Summary
Background
For patients with advanced hepatocellular carcinoma, sorafenib is the only approved drug worldwide, and outcomes remain poor. We aimed to assess the safety and efficacy of nivolumab, a programmed cell death protein-1 (PD-1) immune checkpoint inhibitor, in patients with advanced hepatocellular carcinoma with or without chronic viral hepatitis.
Methods
We did a phase 1/2, open-label, non-comparative, dose escalation and expansion trial (CheckMate 040) of nivolumab in adults (≥18 years) with histologically confirmed advanced hepatocellular carcinoma with or without hepatitis C or B (HCV or HBV) infection. Previous sorafenib treatment was allowed. A dose-escalation phase was conducted at seven hospitals or academic centres in four countries or territories (USA, Spain, Hong Kong, and Singapore) and a dose-expansion phase was conducted at an additional 39 sites in 11 countries (Canada, UK, Germany, Italy, Japan, South Korea, Taiwan). At screening, eligible patients had Child-Pugh scores of 7 or less (Child-Pugh A or B7) for the dose-escalation phase and 6 or less (Child-Pugh A) for the dose-expansion phase, and an Eastern Cooperative Oncology Group performance status of 1 or less. Patients with HBV infection had to be receiving effective antiviral therapy (viral load <100 IU/mL); antiviral therapy was not required for patients with HCV infection. We excluded patients previously treated with an agent targeting T-cell costimulation or checkpoint pathways. Patients received intravenous nivolumab 0·1–10 mg/kg every 2 weeks in the dose-escalation phase (3+3 design). Nivolumab 3 mg/kg was given every 2 weeks in the dose-expansion phase to patients in four cohorts: sorafenib untreated or intolerant without viral hepatitis, sorafenib progressor without viral hepatitis, HCV infected, and HBV infected. Primary endpoints were safety and tolerability for the escalation phase and objective response rate (Response Evaluation Criteria In Solid Tumors version 1.1) for the expansion phase. This study is registered with ClinicalTrials.gov, number NCT01658878.
Findings
Between Nov 26, 2012, and Aug 8, 2016, 262 eligible patients were treated (48 patients in the dose-escalation phase and 214 in the dose-expansion phase). 202 (77%) of 262 patients have completed treatment and follow-up is ongoing. During dose escalation, nivolumab showed a manageable safety profile, including acceptable tolerability. In this phase, 46 (96%) of 48 patients discontinued treatment, 42 (88%) due to disease progression. Incidence of treatment-related adverse events did not seem to be associated with dose and no maximum tolerated dose was reached. 12 (25%) of 48 patients had grade 3/4 treatment-related adverse events. Three (6%) patients had treatment-related serious adverse events (pemphigoid, adrenal insufficiency, liver disorder). 30 (63%) of 48 patients in the dose-escalation phase died (not determined to be related to nivolumab therapy). Nivolumab 3 mg/kg was chosen for dose expansion. The objective response rate was 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase.
Interpretation
Nivolumab had a manageable safety profile and no new signals were observed in patients with advanced hepatocellular carcinoma. Durable objective responses show the potential of nivolumab for treatment of advanced hepatocellular carcinoma.
Funding
Bristol-Myers Squibb.
Introduction
Worldwide, liver cancer accounts for more than 850 000 new cancer cases annually, and approximately 90% of these are hepatocellular carcinoma.1,2 Chronic infection with hepatitis C virus (HCV) or hepatitis B virus (HBV) is the leading cause of hepatocellular carcinoma.3 Hepatocellular carcinoma is often diagnosed at advanced stages of disease for which highly effective therapies are insufficient. At present, sorafenib, a small-molecule multikinase inhibitor, is the only evidence-based systemic treatment option for patients with advanced hepatocellular carcinoma.2,4,5 In previously untreated patients with advanced disease, the median overall survival was 10·7 months in those treated with sorafenib and 7·9 months in those who received placebo (hazard ratio [HR] 0·69, 95% CI 0·55–0·87; p<0·001).6 In selected patients who tolerated sorafenib but progressed while on therapy, another multikinase inhibitor, regorafenib, has been reported to provide an overall survival benefit compared with placebo (10·6 months vs 7·8 months; HR 0·62, 95% CI 0·50–0·78; p<0·001).7
Immunotherapies that inhibit the immune checkpoint interaction between programmed cell death protein-1 (PD-1) and programmed death-ligand 1 (PD-L1) have shown substantial survival benefit in some patients with metastatic carcinomas of multiple tissue origins.8–11 The presence of tumour-infiltrating lymphocytes expressing PD-1 in hepatocellular carcinoma lesions and their correlation with outcome suggest that immunotherapeutic approaches might be useful in this setting.12–15
Nivolumab is a fully human immunoglobulin G4 monoclonal antibody that disrupts PD-1 immune checkpoint signalling and thereby restores the antitumour activity of otherwise suppressed effector T cells. CheckMate 040 is an ongoing, global, phase 1/2 study of nivolumab in patients with advanced hepatocellular carcinoma with or without chronic viral hepatitis who were previously treated or untreated with sorafenib. In this first report of a PD-1 checkpoint inhibitor for the treatment of advanced hepatocellular carcinoma, we detail nivolumab safety and efficacy results from the dose-escalation and dose-expansion phases of CheckMate 040.
Methods
Study design and participants
We did a multicentre, non-comparative, open-label, phase 1/2 study in patients with advanced hepatocellular carcinoma with or without chronic viral hepatitis (HCV or HBV) to evaluate the safety and efficacy of nivolumab as a monotherapy (CheckMate 040). The dose-escalation phase was conducted at seven hospitals or academic centres in four countries or territories (USA, Spain, Hong Kong, and Singapore) and the dose-expansion phase was conducted at 39 sites in 11 countries (Canada, UK, Germany, Italy, Japan, South Korea, Taiwan, and the countries or territories involved in dose escalation).
Eligible patients were at least 18 years old with histologically confirmed advanced hepatocellular carcinoma (not amenable to curative surgery or local treatment); use of archival tissue samples was allowed. Fresh tumour biopsy was required at baseline if no other record of histological diagnosis was available. Patients in the dose-escalation phase and patients in the HCV-infected and HBV-infected cohorts of the expansion phase included those whose disease progressed while receiving at least one previous line of systemic therapy, including sorafenib, or who were intolerant of or refused sorafenib treatment. Patients were also required to have Child-Pugh scores of 7 or less (Child-Pugh A or B7) for the dose-escalation phase and 6 or less (Child-Pugh A) for the dose-expansion phase at screening, and an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or less. Patients with HBV infection were required to be receiving effective antiviral therapy and have a viral load less than 100 IU/mL at screening; antiviral therapy was not required for patients with HCV infection. Patients who had previously been treated with an agent targeting T-cell costimulation or checkpoint pathways (including those targeting PD-1, PD-L1 or PD-L2, CD137, or cytotoxic T-lymphocyte antigen [CTLA-4]) were excluded. Additional eligibility criteria are in the appendix. All patients provided written informed consent, and the study protocol and amendments were approved by each site’s institutional review board or independent ethics committee.
Procedures
Patients received intravenous nivolumab every 2 weeks. In the dose-escalation phase, patients were enrolled into three cohorts on the basis of hepatocellular carcinoma aetiology (without viral hepatitis, HCV-infected, and HBV-infected). Across these cohorts, sequential patient groups (of up to six patients per dose level for doses 0·1–3·0 mg/kg and up to 13 patients for 10 mg/kg) received the following doses of nivolumab: 0·1 mg/kg (patients with HBV infection only), 0·3 mg/kg, 1·0 mg/kg, 3·0 mg/kg, or 10 mg/kg (patients without viral hepatitis [ie, uninfected] only) in a 3+3 design with the intention of determining the maximum-tolerated dose. Dose-limiting toxicities were determined on the basis of the incidence and intensity of adverse events occurring up to 2 weeks after the third nivolumab dose. Patients were treated until a confirmed complete response was achieved (dose-escalation phase only) or until disease progression or unacceptable toxicity occurred.
Safety assessments were done continuously during treatment and up to 100 days after the last dose or until all treatment-related adverse events were resolved to baseline or deemed irreversible by the investigator; adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; version 4.03). Patients were followed up for survival every 3 months.
We analysed investigator-assessed tumour response using the Response Evaluation Criteria In Solid Tumors (RECIST; version 1.1) for key study endpoints.16 Exploratory endpoints included tumour assessments by modified RECIST (mRECIST; assessed by blinded independent central review). RECIST version 1.1 was used for assessment of the primary endpoint because it is well established and provides a more conservative estimation of response than mRECIST. Assessment by RECIST version 1.1 also allows for comparisons of response data with pivotal studies in patients with hepatocellular carcinoma (eg, sorafenib/SHARP trial).6 mRECIST has not been prospectively validated and has not been evaluated for immuno-oncology therapies. Tumour biopsies collected at baseline were retrospectively used for analysis of PD-L1 expression by immunohistochemistry. Details on tumour assessments and measurement of tumour PD-L1 expression are in the appendix. In patients infected with HCV or HBV, HCV RNA and HBV surface antigen (HBsAg), respectively, were measured from patient sera at baseline and on treatment. Serum anti-HBs levels were also measured.
We assessed patient-reported health status in the dose-expansion phase using the three-level version of the European Quality of Life-5 Dimensions utility index (EQ-5D-3L) and visual analogue scale (EQ-5D-VAS).17 Patients completed the EQ-5D-3L at baseline and every 6 weeks through week 25 while on treatment. The analysis population included those who had a baseline EQ-5D-3L assessment and at least one post-baseline assessment. Additional details on the methodology for assessing patient-reported outcomes are in the appendix.
Outcomes
The primary endpoint of the dose-escalation phase was safety and tolerability, based on incidence of adverse events, serious adverse events, adverse events leading to discontinuation, and deaths. The primary endpoint of the dose-expansion phase was objective response rate. Key secondary endpoints included objective response rate (dose-escalation phase only), complete response rate, disease control rate, duration of response, time to response, time to progression, progression-free survival, overall survival, and response stratified by PD-L1 expression. Additionally, patient-reported quality of life measures and tumour response evaluation using mRECIST were exploratory endpoints. Secondary and exploratory endpoints not reported here are provided in the appendix.
Statistical analysis
We used descriptive statistics to characterise safety analyses for all treated patients and to characterise patient-reported quality of life outcomes in patients treated in the dose-expansion phase. We estimated 95% CIs using the Clopper-Pearson method for objective response rate and the conventional Wald method for patient-reported outcomes. We used Kaplan-Meier methodology to determine medians and 95% CIs for duration of response and overall survival. We determined sample sizes for each dose in the dose-escalation phase (3–13 patients) on the basis of observed toxicities, not statistical considerations. For the dose-expansion phase, we chose sample sizes of approximately 50 treated patients per cohort to improve estimations of efficacy. With a minimum of 50 patients, the lower bound of the 95% CI for a hypothetical response rate of 20% would be 10%.
This study is registered with ClinicalTrials.gov, number NCT01658878.
Role of the funding source
The study was designed by the authors in collaboration with the funder (Bristol-Myers Squibb). The authors and funder were responsible for data collection, and the sponsor was responsible for data analysis. The authors and funder were involved in data interpretation, development of the report, and the decision to submit. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
The cutoff date for this analysis was Aug 8, 2016. Between Nov 26, 2012, and Aug 8, 2016, 262 patients with advanced hepatocellular carcinoma with or without HCV or HBV infection were treated: 48 patients in the dose-escalation phase and 214 in the dose-expansion phase (figure 1). Intravenous nivolumab monotherapy doses were 0·1–10 mg/kg every 2 weeks in the dose-escalation phase, and cohorts included 23 patients without viral hepatitis, ten patients with HCV infection, and 15 patients with HBV infection. Across these three cohorts, six patients were assigned to nivolumab 0·1 mg/kg, nine patients to 0·3 mg/kg, ten patients to 1 mg/kg, ten patients to 3 mg/kg, and 13 patients to 10 mg/kg, every 2 weeks. Only patients in the cohort without viral hepatitis were assigned to the maximum dose of 10 mg/kg.
Figure 1: Trial design.
HCV=hepatitis C virus. HBV=hepatitis B virus.
Patient demographics, baseline disease characteristics, and previous treatments are presented in table 1. In the dose-escalation phase, the overall median age was 62 years (IQR 55–69; table 1). Patients were heavily pretreated and 37 (77%) of 48 patients had previously been treated with sorafenib. Extrahepatic metastases were present in 34 (71%) patients and vascular invasion was present in 19 (40%) patients; all patients were reported as Child-Pugh class A with Child-Pugh scores of 5 or 6 at baseline.
Table 1:
Patient demographics, baseline characteristics, and previous treatment
| Escalation phase |
Expansion phase |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Uninfected (n=23) | HCV infected (n=10) | HBV infected (n=15) | All patients (n=48) | Uninfected untreated/intolerant (n=56) | Uninfected progressor (n=57) | HCV infected (n=50) | HBV infected (n=51) | All patients (n=214) | |
| Median age (years) | 61 (54–72) | 67 (60–74) | 62 (46–66) | 62 (55–69) | 66 (59–71) | 65 (60–71) | 65 (61–73) | 55 (42–66) | 64 (56–70) |
| ≥65 years | 8 (35%) | 6 (60%) | 6 (40%) | 20 (42%) | 33 (59%) | 29 (51%) | 25 (50%) | 13 (25%) | 100 (47%) |
| Sex | |||||||||
| Female | 6 (26%) | 4 (40%) | 2 (13%) | 12 (25%) | 8 (14%) | 15 (26%) | 8 (16%) | 12 (24%) | 43 (20%) |
| Male | 17 (74%) | 6 (60%) | 13 (87%) | 36 (75%) | 48 (86%) | 42 (74%) | 42 (84%) | 39 (76%) | 171 (80%) |
| Race | |||||||||
| White | 19 (83%) | 8 (80%) | 1 (7%) | 28 (58%) | 38 (68%) | 34 (60%) | 29 (58%) | 4 (8%) | 105 (49%) |
| Asian | 2 (9%) | 2 (20%) | 14 (93%) | 18 (38%) | 16 (29%) | 22 (39%) | 18 (36%) | 45 (88%) | 101 (47%) |
| Black | 2 (9%) | 0 | 0 | 2 (4%) | 1 (2%) | 1 (2%) | 2 (4%) | 2 (4%) | 6 (3%) |
| Other | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 1 (2%) | 0 | 2 (1%) |
| ECOG performance status 1* | 9 (39%) | 4 (40%) | 6 (40%) | 19 (40%) | 16 (29%) | 22 (39%) | 15 (30%) | 24 (47%) | 77 (36%) |
| Extrahepatic metastases | 16 (70%) | 6 (60%) | 12 (80%) | 34 (71%) | 36 (64%) | 41 (72%) | 25 (50%) | 42 (82%) | 144 (67%) |
| Vascular invasion | 8 (35%) | 5 (50%) | 6 (40%) | 19 (40%) | 13 (23%) | 18 (32%) | 17 (34%) | 15 (29%) | 63 (29%) |
| Child-Pugh score | |||||||||
| 5 | 19 (83%) | 8 (80%) | 14 (93%) | 41 (85%) | 43 (77%) | 37 (65%) | 27 (54%) | 42 (82%) | 149 (70%) |
| 6 | 4 (17%) | 2 (20%) | 1 (7%) | 7 (15%) | 12 (21%) | 20 (35%) | 20 (40%) | 9 (18%) | 61 (29%) |
| 7–9 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 3 (6%) | 0 | 4 (2%) |
| α-fetoprotein ≥400 μg/L† | 6 (26%) | 3 (30%) | 6 (40%) | 15 (31%) | 15 (27%) | 22 (39%) | 17 (34%) | 25 (49%) | 79 (37%) |
| Previous treatment | |||||||||
| Surgical resection | 15 (65%) | 8 (80%) | 13 (87%) | 36 (75%) | 34 (61%) | 36 (63%) | 18 (36%) | 40 (78%) | 128 (60%) |
| Radiotherapy‡ | 6 (26%) | 2 (20%) | 2 (13%) | 10 (21%) | 9 (16%) | 17 (30%) | 4 (8%) | 11 (22%) | 41 (19%) |
| Local treatment for HCC§ | 8 (35%) | 6 (60%) | 10 (67%) | 24 (50%) | 24 (43%) | 28 (49%) | 25 (50%) | 40 (78%) | 117 (55%) |
| Systemic therapy | 19 (83%) | 6 (60%) | 15 (100%) | 40 (83%) | 23 (41%) | 57 (100%) | 32 (64%) | 47 (92%) | 159 (74%) |
| Sorafenib¶ | 17 (74%) | 5 (50%) | 15 (100%) | 37 (77%) | 15 (27%) | 57 (100%) | 30 (60%) | 43 (84%) | 145 (68%) |
Data are median (IQR) or n (%). HCV=hepatitis C virus. HBV=hepatitis B virus. HCC=hepatocellular carcinoma. ECOG=Eastern Cooperative Oncology Group.
All patients had a baseline ECOG performance status of 0 or 1.
Baseline α-fetoprotein levels were not available for ten patients; dose escalation (n=1), dose expansion (n=9).
Internal or external, and could include radioembolisation.
Includes transcatheter arterial chemoembolisation and transcatheter embolisation.
Reasons for previous treatment failure with sorafenib therapy included disease progression (165 [63%] of 262) and sorafenib intolerance (13 [5%] of 262); four (2%) of 262 patients experienced sorafenib treatment failure due to other reasons.
In the dose-escalation phase, 46 (96%) of 48 patients discontinued treatment; 42 (88%) discontinued due to disease progression (table 2). Two patients (4%; both without viral hepatitis) discontinued after achieving a complete response (per study protocol) and entered the follow-up period. Other reasons for discontinuation included study drug-related toxicity in one patient and adverse events unrelated to treatment in another patient. After discontinuation of nivolumab, 23 (48%) patients were treated with a subsequent therapy (appendix). At the time of data cutoff, two of the 48 patients in the dose-escalation phase were continuing treatment with nivolumab.
Table 2:
Patient disposition at data cutoff (Aug 8, 2016)
| Escalation phase |
Expansion phase |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Uninfected (n=23) | HCV infected (n=10) | HBV infected (n=15) | All patients (n=48) | Uninfected untreated/intolerant (n=56) | Uninfected progressor (n=57) | HCV infected (n=50) | HBV infected (n=51) | All patients (n=214) | |
| Continuing treatment | 1 (4%) | 1 (10%) | 0 | 2 (4%) | 20 (36%) | 10 (18%) | 14 (28%) | 14 (27%) | 58 (27%) |
| Discontinued treatment | 22 (96%) | 9 (90%) | 15 (100%) | 46 (96%) | 36 (64%) | 47 (82%) | 36 (72%) | 37 (73%) | 156 (73%) |
| Disease progression | 18 (78%) | 9 (90%) | 15 (100%) | 42 (88%) | 29 (52%) | 42 (74%) | 24 (48%) | 37 (73%) | 132 (62%) |
| Study drug toxicity | 1 (4%) | 0 | 0 | 1 (2%) | 4 (7%) | 0 | 4 (8%) | 0 | 8 (4%) |
| Unrelated adverse event | 1 (4%) | 0 | 0 | 1 (2%) | 0 | 4 (7%) | 4 (8%) | 0 | 8 (4%) |
| Patient decision* | 0 | 0 | 0 | 0 | 2 (4%) | 1 (2%) | 3 (6%) | 0 | 6 (3%) |
| Complete response | 2 (9%) | 0 | 0 | 2 (4%) | 0 | 0 | 0 | 0 | 0 |
| Other/not reported | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 1 (2%) | 0 | 2 (1%) |
Data are n (%). HCV=hepatitis C virus. HBV=hepatitis B virus.
Includes patients who withdrew consent.
One dose-limiting toxicity (grade 2 hepatic impairment) was reported in a patient in the cohort without viral hepatitis who received 10 mg/kg, which resolved within 7 days. A maximum tolerated dose was not reached. Grade 3/4 treatment-related adverse events occurred in 12 (25%) of 48 patients (table 3). Treatment-related adverse events that occurred in more than 10% of patients were rash in 11 (23%) patients, aspartate aminotransferase (AST) increase in ten (21%) patients, alanine aminotransferase (ALT) increase in seven (15%) patients, lipase increase in ten (21%) patients, amylase increase in nine (19%) patients, and pruritus in nine (19%) patients. Treatment-related serious adverse events were reported in three (6%) patients (pemphigoid [n=1], adrenal insufficiency [n=1], liver disorder [n=1]). Grade 3/4 select adverse events, those with a potential inflammatory mechanism requiring more frequent monitoring, were adrenal insufficiency (n=1), diarrhoea (n=1), hepatitis (n=2), infusion hypersensitivity (n=1), and acute kidney injury (n=1; appendix). One patient without viral hepatitis who received nivolumab 3 mg/kg discontinued due to treatment-related ALT and AST increases without concomitant changes in liver function. 30 (63%) of 48 patients in the dose-escalation phase died, and no deaths were determined to be related to nivolumab therapy.
Table 3:
Safety and tolerability of nivolumab in the dose-escalation phase
| 0·1 mg/kg (n=6) |
0·3 mg/kg (n=9) |
1 mg/kg (n=10) |
3 mg/kg (n=10) |
10 mg/kg (n=13) |
All patients (n=48) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Treatment-related serious AEs | 1 (17%)* | 1 (17%)* | 1 (11%)† | 1 (11%)† | 0 | 0 | 0 | 0 | 1 (8%)‡ | 0 | 3 (6%) | 2 (4%) |
| AEs leading to discontinuation | 0 | 0 | 1 (11%)§ | 1 (11%)§ | 0 | 0 | 1 (10%)¶ | 1 (10%)¶ | 1 (8%)∥ | 1 (8%)∥ | 3 (6%) | 3 (6%) |
| Treatment-related deaths | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patients with a treatment-related AE | 4 (67%) | 2 (33%) | 8 (89%) | 3 (33%) | 8 (80%) | 5 (50%) | 9 (90%) | 2 (20%) | 11 (85%) | 0 | 40 (83%) | 12 (25%) |
| Treatment-related AEs** | ||||||||||||
| Rash | 1 (17%) | 0 | 2 (22%) | 0 | 2 (20%) | 0 | 2 (20%) | 0 | 4 (31%) | 0 | 11 (23%) | 0 |
| Pruritus | 2 (33%) | 0 | 3 (33%) | 0 | 0 | 0 | 1 (10%) | 0 | 3 (23%) | 0 | 9 (19%) | 0 |
| Diarrhoea | 0 | 0 | 3 (33%) | 0 | 0 | 0 | 1 (10%) | 0 | 1 (8%) | 0 | 5 (10%) | 0 |
| Decreased appetite | 1 (17%) | 0 | 2 (22%) | 0 | 1 (10%) | 0 | 0 | 0 | 1 (8%) | 0 | 5 (10%) | 0 |
| Fatigue | 1 (17%) | 1 (17%) | 2 (22%) | 0 | 1 (10%) | 0 | 0 | 0 | 0 | 0 | 4 (8%) | 1 (2%) |
| Asthenia | 0 | 0 | 1 (11%) | 0 | 0 | 0 | 1 (10%) | 0 | 1 (8%) | 0 | 3 (6%) | 0 |
| Weight decreased | 0 | 0 | 1 (11%) | 0 | 0 | 0 | 0 | 0 | 2 (15%) | 0 | 3 (6%) | 0 |
| Nausea | 0 | 0 | 1 (11%) | 0 | 0 | 0 | 1 (10%) | 0 | 1 (8%) | 0 | 3 (6%) | 0 |
| Dry mouth | 0 | 0 | 1 (11%) | 0 | 1 (10%) | 0 | 0 | 0 | 1 (8%) | 0 | 3 (6%) | 0 |
| Laboratory treatment-related AEs** | ||||||||||||
| AST increase | 0 | 0 | 2 (22%) | 2 (22%) | 3 (30%) | 2 (20%) | 1 (10%) | 1 (10%) | 4 (31%) | 0 | 10 (21%) | 5 (10%) |
| ALT increase | 0 | 0 | 2 (22%) | 2 (22%) | 1 (10%) | 0 | 2 (20%) | 1 (10%) | 2 (15%) | 0 | 7 (15%) | 3 (6%) |
| Lipase increase | 1 (17%) | 1 (17%) | 1 (11%) | 0 | 4 (40%) | 4 (40%) | 2 (20%) | 1 (10%) | 2 (15%) | 0 | 10 (21%) | 6 (13%) |
| Amylase increase | 1 (17%) | 0 | 0 | 0 | 4 (40%) | 1 (10%) | 2 (20%) | 1 (10%) | 2 (15%) | 0 | 9 (19%) | 2 (4%) |
| Anaemia | 0 | 0 | 1 (11%) | 0 | 1 (10%) | 1 (10%) | 0 | 0 | 2 (15%) | 0 | 4 (8%) | 1 (2%) |
| Hypoalbuminaemia | 0 | 0 | 1 (11%) | 0 | 1 (10%) | 0 | 0 | 0 | 1 (8%) | 0 | 3 (6%) | 0 |
| Hyponatraemia | 0 | 0 | 0 | 0 | 2 (20%) | 0 | 0 | 0 | 1 (8%) | 0 | 3 (6%) | 0 |
Data are n (%). AE=adverse event. AST=aspartate aminotransferase. ALT=alanine aminotransferase.
Pemphigoid (n=1).
Adrenal insufficiency (n=1).
Liver disorder (n=1).
Malignant neoplasm progression (n=1).
Grade 3 ALT increase (n=1), grade 2 AST increase.
Grade 3 blood bilirubin increase (n=1).
Treatment-related AEs reported in ≥5% of all patients, any grade.
The overall objective response rate was 15% (95% CI 6–28; appendix) in the dose-escalation phase, including three complete responses and four partial responses. Responses occurred early in treatment; of the seven patients who achieved an objective response, five responded within 3 months of treatment initiation (figure 2). The disease control rate was 58% (95% CI 43–72) and the median time to progression was 3·4 months (95% CI 1·6–6·9). The median duration of response was 17 months (95% CI 6–24) and the 6-month and 9-month overall survival rates were both 66% (95% CI 51–78). Median overall survival for patients in the dose-escalation phase was 15·0 months (95% CI 9·6–20·2).
Figure 2: Time to response and duration of response.
Duration of response (months) to nivolumab for the 49 patients who achieved a complete or partial response in the dose-escalation or dose-expansion phases. HCV=hepatitis C virus. HBV=hepatitis B virus.
On the basis of the results from the dose-escalation phase and from studies of nivolumab in other tumour types,18 a dose of 3 mg/kg was selected for the dose-expansion phase. 214 patients with advanced hepatocellular carcinoma were treated in the dose-expansion phase in four cohorts: 56 patients were not infected with HCV or HBV and had not been treated with sorafenib previously or were intolerant, 57 had disease progression on sorafenib, 50 patients were infected with HCV, and 51 were infected with HBV (figure 1). Patients enrolled in the dose-expansion phase had comparable demographics and baseline disease characteristics to those in the dose-escalation phase (table 1). 145 (68%) of 214 patients had previously been treated with sorafenib.
As of Aug 8, 2016, 58 (27%) of 214 patients enrolled in the dose-expansion phase were continuing treatment. Disease progression was the most common reason for discontinuation, occurring in 132 (62%) of 214 patients. Eight patients (4%) discontinued after experiencing study drug toxicity (table 2).
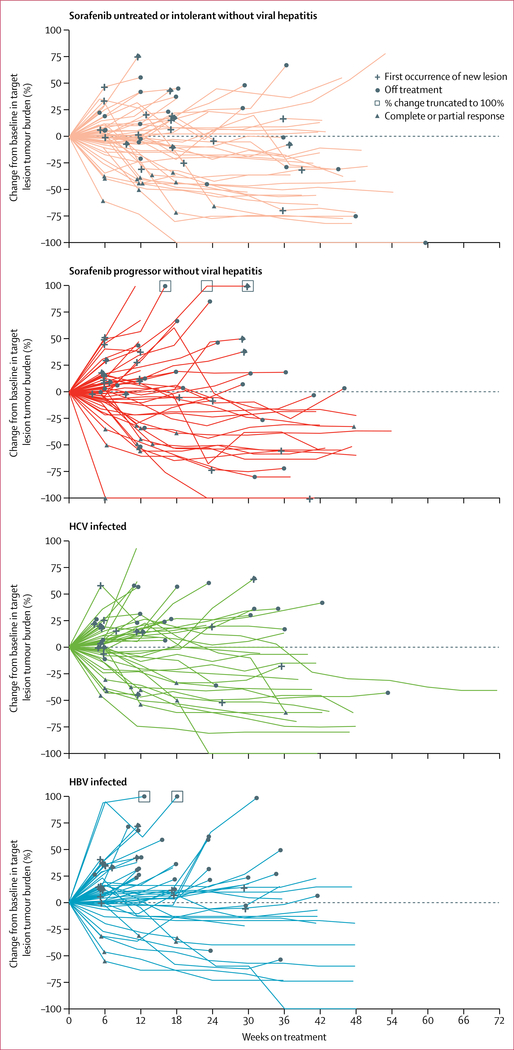
An objective response was observed in 42 patients (20%; 95% CI 15–26) who received nivolumab 3 mg/kg every 2 weeks in the dose-expansion phase (table 4). Objective responses included three complete responses and 39 partial responses. Stable disease was observed in 96 (45%) patients, and thus disease control was observed in 138 patients (64%). Among the 202 patients who were evaluable and had at least one post-baseline target lesion assessment, substantial reductions in tumour burden were observed in all cohorts (figure 3). Best reductions from baseline in tumour burden are shown in figure 4. Most of the objective responses occurred before 3 months (29 of 42; 69%), similar to the time-to-response profile observed in the dose-escalation phase (figure 2). 28 (67%) of the 42 patients with a response had ongoing responses at the time of data cutoff. The median duration of response was 9·9 months (95% CI 8·3 to not estimable [NE]). Most disease stabilisations lasted at least 6 months, as reported in 79 of 138 patients (57%) with disease control. In the dose-expansion phase, the median time to progression was 4·1 months (95% CI 3·7–5·5). The 6-month overall survival rate was 83% (95% CI 78–88) and the 9-month overall survival rate was 74% (95% CI 67–79) with nivolumab 3 mg/kg in patients in the dose-expansion phase (table 4). The 6-month progression-free survival rate was 37% (95% CI 30–43) and the 9-month progression-free survival rate was 28% (95% CI 22–35).
Table 4:
Nivolumab efficacy in the dose-expansion phase
| Uninfected untreated/intolerant (n=56) | Uninfected progressor (n=57) | HCV infected (n=50) | HBV infected (n=51) | All patients (n=214) | |
|---|---|---|---|---|---|
| Objective response* | 13 (23%; 13 to 36) | 12 (21%; 11 to 34) | 10 (20%; 10 to 34) | 7 (14%; 6 to 26) | 42 (20%; 15 to 26) |
| Complete response | 0 | 2 (4%) | 0 | 1 (2%) | 3 (1%) |
| Partial response | 13 (23%) | 10 (18%) | 10 (20%) | 6 (12%) | 39 (18%) |
| Stable disease | 29 (52%) | 23 (40%) | 23 (46%) | 21 (41%) | 96 (45%) |
| Progressive disease | 13 (23%) | 18 (32%) | 14 (28%) | 23 (45%) | 68 (32%) |
| Not evaluable | 1 (2%) | 4 (7%) | 3 (6%) | 0 | 8 (4%) |
| Duration of response* | |||||
| KM median | 8·4 (8·3 to NE) | NR | 9·9 (4·5 to 9·9) | NR | 9·9 (8·3 to NE) |
| Ongoing, n/N (%) | 8/13 (62%) | 7/12 (58%) | 8/10 (80%) | 5/7 (71%) | 28/42 (67%) |
| Disease control* | 42 (75%; 62 to 86) | 35 (61%; 48 to 74) | 33 (66%; 51 to 79) | 28 (55%; 40 to 69) | 138 (64%; 58 to 71) |
| Disease control with stable disease for ≥6 months | 22 (39%; 27 to 53) | 22 (39%; 26 to 52) | 17 (34; 21 to 49) | 18 (35%; 22 to 50) | 79 (37%; 30 to 44) |
| Overall survival | |||||
| 6 months | 89% (77 to 95) | 75% (62 to 85) | 85% (72 to 93) | 84% (71 to 92) | 83% (78 to 88) |
| 9 months | 82% (68 to 90) | 63% (49 to 74) | 81% (66 to 90) | 70% (55 to 81) | 74% (67 to 79) |
| KM median | NR | 13·2 (8·6 to NE) | NR | NR | NR |
| Progression-free survival* | |||||
| KM median | 5·4 (3·9 to 8·5) | 4·0 (2·6 to 6·7) | 4·0 (2·6 to 5·7) | 4·0 (1·3 to 4·1) | 4·0 (2·9 to 5·4) |
Unless otherwise indicated, data are n (%; 95% CI); n (%); months (95% CI); or % (95% CI). HCV=hepatitis C virus. HBV=hepatitis B virus. KM=Kaplan-Meier estimate. NR=not reached. NE=not estimable. RECIST=Response Evaluation Criteria In Solid Tumors.
Determined by investigator assessment using RECIST version 1.1.
Figure 3: Percentage change in tumour burden.
Percentage change in tumour lesion size from baseline over time in the dose-expansion phase (n=202). HCV=hepatitis C virus. HBV=hepatitis B virus.
Figure 4: Best percentage change in tumour burden.
Best percentage change in tumour lesion size from baseline over time in the dose-expansion phase (n=202). Red dash indicates a 30% reduction. HCV=hepatitis C virus. HBV=hepatitis B virus.
Objective responses occurred in 13 (23%) of 56 patients without viral hepatitis who had not previously been treated with sorafenib or were intolerant and 12 (21%) of 57 sorafenib progressors without viral hepatitis (table 4); 15 responses were ongoing. The three complete responses in the dose-expansion phase occurred in two patients without viral hepatitis who had progression on sorafenib therapy and one patient with HBV infection (who had previously been treated with sorafenib). Disease control was seen in 42 (75%) of 56 patients without viral hepatitis who had not previously been treated with sorafenib or were intolerant and 35 (61%) of 57 patients in the sorafenib progressor cohort without viral hepatitis. 6-month overall survival in patients without viral hepatitis who had not previously been treated with sorafenib or were intolerant was 89% (95% CI 77 to 95; 48 at risk) and 75% (95% CI 62 to 85; 43 at risk) in the sorafenib progressor cohort without viral hepatitis (table 4). Median overall survival in the sorafenib progressor without viral hepatitis cohort was 13·2 months (95% CI 8·6 to NE); medians were not reached in the other dose-expansion cohorts.
Objective response rates were ten (20%) of 50 patients infected with HCV and seven (14%) of 51 patients infected with HBV (table 4); 13 responses were ongoing. Disease control was achieved in 33 (66%) patients infected with HCV and 28 (55%) patients infected with HBV. 6-month overall survival was 85% (95% CI 72–93) in the cohort with HCV infection (38 at risk) and 84% (95% CI 71–92) in the cohort with HBV infection (43 at risk). Nivolumab exhibited limited antiviral activity. The kinetics of HCV RNA levels over time were assessed in patients infected with HCV with advanced hepatocellular carcinoma, and no patient achieved a sustained virological response for more than 24 weeks. Some patients infected with HCV had transient reductions in HCV RNA. No patients had reactivation of HBV, and no instances of anti-HBs seroconversion were noted among patients infected with HBV.
In the dose-expansion phase, the objective response rate was analysed using mRECIST by blinded independent central review in the 145 patients who had previously been treated with sorafenib (irrespective of hepatocellular carcinoma aetiology); under these criteria the objective response rate was 27 (19%) of 145 patients, including five patients with a complete response (appendix).
The overall safety profile of nivolumab in patients in the dose-expansion phase was comparable to that observed in the dose-escalation phase. Grade 3/4 treatment-related adverse events were seen in 40 (19%) patients and grade 3/4 treatment-related serious adverse events were seen in nine (4%) patients (appendix). Symptomatic treatment-related adverse events were comparable in patients with and without HCV or HBV infection. Adverse events led to discontinuation in 24 patients, and there were no treatment-related deaths.
As a secondary endpoint, PD-L1 expression levels were retrospectively assessed as a potential biomarker for nivolumab therapy in the 174 (81%) of 214 patients with available data in the dose-expansion phase. Membrane expression of PD-L1 on at least 1% of tumour cells was observed in 34 (20%) of 174 patients at baseline; 140 (80%) patients had PD-L1 expression on less than 1% of tumour cells (table 5). Objective responses were observed in nine (26%) of 34 patients with PD-L1 expression on at least 1% of tumour cells (95% CI 13–44) and in 26 (19%) of 140 patients with PD-L1 on less than 1% of tumour cells (95% CI 13–26).
Table 5:
PD-L1 expression on tumour cells and response
| Escalation phase (n=44)* | Expansion phase (n=174)* | |
|---|---|---|
| PD-L1 ≥1%† | 11 (25%) | 34 (20%) |
| Objective response | 3/11 (27%; 6–61) | 9/34 (26%; 13–44) |
| Complete response | 1 (9%) | 1 (3%) |
| Partial response | 2 (18%) | 8 (24%) |
| Stable disease | 0 | 16 (47%) |
| Progressive disease | 7 (64%) | 9 (26%) |
| Not determined | 1 (9%) | 0 |
| PD-L1 <1%† | 33 (75%) | 140 (80%) |
| Objective response | 4/33 (12%; 3–28) | 26/140 (19%; 13–26) |
| Complete response | 2 (6%) | 2 (1%) |
| Partial response | 2 (6%) | 24 (17%) |
| Stable disease | 19 (58%) | 62 (44%) |
| Progressive disease | 8 (24%) | 46 (33%) |
| Not determined | 2 (6%) | 6 (4%) |
Data are n (%); n/N (%; 95% CI). PD-L1=programmed death-ligand 1.
Four patients in the dose-escalation phase and 40 patients in the dose-expansion phase did not have tumour PD-L1 expression data available.
PD-L1 membrane expression on tumour cells.
Among patients in the dose-expansion phase who were on treatment at the data cutoff, the EQ-5D-3L completion rate exceeded 90% at each timepoint through week 25. EQ-5D-3L index scores were stable while on treatment with no significant changes from baseline (mean 0·856, 95% CI 0·827 to 0·884) to week 25 (0·829, 0·786 to 0·872); mean change from baseline was −0.015 (−0·051 to 0·021). EQ-5D-VAS scores were also stable, with no significant changes from baseline (mean 73·0, 95% CI 69·0 to 77·1) to week 25 (75·4, 70·0 to 80·9); mean change from baseline was 3·2 (−1·2 to 7·5). Comparable results were observed in patients who had previously been treated with sorafenib. For this patient subpopulation, EQ-5D-3L scores were not appreciably changed from baseline (mean 0·853, 95% CI 0·816 to 0·889) through week 25 (0·825, 0·773 to 0·877); mean change from baseline was −0.014 (−0·058 to 0·030). Similarly, EQ-5D-VAS scores were stable from baseline (mean 73·9, 95% CI 69·2–78·6) through week 25 (75·8, 69·3–82·4); mean change from baseline was 3·1 (−1·3 to 7·6).
Discussion
Previous studies6,7,19 in advanced hepatocellular carcinoma of first-line sorafenib have shown response rates of 2–3% and second-line regorafenib has shown a response rate of 7%. In this phase 1/2 study, treatment with nivolumab resulted in substantial tumour reductions and objective response rates of 15–20% irrespective of line of therapy in patients with advanced hepatocellular carcinoma. Notably, the disease control rate was 58% in the dose-escalation phase and 64% in the dose-expansion phase, which could have positively affected overall survival. Baseline tumour cell PD-L1 status did not have an apparent effect on response rates. Median duration of response in both phases of the study (as high as 17 months in the dose-escalation phase) suggests that in the treatment of patients with advanced hepatocellular carcinoma, nivolumab might offer durable responses when other existing therapies have not.6,19 Median overall survival relative to sorafenib was encouraging in a population enriched in patients with metastatic disease and with previous treatment with sorafenib.20,21
In the dose-escalation phase, the safety profile of nivolumab in patients with hepatocellular carcinoma was consistent with that observed in other tumour types.9,10,22–25 To our knowledge, all previous studies of PD-1 inhibitors have excluded patients with chronic viral hepatitis. Hepatic safety events in virally infected patients with hepatocellular carcinoma treated with a CTLA-4 checkpoint inhibitor have been reported.26 Therefore, we evaluated viral aetiologies in separate cohorts in this study to identify any unique safety signals. We noted no new nivolumab safety signals. Safety findings from the dose-escalation phase were consistent with those in a larger group of patients from the dose-expansion phase.
The comparable objective response results in patients who had not previously been treated with sorafenib or were intolerant and in patients with disease progression on sorafenib suggest that nivolumab efficacy is not affected by previous sorafenib treatment status. In addition to potentially supporting nivolumab as a viable second-line therapy for patients with disease progression on multikinase inhibitors (as shown with a median overall survival of more than 13 months in patients without viral hepatitis with previous progression on sorafenib), the objective response rate of 23% and 9-month overall survival of 82% in untreated patients supports the investigation of nivolumab as a first-line therapy for patients with advanced hepatocellular carcinoma. This study was not powered for statistical comparisons between patients who were infected with HCV or HBV, or who did not have viral hepatitis; however, responses were observed irrespective of hepatocellular carcinoma aetiology. Treatment with nivolumab was associated with stable patient-reported outcomes, including indicators of health status and quality of life, irrespective of previous treatment with sorafenib.
A limitation of this study is the lack of randomised control arms. A subsequent randomised cohort-expansion phase of CheckMate 040 is evaluating nivolumab compared with sorafenib in the first-line setting. Although objective responses occurred in this study regardless of PD-L1 expression on tumour cells (using 1% of tumour cells expressing PD-L1 as a cutoff), future studies will need to evaluate the expression of PD-1 and PD-L1 on tumour-infiltrating lymphocytes as potentially valuable biomarkers. Inhibition of PD-L1 signalling by non-tumour cells could contribute to the efficacy of nivolumab in patients who have low (<1%) levels of PD-L1 expression on tumour cells. Although PD-L1 is not yet established as a consistently reliable biomarker across tumour types or lines of therapy, it is also possible that in a larger patient population, patients who have a higher proportion of tumour cells expressing PD-L1 might achieve greater benefit. An in-depth characterisation of tumour-infiltrating T-cell and macrophage subsets, including their expression of PD-1 and PD-L1, could be important for future biomarker assessments in patients with advanced hepatocellular carcinoma. Additionally, for more meaningful median overall survival results in the dose-expansion patient cohorts, longer follow-up will be needed.
Results from subsequent comparative, randomised phases of CheckMate 040 will further inform the therapeutic potential of nivolumab in patients with advanced hepatocellular carcinoma who have few existing treatment options. Nivolumab might provide favourable efficacy with a good safety profile in the context of the available targeted therapies. A phase 3 randomised study of nivolumab monotherapy compared with sorafenib in the first-line setting is ongoing.
Supplementary Material
Research in context.
Evidence before this study
Patients with advanced hepatocellular carcinoma who have tumours that are not amenable to surgical resection or local treatment have few effective treatment options. Although treatment with multikinase inhibitors provides some overall survival benefit—for example, sorafenib in previously untreated patients and regorafenib in sorafenib progressors—an unmet need remains in many patients. Chronic inflammatory conditions in the liver, such as cirrhosis and viral hepatitis, result in some degree of immunosuppression within the hepatocellular carcinoma tumour microenvironment, making immune checkpoints attractive therapeutic targets. We searched PubMed from Sept 1, 2010, to Sept 1, 2016, for articles using search terms “advanced HCC” and “immunotherapy OR immune checkpoint AND HCC”. Non-English articles, review articles, and meta-analysis references were excluded. We identified one relevant phase 1 clinical trial from 2013 evaluating the cytotoxic T-lymphocyte antigen (CTLA-4) checkpoint inhibitor tremelimumab in a small cohort of patients with advanced hepatocellular carcinoma who were infected with hepatitis C virus (HCV), which reported a manageable safety profile as well as preliminary evidence of antitumour and antiviral activity. Several preclinical studies have provided evidence in support of immunotherapeutic approaches for hepatocellular carcinoma, including the immunogenicity of transformed hepatocytes and immunosuppressive tumour microenvironments containing infiltrating lymphocytes. However, evidence showing the usefulness of immune checkpoint inhibitors in the treatment of patients with advanced hepatocellular carcinoma has been very limited. As early as 2010, reports have shown that programmed cell death protein-1 (PD-1) inhibitors can be potent immuno-oncology agents in patients with metastatic melanoma, providing rationale for immune checkpoint therapies in multiple other malignancies. When the CheckMate 040 trial began in 2012, several trials of nivolumab in metastatic tumour settings were ongoing. Whether liver-related toxicities from immune checkpoint inhibitors would be affected by concomitant HCV or hepatitis B virus (HBV) infection in patients with hepatocellular carcinoma was not known.
Added value of this study
To our knowledge, this is the first report of a PD-1 checkpoint inhibitor in patients with advanced hepatocellular carcinoma. The CheckMate 040 trial is a prospective, non-comparative, phase 1/2 dose study of nivolumab that assessed safety and clinical benefit across multiple hepatocellular carcinoma aetiologies, including patients with HCV or HBV infection. The efficacy of nivolumab monotherapy was evaluated as a first-line treatment in patients who had not previously received sorafenib or were intolerant and as a second-line treatment in those with previous disease progression on sorafenib.
Implications of all the available evidence
Since the CheckMate 040 trial began, nivolumab has been approved in the USA and European Union for the treatment of melanoma, refractory non-small cell lung cancer, advanced renal cell carcinoma, and Hodgkin lymphoma; and squamous cell carcinoma of the head and neck and urothelial carcinoma (only in the USA). Studies have shown that nivolumab monotherapy provides improved overall survival or clinical benefit in these approved indications. In this study in patients with advanced hepatocellular carcinoma, nivolumab showed encouraging objective response rates and overall survival. The safety profile of nivolumab was manageable and no new safety signals were observed. These findings support further investigation of nivolumab as a treatment option for patients with advanced hepatocellular carcinoma; a phase 3 randomised study of nivolumab monotherapy compared with sorafenib is underway.
Acknowledgments
This study was supported by Bristol-Myers Squibb (Princeton, NJ, USA) and by Ono Pharmaceutical Co (Osaka, Japan). We thank the patients and their families, and investigators and research staff at all study sites. The PD-L1 immunohistochemistry 28–8 pharmDx assay was developed by Dako North America (Carpinteria, CA, USA). Lisa Dauffenbach of Mosaic Laboratories (Lake Forest, CA, USA) and Cyrus Hedvat from Bristol-Myers Squibb contributed to analyses of PD-L1 immunohistochemical staining. TM is supported by the NIHR Biomedical Research Centre at University College London Hospitals. We acknowledge Jon Wigginton (MacroGenics Inc, Rockville, MD, USA) for early development of the CheckMate 040 protocol and Ashok Gupta (MedImmune) for serving as an early medical lead for CheckMate 040. Editorial assistance was provided by Jeff Bergen of Chrysalis Medical Communications (Hamilton, NJ, USA) and was funded by Bristol-Myers Squibb.
Declaration of interests
ABE-K has received research support from Astex, received personal fees from Merrimack, and served as an adviser for Bristol-Myers Squibb, AstraZeneca, Bayer, Genentech, and Novartis. BS has received speaking and consulting fees from Bristol-Myers Squibb and Bayer and consulting fees from AstraZeneca, Transgene, and Adaptimmune. TY has received speaking fees and research support from Bristol-Myers Squibb and has served as an adviser to Bristol-Myers Squibb. TSC has received research support from Bristol-Myers Squibb. S-PC has received speaking fees from Bristol-Myers Squibb. JT has received speaking and consulting fees from Bristol-Myers Squibb and Bayer. TM has served as a consultant for Bristol-Myers Squibb, Bayer, Ipsen, and Eisai. Y-KK has received consulting fees from Bristol-Myers Squibb, Ono Pharmaceutical Co, Bayer, Blueprint, AstraZeneca, Pfizer, Dicerna, and Mirna. WY has received research support from Bristol-Myers Squibb and has served as an adviser to Bristol-Myers Squibb. ACh has received research support and personal fees from Bristol-Myers Squibb, Bayer, Astellas, MSD, and Boehringer Ingelheim, and has received personal fees from Janssen Oncology, Bayer, Lilly, AstraZeneca, Roche, and Mundipharma. JA, JN, and HBD are employees and stockholders of Bristol-Myers Squibb. CdC, LL, and HT are employees of Bristol-Myers Squibb. IM has received research support and personal fees from Bristol-Myers Squibb. MK, CH, T-YK, and THW declare no competing interests.
Contributor Information
Anthony B El-Khoueiry, USC Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Bruno Sangro, Clinica Universidad de Navarra and CIBEREHD, Pamplona, Spain.
Thomas Yau, University of Hong Kong, Hong Kong Special Administrative Region, China.
Todd S Crocenzi, Providence Cancer Center, Portland, OR, USA.
Masatoshi Kudo, Kindai University Faculty of Medicine, Osaka, Japan.
Chiun Hsu, National Taiwan University Hospital, Taipei, Taiwan.
Tae-You Kim, Seoul National University Hospital, Seoul, South Korea.
Su-Pin Choo, National Cancer Center, Singapore.
Jörg Trojan, Goethe University Hospital and Cancer Center, Frankfurt, Germany.
Theodore H Welling, 3rd, University of Michigan School of Medicine, Ann Arbor, MI, USA.
Tim Meyer, Royal Free Hospital, London, UK.
Yoon-Koo Kang, Asan Medical Center, University of Ulsan, Seoul, South Korea.
Winnie Yeo, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China.
Akhil Chopra, Johns Hopkins Singapore International Medical Centre, Singapore.
Jeffrey Anderson, Bristol-Myers Squibb, Princeton, NJ, USA.
Christine dela Cruz, Bristol-Myers Squibb, Princeton, NJ, USA.
Lixin Lang, Bristol-Myers Squibb, Princeton, NJ, USA.
Jaclyn Neely, Bristol-Myers Squibb, Princeton, NJ, USA.
Hao Tang, Bristol-Myers Squibb, Princeton, NJ, USA.
Homa B Dastani, Bristol-Myers Squibb, Princeton, NJ, USA.
Ignacio Melero, Biomedical Research Network in Oncology (CIBERONC), Pamplona, Spain; Center for Applied Medical Research (CIMA), Pamplona, Spain.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 2: 16018. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015; 19: 223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–43. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers. Version 1.2017. 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed April 3, 2017).
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66.For the phase 3 trial see ClinicalTrials.gov number NCT02576509
- 8.Whiteside TL, Demaria S, Rodriguez-Ruiz ME, et al. Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res 2016; 22: 1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–84. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27: 450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015; 12: 681–700. [DOI] [PubMed] [Google Scholar]
- 13.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011; 128: 887–96. [DOI] [PubMed] [Google Scholar]
- 14.Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014; 59: 1415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol 2011; 54: 830–34. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 17.Group EuroQol. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012; 57: 821–29. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III sorafenib Asia-Pacific trial. Eur J Cancer 2012; 48: 1452–65. [DOI] [PubMed] [Google Scholar]
- 22.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372: 311–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 385: 1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.