doi: 10.1208/pt0801024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fang G, Tang B, Chao Y, Xu H, Gou J, Zhang Y. et al. Cysteine-functionalized nanostructured lipid carriers for oral delivery of docetaxel: a permeability and pharmacokinetic study. Mol Pharm. 2015;12(7):2384–95. doi: 10.1021/acs.molpharmaceut.5b00081. [DOI] [PubMed] [Google Scholar]
- 112.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 113.Zhou X, Zhang X, Ye Y, Zhang T, Wang H, Ma Z. et al. Nanostructured lipid carriers used for oral delivery of oridonin: an effect of ligand modification on absorption. Int J Pharm. 2015;479(2):391–8. doi: 10.1016/j.ijpharm.2014.12.068. [DOI] [PubMed] [Google Scholar]
- 114.Han HK, Lee BJ, Lee HK. Enhanced dissolution and bioavailability of biochanin A via the preparation of solid dispersion: in vitro and in vivo evaluation. Int J Pharm. 2011;415(1-2):89–94. doi: 10.1016/j.ijpharm.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 115.Zhuang CY, Li N, Wang M, Zhang XN, Pan WS, Peng JJ. et al. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm. 2010;394(1-2):179–85. doi: 10.1016/j.ijpharm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 116.Jaiswal P, Gidwani B, Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):27–40. doi: 10.3109/21691401.2014.909822. [DOI] [PubMed] [Google Scholar]
- 117.Ding X, Xu X, Zhao Y, Zhang L, Yu Y, Huang F. et al. Tumor targeted nanostructured lipid carrier co-delivering paclitaxel and indocyanine green for laser triggered synergetic therapy of cancer. RSC Adv. 2017;7(56):35086–95. doi: 10.1039/c7ra06119f. [DOI] [Google Scholar]
- 118.Ranpise NS, Korabu SS, Ghodake VN. Second generation lipid nanoparticles (NLC) as an oral drug carrier for delivery of lercanidipine hydrochloride. Colloids Surf B Biointerfaces. 2014;116:81–7. doi: 10.1016/j.colsurfb.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 119.Liu D, Liu Z, Wang L, Zhang C, Zhang N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf B Biointerfaces. 2011;85(2):262–9. doi: 10.1016/j.colsurfb.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 120.Sun B, Luo C, Li L, Wang M, Du Y, Di D. et al. Core-matched encapsulation of an oleate prodrug into nanostructured lipid carriers with high drug loading capability to facilitate the oral delivery of docetaxel. Colloids Surf B Biointerfaces. 2016;143:47–55. doi: 10.1016/j.colsurfb.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 121.Wang Q, Cheng H, Zhou K, Wang L, Dong S, Wang D. et al. Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv. 2013;20(8):331–7. doi: 10.3109/10717544.2013.838716. [DOI] [PubMed] [Google Scholar]
- 122.How CW, Rasedee A, Manickam S, Rosli R. Tamoxifen-loaded nanostructured lipid carrier as a drug delivery system: characterization, stability assessment and cytotoxicity. Colloids Surf B Biointerfaces. 2013;112:393–9. doi: 10.1016/j.colsurfb.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 123.Shete H, Chatterjee S, De A, Patravale V. Long chain lipid based tamoxifen NLC Part II: pharmacokinetic, biodistribution and in vitro anticancer efficacy studies. Int J Pharm. 2013;454(1):584–92. doi: 10.1016/j.ijpharm.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 124.Zhang T, Chen J, Zhang Y, Shen Q, Pan W. Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur J Pharm Sci. 2011;43(3):174–9. doi: 10.1016/j.ejps.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 125.Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010;15(21-22):958–65. doi: 10.1016/j.drudis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 126.Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res. 1992;9(1):87–93. doi: 10.1023/a:1018987928936. [DOI] [PubMed] [Google Scholar]
- 127.Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12(11):1561–72. doi: 10.1023/a:1016268311867. [DOI] [PubMed] [Google Scholar]
- 128.Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106(1):15–23. doi: 10.1016/0378-5173(94)90271-2. [DOI] [Google Scholar]
- 129.Wei Y, Ye X, Shang X, Peng X, Bao Q, Liu M. et al. Enhanced oral bioavailability of silybin by a supersaturatable self-emulsifying drug delivery system (S-SEDDS) Colloids Surf A Physicochem Eng Asp. 2012;396:22–8. doi: 10.1016/j.colsurfa.2011.12.025. [DOI] [Google Scholar]
- 130.Patel D, Sawant KK. Self micro-emulsifying drug delivery system: formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr Drug Deliv. 2009;6(4):419–24. doi: 10.2174/156720109789000519. [DOI] [PubMed] [Google Scholar]
- 131.Singh B, Bandopadhyay S, Kapil R, Singh R, Katare O. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit Rev Ther Drug Carrier Syst. 2009;26(5):427–521. doi: 10.1615/critrevtherdrugcarriersyst.v26.i5.10. [DOI] [PubMed] [Google Scholar]
- 132.Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 133.Vishwakarma N, Jain A, Sharma R, Mody N, Vyas S, Vyas SP. Lipid-based nanocarriers for lymphatic transportation. AAPS PharmSciTech. 2019;20(2):83. doi: 10.1208/s12249-019-1293-3. [DOI] [PubMed] [Google Scholar]
- 134.Wasan KM. The role of lymphatic transport in enhancing oral protein and peptide drug delivery. Drug Dev Ind Pharm. 2002;28(9):1047–58. doi: 10.1081/ddc-120014573. [DOI] [PubMed] [Google Scholar]
- 135.Trevaskis NL, Porter CJ, Charman WN. The lymph lipid precursor pool is a key determinant of intestinal lymphatic drug transport. J Pharmacol Exp Ther. 2006;316(2):881–91. doi: 10.1124/jpet.105.094094. [DOI] [PubMed] [Google Scholar]
- 136.Porter CJ. Drug delivery to the lymphatic system. Crit Rev Ther Drug Carrier Syst. 1997;14(4):333–93. [PubMed] [Google Scholar]
- 137. Hauss DJ. Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluable Drugs. New York: Informa Healthcare; 2007.
- 138.Gupta S, Kesarla R, Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013;2013:848043. doi: 10.1155/2013/848043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Y, Chen C, Zheng J, Chen Z, Shi Q, Liu H. Development of a solid supersaturatable self-emulsifying drug delivery system of docetaxel with improved dissolution and bioavailability. Biol Pharm Bull. 2011;34(2):278–86. doi: 10.1248/bpb.34.278. [DOI] [PubMed] [Google Scholar]
- 140.Gürsoy RN, Çevik Ö. Design, characterization and in vitro evaluation of SMEDDS containing an anticancer peptide, linear LyP-1. Pharm Dev Technol. 2014;19(4):486–90. doi: 10.3109/10837450.2013.795170. [DOI] [PubMed] [Google Scholar]
- 141.Wu Z, Guo D, Deng L, Zhang Y, Yang Q, Chen J. Preparation and evaluation of a self-emulsifying drug delivery system of etoposide-phospholipid complex. Drug Dev Ind Pharm. 2011;37(1):103–12. doi: 10.3109/03639045.2010.495752. [DOI] [PubMed] [Google Scholar]
- 142.Lu JL, Wang JC, Zhao SX, Liu XY, Zhao H, Zhang X. et al. Self-microemulsifying drug delivery system (SMEDDS) improves anticancer effect of oral 9-nitrocamptothecin on human cancer xenografts in nude mice. Eur J Pharm Biopharm. 2008;69(3):899–907. doi: 10.1016/j.ejpb.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 143.Singh AK, Chaurasiya A, Awasthi A, Mishra G, Asati D, Khar RK. et al. Oral bioavailability enhancement of exemestane from self-microemulsifying drug delivery system (SMEDDS) AAPS PharmSciTech. 2009;10(3):906–16. doi: 10.1208/s12249-009-9281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thakkar H, Nangesh J, Parmar M, Patel D. Formulation and characterization of lipid-based drug delivery system of raloxifene-microemulsion and self-microemulsifying drug delivery system. J Pharm Bioallied Sci. 2011;3(3):442–8. doi: 10.4103/0975-7406.84463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Attivi D, Ajana I, Astier A, Demoré B, Gibaud S. Development of microemulsion of mitotane for improvement of oral bioavailability. Drug Dev Ind Pharm. 2010;36(4):421–7. doi: 10.3109/03639040903225083. [DOI] [PubMed] [Google Scholar]
- 146.Khalid N, Rahman NU, Löbenberg R, Akhtar M, Sarfaraz MK, Hajjar B. Design and evaluation of SMEDDS to enhance solubility and dissolution of anticancer drug using modified cylinder method. Lat Am J Pharm. 2017;36(4):647–57. [Google Scholar]
- 147.Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H. et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371(1-2):148–55. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 148.Wang YH, Wang SH, Yang RP, Ma XJ. [Design, optimization and quality evaluation of curcumin self-emulsifying drug delivery system (SEDDS)] Zhong Yao Cai. 2010;33(12):1933–7. [PubMed] [Google Scholar]
- 149.Huang Y, Tian R, Hu W, Jia Y, Zhang J, Jiang H. et al. A novel plug-controlled colon-specific pulsatile capsule with tablet of curcumin-loaded SMEDDS. Carbohydr Polym. 2013;92(2):2218–23. doi: 10.1016/j.carbpol.2012.11.105. [DOI] [PubMed] [Google Scholar]
- 150.Yan YD, Kim JA, Kwak MK, Yoo BK, Yong CS, Choi HG. Enhanced oral bioavailability of curcumin via a solid lipid-based self-emulsifying drug delivery system using a spray-drying technique. Biol Pharm Bull. 2011;34(8):1179–86. doi: 10.1248/bpb.34.1179. [DOI] [PubMed] [Google Scholar]
- 151.Pawar YB, Purohit H, Valicherla GR, Munjal B, Lale SV, Patel SB. et al. Novel lipid based oral formulation of curcumin: development and optimization by design of experiments approach. Int J Pharm. 2012;436(1-2):617–23. doi: 10.1016/j.ijpharm.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 152.Wu X, Xu J, Huang X, Wen C. Self-microemulsifying drug delivery system improves curcumin dissolution and bioavailability. Drug Dev Ind Pharm. 2011;37(1):15–23. doi: 10.3109/03639045.2010.489560. [DOI] [PubMed] [Google Scholar]
- 153.Yang S, Gursoy RN, Lambert G, Benita S. Enhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors. Pharm Res. 2004;21(2):261–70. doi: 10.1023/b:pham.0000016238.44452.f1. [DOI] [PubMed] [Google Scholar]
- 154.Gao P, Rush BD, Pfund WP, Huang T, Bauer JM, Morozowich W. et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–98. doi: 10.1002/jps.10511. [DOI] [PubMed] [Google Scholar]
- 155.Gursoy N, Garrigue JS, Razafindratsita A, Lambert G, Benita S. Excipient effects on in vitro cytotoxicity of a novel paclitaxel self-emulsifying drug delivery system. J Pharm Sci. 2003;92(12):2411–8. doi: 10.1002/jps.10501. [DOI] [PubMed] [Google Scholar]
- 156.Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212(2):233–46. doi: 10.1016/s0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 157.Cherniakov I, Domb AJ, Hoffman A. Self-nano-emulsifying drug delivery systems: an update of the biopharmaceutical aspects. Expert Opin Drug Deliv. 2015;12(7):1121–33. doi: 10.1517/17425247.2015.999038. [DOI] [PubMed] [Google Scholar]
- 158.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 159.Porter CJ, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60(6):673–91. doi: 10.1016/j.addr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 160.Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 161.Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annu Rev Physiol. 1983;45(1):651–77. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- 162.Jeevana JB, Sreelakshmi K. Design and evaluation of self-nanoemulsifying drug delivery system of flutamide. J Young Pharm. 2011;3(1):4–8. doi: 10.4103/0975-1483.76413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dimitrijevic D, Shaw AJ, Florence AT. Effects of some non-ionic surfactants on transepithelial permeability in Caco-2 cells. J Pharm Pharmacol. 2000;52(2):157–62. doi: 10.1211/0022357001773805. [DOI] [PubMed] [Google Scholar]
- 164.Quan YS, Hattori K, Lundborg E, Fujita T, Murakami M, Muranishi S. et al. Effectiveness and toxicity screening of various absorption enhancers using Caco-2 cell monolayers. Biol Pharm Bull. 1998;21(6):615–20. doi: 10.1248/bpb.21.615. [DOI] [PubMed] [Google Scholar]
- 165.Koga K, Ohyashiki T, Murakami M, Kawashima S. Modification of ceftibuten transport by the addition of non-ionic surfactants. Eur J Pharm Biopharm. 2000;49(1):17–25. doi: 10.1016/s0939-6411(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 166.Dudeja PK, Anderson KM, Harris JS, Buckingham L, Coon JS. Reversal of multidrug-resistance phenotype by surfactants: relationship to membrane lipid fluidity. Arch Biochem Biophys. 1995;319(1):309–15. doi: 10.1006/abbi.1995.1298. [DOI] [PubMed] [Google Scholar]
- 167.Collnot EM, Baldes C, Wempe MF, Kappl R, Hüttermann J, Hyatt JA. et al. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm. 2007;4(3):465–74. doi: 10.1021/mp060121r. [DOI] [PubMed] [Google Scholar]
- 168.Peters WH, Kremers PG. Cytochromes P-450 in the intestinal mucosa of man. Biochem Pharmacol. 1989;38(9):1535–8. doi: 10.1016/0006-2952(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 169.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270(1):414–23. [PubMed] [Google Scholar]
- 170.de Waziers I, Cugnenc PH, Yang CS, Leroux JP, Beaune PH. Cytochrome P 450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990;253(1):387–94. [PubMed] [Google Scholar]
- 171.Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90(5):1871–8. doi: 10.1172/jci116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 173.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80(4):1029–36. doi: 10.1172/jci113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gupta SK, Manfro RC, Tomlanovich SJ, Gambertoglio JG, Garovoy MR, Benet LZ. Effect of food on the pharmacokinetics of cyclosporine in healthy subjects following oral and intravenous administration. J Clin Pharmacol. 1990;30(7):643–53. doi: 10.1002/j.1552-4604.1990.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 175.Wu CY, Benet LZ, Hebert MF, Gupta SK, Rowland M, Gomez DY. et al. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: studies with cyclosporine. Clin Pharmacol Ther. 1995;58(5):492–7. doi: 10.1016/0009-9236(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 176.Akhtar N, Talegaonkar S, Khar RK, Jaggi M. Self-nanoemulsifying lipid carrier system for enhancement of oral bioavailability of etoposide by P-glycoprotein modulation: in vitro cell line and in vivo pharmacokinetic investigation. J Biomed Nanotechnol. 2013;9(7):1216–29. doi: 10.1166/jbn.2013.1613. [DOI] [PubMed] [Google Scholar]
- 177.Seo YG, Kim DH, Ramasamy T, Kim JH, Marasini N, Oh YK. et al. Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int J Pharm. 2013;452(1-2):412–20. doi: 10.1016/j.ijpharm.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 178.Elnaggar YS, El-Massik MA, Abdallah OY. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: design and optimization. Int J Pharm. 2009;380(1-2):133–41. doi: 10.1016/j.ijpharm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 179.Khurana RK, Beg S, Burrow AJ, Vashishta RK, Katare OP, Kaur S. et al. Enhancing biopharmaceutical performance of an anticancer drug by long chain PUFA based self-nanoemulsifying lipidic nanomicellar systems. Eur J Pharm Biopharm. 2017;121:42–60. doi: 10.1016/j.ejpb.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 180.Christie WW. Advances in Lipid Methodology Volume 4. Oily Press. 1997 [Google Scholar]
- 181.Lambert DM. Rationale and applications of lipids as prodrug carriers. Eur J Pharm Sci. 2000;11 Suppl 2:S15–27. doi: 10.1016/s0928-0987(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 182.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 183.Zaro JL. Lipid-based drug carriers for prodrugs to enhance drug delivery. AAPS J. 2015;17(1):83–92. doi: 10.1208/s12248-014-9670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kuznetsova L, Chen J, Sun L, Wu X, Pepe A, Veith JM. et al. Syntheses and evaluation of novel fatty acid-second-generation taxoid conjugates as promising anticancer agents. Bioorg Med Chem Lett. 2006;16(4):974–7. doi: 10.1016/j.bmcl.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 185.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 186.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46(1-3):149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 187.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR Jr, Banaszak Holl MM. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chem Biol. 2007;14(1):107–15. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 188.Jain SK, Chaurasiya A, Gupta Y, Jain A, Dagur P, Joshi B. et al. Development and characterization of 5-FU bearing ferritin appended solid lipid nanoparticles for tumour targeting. J Microencapsul. 2008;25(5):289–97. doi: 10.1080/02652040701799598. [DOI] [PubMed] [Google Scholar]
- 189.Yang XY, Li YX, Li M, Zhang L, Feng LX, Zhang N. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013;334(2):338–45. doi: 10.1016/j.canlet.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 190.Liu B, Han L, Liu J, Han S, Chen Z, Jiang L. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int J Nanomedicine. 2017;12:955–68. doi: 10.2147/ijn.s115136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alshahrani SM, Alshetaili AS, Alalaiwe A, Alsulays BB, Anwer MK, Al-Shdefat R. et al. Anticancer efficacy of self-nanoemulsifying drug delivery system of sunitinib malate. AAPS PharmSciTech. 2018;19(1):123–33. doi: 10.1208/s12249-017-0826-x. [DOI] [PubMed] [Google Scholar]
- 192.Koziara JM, Lockman PR, Allen DD, Mumper RJ. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release. 2004;99(2):259–69. doi: 10.1016/j.jconrel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 193.Liu D, Liu F, Liu Z, Wang L, Zhang N. Tumor specific delivery and therapy by double-targeted nanostructured lipid carriers with anti-VEGFR-2 antibody. Mol Pharm. 2011;8(6):2291–301. doi: 10.1021/mp200402e. [DOI] [PubMed] [Google Scholar]
- 194.Dhumal DM, Kothari PR, Kalhapure RS, Akamanchi KG. Self-microemulsifying drug delivery system of curcumin with enhanced solubility and bioavailability using a new semi-synthetic bicephalous heterolipid: in vitro and in vivo evaluation. RSC Adv. 2015;5(110):90295–306. doi: 10.1039/c5ra18112g. [DOI] [Google Scholar]
- 195.Choo GH, Park SJ, Hwang SJ, Kim MS. Formulation and in vivo evaluation of a self-microemulsifying drug delivery system of dutasteride. Drug Res (Stuttg) 2013;63(4):203–9. doi: 10.1055/s-0033-1334965. [DOI] [PubMed] [Google Scholar]
- 196.Shah M, Ullah N, Choi MH, Kim MO, Yoon SC. Amorphous amphiphilic P(3HV-co-4HB)-b-mPEG block copolymer synthesized from bacterial copolyester via melt transesterification: nanoparticle preparation, cisplatin-loading for cancer therapy and in vitro evaluation. Eur J Pharm Biopharm. 2012;80(3):518–27. doi: 10.1016/j.ejpb.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 197.Danhier F, Lecouturier N, Vroman B, Jérôme C, Marchand-Brynaert J, Feron O. et al. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Control Release. 2009;133(1):11–7. doi: 10.1016/j.jconrel.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 198.Li R, Wu R, Zhao L, Wu M, Yang L, Zou H. P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano. 2010;4(3):1399–408. doi: 10.1021/nn9011225. [DOI] [PubMed] [Google Scholar]
- 199.Varan C, Bilensoy E. Development of implantable hydroxypropyl-beta-cyclodextrin coated polycaprolactone nanoparticles for the controlled delivery of docetaxel to solid tumors. J Incl Phenom Macrocycl Chem. 2014;80(1):9–15. doi: 10.1007/s10847-014-0422-6. [DOI] [Google Scholar]
- 200.Chen J, Shi M, Liu P, Ko A, Zhong W, Liao W. et al. Reducible polyamidoamine-magnetic iron oxide self-assembled nanoparticles for doxorubicin delivery. Biomaterials. 2014;35(4):1240–8. doi: 10.1016/j.biomaterials.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 201.Brown SD, Nativo P, Smith JA, Stirling D, Edwards PR, Venugopal B. et al. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc. 2010;132(13):4678–84. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]