Abstract
A prospective study among adults hospitalized for polymerase chain reaction–confirmed respiratory syncytial virus infections (n = 123) showed frequent occurrence of lower respiratory-tract complications causing respiratory insufficiency (52.8%), requirement for assisted ventilation (16.3%), and intensive care unit admission/death (12.2%). High viral RNA concentration was detected at time of hospitalization, including in patients who presented later than 2 days of illness (day 1–2, 7.29 ± 1.47; day 3–4, 7.28 ± 1.41; day 5–8, 6.66 ± 1.87 log10 copies/mL). RNA concentration was independently associated with risk of complications and respiratory insufficiency (adjusted odds ratio 1.40 per log10 copies/mL increase, 95% confidence interval, 1.03–1.90; P = .034). Our data indicate the need and provide a basis for clinical research on antiviral therapy in this population.
Keywords: respiratory failure, RSV, viral load
Respiratory syncytial virus (RSV) is increasingly recognized as an important cause of severe respiratory-tract infections in older adults, resulting in excessive hospitalizations and deaths annually [1]. However, there is no established antiviral treatment at present, and vaccine remains unavailable. The slow progress is further limited by the lack of understanding of the clinical manifestations, severity, virologic changes, and related pathophysiology in adult diseases [2–5]. This study was conducted to investigate the lower-respiratory manifestations, progression to respiratory failure, and viral load in hospitalized adults with polymerase chain reaction (PCR)–confirmed RSV infections. Such information may provide a basis for RSV therapeutics research in this high-risk population.
METHODS
A prospective, observational study was performed at 2 acute, general hospitals in Hong Kong during a 12-week seasonal RSV outbreak in 2013 (Supplementary 1). Adults aged >18 years who presented with symptoms of acute respiratory infection and were hospitalized because of potentially serious medical conditions, exacerbation of underlying chronic illnesses, or severe symptoms impossible to manage at home were eligible for study [2]. For consenting individuals, nasopharyngeal specimens collected at time of admission were tested for RSV using a quantitative, real-time reverse-transcription PCR (RT-PCR) assay, in addition to an antigen assay performed during routine care irrespective of its result (see below); their clinical course was followed and recorded. Because there was no specific intervention, all recruited patients were managed and discharged according to standard procedures as described [2]. Written, informed consent was obtained from each patient. Ethical approval for the study was obtained from the institutional review boards of the Hospital Authority of Hong Kong and the Chinese University of Hong Kong.
Detection of RSV and quantitation of viral RNA (to provide estimates of the “virus load”) in the nasopharyngeal samples were performed using established methods [6, 7]. Briefly, viral RNA was extracted from the original specimens using PureLinkTM Viral RNA/DNA Mini kit (Life Technologies), followed by TaqMan-based, real-time RT-PCR assays using RSV-A and RSV-B subtype specific primers and probes, which targeted the highly conserved genomic regions of the virus N gene. Copy numbers were inferred from 10-fold serially diluted synthetic RNA standards spanning the amplified region. Using probit analysis, the lower detection limit (at 95% confidence level) for RSV-A and RSV-B RT-PCR assays was calculated to be 29.9 and 9.0 copies per reaction, respectively. Extraction and RT-PCR negative controls were run in parallel for every experiment. Exogenous RNA (TATAA Biocenter) was spiked during extraction to check for downstream amplification inhibition. Results were expressed in copies/mL (equivalents to plaque-forming unit/mL have been described) [4, 5, 7]. Virus genotyping was performed by direct amplicon sequencing of the G gene (Supplementary-2). As part of hospital care/protocol, immunofluorescence assays (IFAs) for a panel of respiratory viruses, including RSV, influenza A/B, and parainfluenza, were performed concomitantly using methods previously described; because IFA has lower sensitivity, eligible patients with negative results were not excluded from study (we recruited 115 IFA-positive and 56 IFA-negative cases for RT-PCR testing) [2, 8, 9]. Clinical data were collected using a standardized research tool, which included demographics, major comorbidities (defined in the Charlson Comorbidity Index, see Table 1), presenting symptoms, lower respiratory-tract complications (including acute bronchitis/bronchiolitis, clinico-radiological pneumonia, exacerbation of chronic airway diseases, or their combinations), cardiovascular complications (including decompensated heart failure, acute coronary syndrome, arrhythmia, cerebrovascular events), requirements for assisted ventilation, supplemental oxygen, bronchodilator therapy, corticosteroid administration, hospitalization duration, and all-cause death [2, 3, 4, 8–10].
Table 1.
Clinical Manifestations, Complications, and Outcomes of 123 Adult Patients Hospitalized for PCR-confirmed RSV Infections
| Variable | No. (%) |
|---|---|
| Patient characteristics | |
| Age, year (mean ± SD) | 78.0 ± 15.0 |
| Male sex | 64 (52.0) |
| Nursing home resident | 50 (40.7) |
| Comorbidity, majora | 99 (80.5) |
| Chronic statin usera | 32 (26.0) |
| Aspirin/NSAID usera | 30 (24.4) |
| Symptoms and complications | |
| Day of illness, at presentation, median (IQR) | 2 (1–3) |
| Fever | 102 (82.9) |
| Cough | 108 (87.8) |
| Shortness of breath | 92 (74.8) |
| Pleuritic chest pain | 10 (8.1) |
| WBC count (×109/L, mean ± SD) | 9.6 ± 3.6 |
| Neutrophil count (×109/L, mean ± SD) | 7.5 ± 3.3 |
| C-reaction protein (mg/L, mean ± SD) | 59.8 ± 52.7 |
| Lower respiratory-tract complicationsb | 110 (89.4) |
| Pneumonia, clinico-radiological | 83 (67.5) |
| Bronchitis/bronchiolitis | 15 (12.2) |
| Exacerbation of chronic airway diseases | 28 (22.8) |
| Cardiovascular complicationsb | 30 (24.4) |
| Bacterial superinfection, community-acquiredb | 25 (20.3) |
| Hospital-acquired | 17 (13.8) |
| Treatments and outcomes | |
| Hypoxemia, requiring supplemental oxygen | 97 (78.9) |
| Bronchoconstriction, requiring bronchodilators | 72 (58.5) |
| Antibiotics use | 121 (98.4) |
| Systemic corticosteroid useb | 44 (35.8) |
| Assisted ventilation, invasive/noninvasive | 20 (16.3) |
| ICU admission/death | 15 (12.2) |
| Death, within 30 d | 11 (8.9) |
| Death, within 60 d | 17 (13.8) |
| Length of stay, median (IQR) | 10 (5–21) |
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; SD, standard deviation; WBC, white blood cell.
a Major comorbidity: congestive heart failure; cerebrovascular, neoplastic, and chronic liver or renal diseases; diabetes mellitus; chronic cardiovascular, autoimmune, and neurological diseases; and immunosuppression (not hypertension or hypercholesterolemia alone); and chronic lung diseases (COPD, asthma, bronchiectasis, pulmonary fibrosis); as defined in the Charlson Index [2, 8]. Chronic statin and aspirin/NSAID use was defined as regular use of these medications for any length of time prior to and at the time of hospitalization [8].
b Lower respiratory-tract complications [2, 8]: clinico-radiographical pneumonia, acute bronchitis/bronchiolitis (wheezing breathing alone, without pneumonia), and acute exacerbations of chronic airway diseases (eg, COPD/asthma), or their combinations. Cardiovascular complications (see text). Bacterial superinfection: evidenced by positive culture of a pathogenic bacteria from respiratory (sputum, bronchial/tracheal aspirates; gram stain and culture of adequate samples on the basis of standard criteria) and/or blood samples; infections with onset >2 days after admission were considered hospital-acquired; oral commensals or skin contaminants were excluded. Systemic corticosteroid use was defined as corticosteroids given via the parental/enteral routes for the treatment of bronchoconstriction [2].
Clinical manifestations, severity, and outcomes of PCR-confirmed RSV cases were reported. RSV RNA quantitation results were described, and examined for associations with clinical variables [4, 9]. Univariate associations with development of severe disease, defined as lower respiratory-tract complications causing respiratory insufficiency, evidenced by requirements for both bronchodilator (to relieve bronchoconstriction) and supplemental oxygen (to maintain oxygen saturation >95%) therapies, were examined [2, 3, 10]. Variables with P values <.2 were entered into a backward, stepwise, logistic regression model for analysis [9]. Adjusted odds ratio (AOR) and the 95% confidence interval (CI) of each explanatory variable were reported. Further, independent factors associated with survival within 30 days were analyzed using Cox proportional hazards models (backward, stepwise regression); adjusted hazard ratio (AHR) and 95% CI were reported [2, 8]. All probabilities were 2-tailed, and a P value of <.05 was considered to indicate statistical significance. Statistical analysis was performed using PASW Statistics software, version 18.0.
RESULTS
Altogether, 123 patients were confirmed to have RSV infection by RT-PCR. There were 9 RSV-A and 114 RSV-B viruses; phylogenetic analysis showed that the circulating RSV-A strain belonged to the novel ON1 genotype, which contained a 72-nucleotide duplication in the G gene, and the RSV-B strains belonged to the BA genotypes (Supplementary-2). The mean ± standard deviation (SD) age of RSV patients was 78.0 ± 15.0 years; 80.5% had major comorbidities; mean ± SD time of presentation was 2.6 ± 1.9 days of illness. Lower respiratory-tract and cardiovascular complications were documented in 89.4% and 24.4% of patients, respectively. About half (52.8%) of RSV patients had severe disease and respiratory insufficiency, necessitating the use of both bronchodilator and supplemental oxygen therapies; and 16.3% had required assisted ventilation (invasive/noninvasive; started on 4.4 ± 5.4 days of illness) (Table 1). Among those with severe disease, 66.2% had acute pulmonary infiltrates evident on chest radiographs. The adverse outcomes of intensive care unit (ICU) admission/death, and deaths at 30 and 60 days, occurred in 12.2%, 8.9%, and 13.8% of patients, respectively. Nearly all (98.4%) hospitalized RSV patients had received initial antibiotics and 35.8% received systemic corticosteroid treatment.
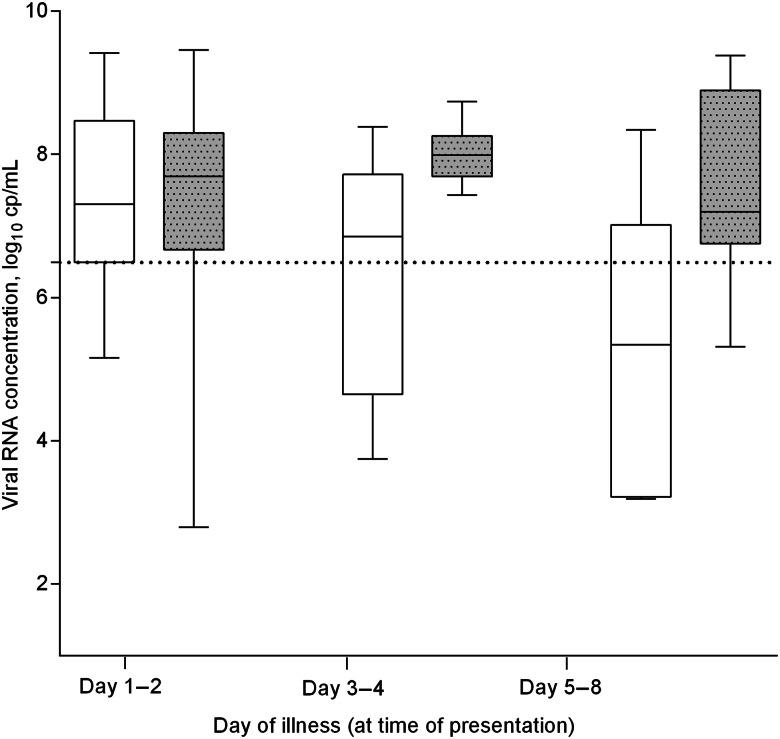
Patients hospitalized with RSV infection were found to have high viral RNA concentration at time of presentation (mean ± SD, 7.21 ± 1.52 log10 copies/mL; median [interquartile range], 7.66 [6.49–8.27] log10 copies/mL), even on >2 days of illness (day 1–2, 7.29 ± 1.47; day 3–4, 7.28 ± 1.41; day 5–8, 6.66 ± 1.87 log10 copies/mL) (Figure 1). A significantly higher viral RNA concentration was found in association with underlying major comorbidities (7.36 ± 1.40 vs 6.57 ± 1.83 log10 copies/mL; P = .021) and development of severe disease (lower respiratory-tract complications causing respiratory insufficiency, 7.53 ± 1.36 vs 6.84 ± 1.60 log10 copies/mL; P = .011); cases with fatal outcomes tended to have higher viral concentrations but it did not reach statistical significance (7.79 ± 1.34 vs 7.14 ± 1.53 log10 copies/mL; P = .163). We did not observe significant difference between RSV subtypes (RSV-B vs RSV-A, 7.27 ± 1.47 vs 6.45 ± 1.92 log10 copies/mL; P = .121), nor types of specimen collected (nasopharyngeal aspirates vs flocked swabs, 7.19 ± 1.57 vs 7.29 ± 1.28 log10 copies/mL; P = .774). IFA-negative samples were shown to have lower viral RNA concentrations (3.92 ± 1.30 vs 7.44 ± 1.24 log10 copies/mL; P < .001).
Figure 1.
RSV RNA concentration at time of presentation, shown according to presence (gray box-plot) or absence (white box-plot) of underlying major comorbidities. Dotted line indicates the 25th percentile of viral RNA concentration in this cohort (6.49 log10 copies/mL); day 1 (n = 44), day 2 (n = 36), day 3–4 (n = 27), day 5–8 (n = 16). “Major comorbidities,” as defined in the Charlson Index (see Table 1). Specimen used for diagnosis: nasopharyngeal aspirate, n = 99; nasopharyngeal flocked swab, n = 24; RNA concentrations measured were not significantly different. Abbreviations: cp, copies; RSV, respiratory syncytial virus.
We found that high viral RNA concentration (AOR 1.40 per unit increase, 95% CI, 1.03–1.90; P = .034), underlying chronic lung diseases (AOR 11.73, 95% CI, 4.23–32.49; P < .001), and older age (AOR 1.03, 95% CI, 1.00–1.06; P = .048) were independent factors associated with development of severe disease (adjusted for gender, other major comorbidities, and time from illness onset). Requirement of assisted ventilation because of respiratory failure progression (AHR 7.90, 95% CI, 1.93–32.31; P = .004), bacterial superinfection (AHR 4.31, 95% CI, 1.12–16.52; P = .033), older age (AHR 1.10, 95% CI, 1.01–1.19; P = .030), and presence of major comorbidities were associated with lower survival (adjusted for viral RNA concentration and corticosteroid administration). There was an increased rate of bacterial superinfections among corticosteroid recipients during the hospital course (20.5% vs 10.1%; P = .112).
DISCUSSION
We found frequent occurrence of lower respiratory-tract complications and respiratory insufficiency, requirement for assisted ventilation, ICU admissions, and deaths among adults hospitalized for RSV infections. High viral load was detected at presentation, which significantly correlated with clinical severity. Our data support the study of antiviral treatment in this population.
The clinical manifestations and outcomes reported in this prospective cohort (n = 123) are highly consistent with our recent retrospective study on >600 hospitalized RSV patients [2]. We confirm that serious lower respiratory and cardiovascular complications are frequent among these older adults, and the mortality is high (9%–14%) [2, 3]. Bronchoconstriction and hypoxemia are the major manifestations, as a result of acute bronchitis/bronchiolitis, pneumonia, or their combinations, not unlike pediatric RSV diseases [1–3, 5, 10]. In this cohort, almost 79%, 59%, and 53% of patients had required supplemental oxygen, bronchodilators, and both therapies, respectively, because of respiratory insufficiency; and ventilatory support was necessary in 16%. Importantly, this study adds that high viral load is significantly associated with development of severe, complicated RSV disease (AOR 1.40 per log10 copies/mL RNA increase, 95% CI, 1.03–1.90), which is independent of known risk factors such as chronic lung diseases and advanced age. Progression of respiratory failure predicts a high risk of death [1, 3]. Our findings are supported by recent studies showing correlations between RSV concentration and symptom scores in healthy adult volunteers [4], and a trend toward higher viral concentration in hospitalized RSV patients when compared with the nonhospitalized [11]. In one report, RSV concentration in 8 patients who required mechanical ventilation was higher than 23 nonventilated cases [12]. Emerging data from young children similarly suggest high viral load as a driving force in severe manifestations/outcomes, thus a potential role of antiviral treatment [5]. Additionally, we found high viral RNA concentration in most hospitalized patients (25th, 75th, 95th percentile: 6.5, 8.3, 9.3 log10 copies/mL, respectively), including those who presented later than 2 days of illness, and particularly if they had underlying compromising conditions. Recent data suggest that viral shedding duration in adult RSV infection may last for at least 7 days (detected by culture and PCR) [4, 11], which could be longer than seasonal influenza [9, 13]. Taken together with the seriousness of disease, the high viral load, and its significance, our results strongly indicate the need and provide basis for clinical research on potential antiviral therapies in the hospitalized adults (eg, fusion inhibitors, polymerase inhibitors, small interfering RNA, antibody-based interventions) [1, 4, 5, 12, 14, 15]. As viral replication may continue for several days, a therapeutic time window of more than 48 hours seems possible and warrant study [8, 9, 13, 15].
Our study's strengths include a prospective design, larger sample size, examination of viral concentration by real-time RT-PCR (for RSV-A/B) and its relationships with key host/disease factors (patient characteristics, time of illness, severity), and adjustment of confounders in multivariable analyses [5, 11, 12]. Quantitative culture, serial measurements, and lower respiratory-tract sampling have been planned to provide further information on virokinetics [4, 5, 7, 9, 11, 13, 14]. Further, data generated in this study can assist planning and design of future clinical trials on antivirals in the population of hospitalized adults (eg, patient characteristics and manifestations; clinical/virological study parameters; outcome measures).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank Dr Cao Bin, Capital Medical University, Beijing, China, for his critical review of the manuscript.
Financial support. This work was supported by the Research Fund for the Control of Infectious Diseases (CU-12-01-01), Food and Health Bureau of the Hong Kong Special Administrative Region Government; and a Departmental research fund, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lee N, Qureshi ST. Other viral pneumonias: coronavirus, respiratory syncytial virus, adenovirus, hantavirus. Crit Care Clin 2013; 29:1045–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee N, Lui GC, Wong KT et al. . High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis 2013; 57:1069–77. [DOI] [PubMed] [Google Scholar]
- 3. Walsh EE, Peterson DR, Falsey AR. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis 2007; 195:1046–51. [DOI] [PubMed] [Google Scholar]
- 4. DeVincenzo JP, Wilkinson T, Vaishnaw A et al. . Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 2010; 182:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasegawa K, Jartti T, Mansbach JM et al. . Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 2015; 211:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Elden LJ, van Loon AM, van der Beek A et al. . Applicability of a real-time quantitative PCR assay for diagnosis of respiratory syncytial virus infection in immunocompromised adults. J Clin Microbiol 2003; 41:4378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol 2003; 41:4160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee N, Leo YS, Cao B et al. . NAI treatment, superinfection, and corticosteroids affect survival of influenza patients. Eur Respir J 2015; 10.1183/09031936.00169714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee N, Chan PK, Hui DS et al. . Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pickles RJ, DeVincenzo JP. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol 2015; 235:266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis 2013; 207:1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duncan CB, Walsh EE, Peterson DR et al. . Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis 2009; 200:1242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bagga B, Woods CW, Veldman TH et al. . Comparing influenza and RSV viral and disease dynamics in experimentally infected adults predicts clinical effectiveness of RSV antivirals. Antivir Ther 2013; 18:785–91. [DOI] [PubMed] [Google Scholar]
- 14. DeVincenzo JP, Whitley RJ, Mackman RL et al. . Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 2014; 371:711–22. [DOI] [PubMed] [Google Scholar]
- 15. Simões EA, DeVincenzo JP, Boeckh M et al. . Challenges and opportunities in developing respiratory syncytial virus therapeutics. J Infect Dis 2015; 211(suppl 1):S1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.