Abstract
A pedigree of subjects presented with frontonasal dysplasia (FND). Genome sequencing and analysis identified a p.L165F missense variant in the homeodomain of the transcription factor ALX1 which was imputed to be pathogenic. Induced pluripotent stem cells (iPSC) were derived from the subjects and differentiated to neural crest cells (NCC). NCC derived from ALX1L165F/L165F iPSC were more sensitive to apoptosis, showed an elevated expression of several neural crest progenitor state markers, and exhibited impaired migration compared to wild‐type controls. NCC migration was evaluated in vivo using lineage tracing in a zebrafish model, which revealed defective migration of the anterior NCC stream that contributes to the median portion of the anterior neurocranium, phenocopying the clinical presentation. Analysis of human NCC culture media revealed a change in the level of bone morphogenic proteins (BMP), with a low level of BMP2 and a high level of BMP9. Soluble BMP2 and BMP9 antagonist treatments were able to rescue the defective migration phenotype. Taken together, these results demonstrate a mechanistic requirement of ALX1 in NCC development and migration.
Keywords: ALX1, frontonasal dysplasia, iPSC, neural crest cells, zebrafish
Subject Categories: Development & Differentiation; Genetics, Gene Therapy & Genetic Disease; Regenerative Medicine
Variants of the transcription factor ALX1 are implicated in the development of Frontonasal Dysplasia. This study explores the role of ALX1 in neural crest cell development and migration in patient‐derived induced pluripotent stem cells and zebrafish.
The paper explained.
Problem
The causes of malformations of the human face remain poorly understood. This lack of understanding results in limited treatment and counseling options, specifically in families affected by malformations linked to a genetic cause. One such gene is called ALX1. This study aimed to understand the role of this gene in the development of the face and the effect of mutations of the gene in the genesis of malformation. To do so, we reprogrammed blood cells from children affected by ALX1‐related malformations of the face into stem cells which allow us to retrace development. Additionally, we created a disruption of the gene in zebrafish in order to model the malformation in an animal and understand the role of the gene in development more broadly.
Results
ALX1 was found to be crucial to the development of a cell population which exists only during a limited time of early development, termed neural crest cells. These cells form while the early structures which will come to form the nervous system grow. They migrate to the front of the embryo to form the face. The cells of patients bearing a mutation of ALX1 were found to be more likely to die when compared with cells derived from healthy donors. They were also found to show a migration defect. Similar differences were observed in the zebrafish models of the disease created by a disruption of the same gene.
Impact
Understanding the causes of malformations of the face will give us the tools to innovate and transform the insufficient treatment options currently available to patients.
Introduction
The central part of the human face contains key anatomic features and sensory organs that enable us to interact with the environment and each other. The embryologic processes that form midface structures, including the eyes, nose, upper lip, and maxilla, are tightly regulated (Johnston, 1966; Minoux & Rijli, 2010; Rada‐Iglesias et al, 2012). The midface structures form as the centrally located frontonasal prominence extends anteriorly, coalescing with elements derived from the paired maxillary prominences (Johnston, 1966, 1975; Le Lièvre & Le Douarin, 1975; Le Lièvre, 1978; Sadaghiani & Thiébaud, 1987). The embryonic facial prominences are derived from distinct migrating streams of cranial neural crest cells (NCC) that are conserved across vertebrates (Le Douarin et al, 1993; Schilling et al, 1996; Chai et al, 2000; Olsson, et al, 2002; Trainor et al, 2002; Barrallo‐Gimeno et al, 2004; Wada, 2005; Dougherty et al, 2012). NCC migration and differentiation are highly coordinated and are associated with dynamic gene expression patterns (Simoes‐Costa & Bronner, 2015). Key signaling pathways that regulate NCC development involve BMP, Wnt, FGF, or Notch which activate the expression of transcription factors such as PAX3, ZIC1, TFAP2a, MSX1/2, and DLX5 (Meulemans & Bronner‐Fraser, 2002; Khudyakov & Bronner‐Fraser, 2009; Stuhlmiller & Garcia‐Castro, 2012; Rada‐Iglesias et al, 2013; Simoes‐Costa & Bronner, 2015). Disruptions of NCC development contribute to a number of congenital malformations such as Waardenburg syndrome (WS), velocardiofacial syndrome/DiGeorge syndrome, Hirschsprung's disease, congenital heart conditions, and craniofacial anomalies (Sedano et al, 1970; Fox et al, 1976; Pierpont et al, 2007; Uz et al, 2010)
Frontonasal dysplasia (FND) is considered a rare “orphan” disease (ORPHA250), with very few cases reported in the literature. The true prevalence of FND and its etiology remain unknown. To date, six genetic causes of subtypes of FND with varying patterns of inheritance have been described in individual case reports: EFNB1 (MIM 300035) in X‐linked craniofrontonasal syndrome (MIM 304110); ALX3 (MIM 606014) in FND type 1 (MIM 136760); ALX4 (MIM 605420) in FND type 2 (MIM 613451); ALX1 (MIM 601527) in FND type 3 (MIM 613456); ZSWIM6 (MIM 615951) in dominant acromelic frontonasal dysostosis (MIM 603671); and SPECC1L (MIM 614140) in Teebi syndrome (MIM 145420; Bhoj et al, 2015; Kayserili et al, 2009; Smith et al, 2014; Twigg et al, 2004, 2009; Ullah et al, 2016; Uz et al, 2010; Wieland et al, 2004). The heterogeneity of clinical phenotypes, including a wide range of possible ocular and craniofacial components, likely corresponds to different underlying genetic variants, genetic environments, and epigenetic modifications.
This study examined a pedigree of FND and identified a pathogenic variant in the homeodomain of transcription factor ALX1 resulting in a likely loss of function. A human stem cell model of FND was generated in order to investigate the effect of ALX1 mutations on NCC behavior. Cellular and molecular characterizations identified a number of differences between subject‐derived and control NCC that shed light on the developmental processes that are disrupted in FND. In vivo characterization of alx1 in zebrafish revealed defective migration of the most anterior cranial NCC. This study underscores the utility of complementary human iPSC and zebrafish models to gain mechanistic insight into the molecular and cellular basis of ALX1‐related FND.
Results
Clinical features of ALX1‐related FND in a consanguineous pedigree
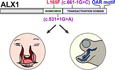
A family with four children born of consanguineous parents of Amish heritage presented with complex FND. The FND phenotype was inherited in a Mendelian recessive fashion. Both parents, one unaffected sibling and three affected children (one male and two females), were consented and enrolled in the study (Fig 1A, subject numbers indicated in red). The parents (subjects 1 and 2) and nine unaffected siblings (including subject 3) had normal facial structures without clinical stigmata suggestive of mild FND. All four affected children presented with bilateral oblique facial clefts, extending from either side of the nasal bone, involving both the primary and secondary palate. Among the affected children, there was some variability of the ocular phenotype, where the older affected girl (subject 4) presented with bilateral coloboma and asymmetric microphthalmia, whereas the three other affected children (including subjects 5 and 6) exhibited bilateral anophthalmia, with deficient upper and lower eyelids covering a shallow orbit. Subject 6 was the most severely affected and presented with bilateral oblique facial clefts and anophthalmia as well as no upper and lower eyelids, leaving the mucous membranes of both orbits exposed. Her nasal remnant also lacked the lateral alar subunits and is surrounded by several nodular skin tags.
Figure 1. Clinical presentation of the FND pedigree and generation of control, father, and subject‐derived iPSC .
-
AThe pedigree family tree includes two unaffected parents, four unaffected male siblings, five unaffected female siblings, and two each female and male affected sibling. Subjects 1–6, indicated in red, were enrolled in the study. Subjects 4–6 show complex FND with ocular involvement. The eldest affected sibling (subject 4) presented with right coloboma, left microphthalmia, and bilateral Tessier 4 oblique facial clefts. Subject 5 presented with bilateral anophthalmia with fused eyelids and shallow orbits, with bilateral oblique facial clefts. Subject 6 presented with bilateral anophthalmia with open shallow orbits, absent upper and lower eyelids, exposed orbital mucosa, bilateral oblique facial clefts, and malformed nasal ala with nodular skin tags. iPSCs were generated using blood samples collected from subjects 1, 5, and 6.
-
BWhole‐exome sequencing was carried out and analysis revealed a missense p.L165F variant (c.493 C>T) in the ALX1 homeodomain, heterozygous in the parents (ALX1 165L/165F), wild type in the unaffected sibling (ALX1 165L/165L), and homozygous in both affected subjects (ALX1 165F/165F).
- C
- D
-
EImmunofluorescence staining for pluripotent markers SSEA4, OCT4, SOX2, and TRA‐1-60 and alkaline phosphatase staining of iPSC clones. One representative iPSC clone is shown for each genotype. Scale bar: 400 μm.
-
FExpression of pluripotent (OCT4, NANOG), endoderm (Endo., AFP, GATA4, FOXA2), ectoderm (Ecto., NESTIN, GFAP, SOX1), and mesoderm (Meso., BRACH. (BRACHYURY), RUNX1, CD34) gene markers for ALX1 165L/165L (green), ALX1 165L/165F(red), and ALX1 165F/165F (blue) iPSC relative to undifferentiated cells (UND). Data are represented as pooled mean ± SEM of three experiments on three clones from each genotype. Significance: P = 0.0167 for OCT4, P = 0.0005 for NANOG, P = 0.000004 for AFP, P = 0.0082 for GATA4, P = 0.0137 for FOXA2, P = 0.00002 for NESTIN, P = 0.0167 for GFAP, P = 0.0014 for SOX1, P = 0.0117 for BRACHYURY, P = 0.0008 for RUNX1 and P = 0.0068 for CD34 when comparing undifferentiated and differentiated ALX1 165L/165L iPSC. P = 0.0013 for OCT4, P = 0.0011 for NANOG, P = 0.0000003 for AFP, P = 0.0003 for GATA4, P = 0.0063 for FOXA2, P = 0.0001 for NESTIN, P = 0.027 for GFAP, P = 0.000002 for SOX1, P = 0.000009 for BRACHYURY, P = 3e−9 for RUNX1 and P = 0.000006 for CD34 when comparing undifferentiated and differentiated ALX1165F/165L iPSC. P = 0.0201 for OCT4, P = 0.006 for NANOG, P = 1 × 10−12 for AFP, P = 5 × 10‐13 for GATA4, P = 0.0031 for FOXA2, P = 0.0292 for NESTIN, P = 0.00001 for GFAP, P = 6 × 10‐7 for SOX1, P = 0.0204 for BRACHYURY, P = 0.0009 for RUNX1 and P = 0.000003 for CD34 when comparing undifferentiated and differentiated ALX1 165F/165F iPSC. Data from each clone were pooled, and the mathematical mean was calculated. SEM was used to determine the standard error. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant.
Identification of pathogenic ALX1 variant
Whole‐exome sequencing (WES) was performed on blood samples collected from subjects 1–5, which corresponded to both parents, one unaffected sibling, and two affected children. A missense p.L165F variant (c.493C>T) was identified in the homeodomain of ALX1, which was heterozygous in the parents (ALX1165L/165F), wild type in the unaffected sibling (ALX1165L/165L), and homozygous in both affected subjects (ALX1165F/165F; Fig 1B). WES results were confirmed by Sanger sequencing of the entire ALX1 coding sequence. The ALX1 p.L165F missense variant has not been reported in connection with an ALX1‐related instance of FND in the literature nor been recorded as a variant in the gnomAD database (preprint: Karczewski et al, 2019; Fig 1C and D). The ALX1 p.L165F amino acid substitution was predicted to be damaging and disease causing by in silico tools (Sift, Polyphen, muttaster, fathmm) and consistent with the observed autosomal recessive inheritance pattern of this pedigree (Lowe, 1999; Adzhubei et al, 2010; Schwarz et al, 2014; Shihab et al, 2014).
Generation of patient‐derived iPSC model of ALX1‐related FND
Induced pluripotent stem cell lines were generated using peripheral blood mononuclear cells (PBMC) obtained from whole blood samples that were collected from three unrelated wild‐type individuals (ALX1 165L/165L), the heterozygous father (ALX1 165L/165F), and two of the four affected children (Subjects 5 and 6; ALX1 165F/165F). PBMC were subsequently reprogrammed into iPSC (Fig EV1). Overall, 22 mutant ALX1 165F/165F iPSC clones were successfully isolated and expanded from the two affected subjects, 13 ALX1 165L/165F clones were isolated and expanded from the heterozygous father, and 35 ALX1 165L/165L clones were isolated and expanded from healthy controls. Six ALX1 165F/165F mutant clones (3 for each affected subject), 3 ALX1 165L/165F clones from the heterozygous father, and 9 ALX1 165L/165L clones from healthy controls (3 from each control) were fully characterized to confirm their pluripotency (Fig 1E) and ability to generate the three germ layers (Fig 1F). Sanger sequencing confirmed that the affected ALX1 165F/165F and the heterozygous ALX1 165L/165F ‐derived iPSC clones retained the ALX1 p.L165F variant through reprogramming. Copy number variant analysis did not show any amplifications or deletions.
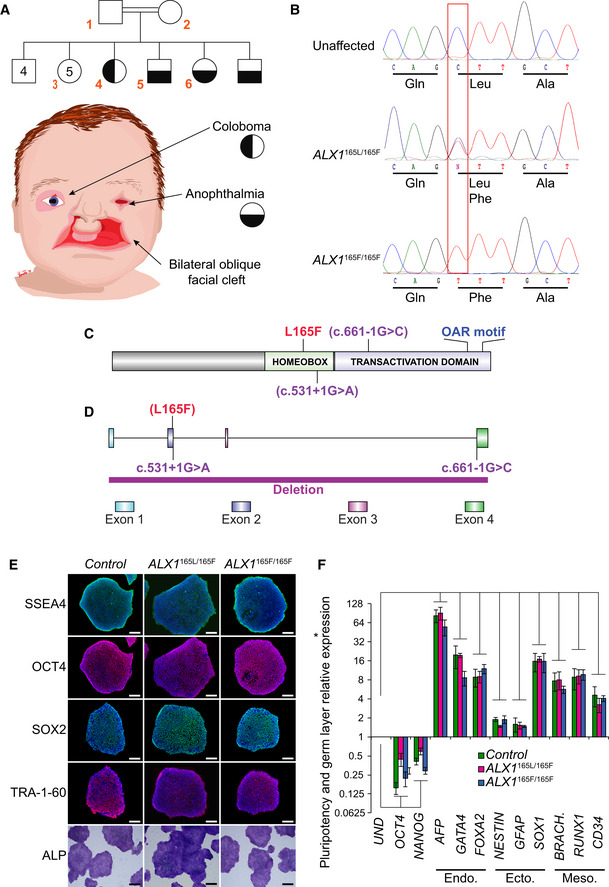
Figure EV1. Induced pluripotent stem cells (iPSC) derivation and EB generation.
-
ASchematic representation of the strategy used to generate iPSC. Blood samples from an unrelated normal individual, unaffected father (subject 1), and two of the affected children (subjects 5 and 6) were processed. Isolated PBMC were infected with Sendai virus, and individual clones were picked 21 days after the infection. Following expansion until passage 10, iPSC were characterized and embryoid bodies were formed by suspension culture for 14 days.
-
BThe reprogramming process of the PBMC showed that all cells underwent similar morphological changes leading to the formation of iPSC clones by day 21. These clones still displayed embryonic stem cells morphology at passage 10, indicating that the cells are able to self‐renew. All iPSC clones were able to form EBs. One clone of each subject is represented. Scale bar is 400 μm.
Generation and characterization of iPSC‐derived NCC
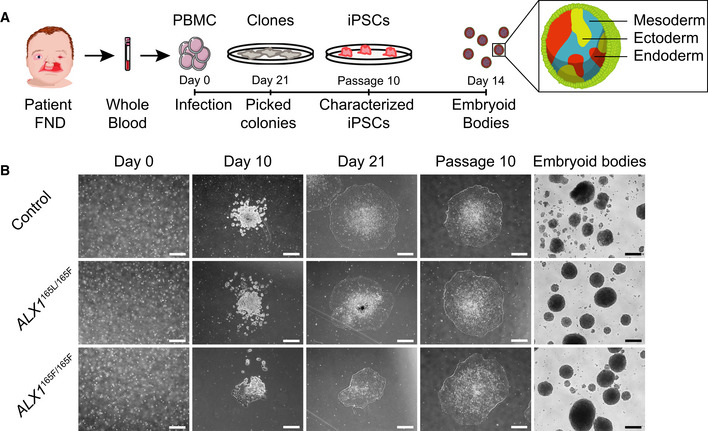
Given the primary role of neural crest cells in midface morphogenesis, the iPSC clones were differentiated into NCC using a protocol adapted from a previous study (Pini et al, 2018; Fig 2A). All NCC displayed similar morphological features and were indistinguishable at the colony level immediately following differentiation at passage 1 (Fig 2B).
Figure 2. Generation of iPSC‐derived NCC.
-
ASchematic of the differentiation protocol timeline. Maintenance Medium (MM) = iPSC medium (StemFlex with 1× penicillin/streptomycin), NCC differentiation medium = DMEM‐F12, 10% fetal bovine serum, 1 mM sodium pyruvate, 1 mM penicillin/streptomycin, 1 mM nonessential amino acids, 110 μM 2‐mercaptoethanol, 10 ng/ml epidermal growth factor.
-
BImages of iPSC and iPSC‐derived NCC at Days 0, 14, and passage 4 following differentiation. Scale bars: 400 μm (Day 0), 200 μm (Day 14, passage 4).
Overexpression of neural plate border specifier genes in ALX1165F/165F NCC
A panel of marker genes at the center of the gene regulatory network required for NCC development and differentiation was selected to be examined in detail across the 14 days of the neural crest differentiation protocol (Fig 3; Barrallo‐Gimeno et al, 2004; Sauka‐Spengler & Bronner‐Fraser, 2008; Sauka‐Spengler et al, 2007; Simoes‐Costa & Bronner, 2015). The NCC gene expression results can broadly be divided into three groups. The first group includes genes that did not significantly differ between affected, heterozygous, and unaffected controls. This group of genes comprises the neural crest specifiers FOXD3 and P75, as well as the lineage specifier HAND2. The second group includes genes where the affected cells exhibited expression patterns that differed significantly from the heterozygous and the unaffected control cells, with no difference between the heterozygote and the control. This group of genes includes the neural plate border specifiers ZIC1, PAX7, PAX3, MSX1, and DLX5 and the neural crest specifiers SNAI2 and TWIST1 (P < 0.05 between days 2–8 when comparing subjects’, father, and control NCC for ZIC1, PAX7, DLX5; P < 0.05 between days 2–14 when comparing subjects’, father, and control NCC for PAX3, MSX1, SNAI2, and TWIST1). The final group includes genes that were significantly differentially expressed between the affected homozygous, heterozygous, and unaffected control cells. This group comprised the neural plate border specifier MSX2, DLX5, and the neural crest specifier TFAP2A (P < 0.05 between days 2–14 when comparing subjects’, father, and control NCC for MSX2, DLX5, and TFAP2A). Of note, all significantly differentially expressed genes in the affected cells were overexpressed above the levels observed in the heterozygous and unaffected control cells, consistent with a putative role of ALX1 as a transcriptional repressor.
Figure 3. Timeline of key NCC‐associated genes during differentiation.
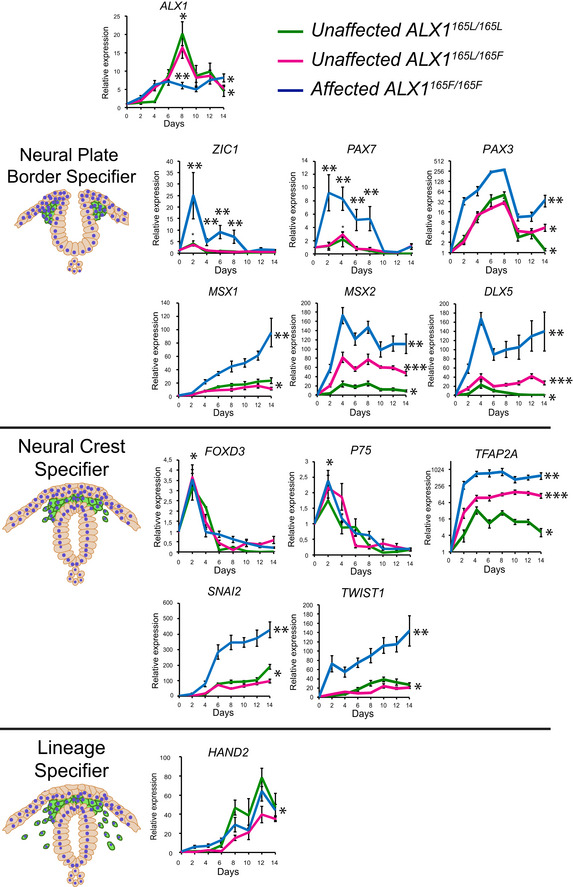
Gene expression analysis across NCC differentiation of unaffected control ALX1 165L/165L (green), heterozygous ALX1 165L/165F (magenta), and homozygous ALX1 165F/165F iPSC:ALX1, neural plate border specifier genes ZIC1, PAX7, PAX3, MSX1, MSX2, DLX5; neural crest specifier genes FOXD3, P75, TFAP2A, SNAI2, TWIST1; and lineage specifier gene HAND2. The RT–qPCR relative expression values were normalized to RPLP0 and GAPDH expression. Data are represented as pooled mean ± SEM of three experiments on three clones from each genotype. Exact P‐values are provided in Table EV1. Data from each clone were pooled, and the mathematical mean was calculated. SEM was used to determine the standard error. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant.
ALX1 itself was found to be differentially expressed between affected cells when compared to the heterozygous and unaffected control cells at day 8 during NCC differentiation. The unaffected control and heterozygous cells exhibited similar ALX1 expression levels, with peak expression level reached at day 8 where unaffected cells exhibited a plateaued, lower level of expression. The greatest difference in gene expression levels was observed early in the NCC differentiation process, around days 2–8 (such as in the cases of ZIC1, PAX3, PAX7, DLX5, and TWIST1). This characterization suggests an early function for ALX1 in NCC differentiation and identifies the 2–8 day window for in‐depth transcriptome analysis in future studies.
Increased sensitivity to apoptosis in ALX1165F/165F NCC
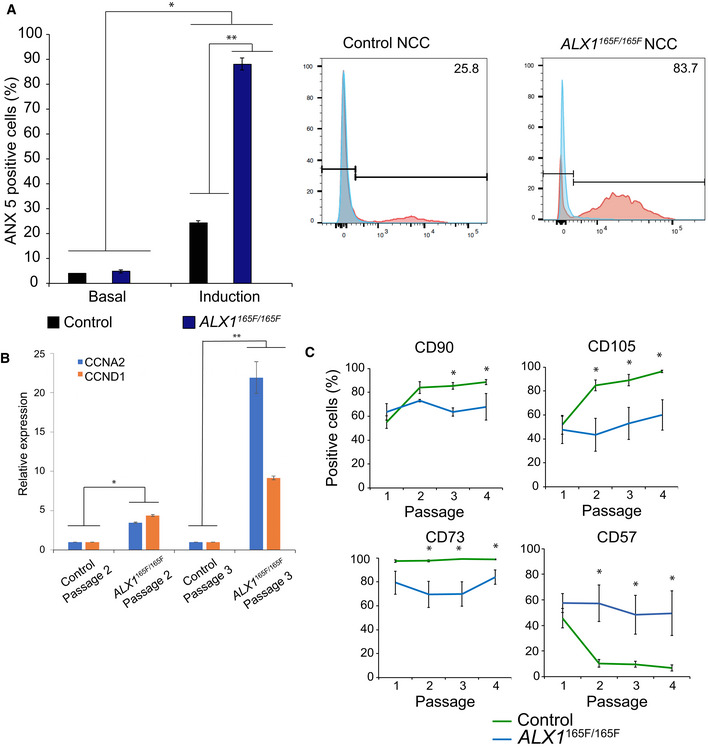
Since anomalies in cell cycle progression predispose cells to apoptosis and given the importance of apoptosis in regulating craniofacial development, the impact of the ALX1 165F/165F gene variant on the sensitivity of the iPSC‐derived NCC to apoptosis was analyzed. Basal apoptosis levels, determined by the percentage of Annexin V‐positive cells, did not differ between control NCC (4 ± 0.2%) and ALX1 165F/165F NCC (4.82 ± 0.65%; Fig 4A). After apoptosis induction via heat shock, the percentage of Annexin V‐positive cells significantly increased specifically in ALX1 165F/165F NCC (87.97 ± 2.44%) versus control NCC (24.15 ± 0.96%). These findings suggest that the affected subject's ALX1 165F/165F NCC are more sensitive to apoptosis.
Figure 4. NCC apoptosis, cell cycle, and differentiation.
-
AHomozygous ALX1 165F/165F NCC (blue) showed an increase in sensitivity to apoptosis when compared to control ALX1 165L/165L NCC (black). The data on the left represent the mean percentage of Annexin V‐positive cells, indicative of apoptosis, as determined by FACS analysis, with the data on the right being an example of one such experiment. Apoptosis was induced by immersion in a 55°C water bath for 10 min. Representative experiment for each condition is shown. Data are represented as pooled mean ± SEM of three independent experiments. Data obtained of each clone from three independent experiments were pooled, and the mathematical mean was calculated. SEM was used to determine the standard error. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant. *: Significantly different from the basal apoptosis rate: P = 3 × 10−12 between control NCC basal apoptosis and induced apoptosis, and between ALX1 165F/165F NCC basal apoptosis and induced apoptosis. **Significantly different from control NCC (P = 0.0004).
-
BExpression levels of cyclins CCNA2 (blue) and CCND1 (orange) in NCC at passages 2 and 3 of ALX1 165L/165L and ALX1 165F/165F NCC. The RT–qPCR relative expression values were normalized to RPLP0 and GAPDH expression. Data are represented as pooled mean ± SEM of three experiments on three clones from each genotype. *Significantly different from control NCC at passage 2 (P = 0.001 between control and ALX1 165F/165F NCC at passage 2 for CCNA2, P = 0.0052 for CCND1). **Significantly different from control NCC at passage 3 (P = 0.0494 between control and ALX1 165F/165F NCC for CCNA2, P = 0.0008 for CCND1).
-
CFluorescence activated cell sorting (FACS) experiments showed that control ALX1 165L/165L NCC (green) exhibited increased expression of mesenchymal markers CD90, CD105, and CD73 with culture time (passages 1 through 4), whereas homozygous ALX1 165F/165F NCC (blue) showed a consistent expression of the markers expressed at passage 1 throughout. Further, control ALX1 165L/165L NCC showed a downregulation of CD57 expression with culture time, while ALX1 165F/165F NCC maintained the same level of CD57 across passages. Data are presented as the mean percentage of positive‐stained cells across passage numbers. Data obtained of each clone from three independent experiments were pooled, and the mathematical mean was calculated. SEM was used to determine the standard error. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant. *Significantly different from control NCC. For CD90, P = 0.0013 at passage 3 and P = 0.0207 at passage 4. For CD105, P = 0.0016 at passage 2, P = 0.00004 at passage 3 and P = 0.0021 at passage 4. For CD73, P = 0.0060 at passage 2, P = 0.00004 at passage 3 and P = 0.0114 at passage 4. For CD57, P = 0.0026 at passage 2, P = 0.000003 at passage 3 and P = 0.000007 at passage 4.
These findings also indicate that ALX1 functions in proliferating NCC. To determine whether ALX1 function is required for cell cycle progression, we investigated expression of Cyclin D1 (CCND1), required for the cell cycle G1/S transition, and Cyclin A2 (CCNA2), required for the DNA synthesis during the S‐phase. Both cyclins are expressed throughout the active cell cycle, from the G1/S transition to the G2/M transition (Pagano et al, 1992; Minarikova et al, 2016). Expression levels of CCND1 and CCNA2 were compared between control and ALX1165F/165F NCC at passages 2 and 3 post‐differentiation. The ALX1 165F/165F NCC were found to express significantly more CCNA2 and CCND1 at both passage 2 and passage 3 compared to the control NCC, consistent with a greater degree of active cellular proliferation (Fig 4B).
ALX1165F/165F NCC do not undergo mesenchymal marker transition
As NCC clones derived from the control ALX1 165L/165L, heterozygous ALX1 165L/165F, and homozygous ALX1 165F/165F iPSC were maintained in culture, consistent qualitative morphologic differences were observed across cell passages. While control‐derived NCC became progressively spindle‐shaped and elongated, the mutant ALX1 165F/165F NCC remained rounded (Fig 2B). In order to investigate these differences more fully, flow cytometry was performed across different cell passage cycles in order to investigate the effect of the in vitro maturation of the NCC via an examination of NCC marker expression. At passage cycles 1–4, a number of key surface markers were examined. Expression of CD57 (synonym: HNK‐1), indicative of NCC precursors before their commitment to downstream cell lineages (Minarcik & Golden, 2003), as well as markers of mesenchymal differentiation, CD105, CD73, and CD90, was assessed (Fig 4C).
Table 1 contains the precise percentage values of the FACS analysis of NCC at varying passage numbers. At passage 1 following differentiation, control and homozygous ALX1 165F/165F NCC expressed similar levels of neural crest precursor marker CD57. The control and homozygous ALX1165F/165F NCC also expressed similar levels of mesenchymal markers CD90, CD105, and CD73. No significant differences were observed in marker expression between control and homozygous ALX1 165F/165F NCC at this stage (P > 0.05). However, by passage 4, control NCC exhibited decreased CD57 expression and increased expression of CD90, CD105, and CD73, consistent with a progression to MSC differentiation. In contrast, homozygous ALX1 165F/165F NCC displayed a similar expression of the aforementioned NCC and MSC markers at passage 4 as they did at passage 1 (Fig 4C).
Table 1.
Comparative FACS analysis of subject‐derived ALX1 165F/165F and control NCC at passages 1 and 4
| Passage 1 | Passage 4 | |||
|---|---|---|---|---|
| Control (ALX1 165L/165L) | ALX1 165F/165F | Control (ALX1 165L/165L) | ALX1 165F/165F | |
| CD57 | 45.6 ± 7.7% | 57.4 ± 7.3% | 6.78 ± 2.36% | 49.55 ± 17.53% |
| CD90 | 55.05 ± 5.24% | 63.6 ± 6.9% | 88.46 ± 2.05% | 67.8 ± 11.07% |
| CD105 | 51.7 ± 7.8% | 47.6 ± 11.4% | 96.31 ± 0.95% | 60.02 ± 12.66% |
| CD73 | 97.4 ± 1.08% | 79.2 ± 9.6% | 98.8 ± 0.44% | 83.95 ± 6.05% |
The persistent CD57 expression in the homozygous ALX1 165F/165F NCC, taken together with the elevated expression of neural crest specifier genes ZIC1, PAX7, PAX3, MSX1, MSX2, and DLX5, suggests that the mutant NCC may be unable to progress from the progenitor to the differentiating state. To understand whether the persistent CD57 expression had an effect on the ability of the homozygous ALX1 165F/165F NCC to differentiate into downstream cell types, multilineage differentiation experiment was performed. Control and homozygous ALX1 165F/165F NCC demonstrated equal ability to differentiate into Schwann cells (GFAP and S100B‐positive expression), adipocytes (oil Red O. staining), chondrocytes (Alcian Blue, Safranin O. and Toluidine Blue staining), and osteoblasts (Alizarin Red S., Von Kossa staining and strong alkaline phosphatase activity; Fig EV2). The maintenance of CD57 and lack of elevation of CD90 / CD105 / CD73 and the same ability to differentiate into NCCs derivatives suggest that the homozygous ALX1 165F/165F failed to progress through the process of NCCs differentiation despite multiple cell passages and are blocked into the progenitor state.
Figure EV2. Characterization of NCC .
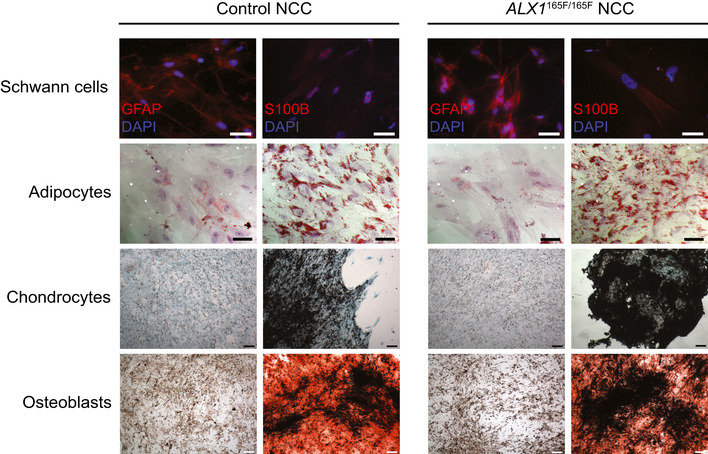
Multilineage differentiation experiments revealed that both control and subject‐derived NCC are able to differentiate into Schwann cells, shown by the GFAP and S100B‐positive immunofluorescence staining; adipocytes, demonstrated by the Oil Red O. positive lipidic droplets; osteoblasts, shown by Alizarin Red S. positive mineralized nodules and chondrocytes, assessed by Alcian Blue‐positive cartilaginous matrix. Scale bar is 200 μm for images of Schwann cells and adipocytes, and 400 μm for images of chondrocytes and osteoblasts.
Homozygous ALX1165F/165F NCC display a migration defect
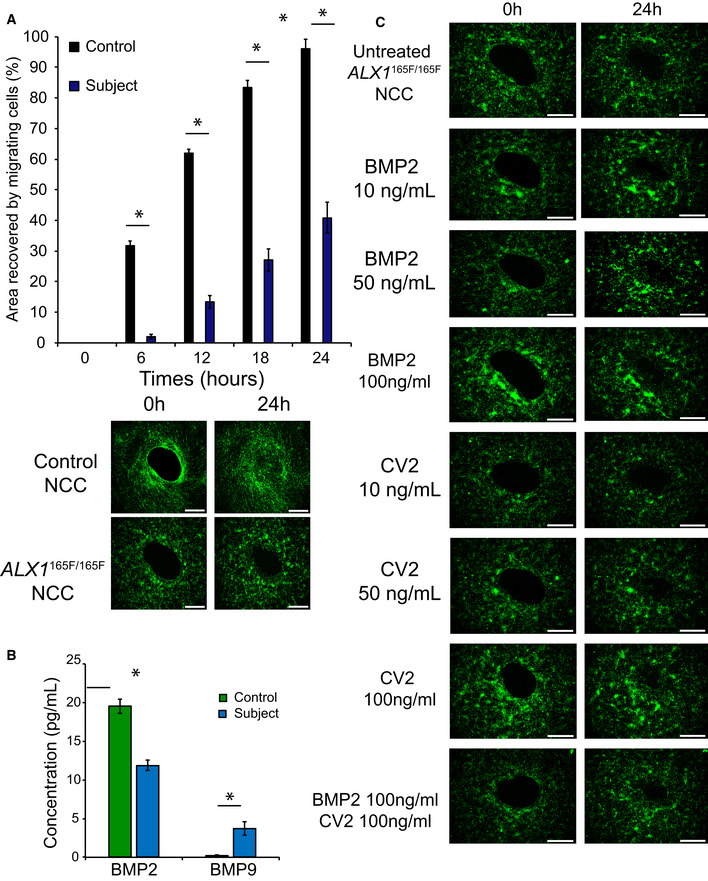
During embryonic development, NCC migrate to specific locations in order to form the prominences that coalesce to shape the face. To investigate the migratory properties of the iPSC‐derived NCC in vitro, a wound healing assay with a central clearing was used. A significant migration defect was observed in the homozygous ALX1 165F/165F NCC when compared with control NCC (Fig 5A, Movie EV1). Control NCC were able to migrate and fully cover the central clearing area of the wound healing assay after 24 h (recovery of 95.99 ± 3.22% of the surface area). In contrast, the homozygous ALX1 165F/165F NCC covered less than half of the central clearing surface area (38.79 ± 3.22% for ALX1 165F/165F NCC).
Figure 5. ALX1 165F/165F NCC show a migration defect and a difference in BMP secretion.
-
AMutant ALX1 165F/165F NCC (blue) exhibited a migration defect in timed coverage of the central clearing of the wound assay when compared with control ALX1 165L/165L NCC (black). Data are presented as percent area recovery of the central circular clear area of the wound assay by migrating NCC at the end of a 24‐h period. For fluorescent pictures, cells were stained in serum‐free media containing 3.6 μM CellTracker Green CMFDA (Life Technologies) for 30 min at 37°C and allowed to recover for 30 min before starting the experiment. Images were acquired every 6 h using a Keyence BZ‐X800 microscope. Surface area analyses and percentages of coverage were measured using ImageJ software (NIH). The NCC were monitored over 24 h. The data are represented as the average of the percentage of closure ± SEM. Scale bar = 200 μm. Data obtained of each clone from three independent experiments were pooled, and the mathematical mean was calculated. SEM was used to determine the standard error. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant. *Significantly different from control NCC (P < 0.0001).
-
BMultiplex analysis of BMP2 and BMP9 in the supernatant of cultured NCC showed that ALX1 165F/165F NCC (blue) secrete less BMP2 and more BMP9 compared to control ALX1 165L/165L NCC (green). Data are represented as pooled mean ± SEM of three clones from each genotype. Statistical significance was determined with an ANOVA test. A P‐value < 0.05 was considered significant. *Significantly different from control NCC (P = 0.0424 for BMP2 and P = 0.0192 for BMP9).
-
CAddition of soluble BMP2 or CV2, a BMP9 antagonist, to the culture medium could partially rescue the migration defect of ALX1 165F/165F NCC. At the beginning of the assay, 10, 50, or 100 ng/ml of soluble BMP2, CV2, or a combination of the two at 100 ng/ml each were added to the culture medium, and the cells were monitored over the next 24 h. The data are represented as the average of the percentage of closure ± SEM. Scale bar: 400 μm.
ALX1165F/165F NCC show differences in BMP secretion
The family of BMP family of growth factors plays a critical role in NCC migration (Tribulo et al, 2003; Sato et al, 2005). This, in combination with the increased expression of TWIST1 in ALX1 165F/165F NCC, a known BMP inhibitor, led to us to hypothesize that ALX1 165F/165F NCC might display abnormal levels of secreted BMP when compared to healthy control NCC (Hayashi et al, 2007). To test this hypothesis, the levels of secreted BMP in the culture medium of ALX1 165F/165F and control NCC were measured via multiplex analysis. The concentration of BMP2 was found to be significantly reduced in control ALX1 165F/165F NCC (11.9 ± 0.65 pg/ml) compared to control NCC (19.52 ± 0.9 pg/ml; P < 0.05; Fig 5B). In contrast, the BMP9 concentration was significantly increased in mutant ALX1 165F/165F NCC (3.72 ± 0.85 pg/ml) compared to control NCC (0.25 ± 0.02 pg/ml). BMP4, BMP7, and BMP10 levels were undetectable.
To follow‐up on the observed dysregulation of BMPs, we hypothesized that treatments to counteract BMP2 reduction or BMP9 elevation could result in an improved migration phenotype. The ALX1 165F/165F NCC were treated with different concentrations of soluble BMP2, the BMP9 antagonist Crossveinless (CV2), or a combination of the two (Fig 5C, Fig EV3, [Link], [Link]). Treatment with an increasing concentration of BMP2 from 10 to 50 ng/ml was able to restore the migration of homozygous ALX1 165F/165F NCC in a dose‐dependent manner. However, no difference was observed between 50 and 100 ng/ml of BMP2, suggesting a saturation effect (64.53 ± 3.17% for 50 ng/ml BMP2, and 67.31 ± 3.25% for 100 ng/ml BMP2).
Figure EV3. Effect of BMP2 and CV2 on NCC migration.
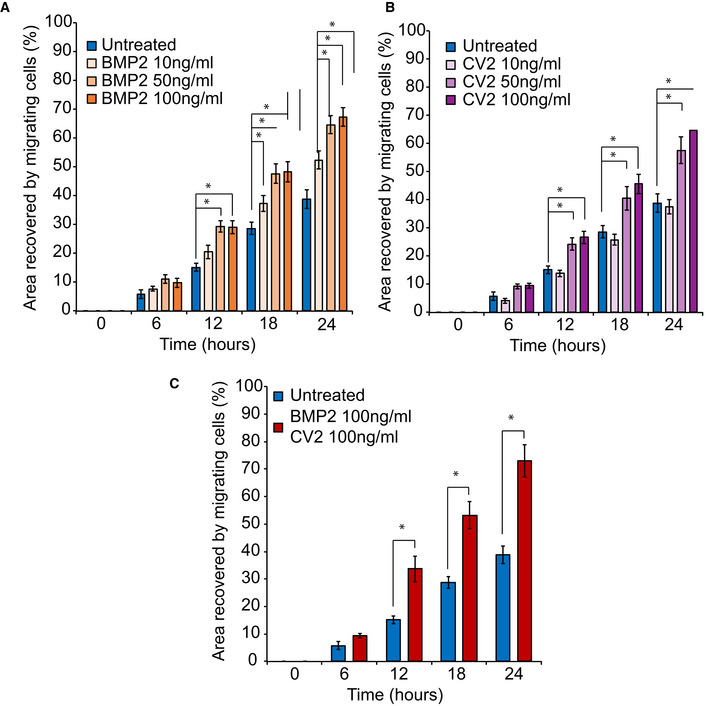
-
A10, 50, or 100 ng/ml of soluble BMP2 was added to the culture medium. Surface area analyses and percentages of coverage were measured using ImageJ software (NIH). The data of NCC migration following the treatment with 10, 50, and 100 ng/ml soluble BMP2 are represented as the average of the percentage of closure ± SEM from three independent experiments performed with each clone. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant *: Significantly different from untreated ALX1 165F/165F NCC: at 12 h, P = 0.0038 when comparing BMP2 50 ng/ml and untreated ALX1 165F/165F NCC, and P = 0.0045 when comparing BMP2 100 ng/ml and untreated ALX1 165F/165F NCC. At 18 h, P = 0.0337 when comparing BMP2 10 ng/ml and untreated ALX1 165F/165F NCC; P = 0.0009 when comparing BMP2 50 ng/ml and untreated ALX1 165F/165F NCC; and P = 0.0006 when comparing BMP2 100 ng/ml and untreated ALX1 165F/165F NCC. At 24 h, P = 0.005 when comparing BMP2 10 ng/ml and untreated ALX1 165F/165F NCC; P < 0.0001. when comparing BMP2 50 ng/ml and untreated ALX1 165F/165F NCC; and P < 0.0001 when comparing BMP2 100 ng/ml and untreated ALX1 165F/165F NCC.
-
B10, 50, or 100 ng/ml of soluble CV2 was added to the culture medium. Surface area analyses and percentages of coverage were measured using ImageJ software (NIH). The data of NCC migration following the treatment with 10, 50, and 100 ng/ml soluble CV2 are represented as the average of the percentage of closure ± SEM. Scale bar = 400 μm. *: Significantly different from untreated ALX1 165F/165F NCC: at 12 h, P = 0.0146 when comparing CV2 50 ng/ml and untreated ALX1 165F/165F NCC, and P = 0.0262 when comparing CV2 100 ng/ml and untreated ALX1 165F/165F NCC. At 18 h, P = 0.0028 when comparing CV2 50 ng/ml and untreated ALX1 165F/165F NCC, and P = 0.0035 when comparing CV2 100 ng/ml and untreated ALX1 165F/165F NCC. At 24 h, P = 0.0002 when comparing CV2 50 ng/ml and untreated ALX1 165F/165F NCC, and P < 0.0001 when comparing CV2 100 ng/ml and untreated ALX1 165F/165F NCC.
-
CRecovery of subject‐derived NCC migration in a migration assay following the combined treatment with 100 ng/ml each of soluble BMP2 and CV2. The data are represented as the average of the percentage of closure ± SEM from three independent experiments performed with each clone. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant * : Significantly different from ALX1 165F/165F NCC: at 12 h, P = 0.0031, at 18 h P = 0.0001, and at 24 h P < 0.0001).
Likewise, treatment with the BMP9 antagonist CV2 was able to partially rescue the migration defect of homozygous ALX1165F/165F NCC. The low dose of 10 ng/ml of CV2 did not show a significant effect on the migration defect of treated and untreated ALX1 165F/165F NCC (37.5 ± 2.5% for 10 ng/ml CV2). As observed with BMP2, treatments with both 50 ng/ml and 100 ng/ml of CV2 were able to partially restore the ability of the homozygous ALX1 165F/165F NCC to migrate, with no difference found between these two concentrations (57.6 ± 4.77% for 50 ng/ml of CV2, and 64.64 ± 3.36% for 100 ng/ml of CV2). Finally, we asked whether treatment with a combination of BMP2 and CV2 would exert an additive or synergistic effect to restore cell migration than single compound treatment. No additive effect was identified, as BMP2 and CV2 cotreatment at 100 ng/ml was able to rescue the migration defect phenotype of mutant ALX1 165F/165F NCC at a similar level to what was observed with the individual treatments (73 ± 5.89% for BMP2 and CV2 cotreatment).
Evaluation of alx1 function in the zebrafish
We and others previously showed that the zebrafish anterior neurocranium (ANC) forms from the convergence of the frontonasal prominence and the paired maxillary prominences (Wada, 2005; Eberhart, 2006; Dougherty et al, 2012). Since FND malformation is characterized by facial cleft affecting fusion of the frontonasal and maxillary structures, examination of the ANC morphology in zebrafish would be a sensitive assay for frontonasal development.
To generate an in vivo model of alx1, we employed CRISPR/Cas9‐mediated targeted mutagenesis of the alx1 locus in zebrafish. This approach produced a frameshift mutation harboring a 16‐base pair (bp) deletion in exon 2 of alx1 (NM_001045074), named alx1 uw2016 (Fig 6A, Fig EV4). The alx1 uw2016 mutation is likely to cause complete loss of function, since the encoded truncated protein lacks both the homeobox and transactivation domains. While the majority of alx1 uw2016 homozygous zebrafish developed normally and were viable as adults, approximately 5% displayed specific craniofacial defects (Fig EV5). Alcian blue staining of alx1 uw2016 homozygous larvae at 5 days post‐fertilization (dpf) revealed that the posterior neurocranium and ventral cartilages and Meckel's cartilage were formed but smaller in size in a subset of zebrafish. In contrast, the ANC appeared dysmorphic, i.e., narrower in the transverse dimension and linear, rather than fan‐shaped (Fig 6A). The chondrocytes of the ANC appeared cuboidal in mutant larvae, whereas wild‐type ANC chondrocytes were lenticular and stacked in an intercalated pattern (Fig 6A).
Figure 6. Alx1 function in zebrafish.
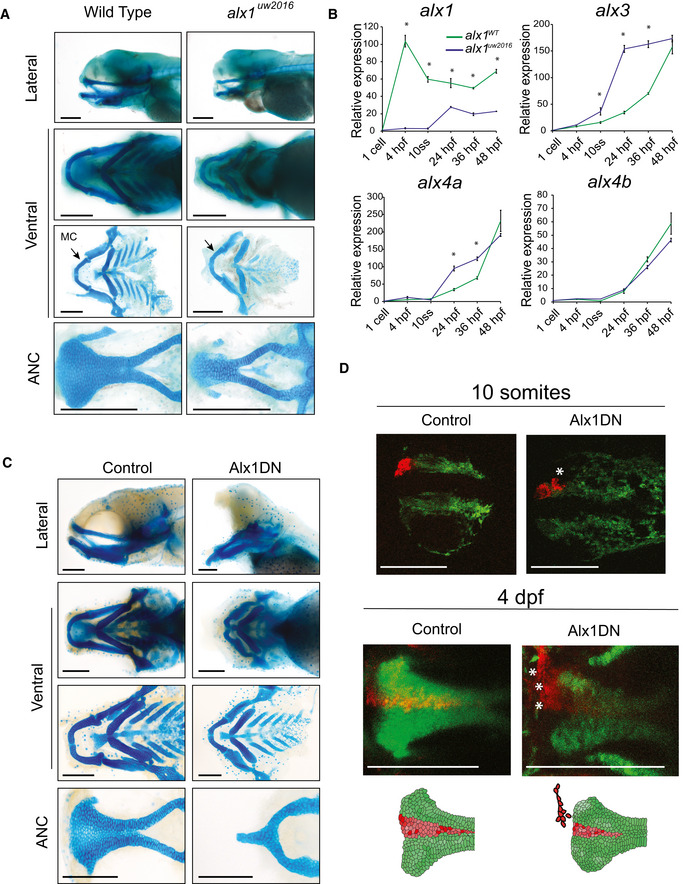
-
ADissected flatmount wild‐type and alx1 −/− zebrafish larvae craniofacial cartilages after Alcian blue staining, the anterior points to the left of the page in all images. The ventral cartilages appear normal, but the alx1 −/− anterior neurocranium (ANC) appears narrow, with the midline element that is derived from the frontonasal NCC being absent. Meckel's cartilage (arrow, MC) is also diminutive. Scale bar: 200 μm.
-
BZebrafish alx1 mutants (blue) show reduced detectable expression of alx1 but increased expression of alx3, alx4a compared to wild‐type controls (green). alx4b expression levels are similar between wild‐type and alx1 −/− lines. Data are represented as the mean of all pooled embryos from three different clutches. The RT–qPCR relative expression values were normalized to elfa and 18S expression using the ΔΔCT method. Data from each clutch were pooled, and the mathematical mean was calculated. SEM was used to determine the standard error. To test statistical significance, an ANOVA test was performed. A P‐value < 0.05 was considered to be statistically significant. Statistical significance denoted by *; P < 0.0001 between WT zebrafish and alx1 −/− at all measured time points; at 10 ss, 24 hpf and 36 hpf for alx3; and at 24 hpf and 36 hpf for alx4a. Refer to Table EV2 for P‐values.
-
CDissected flatmount of zebrafish embryos injected with Alx1DN, after Alcian blue staining. The embryos developed an absence of the frontonasal‐derived median portion of the anterior neurocranium (ANC) and a profound hypoplasia of Meckel's and ventral cartilages. In the most severely affected zebrafish, a nearly abrogated ANC was observed. Scale bar: 200 μm.
-
DLineage tracing experiments in control and Alx1DN mutant embryos revealed aberrant migration of anterior cranial NCC when alx1 is disrupted. In the control animal, the anterior cranial NCC always migrate to contribute to the median portion of the ANC. In contrast, the anterior cranial NCC labeled in the Alx1DN animals fail to migrate to the median ANC, where the ANC structure is narrower and the labeled cranial NCC are found in an anterior and lateral ectopic location (white asterisks). Scale bar: 250 μm.
Figure EV4. CRISPR/Cas9 targeted mutagenesis of alx1 in zebrafish.
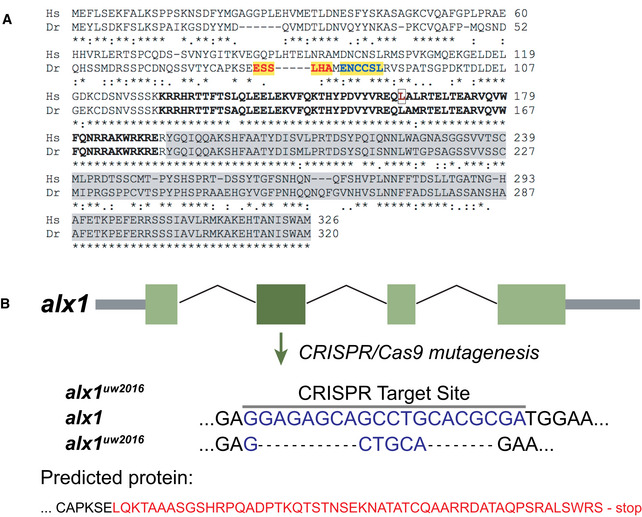
-
AHuman ALX1 and zebrafish alx1 protein sequences were obtained from Ensembl and aligned using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) under the default settings. The homeobox DNA‐binding domain is shown in bold, with the amino acid residue mutated in the subjects indicated by an outline. The transactivation domain is shaded in gray. Zebrafish alx1 CRISPR sites #1 and #2 are highlighted in yellow. The red and blue letters visually demarcate the sites.
-
BSchematic diagram shows the effect of the mutant allele resulting from our choice of target site #1. The allele, termed, alx1 uw2016, has a net deletion of 16 nucleotides. Red letters denote the abnormal sequence that results from the frameshift mutation.
Figure EV5. Qualitative and quantitative characterization of zebrafish mutants.
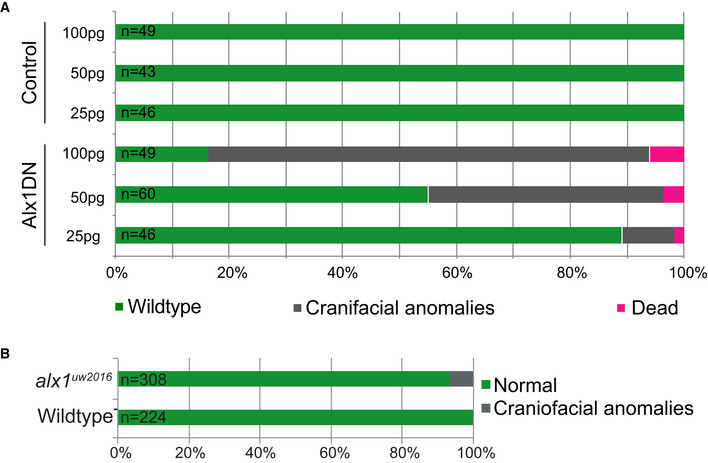
-
AThe number of embryos displaying craniofacial phenotypes increased with increasing concentration of Alx1DN mRNA injected into the single cell stage embryo. Overview of the relationship of the results of injections of 25, 50, and 100 pg of control mRNA and Alx1DN mRNA with the outcomes of wild‐type zebrafish (green), a craniofacial phenotype (gray), and dead zebrafish (magenta).
-
BThe number of embryos displaying craniofacial phenotypes injected with alx1 uw2016 mRNA was very low. Overview of the percent of injected wild‐type zebrafish displaying a craniofacial phenotype (gray), compared with uninjected wild‐type zebrafish from the identical clutches (gray). Data of the injections are presented as a comparative percentage.
The low penetrance of the ANC defect suggests the possibility of gene compensation by other alx family members (also see Discussion). To test this hypothesis, alx transcripts were quantified by qRT–PCR at several stages of embryogenesis in alx1 uw2016 homozygotes. We observed that alx1 mRNA level was significantly decreased in alx1 uw2016 mutants across several time points, likely because the mutation triggers nonsense‐mediated decay (Fig 6B; El‐Brolosy et al, 2019). Consistent with transcriptional gene compensation, alx3 and alx4a mRNA levels were significantly increased in the alx1 uw2016 mutant embryos compared to wild‐type embryos (P < 0.05 for 10 ss, 24 hpf and 36 hpf embryos for alx3; P < 0.05 for 24 hpf and 36 hpf embryos for alx4a). These data suggest that alx3 and alx4a functions may compensate for the loss of alx1 during zebrafish development (Fig 6B).
Given the likely genetic compensation between different alx family members, we utilized a dominant‐negative approach to interrogate the function of alx genes in embryonic development. It has been reported that ALX1 protein homodimerizes to be fully functional (Furukawa et al, 2002). In order to circumvent the genetic compensation by alx3 and alx4a, a truncated form of alx1 containing the N‐terminal domain (Alx1DN) was generated. The Alx1DN truncation contains the DNA‐binding homeodomain but is missing the transactivation domain (Herskowitz, 1987). Additionally, an alternative truncated protein that lacks the DNA‐binding homeodomain and contains the transactivation domain, termed Alx1CT, was generated. We reasoned that, if a truncated Alx1 protein can occupy its binding sites but fail to dimerize or associate with its coactivators, it may function in a dominant‐negative manner.
When Alx1DN was overexpressed by mRNA injection in wild‐type zebrafish embryos, embryos displayed significant craniofacial defects (Fig 6C), while Alx1CT‐expressing embryos developed normally. On closer inspection with Alcian blue staining, Alx1DN‐expressing embryos showed complete abrogation of the frontonasal‐derived median section of the ANC and Meckel's cartilage, a similar but more severe phenotype as expected due to the interference with all alx proteins than that observed in alx1 uw2016 mutant embryos. Both alx1 uw2016 and the Alx1DN mutant phenotypes suggest that alx1 regulates the migration of the anterior frontonasal NCC stream that contributes to the median portion of the ANC.
Lineage tracing reveals migration defect of NCC
To elucidate whether anterior cranial NCC migration is specifically affected in Alx1DN embryos, lineage tracing of the migrating NCC was carried out using the Tg(sox10:kaede) reporter line (Wada, 2005; Eberhart, 2006; Dougherty et al, 2012; McGurk et al, 2014). The anteromost stream of NCC in wild‐type and Alx1DN injected embryos was labeled at the 10‐somites stage and followed to 96 h post‐fertilization (Fig 6D). In the wild‐type embryos, at 4 days post‐fertilization (dpf), the NCC were able to migrate into the frontonasal region of the palate (Fig 6D). In contrast, the anterior cranial NCC of Alx1DN injected embryos were unable to reach their final location of the median ANC. Instead, the NCC in the AlxDN injected zebrafish were found in an ectopic anterior location outside of the frontonasal domain (Fig 6D, Movie EV3). While it is possible that increased cell death and altered cell division that were observed in the iPSC model are also operating here in the embryo, these cellular derangements are likely minor contributors to explain the ectopic localization of the cranial NCC, whereas altered cell migration is the dominant mechanism.
Discussion
We report the identification of a novel missense variant of human ALX1 associated with FND. Analysis of this putative loss‐of‐function variant in patient‐derived iPSC and NCC showed a lack of cellular maturation, an increase in apoptosis, and a migration defect. We identified an overexpression of neural plate border specifiers in subject‐derived cells, and an imbalance of BMP levels which, when addressed, was capable of mitigating the migration defect discovered in the subject's NCC. A delay of NCC migration was also recognized as key to the morphologic consequences of a loss of alx1 in zebrafish models. We could identify genetic compensation between different members of the alx gene family.
Human genetics of ALX1
Genes of the ALX family encode homeodomain transcription factors and are associated with craniofacial malformations. Like other members of this family, the ALX1 protein is composed of two main functional domains: the N‐terminal portion containing the DNA‐binding homeodomain with two nuclear localization signals, and the C‐terminal portion containing an OAR/aristaless domain required for transactivation and protein interaction (Furukawa et al, 2002). In this study's pedigree, a novel missense variant p.L165F within the conserved homeodomain was identified. Leucine is an aliphatic, branched amino acid, while phenylalanine is an aromatic, neutral, and nonpolar amino acid. Due to properties of leucine, the substitution itself is likely disruptive to helix II in the DNA‐binding element of the homeodomain within which it resides. Disruptive leucine to phenylalanine substitutions have been described in a number of published, genotyped disorders (Miller et al, 1992; Gomez & Gammack, 1995). Pathogenic missense variants within the homeodomains of both ALX3 and ALX4 have previously been identified as the causes of FND types 1 and 2, respectively (Wuyts et al, 2000; Kayserili et al, 2009; Twigg et al, 2009).
Pathogenic ALX1 gene variants in FND have been reported in two case studies in the literature. The first study described two families (Uz et al, 2010). In the first, three siblings of consanguineous parents were described to suffer from a midline defect of the cranium, bilateral extreme microphthalmia, bilateral oblique cleft lip and palate, a caudal appendage in the sacral region, and agenesis of the corpus callosum. A homozygous 3.7 Mb deletion spanning ALX1 was identified in all affected subjects. In a second family, one affected subject was born with a midline defect of the cranium, bilateral microphthalmiamicrophthalmia, bilateral oblique cleft lip and palate, and a thin corpus callosum. A donor splice‐site mutation c.531+1G>A of ALX1, homozygous in the child and heterozygous in the parents, was identified to be the likely cause of the child's phenotype.
None of the affected subjects from the pedigree reported in this study demonstrated midline defects of the cranium or a cerebral phenotype. This is in spite of the fact that the missense mutation of the affected subjects in our study lies just proximal to that of family 2, within helix II, within the homeodomain.
A second case report described one family with FND (Ullah et al, 2016). It reported on four children born of consanguineous parents that presented with a broad nasal root, smooth philtrum, and mouth protrusion. An ALX1 gene variant c.661‐1G>C was found to be heterozygous in the parents and homozygous in the affected children. The skipping of the exon via alternative splicing likely resulted in a mutant protein with some residual function, explaining the relatively mild phenotype.
The ALX gene family: ALX1, ALX3, and ALX4
The ALX gene family consists of three members: ALX1, ALX3, and ALX4 (McGonnell et al, 2011). In humans, mutations of each ALX gene have been associated with craniofacial malformations of the frontonasal‐derived structures with variable phenotypic severity (Wu et al, 2000; Wuyts et al, 2000; Mavrogiannis et al, 2001; Kayserili et al, 2009; Twigg et al, 2009; Uz et al, 2010). FND is a descriptive term that broadly describes a number of malformations of the midface. Previously classified based on appearance (see Tessier, Sedano, De Myer classifications), FND related to variants within the ALX gene family has recently been reordered on the basis of genetics: Type I is caused by mutations of ALX3; type 2 is caused by mutations of ALX4; and type 3 is caused by mutations of ALX1. FND types 1 and 2 appear milder than type 3, frequently presenting with altered appearance of the nasal soft tissue (Twigg et al, 2009).
In mouse and zebrafish, Alx1, Alx3, and Alx4 have been shown to be expressed in spatiotemporally restricted regions of the craniofacial mesenchyme (Zhao et al, 1994; Qu et al, 1997; ten Berge et al, 1998; Beverdam & Meijlink, 2001; Dee et al, 2013; Lours‐Calet et al, 2014). Evidence of gene compensation has previously been reported in animal studies (Beverdam et al, 2001; Dee et al, 2013). In studies of sea urchins, Alx4 was shown to be directly regulated by Alx1 (Rafiq et al, 2012; Khor et al, 2019). The question remains how the different ALX family members regulate craniofacial development, through transcriptional activation or repression of shared and unique target genes.
iPSC‐derived NCC for craniofacial disease modeling
Most craniofacial structures are derived from a transient multipotent embryonic population called NCC. The NCC progenitors give rise to a wide variety of cell lineages including peripheral neurons, melanocytes, and craniofacial mesenchyme (Betancur et al, 2010; Stuhlmiller & Garcia‐Castro, 2012). NCC exhibit a restricted expression of ALX1 in the rostral domain during early developmental stages (Zhao et al, 1996; Dee et al, 2013). Cellular and genetic mechanisms that drive frontonasal NCC formation are poorly understood. In order to gain insight into the functional consequences of the clinically pathogenic ALX1 gene variant identified in this study's pedigree, iPSC were differentiated into NCC.
While a number of sophisticated protocols using chemically defined mediums and a combination of adherent and/or suspension culturing approaches have been published in recent years, no consensus has been established on a number of controversial issues (Bajpai et al, 2010; Leung et al, 2016; Tchieu et al, 2017).
First, the definition of what a NCC in fact is remains based entirely on the transcriptomic profiling performed in animal studies. While we succeeded in identifying distinctive differences between the ALX1 165F/165F NCC and healthy controls, the results suffer from an obvious limitation: a lack of understanding which stage of development the NCC represent. The central challenge of the work with iPSC models of human disease remains the lack of available human transcriptomic cell data to allow for an understanding which stage of development is modeled by the cellular lineages derived. NCC are characterized in vitro by the expression of markers identified to be specific to this cell population, namely P75, CD57, CD90, CD73, and CD105 (Minarcik & Golden, 2003; Billon et al, 2007; Kawano et al, 2017) as well as their multilineage differentiation ability. NCC formation is a stepwise process coordinated by a spatiotemporally specific gene expression pattern. In this study, a putative loss of ALX1 function did not impair NCC differentiation itself or the ability of NCC to differentiate into multiple cell lineages. Rather, it appeared that the clinically pathogenic ALX1 165F/165F variant maintained the NCC in a precursor state. While control cells progressively became craniofacial mesenchymal cells by CD57 downregulation and increases in MSC associated marker expression, ALX1 165F/165F NCC did not undergo changes of their morphology or show a transition of progenitor to mesenchymal markers. Second, a lack of a 3D representation of NCC migration in vitro based studies force scientists to either transplant human iPSC‐derived NCC into model organisms or retain a 2D system of representation (Bajpai et al, 2010; Okuno et al, 2017). We focused on the validation of the findings in human iPSC in zebrafish. Third, the development of craniofacial mesenchyme is a product of the interactions of derivatives of all three germ layers. This left the role of ALX1 in other developmental derivatives unexplored in this study.
To allow for some insight into the expression profile of key NCC markers during the in vitro differentiation process, we surveyed relative expression via qPCR every 2 days throughout our differentiation protocol. We found the greatest differences between the unaffected father and the affected children in the expression of PAX7, PAX3, DLX5, SNAI2, and TWIST1. ALX1 has been described as a transcription modulator capable of both activating and repressing target gene expression, adapting its activity to different cell types and environments (Gordon et al, 1996; Cai, 1998; Damle & Davidson, 2011). Its activity as a repressor, for example, has been documented with prolactin, as ALX1 binds directly to the prolactin promoter (Gordon et al, 1996). In this study, all of the genes were substantially upregulated in the affected children, with the greatest changes found in the earlier time points of differentiation. ALX1 appears to play the role of a transcriptional repressor in NCC‐based craniofacial development.
Neural crest cells delamination and migration depend on signals from the surrounding epidermis, including BMPs, which induce expression of neural plate border specifier genes such as PAX3, TFAP2a, MSX1/2 or ZIC1 (Tribulo et al, 2003; Sato et al, 2005; Garnett et al, 2012; Dougherty et al, 2013; Simoes‐Costa & Bronner, 2015). Fine temporospatial regulation of the level of these signaling molecules is critical to allow for delamination and migration of NCC craniofacial development. BMPs belong to the transforming growth factor beta (TGFβ) superfamily and can be divided into several subcategories based on molecular similarities. The two BMPs showing dysregulation in this study, BMP2 and BMP9, belong to different subcategories which exhibit different expression patterns and receptors. BMP2 was identified as an important factor in migratory NCC development, with a depletion of mobile NCC in knockout models in transgenic mice resulting in hypomorphic branchial arches. (Kanzler et al, 2000). In a complementary mouse model, BMP2 increased migration of NCC when added as a supplement to culture medium (Anderson et al, 2006). BMP2 was also found to be required for the migration of NCC in the enteric nervous system in the zebrafish and to be significantly decreased in the gut of patients affected by Hirschsprung's disease, a disease characterized by deficient enteric NCC migration (Huang et al, 2019a). BMP9 on the other hand was shown to be required for tooth development in mice (Huang et al, 2019b). It was previously identified as a potent inducing factor of osteogenesis, chondrogenesis, and adipogenesis during development (Luther et al, 2011; Lamplot et al, 2013). Opposed to other BMPs, including BMP2, BMP9 was found to be resistant to feedback inhibition by BMP3 and noggin (Wang et al, 2013).
The relationship of BMP2 and BMP9 in NCC development, migration, and differentiation has yet to be examined. Why BMP2 and BMP9 appeared to play antagonistic roles in the NCC modeling of ALX1‐related FND presented in this study remains unclear. On the basis of the qPCR data and the multiplex assay revealing a decrease in BMP2 and an increase in BMP9 in NCC supernatant, we hypothesized that a lack of fully functional ALX1 may account for the overexpression of neural plate border specifiers, and the change of BMP signaling. In substituting or repressing BMP2 and BMP9, respectively, an almost complete rescue of the migration defect of the mutant ALX1 165F/165F NCC was achieved. Pretreatment of subject‐derived NCC could perhaps result in a complete rescue of migration.
Animal models of ALX1‐related FND
Studies in sea urchins have contributed meaningful knowledge to the regulatory functions of Alx1 as a transcription factor. In the sea urchin Strongylocentrotus pupuratus, the alx1 gene was found to activate itself in a self‐regulatory loop at lower levels. Once its level exceeds a certain threshold, alx1 reverses its activity and becomes a repressor of its own transcription (Damle & Davidson, 2011). As a transcription factor, alx1 was found to be essential for the regulation of epithelial–mesenchymal transition, a process of great importance for the ability of NCC to delaminate and initiate migration (Ettensohn et al, 2003).
The specific role of Alx1 in craniofacial development was investigated in different animal models. Targeted gene ablation of Alx1 in mice resulted in neural tube closure defects in the majority of the pups, a phenotype not observed in any reported case report of ALX1‐related FND type 3 (Zhao et al, 1996). A previously published morpholino knockdown of alx1 in zebrafish suggested that the gene is essential for the migration of NCC into the frontonasal prominence, with a disorganization of NCC in the frontonasal stream, and reduction both in the number of NCC and its cellular projections (Dee et al, 2013). A major weakness of morpholino gene disruption is nonspecific or off target effects. This study utilized germline alx1 mutant allele to investigate the effect of alx1 loss‐of‐function, complemented by a dominant‐negative disruption approach to address gene compensation of other alx family members. These approaches corroborate a requirement for alx1 in median ANC morphogenesis, corresponding to formation of the midface in humans.
In summary, this work describes a novel ALX1 gene variant associated with FND. Using complementary human iPSC and zebrafish models, this study showed that ALX1 is required for coordinated NCC differentiation and migration. Discordance of NCC differentiation from cell migration during midface morphogenesis results in FND. Future work will be directed at identifying ALX1 downstream targets and characterize the ALX regulated pathways in craniofacial development.
Materials and Methods
Approvals to perform research with human samples and zebrafish
The collection of human blood and discard specimens, genome sequencing, and generation of iPSC was approved by the Institutional Review Board of Partners Healthcare (IRB No. 2015P000904). Informed consent was obtained from the parents of the patients prior to all sample collections. All experimental protocols using zebrafish were approved by the Animal Care and Use Committees of Massachusetts General Hospital (IACUC No. 2010N000106) and the University of Wisconsin and carried out in accordance with institutional animal care protocols.
iPSC and EB generation
Peripheral blood mononuclear cells were isolated using whole blood from two individuals (subjects 5 and 6: ALX1 165F/165F), the unaffected father (subject 1: ALX1 165L/165F), and three unrelated healthy individuals (controls: ALX1 165L/165L). Samples were diluted in an equal volume of PBS and gently transferred to a tube containing 4 ml of FICOLL. After centrifuging the sample for 10 min at 350 g, the FICOLL‐plasma interface containing the PBMCs was recovered and washed several times in PBS. After 24 h of recovery in StemPro‐34 SFM medium (Invitrogen) supplemented with 100 ng/ml Stem Cell Factor (SCF, PeproTech, Rocky Hill, NJ), 100 ng/ml Fms‐related tyrosine kinase 3 ligand (Flt3L, PeproTech), 20 ng/ml Interleukin‐3 (IL‐3, PeproTech) and 20 ng/ml IL‐6 (PeproTech), 1 million PBMC were processed using the CytoTune‐iPS 2.0 Sendai Reprogramming Kit (Invitrogen, Carlsbad, CA), following manufacturers instruction, for iPSC generation. One million PBMCs were infected with three different Sendai Viruses containing the Yamanaka reprogramming factors, OCT4, SOX2, KLF4, and c‐MYC, in StemPro‐34 SFM medium supplemented with cytokines. Starting on day 21, individual iPSC clones were picked based on morphologic criteria. Subsequently, the iPSC were maintained in StemFlex medium and passaged 1–2 a week using ReLSR (STEMCELL Technologies, Vancouver, BC, Canada) dissociation buffer. Since iPSC can exhibit genetic instability after reprogramming, the clones were expanded up to passage 10 before characterizing each cell line. The genetic stability of the cells was assessed analyzing copy number variants through genome‐wide microarray analysis (Thermo Fisher). Epigenetic differences were controlled for in a limited manner by ensuring that all major experiments were performed in both biologic and technical triplicate at the identical passage number.
To form embryoid bodies (EB), iPSC were harvested using ReLSR dissociation buffer and clumps were transferred to a low adherent 6‐well plate in differentiation medium containing 80% DMEM‐F12, 20% Knock out Serum Replacer (Invitrogen), 1 mM nonessential amino acids, 1 mM Penicillin/Streptomycin, and 100 μM 2‐mercaptoethanol. The medium was changed daily. After 14 days of differentiation, cells were recovered for RNA extraction and subsequent qPCR analysis of markers of the ectoderm, endoderm, and mesoderm.
Derivation of NCC and multilineage differentiation
In order to derive NCC, a previously published protocol for mesenchymal differentiation was adapted (Pini et al, 2018). iPSC medium was replaced by NCC‐inducing medium containing DMEM‐F12, 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 1 mM Penicillin/Streptomycin, 1 mM nonessential amino acid, 110 μM 2‐mercaptoethanol, and 10 ng/ml epidermal growth factor (EGF). The medium was changed every 2 days. After 1 week, cells were recovered using 0.25% trypsin‐EDTA and transferred to new cultureware for an additional week. Following this, cells were harvested, phenotypically characterized by flow cytometry for their expression of NCC markers and assayed for their mesenchymal differentiation ability.
Schwann cell differentiation was performed as previously described (Kawano et al, 2017). NCC were plated on glass coverslips in 24‐well tissue culture plates (0.2 × 105 cells per well) in neuronal differentiation medium consisting of a 3:1 ratio of DMEM‐F12 and neurobasal medium supplemented with 0.25× B‐27, 1 mM glutamine, and 1 mM Penicillin/Streptomycin for 5 weeks. The medium was changed weekly. At the end of the differentiation, cells were fixed in 4% formaldehyde and analyzed by immunohistochemistry for S100B (Thermo Fisher, Waltham, MA) and GFAP expression (Abcam, Cambridge, United Kingdom).
Adipocyte and chondrocyte differentiation was performed as previously described (Pini et al, 2018). Adipogenesis was investigated using the StemPro adipogenesis differentiation kit (Life Technologies, Carlsbad, CA). NCC were seeded at 5 × 104 per well, in a 24‐well plate, and cultured for 2 weeks, in complete adipogenesis differentiation medium. Lipid deposits were observed following staining with Oil Red O (MilliporeSigma, St. Louis, MO), according to manufacturers’ instructions. After washing, cells were counterstained with Mayer's hematoxylin.
Chondrogenic differentiation was performed using the StemPro chondrogenesis differentiation kit (Life Technologies). NCC were seeded in a 12‐well plate, in aggregates containing 8 × 104 cells, in 5 μl of NCC medium, and placed in a 37°C, 5% CO2 incubator for 1 h. Following this, the NCC medium was replaced by chondrogenesis differentiation medium, and cultured for 20 days. The medium was changed once a week. Chondrogenic matrix formation was observed following Alcian blue, Safranin O and Toluidine Blue staining.
Osteoblast differentiation was performed using the StemPro osteogenesis differentiation kit (Life Technologies). NCC were plated in 12‐well tissue culture plates (5 × 105 cells per well) in osteogenesis differentiation medium for 14 days. At the end of the differentiation, the presence of mineralized nodules was assessed using Alizarin Red S, Von Kossa (silver nitrate) and Alkaline Phosphatase staining.
Images were acquired using the RETIGA OEM fast camera and Qcapture software (Teledyne QImaging, Surrey, BC, Canada).
Genomic DNA extraction and sequencing
Genomic DNA was extracted with the REDExtract‐N‐Amp tissue PCR kit (MilliporeSigma), following the manufacturer's instructions. Sanger sequencing of ALX1 to ensure ALX1 sequence integrity in all iPSCs clones was carried out as previously described (Umm‐e‐Kalsoom et al, 2012). The four ALX1 exons encoding the open reading frame were amplified using the CloneAmp HiFi PCR premix (Takara Bio Inc., Kusatsu, Shiga, Japan) and exon‐specific ALX1 oligonucleotides. All exon‐specific PCR products were purified using the Qiaquick PCR purification kit (Qiagen, Hilden, Germany) prior to sequencing.
Whole‐exome sequencing and analysis
Whole‐exome sequencing (WES) of the affected subjects 3 and 4, an unaffected sibling, and the parents was performed and analyzed assuming a recessive mode of inheritance given the presence of multiple affected siblings. Three compound heterozygous variants and one homozygous recessive variant were identified in the affected siblings (ALX1 c.493C>T). This variant was predicted to be causative of the phenotype based on known gene function, the previously identified role of ALX1 in frontonasal development, and the effect of the variant (substitution of phenylalanine for the highly conserved leucine in a DNA‐binding domain). Polymorphism Phenotyping v2, Sorting Intolerant from Tolerant, MutationTaster, and Functional Analysis through Hidden Markov Models (v2.3) were used for functional variant consequence prediction (Lowe, 1999; Adzhubei et al, 2010; Schwarz et al, 2014; Shihab et al, 2014). The gnomAD platform was used to identify any other missense variants at the location identified in the subjects (preprint: Karczewski et al, 2019). Clustal Omega was used for multiple sequence alignment (Sievers et al, 2011). Domain Graph was used to create the annotated schematic diagrams of ALX1 and ALX1 (Ren et al, 2009).
RNA extraction and processing
RNA was isolated using the RNAeasy Plus mini kit (Qiagen), following the manufacturer's recommendations. One μg of RNA was reverse‐transcribed using the SuperScript III first‐strand synthesis system (Thermo Fisher). All PCR reactions on cDNA were performed using the GoTaq DNA polymerase (Promega, Madison, WI) unless otherwise noted. For zebrafish RNA extraction, 24 hpf Tübingen zebrafish embryos were harvested and homogenized using a micropestle in TRIzol reagent (Thermo Fisher), following manufacturer's instructions. Total RNA was then purified using phenol‐chloroform. One μg of total RNA was reverse‐transcribed using the SuperScript III First‐Strand Synthesis Kit (Thermo Fisher), following manufacturer's recommendations.
Flow cytometry analysis
Neural crest cells were harvested and suspended in FACS buffer solution consisting of PBS with Ca2+ and Mg2+, 0.1% bovine serum albumin (BSA), and 0.1% sodium azide. Approximately 2 × 105 cells were incubated with the desired cell surface marker antibodies or isotype controls at 4°C for 15 min. Specific antibodies for CD90, CD73, CD105, and CD57 (BD Biosciences, San Jose, CA), and isotype control immunoglobulin IgG1 (BD Biosciences) were used for labeling. Antibodies were diluted in FACS buffer. After three washes in FACS buffer, samples were fixed in 0.4% formaldehyde and processed using an LSR II flow cytometer (BD Biosciences). The data acquired were analyzed using FlowJo software (FlowJo, LLC).
Immunohistochemical analysis of iPSC
Cells were fixed with 4% formaldehyde in PBS for 15 min at room temperature, permeabilized with 1% saponin in PBS, and blocked using 3% BSA in PBS for 30 min at room temperature. The cells were then incubated with the primary antibodies for 3 h at room temperature. The following primary antibodies and dilutions were used: rabbit anti‐OCT4 (1:100, Life Technologies), mouse anti‐SSEA4 (1:100, Life Technologies); rat anti‐SOX2 (1:100, Life Technologies), mouse anti‐TRA‐1‐60 (1:100, Life Technologies), rabbit anti‐GFAP (1:500, Abcam), and rabbit anti‐S100B (1:500, Thermo Fisher). The cells were then incubated with the secondary antibodies (1:1,000, MilliporeSigma) for 1 h at room temperature, washed with PBS, and counterstained with DAPI (MilliporeSigma). Secondary antibodies were Alexa 594 donkey anti‐rabbit, Alexa 488 goat anti‐mouse, and Alexa 488 donkey anti‐rat and Alexa 594 goat anti‐mouse (Thermo Fisher). Images were acquired using the RETIGA OEM fast camera and Qcapture software (Teledyne QImaging).
Staining of iPSC and mesenchymal NCC derivatives
Alkaline phosphatase activity was measured using the leukocyte alkaline phosphatase staining kit (MilliporeSigma), following the manufacturer instructions. Cells were first fixed using a citrate/acetone/formaldehyde solution for 30 s, washed several times, and stained with Fast Blue for 30 min. After further washing, these cells were counter stained with Mayer's hematoxylin. Alizarin Red S., Von Kossa, Alcian Blue, and Toluidine Blue staining were performed as previously described (Pini et al, 2018). Cells were first fixed in 4% formaldehyde at room temperature for 15 min. Following a wash, cells were incubated in either 1% Alizarin Red, 1% Silver nitrate, 0.1% Toluidine Blue, 0.02% Alcian Blue, or 0.1% Safranine O solution. For Von Kossa staining, cells were exposed to UV light until dark staining appeared. Images were acquired using the RETIGA OEM fast camera and Qcapture software (Teledyne QImaging).
Quantitative and nonquantitative polymerase chain reaction
Real‐time PCR assays were conducted on a StepOnePlus real‐time PCR system, using PowerUp SYBR Green Master Mix (Applied Biosystems, Waltham, MA). Transcript expression levels were evaluated using a comparative CT process (ΔΔCT) with human RPLP0 and GAPDH used as reference genes. For zebrafish, elfa and 18S were used as reference genes. Specific primers were used for amplification as noted (Table EV1).
Apoptosis assay
2 × 105 cells were incubated for 30 min in the dark in 1× Fixable Viability Dye (FVD, Invitrogen) solution. After two washes in FACS buffer and one wash in binding buffer, cells were incubated 10–15 min in 1× Annexin V (BioLegend, San Diego, CA) solution in binding buffer composed of 0.1 M HEPES (pH 7.4), 1.4 M NaCl, and 25 mM CaCl2. After one wash in binding buffer, cells were suspended in 200 μl of binding buffer and immediately processed using an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (FlowJo LLC, Ashland, OR). Apoptosis was induced by placing the cell suspension in a water bath at 55°C for 10 min.
Wound healing assay and analysis
Migration was investigated using the Radius™ 48‐Well Cell Migration Assay (Cell Biolabs, Inc., San Diego, CA), following manufacturer's instructions. Control or ALX1 165F/165F NCC (1 × 105 cells/well) were plated in a 48‐well plate containing a Radium™ Gel spot. Before beginning the migration assay, cells were washed three times with medium and incubated with gel removal solution for 30 min at 37°C. Following three subsequent washes in medium, the NCC were placed in a culture chamber for live cell imaging at 37°C and 5% CO2. Rescue experiments were performed through the addition of soluble BMP2, CV2, or a combination of the two at a concentration of 10, 50, or 100 ng/ml to the medium at the beginning of the assay. For fluorescent pictures, cells were stained in serum‐free media containing 3.6 μM CellTracker Green CMFDA (Life Technologies) for 30 min at 37°C and allowed to recover for 30 min before starting the experiment. All images were acquired using a Keyence BZ‐X800 microscope. The time‐lapse film was made by acquiring images every 15 min for 24 h. The fluorescent images were acquired every 6 h. Surface area analyses and percentages of recovery were measured using ImageJ software (NIH, Bethesda, MD).
Multiplex analysis of BMP concentration
The concentration of the BMP family in the supernatant of ALX1 165F/165F NCC was measured using a bead‐based multiplex array (Forsyth Institute, Cambridge, MA). Manufacturers’ protocols were followed for all panels. Reagents were prepared as per kit instructions. Assay plates (96‐well) were loaded with assay buffer, standards, supernatant from the ALX1 165F/165F NCC, and beads and then covered and incubated on a plate shaker (500 rpm) overnight at 4°C. After primary incubation, plates were washed twice. Following this, the detection antibody cocktail, consisting of BMP‐specific antibody with biotin (1:10) and the detection antibody streptavidin conjugated with PE (1:25), was added to all wells; the plates were covered and left to incubate at room temperature for 1 h on a plate shaker. After the incubation, streptavidin‐phycoerythrin fluorescent reporter was added to all wells, and the plate was covered and incubated for 30 min at room temperature on a plate shaker. Plates were then washed twice, and beads were resuspended in sheath fluid, placed on shaker for 5 min, and then read on a Bio‐Plex® 200 following manufacturers’ specifications and analyzed using Bio‐Plex Manager software v6.0 (Bio‐Rad, Hercules, CA).
Plasmid construct generation
The In‐Fusion Cloning Kit (Takara) and the In‐Fusion Cloning primer design tool were used for primer design. Tübingen zebrafish alx1 was amplified via PCR. Zebrafish alx1 as cloned into the SpeI and PacI (NEB, Ipswich, MA) restriction sites of pCS2 + 8 (Promega). The subsequent reaction product was used to transform One Shot TOP10 competent cells (Thermo Fisher) or Stellar competent cells (Takara).
For the generation of truncated alx1 constructs, the genes were divided into N‐terminal and C‐terminal sections, with aa181 being designated as the beginning of the C‐terminal portion in zebrafish alx1.
For all plasmid constructs, individual clones were picked, DNA purified (Qiagen), and validated using Sanger and Next Generation whole plasmid sequencing.
CRISPR‐Cas9 directed mutagenesis of zebrafish
A CRISPR site on exon 2 of the alx1 gene was selected using the Burgess lab protocol (Varshney et al, 2015, 2016). The single guide RNA (sgRNA) targeting this site (sequence: GGAGAGCAGCCTGCACGCGA), and Cas9 or nCas9n mRNA, were prepared as previously described (Gagnon et al, 2014; Shah et al, 2016a,b). Genetically defined wild‐type (NIHGRI‐1; LaFave et al, 2014) embryos were injected at the one‐cell stage with 50–100 pg of sgRNA and 360–400 pg of Cas9 or nCas9n mRNA. Adult F0 animals were intercrossed to produce the F1 generation. F1 mutant carriers were identified by PCR using forward primer CGTGACTTACTGCGCTCCTA and reverse primer CGAGTTCGTCGAGGTCTGTT. The PCR products were resolved on a MetaPhor gel (Lonza, Basel, Switzerland) and sequenced. A frameshift allele was identified: a deletion of 16 nucleotides, termed alx1 uw2016 (Fig EV4).
Alx1DN expression in zebrafish embryos
The validated Alx1DN (N‐terminal portion of protein product containing homeodomain and nuclear localization domains) clones in pCS2+8 were purified via miniprep (Qiagen) alongside a control (C‐terminal portion containing transactivation domain) and digested using NotI (NEB), before being gel purified using the Zymoclean Gel DNA Recovery Kit (Zymo Research, Irvine, CA). Five hundred microgram of purified, digested plasmid DNA served as the input for the mMessage mMachine SP6 Transcription Kit (Thermo Fisher). The resulting mRNA was then further purified using the RNeasy Mini Kit (Qiagen) and frozen in 100 ng/μl aliquots at −80°C. mRNA overexpression was accomplished using microinjections. mRNA stock aliquots were first diluted to the desired concentration with 0.125% Phenol Red in ultrapure water (Invitrogen). A 2 nl drop was then injected into fertilized Tübingen embryos at the single cell stage. At 4 h post‐fertilization (hpf), all unfertilized and visibly damaged embryos were removed.
Alcian blue staining
All injected and uninjected zebrafish were incubated at 28.5°C for 5 dpf in E3 buffer with 0.0001% methylene blue. At 4 days post‐fertilization (dpf), injected and uninjected embryos were fixed overnight at 4°C in 4% formaldehyde, washed stepwise with 1 × PBS and 50% EtOH in PBS before being stained in a solution of 0.02% Alcian blue, 70% EtOH, and 190 mM MgCl2 overnight at room temperature on a rotating platform. Following this, embryos were washed with ddH2O before being bleached in a solution of 0.9% H2O2, 0.8% KOH, and 0.1% Tween20 for 20 min. Stained embryos were then imaged in 4% methylcellulose in E3 solution and stored in 4% PFA at 4°C. Images were captured using a Nikon DS‐Fi3 digital camera.
Lineage tracing
The application of Tg:sox10:kaede in cell labeling has previously been reported, where photoconversion of the kaede protein from green to red in selected cells under confocal microscopy can be used to follow distinct NCC migration patterns across time.
Alx1DN injected or control sox10:KAEDE transgenic embryos were imaged using a Leica SP8 confocal microscope at 19 to 20‐somite stage to identify neural crest structures in a manner previously described (Dougherty et al, 2012, 2013). The most distal population of the migrating stream of cranial neural crest cells was excited for 15 s for photoconversion with the FRAP module and a 405 nm laser at 25% power. Embryos were then placed back into a 28.5°C incubator. Both the photoconverted red (488 nm) and nonphotoconverted green (572 nm) neural crest populations were captured at 4 days post‐fertilization (dpf) using a Leica Sp8 and analyzed with the Leica Application Suite X (Leica Microsystems, Buffalo Grove, IL) software for image capturing. A composite image was subsequently generated using ImageJ (NIH; Fig 8D, Film 3).
Statistical analysis
Each experiment was performed on six independent healthy control ALX1 165L/165L clones, three heterozygous ALX1 165L/165F clones, and nine homozygous ALX1 165F/165F clones, and repeated at least three times. The qualitative craniofacial analysis of alx1 −/− zebrafish and Alx1DN injections was performed three times, on three different clutches of embryos. For RT–qPCR experiments, data from each clone were pooled and the mathematical mean was calculated. SEM was used to determine the standard error. To test statistical significance, Student's t‐test for paired data was used. Statistical analysis of the significance of the qPCR results was performed with an ANOVA test. A P‐value < 0.05 was considered to be statistically significant. Statistical analysis was performed using GraphPad Prism 7.0 software. The D'Agostino and Pearson normality test was performed to verify normality. For groups that fulfilled normality and equal variance requirements, a one‐way ANOVA with a Sidak comparison test (95% confidence interval to compare all the different groups) was performed. For data sets that did not fulfill normality and equal variance requirements, a Kruskal–Wallis test was performed. Mean values for each group were compared using two‐tailed Student's test for comparisons of two independent groups.
Author contributions
JP, JK, and ECL conceived of the project and designed the research studies. JP, JK, YDH, CT, KK, PY, BY, NC, RM, JC, and YG conducted the experiments. JP, JK, CT, NC, RM, JC, YG, and ECL prepared the manuscript. JK, CT, and ECL worked on the revision of the manuscript.
Conflict of interest
The author declares that he has no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Table EV4
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Source Data for Expanded View and Movies
Review Process File
Source Data for Figure 1E
Source Data for Figure 2B
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
We are grateful for Shriners Hospital for Children (85112) and National Institute of Health (U01DE024443) for funding that supported this work. ECL is a recipient of the Massachusetts General Laurie and Mason Tenaglia Research Scholar award. JK is a recipient of the Shiners Hospital Research Fellowship (84701‐BOS‐19). We thank Jessica Bathoney for excellent management of our aquatics facility.
EMBO Mol Med (2020) 12: e12013
Data availability
The raw sequencing data sets produced in this study are available in the following database: FaceBase Record ID: 25J0 Accession: FB00000907 (https://www.facebase.org/).
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Stottmann RW, Choi M, Klingensmith J (2006) Endogenous bone morphogenetic protein antagonists regulate mammalian neural crest generation and survival. Dev Dyn 235: 2507–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada‐Iglesias A, Zhang J, Xiong Y, Helms J, Chang CP, Zhao Y, Swigut T, Wysocka J (2010) CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463: 958–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrallo‐Gimeno A, Holzschuh J, Driever W, Knapik EW (2004) Neural crest survival and differentiation in zebrafish depends on mont blanc/tfap2a gene function. Development 131: 1463–1477 [DOI] [PubMed] [Google Scholar]
- ten Berge D, Brouwer A, el Bahi S, Guenet JL, Robert B, Meijlink F (1998) Mouse Alx3: an aristaless‐like homeobox gene expressed during embryogenesis in ectomesenchyme and lateral plate mesoderm. Dev Biol 199: 11–25 [DOI] [PubMed] [Google Scholar]
- Betancur P, Bronner‐Fraser M, Sauka‐Spengler T (2010) Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol 26: 581–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverdam A, Brouwer A, Reijnen M, Korving J, Meijlink F (2001) Severe nasal clefting and abnormal embryonic apoptosis in Alx3/Alx4 double mutant mice. Development 128: 3975–3986 [DOI] [PubMed] [Google Scholar]
- Beverdam A, Meijlink F (2001) Expression patterns of group‐I aristaless‐related genes during craniofacial and limb development. Mech Dev 107: 163–167 [DOI] [PubMed] [Google Scholar]
- Bhoj EJ, Li D, Harr MH, Tian L, Wang T, Zhao Y, Qiu H, Kim C, Hoffman JD, Hakonarson H et al (2015) Expanding the SPECC1L mutation phenotypic spectrum to include Teebi hypertelorism syndrome. Am J Med Genet A 167A: 2497–2502 [DOI] [PubMed] [Google Scholar]
- Billon N, Iannarelli P, Monteiro MC, Glavieux‐Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E (2007) The generation of adipocytes by the neural crest. Development 134: 2283–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai RL (1998) Human CART1, a paired‐class homeodomain protein, activates transcription through palindromic binding sites. Biochem Biophys Res Comm 250: 305–311 [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127: 1671–1679 [DOI] [PubMed] [Google Scholar]
- Damle S, Davidson EH (2011) Precise cis‐regulatory control of spatial and temporal expression of the alx‐1 gene in the skeletogenic lineage of S. purpuratus . Dev Biol 357: 505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee CT, Szymoniuk CR, Mills PE, Takahashi T (2013) Defective neural crest migration revealed by a Zebrafish model of Alx1‐related frontonasal dysplasia. Hum Mol Genet 22: 239–251 [DOI] [PubMed] [Google Scholar]
- Dougherty M, Kamel G, Shubinets V, Hickey G, Grimaldi M, Liao EC (2012) Embryonic fate map of first pharyngeal arch structures in the sox10: kaede zebrafish transgenic model. J Craniofac Surg 23: 1333–1337 [DOI] [PubMed] [Google Scholar]
- Dougherty M, Kamel G, Grimaldi M, Gfrerer L, Shubinets V, Ethier R, Hickey G, Cornell RA, Liao EC (2013) Distinct requirements for wnt9a and irf6 in extension and integration mechanisms during zebrafish palate morphogenesis. Development 140: 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK (2006) Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development 133: 1069–1077 [DOI] [PubMed] [Google Scholar]
- El‐Brolosy MA, Kontarakis Z, Rossi A, Kuenne C, Gunther S, Fukuda N, Kikhi K, Boezio GLM, Takacs CM, Lai SL et al (2019) Genetic compensation triggered by mutant mRNA degradation. Nature 568: 193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettensohn CA, Illies MR, Oliveri P, De Jong DL (2003) Alx1, a member of the Cart1/Alx3/Alx4 subfamily of Paired‐class homeodomain proteins, is an essential component of the gene network controlling skeletogenic fate specification in the sea urchin embryo. Development 130: 2917–2928 [DOI] [PubMed] [Google Scholar]
- Fox JW, Golden GT, Edgerton MT (1976) Frontonasal dysplasia with alar clefts in two sisters. Genetic considerations and surgical correction. Plast Reconstr Surg 57: 553–561 [DOI] [PubMed] [Google Scholar]
- Furukawa K, Iioka T, Morishita M, Yamaguchi A, Shindo H, Namba H, Yamashita S, Tsukazaki T (2002) Functional domains of paired‐like homeoprotein Cart1 and the relationship between dimerization and transcription activity. Genes Cells 7: 1135–1147 [DOI] [PubMed] [Google Scholar]
- Gagnon JA, Valen E, Thyme SB, Huang P, Akhmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF (2014) Efficient mutagenesis by Cas9 protein‐mediated oligonucleotide insertion and large‐scale assessment of single‐guide RNAs. PLoS ONE 9: e98186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett AT, Square TA, Medeiros DM (2012) BMP, Wnt and FGF signals are integrated through evolutionarily conserved enhancers to achieve robust expression of Pax3 and Zic genes at the zebrafish neural plate border. Development 139: 4220–4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CM, Gammack JT (1995) A leucine‐to‐phenylalanine substitution in the acetylcholine receptor ion channel in a family with the slow‐channel syndrome. Neurology 45: 982–985 [DOI] [PubMed] [Google Scholar]
- Gordon DF, Wagner J, Atkinson BL, Chiono M, Berry R, Sikela J, Gutierrez‐Hartmann A (1996) Human Cart‐1: structural organization, chromosomal localization, and functional analysis of a cartilage‐specific homeodomain cDNA. DNA Cell Biol 15: 531–541 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nimura K, Kashiwagi K, Harada T, Takaoka K, Kato H, Tamai K, Kaneda Y (2007) Comparative roles of Twist‐1 and Id1 in transcriptional regulation by BMP signaling. J Cell Sci 120: 1350–1357 [DOI] [PubMed] [Google Scholar]
- Herskowitz I (1987) Functional inactivation of genes by dominant negative mutations. Nature 329: 219–222 [DOI] [PubMed] [Google Scholar]
- Huang S, Wang Y, Luo L, Li X, Jin X, Li S, Yu X, Yang M, Guo Z (2019a) BMP2 is related to Hirschsprung's disease and required for enteric nervous system development. Front Cell Neurosci 13: 523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wang F, Zhao C, Yang S, Cheng Q, Tang Y, Zhang F, Zhang Y, Luo W, Wang C et al (2019b) Dentinogenesis and tooth‐alveolar bone complex defects in BMP9/GDF2 knockout mice. Stem Cells Dev 28: 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MC (1966) A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat Rec 156: 143–155 [DOI] [PubMed] [Google Scholar]
- Johnston MC (1975) The neural crest in abnormalities of the face and brain. Birth Defects Orig Artic Ser 11: 1–18 [PubMed] [Google Scholar]
- Kanzler B, Foreman RK, Labosky PA, Mallo M (2000) BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development 127: 1095–1104 [DOI] [PubMed] [Google Scholar]
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP et al (2019) The mutational constraint spectrum quantified from variation in 141,456 humans. bioRxiv 10.1101/531210 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano E, Toriumi T, Iguchi S, Suzuki D, Sato S, Honda M (2017) Induction of neural crest cells from human dental pulp‐derived induced pluripotent stem cells. Biomed Res 38: 135–147 [DOI] [PubMed] [Google Scholar]
- Kayserili H, Uz E, Niessen C, Vargel I, Alanay Y, Tuncbilek G, Yigit G, Uyguner O, Candan S, Okur H et al (2009) ALX4 dysfunction disrupts craniofacial and epidermal development. Hum Mol Genet 18: 4357–4366 [DOI] [PubMed] [Google Scholar]
- Khor JM, Guerrero‐Santoro J, Ettensohn CA (2019) Genome‐wide identification of binding sites and gene targets of Alx1, a pivotal regulator of echinoderm skeletogenesis. Development 146: dev180653 [DOI] [PubMed] [Google Scholar]
- Khudyakov J, Bronner‐Fraser M (2009) Comprehensive spatiotemporal analysis of early chick neural crest network genes. Dev Dyn 238: 716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFave MC, Varshney GK, Vemulapalli M, Mullikin JC, Burgess SM (2014) A defined zebrafish line for high‐throughput genetics and genomics: NHGRI‐1. Genetics 198: 167–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamplot JD, Qin J, Nan G, Wang J, Liu X, Yin L, Tomal J, Li R, Shui W, Zhang H et al (2013) BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells 2: 1–21 [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Ziller C, Couly GF (1993) Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev Biol 159: 24–49 [DOI] [PubMed] [Google Scholar]
- Le Lièvre CS, Le Douarin NM (1975) Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 34: 125–154 [PubMed] [Google Scholar]
- Le Lièvre CS (1978) Participation of neural crest‐derived cells in the genesis of the skull in birds. J Embryol Exp Morphol 47: 17–37 [PubMed] [Google Scholar]
- Leung AW, Murdoch B, Salem AF, Prasad MS, Gomez GA, Garcia‐Castro MI (2016) WNT/beta‐catenin signaling mediates human neural crest induction via a pre‐neural border intermediate. Development 143: 398–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lours‐Calet C, Alvares LE, El‐Hanfy AS, Gandesha S, Walters EH, Sobreira DR, Wotton KR, Jorge EC, Lawson JA, Kelsey Lewis A et al (2014) Evolutionarily conserved morphogenetic movements at the vertebrate head‐trunk interface coordinate the transport and assembly of hypopharyngeal structures. Dev Biol 390: 231–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D (1999) Object recognition from local scale‐invariant features In Proceedings of the International Conference on Computer Vision 2 [Google Scholar]
- Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, Bi Y, Luo X, Luo J, Teven C et al (2011) BMP‐9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther 11: 229–240 [DOI] [PubMed] [Google Scholar]
- Mavrogiannis LA, Antonopoulou I, Baxova A, Kutilek S, Kim CA, Sugayama SM, Salamanca A, Wall SA, Morriss‐Kay GM, Wilkie AO (2001) Haploinsufficiency of the human homeobox gene ALX4 causes skull ossification defects. Nat Genet 27: 17–18 [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Graham A, Richardson J, Fish JL, Depew MJ, Dee CT, Holland PWH, Takahashi T (2011) Evolution of the Alx homeobox gene family: parallel retention and independent loss of the vertebrate Alx3 gene: Evolution of vertebrate Alx homeobox genes. Evol Dev 13: 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk PD, Lovely CB, Eberhart JK (2014) Analyzing craniofacial morphogenesis in zebrafish using 4D confocal microscopy. J Vis Exp 83: e51190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans D, Bronner‐Fraser M (2002) Amphioxus and lamprey AP‐2 genes: implications for neural crest evolution and migration patterns. Development 129: 4953–4962 [DOI] [PubMed] [Google Scholar]
- Miller JL, Lyle VA, Cunningham D (1992) Mutation of leucine‐57 to phenylalanine in a platelet glycoprotein Ib alpha leucine tandem repeat occurring in patients with an autosomal dominant variant of Bernard‐Soulier disease. Blood 79: 439–446 [PubMed] [Google Scholar]
- Minarcik JC, Golden JA (2003) AP‐2 and HNK‐1 define distinct populations of cranial neural crest cells. Orthod Craniofac Res 6: 210–219 [DOI] [PubMed] [Google Scholar]
- Minarikova P, Benesova L, Halkova T, Belsanova B, Tuckova I, Belina F, Dusek L, Zavoral M, Minarik M (2016) Prognostic importance of Cell Cycle Regulators Cyclin D1 (CCND1) and Cyclin‐Dependent Kinase Inhibitor 1B (CDKN1B/p27) in sporadic gastric cancers. Gastroenterol Res Pract 2016: 9408190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoux M, Rijli FM (2010) Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137: 2605–2621 [DOI] [PubMed] [Google Scholar]
- Okuno H, Renault Mihara F, Ohta S, Fukuda K, Kurosawa K, Akamatsu W, Sanosaka T, Kohyama J, Hayashi K, Nakajima K et al (2017) CHARGE syndrome modeling using patient‐iPSCs reveals defective migration of neural crest cells harboring CHD7 mutations. eLife 6: e21114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson L, Moury DJ, Carl TF, Hastad O, Hanken J (2002) Cranial neural crest‐cell migration in the direct‐developing frog, Eleutherodactylus coqui: molecular heterogeneity within and among migratory streams. Zoology 105: 3–13 [DOI] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G (1992) Cyclin A is required at two points in the human cell cycle. EMBO J 11: 961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL et al (2007) Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115: 3015–3038 [DOI] [PubMed] [Google Scholar]
- Pini J, Giuliano S, Matonti J, Gannoun L, Simkin D, Rouleau M, Bendahhou S (2018) Osteogenic and chondrogenic master genes expression is dependent on the Kir2.1 potassium channel through the bone morphogenetic protein pathway. J Bone Miner Res 33: 1826–1841 [DOI] [PubMed] [Google Scholar]
- Qu S, Li L, Wisdom R (1997) Alx‐4: cDNA cloning and characterization of a novel paired‐type homeodomain protein. Gene 203: 217–223 [DOI] [PubMed] [Google Scholar]
- Rada‐Iglesias A, Bajpai R, Prescott S, Brugmann SA, Swigut T, Wysocka J (2012) Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell 11: 633–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada‐Iglesias A, Prescott SL, Wysocka J (2013) Human genetic variation within neural crest enhancers: molecular and phenotypic implications. Philos Trans R Soc Lond B Biol Sci 368: 20120360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq K, Cheers MS, Ettensohn CA (2012) The genomic regulatory control of skeletal morphogenesis in the sea urchin. Development 139: 579–590 [DOI] [PubMed] [Google Scholar]
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X (2009) DOG 1.0: illustrator of protein domain structures. Cell Res 19: 271–273 [DOI] [PubMed] [Google Scholar]
- Sadaghiani B, Thiébaud CH (1987) Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev Biol 124: 91–110 [DOI] [PubMed] [Google Scholar]
- Sato T, Sasai N, Sasai Y (2005) Neural crest determination by co‐activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development 132: 2355–2363 [DOI] [PubMed] [Google Scholar]
- Sauka‐Spengler T, Meulemans D, Jones M, Bronner‐Fraser M (2007) Ancient evolutionary origin of the neural crest gene regulatory network. Dev Cell 13: 405–420 [DOI] [PubMed] [Google Scholar]
- Sauka‐Spengler T, Bronner‐Fraser M (2008) A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol 9: 557–568 [DOI] [PubMed] [Google Scholar]
- Schilling TF, Walker C, Kimmel CB (1996) The chinless mutation and neural crest cell interactions in zebrafish jaw development. Development 122: 1417–1426 [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep‐sequencing age. Nat Methods 11: 361–362 [DOI] [PubMed] [Google Scholar]
- Sedano HO, Cohen MM Jr, Jirasek J, Gorlin RJ (1970) Frontonasal dysplasia. J Pediatr 76: 906–913 [DOI] [PubMed] [Google Scholar]
- Shah AN, Davey CF, Whitebirch AC, Miller AC, Moens CB (2016a) Rapid reverse genetic screening using CRISPR in zebrafish. Zebrafish 13: 152–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AN, Moens CB, Miller AC (2016b) Targeted candidate gene screens using CRISPR/Cas9 technology. Methods Cell Biol 135: 89–106 [DOI] [PubMed] [Google Scholar]
- Shihab HA, Gough J, Mort M, Cooper DN, Day IN, Gaunt TR (2014) Ranking non‐synonymous single nucleotide polymorphisms based on disease concepts. Hum Genomics 8: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J et al (2011) Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes‐Costa M, Bronner ME (2015) Establishing neural crest identity: a gene regulatory recipe. Development 142: 242–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Hing AV, Clarke CM, Johnson NM, Perez FA, Park SS, Horst JA, Mecham B, Maves L, Nickerson DA et al (2014) Exome sequencing identifies a recurrent de novo ZSWIM6 mutation associated with acromelic frontonasal dysostosis. Am J Hum Genet 95: 235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, Garcia‐Castro MI (2012) Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci 69: 3715–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchieu J, Zimmer B, Fattahi F, Amin S, Zeltner N, Chen S, Studer L (2017) A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell 21: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA, Sobieszczuk D, Wilkinson D, Krumlauf R (2002) Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways. Development 129: 433–442 [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R (2003) Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 130: 6441–6452 [DOI] [PubMed] [Google Scholar]
- Twigg SR, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, Morriss‐Kay GM, Wilkie AO (2004) Mutations of ephrin‐B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci USA 101: 8652–8657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg SR, Versnel SL, Nurnberg G, Lees MM, Bhat M, Hammond P, Hennekam RC, Hoogeboom AJ, Hurst JA, Johnson D et al (2009) Frontorhiny, a distinctive presentation of frontonasal dysplasia caused by recessive mutations in the ALX3 homeobox gene. Am J Hum Genet 84: 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A, Kalsoom UE, Umair M, John P, Ansar M, Basit S, Ahmad W (2016) Exome sequencing revealed a novel splice site variant in the ALX1 gene underlying frontonasal dysplasia. Clin Genet 91: 494–498 [DOI] [PubMed] [Google Scholar]
- Umm‐e-Kalsoom BS, Kamran‐ul-Hassan Naqvi S, Ansar M, Ahmad W (2012) Genetic mapping of an autosomal recessive postaxial polydactyly type A to chromosome 13q13.3–q21.2 and screening of the candidate genes. Hum Genet 131: 415–422 [DOI] [PubMed] [Google Scholar]
- Uz E, Alanay Y, Aktas D, Vargel I, Gucer S, Tuncbilek G, von Eggeling F, Yilmaz E, Deren O, Posorski N et al (2010) Disruption of ALX1 causes extreme microphthalmia and severe facial clefting: expanding the spectrum of autosomal‐recessive ALX‐related frontonasal dysplasia. Am J Hum Genet 86: 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney GK, Sood R, Burgess SM (2015) Understanding and editing the zebrafish genome. Adv Genet 92: 1–52 [DOI] [PubMed] [Google Scholar]
- Varshney GK, Zhang S, Pei W, Adomako‐Ankomah A, Fohtung J, Schaffer K, Carrington B, Maskeri A, Slevin C, Wolfsberg T et al (2016) CRISPRz: a database of zebrafish validated sgRNAs. Nucleic Acids Res 44: D822–D826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N (2005) Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development 132: 3977–3988 [DOI] [PubMed] [Google Scholar]
- Wang Y, Hong S, Li M, Zhang J, Bi Y, He Y, Liu X, Nan G, Su Y, Zhu G et al (2013) Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res 31: 1796–1803 [DOI] [PubMed] [Google Scholar]
- Wieland I, Jakubiczka S, Muschke P, Cohen M, Thiele H, Gerlach KL, Adams RH, Wieacker P (2004) Mutations of the ephrin‐B1 gene cause craniofrontonasal syndrome. Am J Hum Genet 74: 1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YQ, Badano JL, McCaskill C, Vogel H, Potocki L, Shaffer LG (2000) Haploinsufficiency of ALX4 as a potential cause of parietal foramina in the 11p11.2 contiguous gene‐deletion syndrome. Am J Hum Genet 67: 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuyts W, Cleiren E, Homfray T, Rasore‐Quartino A, Vanhoenacker F, Van Hul W (2000) The ALX4 homeobox gene is mutated in patients with ossification defects of the skull (foramina parietalia permagna, OMIM 168500). J Med Genet 37: 916–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Eberspaecher H, Seldin MF, de Crombrugghe B (1994) The gene for the homeodomain‐containing protein Cart‐1 is expressed in cells that have a chondrogenic potential during embryonic development. Mech Dev 48: 245–254 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Behringer RR, de Crombrugghe B (1996) Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet 13: 275–283 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Table EV4
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Source Data for Expanded View and Movies
Review Process File
Source Data for Figure 1E
Source Data for Figure 2B
Source Data for Figure 5
Source Data for Figure 6
Data Availability Statement
The raw sequencing data sets produced in this study are available in the following database: FaceBase Record ID: 25J0 Accession: FB00000907 (https://www.facebase.org/).


