Abstract
Overexpression of somatostatin receptors (SSTRs) in patients with neuroendocrine neoplasms (NENs) is used for both diagnosis and treatment. Receptor density may reflect tumor differentiation and thus be associated with prognosis. Noninvasive visualization and quantification of SSTR density is possible by SSTR imaging (SRI) using PET. Recently, we introduced 64Cu-DOTATATE for SRI, and we hypothesized that uptake of this tracer could be associated with overall survival (OS) and progression-free survival (PFS). Methods: We evaluated patients with NENs who underwent 64Cu-DOTATATE PET/CT SRI in 2 prospective studies. Tracer uptake was determined as the maximal SUV (SUVmax) for each patient. Kaplan–Meier analysis with log-rank was used to determine the predictive value of 64Cu-DOTATATE SUVmax for OS and PFS. Specificity, sensitivity, and accuracy were calculated for prediction of outcome at 24 mo after 64Cu-DOTATATE PET/CT. Results: In total, 128 patients with NENs were included and followed for a median of 73 mo (range, 1–112 mo). During follow-up, 112 experienced disease progression, and 69 died. The optimal cutoff for 64Cu-DOTATATE SUVmax was 43.3 for prediction of PFS, with a hazard ratio of 0.56 (95% confidence interval, 0.38–0.84) for patients with an SUVmax of more than 43.3. However, no significant cutoff was found for prediction of OS. In multiple Cox regression adjusted for age, sex, primary tumor site, and tumor grade, the SUVmax cutoff hazard ratio was 0.50 (range, 0.32–0.77) for PFS. The accuracy was moderate for predicting PFS (57%) at 24 mo after 64Cu-DOTATATE PET/CT. Conclusion: In this first study to report the association of 64Cu-DOTATATE PET/CT and outcome in patients with NENs, tumor SSTR density as visualized with 64Cu-DOTATATE PET/CT was prognostic for PFS but not OS. However, the accuracy of prediction of PFS at 24 mo after 64Cu-DOTATATE PET/CT SRI was moderate, limiting the value on an individual-patient basis.
Keywords: 64Cu-DOTATATE PET/CT, maximal SUV (SUVmax), neuroendocrine neoplasm, progression-free survival, overall survival
Patients diagnosed with neuroendocrine neoplasms (NENs) have a highly variable survival ranging from a few months to decades (1). The origin of NENs is frequently gastroenteropancreatic (∼75%) or pulmonary (∼25%). Gastroenteropancreatic NENs are pathologically graded by mitotic rate and proliferation index (Ki-67) as grade 1 (<3%), grade 2 (3%–20%), or grade 3 (>20%) and by differentiation in well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinoma (NECs). NECs are further classified as being of small or large cell type (2,3). Lung NENs are pathologically classified as typical or atypical carcinoids, large cell NECs, or small cell lung carcinomas. Most patients with NENs have low-grade disease and experience long-term survival (1). Treatment is based on resectability, the presence of metastases, the location of the primary tumor and metastases, and the histology of tumor biopsy samples (4). However, as evaluation based on histology is prone to sampling error, more precise prognostic methods are needed. PET allows for a whole-body molecular examination and is well suited for noninvasive longitudinal monitoring of the entire tumor burden. Although 18F-FDG PET is widely used for cancer imaging and is prognostic in NENs of all grades (5,6), it is currently not recommended as a routine in low-grade NENs. However, a feature of most NENs is overexpression of somatostatin receptors (SSTRs). The SSTR density on the tumor cell surface may reflect the degree of differentiation and proliferative index, that is, most SSTRs on well-differentiated tumors with a low proliferation index (7,8). SSTRs are used as the target in SSTR imaging (SRI), which plays an essential role in diagnosis, treatment decisions, and follow-up for NENs (9). The general principle applied involves a radioisotope conjugated via a chelator (e.g., tetraxetan [DOTA]) to a somatostatin analog (e.g., Tyr3-octreotate [TATE]). In diagnostics, PET tracers (68Ga- or 64Cu-conjugated somatostatin analogs) are used, and for therapy, 177Lu-conjugated somatostatin analogs are used (10). PET tracer uptake can be quantified as SUV. Preliminary results in smaller cohorts have indicated that maximal SUV (SUVmax) in 68Ga SRI as a measure of SSTR density is predictive of progression-free survival (PFS) in NETs (11–13). However, the prognostic implications for overall survival (OS) have not previously been reported. We recently introduced 64Cu-labeled DOTATATE for NENs. This agent provides excellent image resolution and can be centrally produced because of its long half-life (14). Hence, the aim of the present study was to examine in a large cohort the ability of 64Cu-DOTATATE PET/CT to predict OS and PFS. We hypothesized that increasing lesion SUVmax was associated with longer OS and PFS.
MATERIALS AND METHODS
Patients
Our group performed 2 prospective clinical studies with 64Cu-DOTATATE PET/CT that included patients seen between November 2009 and March 2013 and for whom a follow-up study was planned (15,16). The study was approved by the Regional Scientific Ethical Committee (reference no. H-D-2008-045), and all participating patients signed an informed consent form. The included patients had histopathologically confirmed NENs and were referred for PET/CT for staging, restaging, or follow-up. All scans were reviewed for inclusion in the present follow-up study. If more than 1 64Cu-DOTATATE PET/CT scan was available for a patient, the earliest scan was used. We excluded patients with no signs of NENs because of previous radical surgery. Thus, only patients with the presence of NENs detectable by PET and/or CT were included. Patients were followed and treated with the standard of care at the ENETS Neuroendocrine Tumor Center of Excellence, Rigshospitalet, Copenhagen, Denmark. Treatment decisions were masked to the 64Cu-DOTATATE PET/CT results but guided by 111In-octreotide scintigraphy (clinical routine throughout the inclusion period). The baseline characteristics collected were age, sex, time of diagnosis, site of primary tumor, Ki-67 index (%), World Health Organization (WHO) grade, and NEN-specific treatment before SRI. Follow-up with diagnostic CT was undertaken biannually. At the discretion of the treating physician, SRI, MRI, or ultrasound was also performed during follow-up.
Radiotracer and Image Acquisition
Radiotracer production, PET/CT image acquisition, and the reconstruction methodology have been published previously (14–16). In short, 64Cu-DOTATATE was produced in-house, and patients underwent a whole-body PET/CT scan 62 ± 1 min (range, 43–99 min) after injection of 202.7 ± 1.0 MBq (range, 174–245 MBq) of 64Cu-DOTATATE. A Siemens Biograph 40 or 64 TruePoint PET/CT was used, and all images were reconstructed with the same reconstruction parameters and algorithm (TrueX; Siemens Medical Solutions). To ensure quantitatively accurate measurements between the different PET/CT scanners, we performed a quality control every 2 wk, testing that they were calibrated to measure within our acceptance range (5%). Furthermore, a diagnostic CT scan with iodine intravenous contrast material was performed before the PET scan unless contraindicated.
Image Analysis
An experienced board-certified nuclear medicine physician together with an experienced board-certified radiologist, working side by side, analyzed the PET/CT scans. SUV was calculated as the decay-corrected measured radioactivity concentration/(injected activity/body weight). The SUVmax was obtained by drawing spheric volumes encompassing the entire lesion. Within each lesion site (divided into regions or groups: lung, pancreas, liver, intestines, bones, lymph nodes, and other), the SUVmax was noted. For each patient, the largest SUVmax obtained in any region or group was used as the predictor variable and, for simplicity, is referred to as SUVmax in this article.
Endpoints
Follow-up was performed on March 28, 2019. Routine CT images or MR images were used for evaluation of PFS in accordance with Response Evaluation Criteria in Solid Tumors, version 1.1 (17). PFS was calculated as the time from 64Cu-DOTATATE PET/CT to progression (if any) or tumor-related death. If no progression or tumor-related death occurred within the follow-up interval, the patient was censored at the time of the last available diagnostic imaging examination. OS was calculated as the time from 64Cu-DOTATATE PET/CT to death by any cause. Patients alive at follow-up were censored to the date of follow-up, that is, March 28, 2019.
Statistics
Continuous variables are reported as mean and SEM or median and range. Independent t tests and 1-way ANOVA with post hoc analysis by Tukey were used for comparison of means. Kaplan–Meier analyses were used to estimate time to outcome (PFS and OS). The optimal cutoff for SUVmax was determined by log-rank testing, as the point yielding the most significant log-rank test split (as determined using the Cutoff Finder web application (18)). Multiple Cox regression analyses for outcome, with the predictor variables being age, sex, primary tumor site, SUVmax, and WHO grade, were performed. Furthermore, specificity, sensitivity, and accuracy in predicting outcome (PFS and OS) at 24 mo by the determined SUVmax cutoff were calculated. The time cutoff of 24 mo after the 64Cu-DOTATATE PET/CT scan was chosen to put the performance of prognostication into a clinically relevant timeline, with most patients undergoing PET/CT biannually or annually and some undergoing PET/CT every other year. A P value of less than 0.05 was considered statistically significant. R statistical software (R Foundation for Statistical Computing) was used for the analyses.
RESULTS
Patients
In total, 172 64Cu-DOTATATE PET/CT scans were performed in relation to the 2 studies; 25 patients participated in both studies. In the 147 unique patients scanned, 128 patients had NENs present at the time of the scan and were thus included in the study; baseline characteristics are shown in Table 1.
TABLE 1.
Baseline Characteristics of 128 Patients with NENs
| Characteristic | Value |
| Age (y) | Median, 63; range, 29–83 |
| Male/female (n) | 72/56 (56%/44%) |
| Time from diagnosis to scan (mo) | Median, 18; range, 0–208 |
| Primary tumor site (%) | |
| Lung | 7 (5) |
| Unknown primary NEN | 23 (18) |
| Stomach | 1 (1) |
| Small intestine | 60 (47) |
| Pancreas | 25 (20) |
| Cecum | 8 (6) |
| Extrahepatic biliary tract | 2 (2) |
| Esophagus | 1 (1) |
| Other | 1 (1) |
| Primary tumor site, grouped (n) | |
| Lung | 7 (5%) |
| Unknown primary NEN | 23 (18%) |
| Gastrointestinal tract* | 73 (57%) |
| Pancreas | 25 (20%) |
| Ki-67 index (%) | Median, 5; range, 1–100 |
| WHO grading (n)† | |
| Grade 1 | 31 (26%) |
| Grade 2 | 84 (69%) |
| Grade 3 | 6 (5%) |
| Treatment before 64Cu-DOTATATE PET/CT (n)‡ | |
| No treatment | 18 (14%) |
| Localized treatment | 13 (10%) |
| Systemic treatment | 43 (34%) |
| Localized and systemic treatment | 54 (42%) |
Gastrointestinal collectively refers to primaries originating from stomach, small intestine, cecum, extrahepatic biliary tract, esophagus, or other.
Not available in 7 patients.
Localized treatment: surgery (n = 59), hepatic artery embolization (n = 9), radiofrequency ablation (n = 7), and/or external radiation (n = 2). Systemic treatment: interferon (n = 57), somatostatin analog (n = 49), chemotherapy (n = 49), and/or peptide receptor radionuclide therapy (n = 43).
64Cu-DOTATATE SUVmax
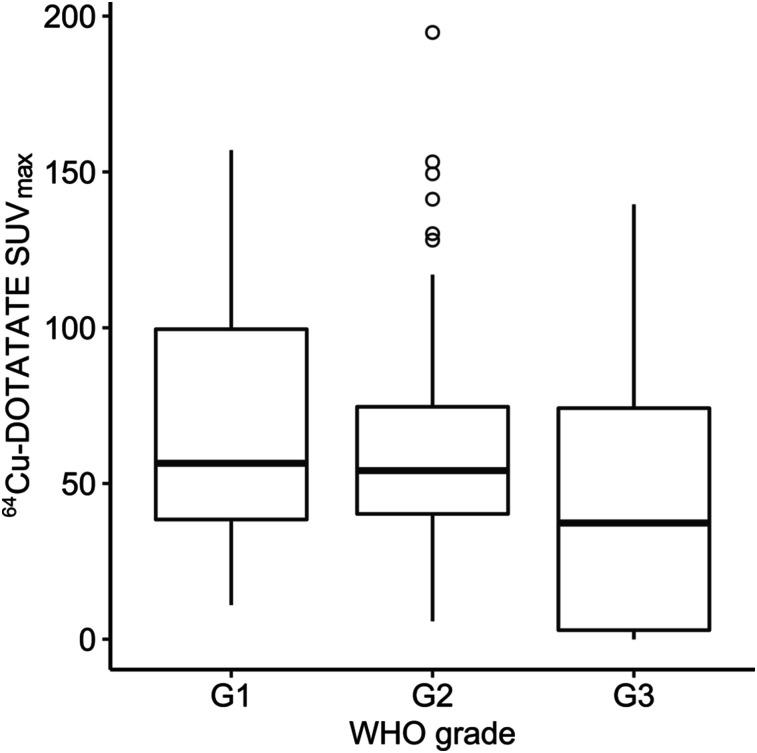
The mean SUVmax ± SEM of the primary tumor or metastasis was 62.2 ± 3.3 in the entire cohort; values for the subgroups are reported in Table 2. A tendency toward a lower SUVmax was observed for patients with a poor outcome (deceased [P = 0.06] or progressive disease [P = 0.17]) at the defined 24-mo cutoff after 64Cu-DOTATATE PET/CT. SUVmax differed significantly according to primary tumor site, with a higher SUVmax for lesions originating from pancreas than for lesions originating from gastrointestinal sites. No significant difference in SUVmax was observed for patients with grade 1 vs. grade 2 vs. grade 3 NENs (Fig. 1).
TABLE 2.
64Cu-DOTATATE SUVmax in Tumor or Metastases in 128 Patients with NEN
| Parameter | Mean | P |
| Overall | 62.2 (3.3) | — |
| Primary tumor site, grouped | 0.001* | |
| Lung (n = 7) | 63.2 (21.4) | |
| Pancreas (n = 25) | 83.8 (10.1) | |
| Gastrointestinal (n = 73) | 51.5 (2.9) | |
| Unknown primary NEN (n = 23) | 72.3 (7.5) | |
| WHO grade | 0.52 | |
| Grade 1 (n = 31) | 67.4 (7.5) | |
| Grade 2 (n = 84) | 62.0 (3.8) | |
| Grade 3 (n = 6) | 48.7 (22.7) | |
| Overall survival at 24 mo | 0.06 | |
| Alive (n = 99) | 65.6 (3.8) | |
| Deceased (n = 29) | 50.7 (5.7) | |
| PFS at 24 mo | 0.17 | |
| No progression (n = 62) | 66.9 (5.0) | |
| Progression (n = 66) | 57.8 (4.3) |
Post hoc analysis by Tukey identified P < 0.001 for pancreas vs. gastrointestinal SUVmax.
Data in parentheses are SEM.
PFS = progression-free survival.
FIGURE 1.
Box plot of 64Cu-DOTATATE SUVmax by WHO grade for 121 patients with NEN. G1, G2, and G3 = grades 1, 2, and 3, respectively.
Prediction of OS and PFS
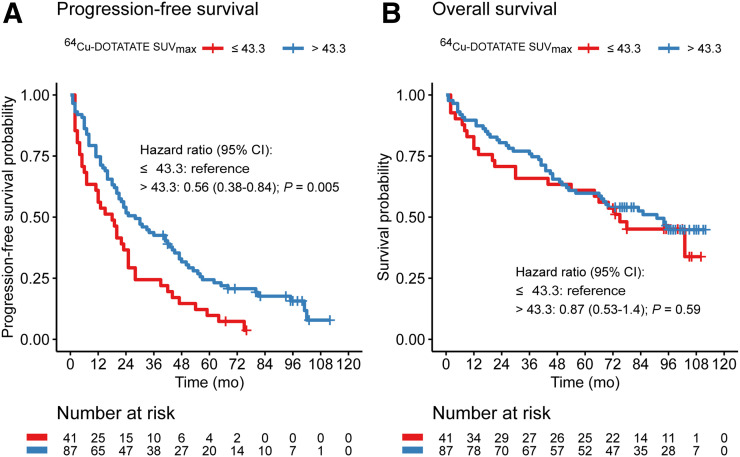
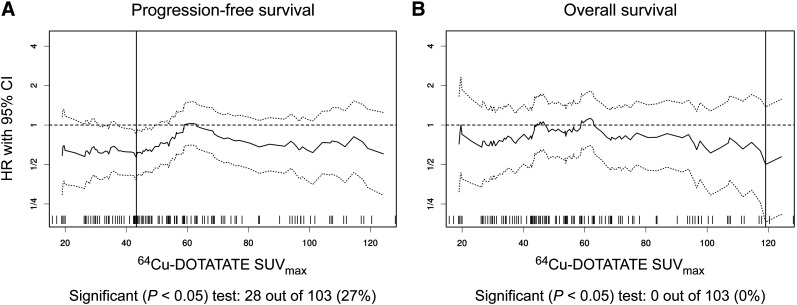
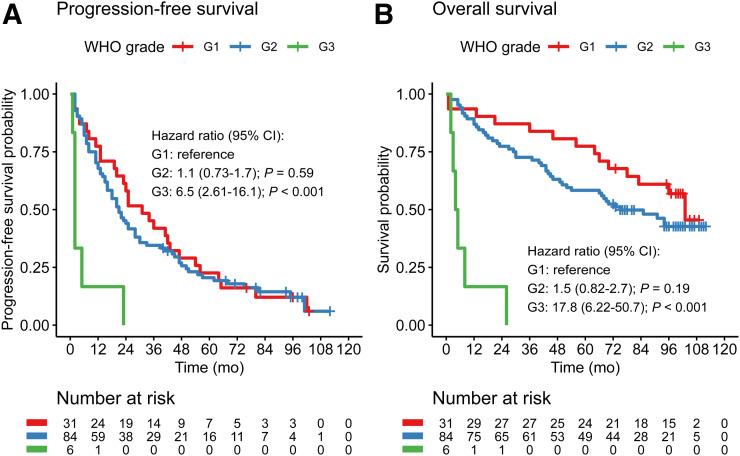
After the 64Cu-DOTATATE scan, patients were followed for a median of 73 mo (range, 1–112mo); 112 had progressive disease, and 69 died during follow-up. The median PFS was 23 mo (95% confidence interval, 19–30 mo), and the median OS was 85 mo (66 mo was the lower limit of the 95% confidence interval, and the upper limit was not reached). Applying the Cutoff Finder method (18), a cutoff SUVmax of 43.3 yielded a hazard ratio of 0.56 (range, 0.38–0.84) for prediction of PFS (reference SUVmax ≤ 43.3). No significant cutoff for prediction of OS was identified; thus, the 43.3 cutoff was used in the Kaplan–Meier plot for OS (Figs. 2–3). A significantly higher risk was found for WHO grade 3 than for grades 2 and 1 for OS and PFS. However, no significant difference was found between grade 1 and grade 2 (Fig. 4).
FIGURE 2.
Kaplan–Meier plots of outcome for 128 patients with NENs, stratified by 64Cu-DOTATATE SUVmax (reference: SUVmax ≤ 43.3). (A) PFS. (B) OS. CI = confidence interval.
FIGURE 3.
Plot of hazard ratios (solid lines) with 95% confidence intervals (dotted lines) at several cutoffs for 64Cu-DOTATATE SUVmax. Vertical solid line indicates most significant cutoff for SUVmax (43.3 for PFS [A] and no significant cutoff identified for OS [B]). CI = confidence interval; HR = hazard ratio.
FIGURE 4.
Kaplan–Meier plots of outcome for 121 patients with NENs, stratified by WHO grade (reference: grade 1). (A) PFS. (B) OS. CI = confidence interval; G1, G2, and G3 = grades 1, 2, and 3, respectively.
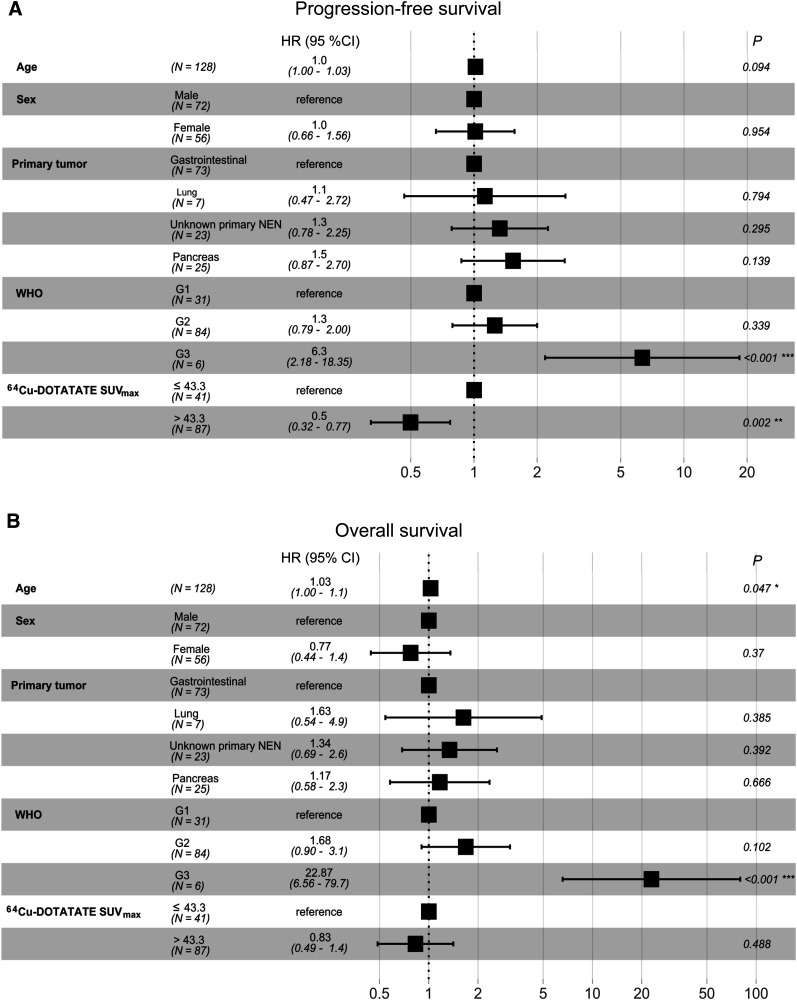
In a multiple Cox regression analysis with the outcome being PFS, both the identified SUVmax cutoff and WHO grade were significantly associated with PFS, whereas age, sex, and primary tumor site were not. In a multiple Cox regression analysis with the outcome being OS, only WHO grade and age were significantly associated with OS (Fig. 5).
FIGURE 5.
Forest plots of multiple Cox regression analyses to predict outcome in 128 patients with NENs. (A) PFS. (B) OS. CI = confidence interval; G1, G2, and G3 = grades 1, 2, and 3, respectively; HR = hazard ratio.
Prediction of PFS and OS 24 mo after 64Cu-DOTATATE PET/CT had a sensitivity of 39% and 41%, respectively; a specificity of 76% and 71%, respectively; and an accuracy of 57% and 64%, respectively.
DISCUSSION
This is the first study to report the association between 64Cu-DOTATATE PET/CT and outcome in patients with NENs. The main finding of our study in a cohort of 128 patients with NENs of all WHO grades and followed for up to more than 9 y was that the 64Cu-DOTATATE SUVmax in tumor lesions was significantly associated with PFS but not with OS. A 64Cu-DOTATATE SUVmax above 43.3 was associated with only half the likelihood of progression (hazard ratio, 0.56; 95% confidence interval, 0.38–0.84) of a 64Cu-DOTATATE SUVmax below or equal to 43.3.
SRI, by 68Ga- or 64Cu-conjugated somatostatin analogs, is regarded as key in initial diagnosis, staging, follow-up, and patient selection for peptide receptor radionuclide therapy in NET patients (19,20). The addition of PET to CT increases both the sensitivity and the specificity of NET detection (21). Several SRI PET tracers exist, and in general they all show high sensitivity and specificity (21). In a head-to-head comparison of 64Cu-DOTATATE versus 68Ga-DOTATOC, we showed that 64Cu-DOTATATE identified significantly more true lesions than 68Ga-DOTATOC (16). However, SRI is not routinely performed in patients with NEC because of lower sensitivity. In patients with NEC, 18F-FDG is often used to visualize the increased glucose metabolism in tumor lesions (22).
Determination of the Ki-67 index in tissue samples is essential in the WHO classification of NENs and has great implications on management of patient treatment. However, as histology is prone to sampling error, methods for whole-body examinations are needed. Furthermore, since most patients with NENs experience long-term survival, methods that allow for easy reexaminations are required. SRI is routinely performed on patients with NETs at the time of diagnosis and for follow-up evaluations. To the best of our knowledge, studies on prediction of OS based on SRI SUVmax have not been reported so far. Previously, it was reported that 68Ga-DOTANOC SUVmax (11,12) and 68Ga-DOTATATE SUVmax (13) are predictive for PFS. Our findings confirm these observations in a substantially larger cohort (128 vs. 46/43/30 patients). However, the specific cutoff varies among the studies, for several possible reasons. First, the study populations varied with regard to the origin of the primary NEN, with 1 study having only pancreatic tumors (11), 1 study having only ileal tumors (13), and 1 study including primarily pancreatic tumors (12). In comparison, our cohort had more patients with primary gastrointestinal tumors than pancreatic tumors and also included patients with unknown primary NENs and lung NENs. Our study confirmed the previous finding that primary pancreatic NENs have a higher SUVmax than primary gastrointestinal NENs—a fact that may affect the obtained cutoff (12). Second, the use of different PET isotopes and somatostatin analogs may affect the specific cutoff. The 64Cu-DOTATATE SUVmax reported here was greater than what has been reported for 68Ga-DOTANOC and 68Ga-DOTATATE (23,24), as was also evident in a previous head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC, in which SUVmax was higher for 64Cu-DOTATATE (16). Collectively, the predictive value of SRI by either 68Ga- or 64Cu-conjugated somatostatin analog for PFS is likely to be similar for the different SRI tracers, that is, a class effect.
Having demonstrated the predictive value of 64Cu-DOTATATE SUVmax for PFS, the question arises of whether this information can be used on an individual basis. Using the identified optimal SUVmax cutoff of 43.3 for prediction of PFS at 24 mo, a moderate accuracy of 57% was found. The previous studies also reported moderate to low accuracy in prediction of PFS on an individual-patient level (11–13).
In contrast to what was the case for PFS, our study could not demonstrate that 64Cu-DOTATATE SUVmax was predictive for OS. Currently, there are no other studies that have looked into SRI and OS. We would have expected that inclusion of NENs of grade 3, which have a substantially poorer prognosis and lower SSTR expression, would have revealed an association between 64Cu-DOTATATE SUVmax and OS. However, patients with NEN grade 3 made up only a small part of the study population, and our study therefore may have been underpowered to show the relation regarding OS.
Supporting the prognostic implications of SSTR are immunohistochemistry histologic examinations of NEN samples reporting variations in the density of SSTR expression according to tumor proliferation and differentiation (7,8,25–27). SRI SUVmax measured in biopsied lesions shows a close correlation with SSTR2 gene expression (28) and with SSTR expression assessed using immunohistochemistry (29,30). In well-differentiated tumors, the density of SSTR may be greater than in poorly differentiated NENs, and SSTR density has been associated with survival (25,31,32). However, a large dataset from 163 patients with NENs with Ki-67 of more than 20% shows that strong SSTR2a expression is present in a great proportion of grade 3 NENs, especially in pancreatic NECs, and SSTR2a density was not prognostic (33). Hence, the notion that SSTR is a trait restricted to low-grade NETs, and thus an indicator of differentiation, may not hold true.
Ideally, the minimum SUV should be applied in SRI, as the tumor or metastasis with the lowest SSTR density, that is, the most dedifferentiated part of the cancer, would then theoretically become the predictor for outcome. However, the accuracy and reproducibility of such a measure are questionable, and in a previous attempt, minimum SUV was not predictive of PFS (13). With SUVmax, the greatest SSTR density is reported, and the prediction is therefore likely based on the most differentiated tumor area, omitting more dedifferentiated areas. However, it is possible that there is a correlation between the minimum SUV and SUVmax, and SUVmax is the only measure that can realistically be obtained in a reproducible way in everyday practice. Other aspects of SRI may be exploited; for example, a quantitative measure of the total tumor burden based on SRI has also been reported as prognostic for PFS (34). In general, a greater use of the vast information embedded in SRI is warranted.
An alternative to SRI for prognostication in NENs could be 18F-FDG PET/CT. The 18F-FDG tracer reflects glucose uptake, and due to the Warburg effect in tumor cells, leading to greatly increased glucose metabolism, a high SUVmax is seen in tumors with high glycolytic activity. A strong correlation between 18F-FDG tumor uptake and prognosis in patients with NENs has been reported (5,6), with patients who have 18F-FDG–avid tumor lesions showing a markedly worse prognosis (hazard ratio of approximately 10 for both PFS and OS). However, compared with 18F-FDG, the advantage of SRI prognostication is the availability in almost all NET patients. In this cohort, 12 of 128 patients had 18F-FDG PET/CT performed within 100 d of the 64Cu-DOTATATE PET/CT, and we therefore abstained from comparative analysis.
This study had some limitations. The patient population was a representative population at a NET center. Accordingly, the patients may range from newly diagnosed to heavily pretreated. Although SRI is widely used for NETs, 18F-FDG PET/CT is preferred for high-grade NENs (20). Therefore, our cohort included relatively few patients with high-grade NENs—a limitation that may have affected the study’s ability to investigate the hypothesized inverse correlation between SRI and OS. Classification of high-grade NENs has changed considerably since the patients in this study were included; hence, the distinction between NET grade 3 and NEC (small and large cell) was not available. However, only 6 patients had a Ki-67% of more than 20%, making further subgrouping of limited value.
CONCLUSION
64Cu-DOTATATE PET/CT SUVmax in tumor or metastases was predictive of PFS in our large cohort of 128 patients with NENs who were followed for up to more than 9 y. However, the accuracy of predicting PFS for the individual patient was modest. Regarding OS, we found SRI to have no predictive value. Compared with 18F-FDG PET/CT, which has been shown strongly prognostic (PFS and OS) across the NEN grades, there seems little or no role for SRI in individual patient prognostication. This finding does not rule out potential prognostic value for SRI when used in predicting the outcome of SSTR-targeted therapies such as peptide receptor radionuclide therapy.
DISCLOSURE
Andreas Kjaer and Ulrich Knigge are inventors on a filed patent application: “PET tracer for imaging of neuroendocrine tumors” (WO 2013029616 A1). This project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreements 670261 (ERC Advanced Grant) and 668532 (Click-It), the Lundbeck Foundation, the Novo Nordisk Foundation, the Innovation Fund Denmark, the Danish Cancer Society, the Arvid Nilsson Foundation, the Svend Andersen Foundation, the Neye Foundation, the Research Foundation of Rigshospitalet, the Danish National Research Foundation (grant 126), the Research Council of the Capital Region of Denmark, the Danish Health Authority, the John and Birthe Meyer Foundation, and the Research Council for Independent Research. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 64Cu-DOTATATE tumor uptake on PET/CT associated with prognosis in patients with NENs?
PERTINENT FINDINGS: Patients with higher tracer uptake had a significantly lower risk of progression, but no significant association with survival was evident. However, the accuracy of predicting prognosis for the individual patient was modest.
IMPLICATIONS FOR PATIENT CARE: A high SUVmax on 64Cu-DOTATATE PET/CT is predictive of a longer PFS. However, on an individual-patient basis, the value seems limited. Nevertheless, 64Cu-DOTATATE SUVmax may have value on an individual basis in predicting the outcome of peptide receptor radionuclide therapy.
Acknowledgments
We are grateful to the staff at the Department of Clinical Physiology, Nuclear Medicine & PET for help in providing the PET tracers and performing the PET/CT studies.
REFERENCES
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2.Kloppel G, Couvelard A, Hruban RH, et al. In: Lloyd RV, Osamura RY, Klöppel G, Rosai J, eds. WHO Classification of Tumours of Endocrine Organs. 4th ed Lyon, France: International Agency for Research on Cancer; 2017:211–214. [Google Scholar]
- 3.WHO Classification of Tumours Editorial Board. Digestive System Tumours. 5th ed Lyon, France: International Agency for Research on Cancer; 2019:16–19. [Google Scholar]
- 4.Janson ET, Sorbye H, Welin S, et al. Nordic guidelines 2014 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. 2014;53:1284–1297. [DOI] [PubMed] [Google Scholar]
- 5.Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–985. [DOI] [PubMed] [Google Scholar]
- 6.Johnbeck CB, Knigge U, Langer SW, et al. Prognostic value of 18F-FLT PET in patients with neuroendocrine neoplasms: a prospective head-to-head comparison with 18F-FDG PET and Ki-67 in 100 patients. J Nucl Med. 2016;57:1851–1857. [DOI] [PubMed] [Google Scholar]
- 7.Kaemmerer D, Trager T, Hoffmeister M, et al. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget. 2015;6:27566–27579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Righi L, Volante M, Tavaglione V, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann Oncol. 2010;21:548–555. [DOI] [PubMed] [Google Scholar]
- 9.Kjaer A, Knigge U. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand J Gastroenterol. 2015;50:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrosini V, Campana D, Polverari G, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med. 2015;56:1843–1848. [DOI] [PubMed] [Google Scholar]
- 12.Campana D, Ambrosini V, Pezzilli R, et al. Standardized uptake values of 68Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010;51:353–359. [DOI] [PubMed] [Google Scholar]
- 13.Koch W, Auernhammer CJ, Geisler J, et al. Treatment with octreotide in patients with well-differentiated neuroendocrine tumors of the ileum: prognostic stratification with Ga-68-DOTA-TATE positron emission tomography. Mol Imaging. 2014;13:1–10. [PubMed] [Google Scholar]
- 14.Pfeifer A, Knigge U, Mortensen J, et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: first-in-humans study. J Nucl Med. 2012;53:1207–1215. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifer A, Knigge U, Binderup T, et al. 64Cu-DOTATATE PET for neuroendocrine tumors: a prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J Nucl Med. 2015;56:847–854. [DOI] [PubMed] [Google Scholar]
- 16.Johnbeck CB, Knigge U, Loft A, et al. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. J Nucl Med. 2017;58:451–457. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 18.Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks RJ, Kwekkeboom DJ, Krenning E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology. 2017;105:295–309. [DOI] [PubMed] [Google Scholar]
- 20.Sundin A, Arnold R, Baudin E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine & hybrid imaging. Neuroendocrinology. 2017;105:212–244. [DOI] [PubMed] [Google Scholar]
- 21.Johnbeck CB, Knigge U, Kjaer A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259–2277. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103:186–194. [DOI] [PubMed] [Google Scholar]
- 23.Wild D, Bomanji JB, Benkert P, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54:364–372. [DOI] [PubMed] [Google Scholar]
- 24.Kabasakal L, Demirci E, Ocak M, et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39:1271–1277. [DOI] [PubMed] [Google Scholar]
- 25.Corleto VD, Falconi M, Panzuto F, et al. Somatostatin receptor subtypes 2 and 5 are associated with better survival in well-differentiated endocrine carcinomas. Neuroendocrinology. 2009;89:223–230. [DOI] [PubMed] [Google Scholar]
- 26.Papotti M, Bongiovanni M, Volante M, et al. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors: a correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461–475. [DOI] [PubMed] [Google Scholar]
- 27.Zamora V, Cabanne A, Salanova R, et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis. 2010;42:220–225. [DOI] [PubMed] [Google Scholar]
- 28.Olsen IH, Langer SW, Federspiel BH, et al. 68Ga-DOTATOC PET and gene expression profile in patients with neuroendocrine carcinomas: strong correlation between PET tracer uptake and gene expression of somatostatin receptor subtype 2. Am J Nucl Med Mol Imaging. 2016;6:59–72. [PMC free article] [PubMed] [Google Scholar]
- 29.Miederer M, Seidl S, Buck A, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52. [DOI] [PubMed] [Google Scholar]
- 30.Kaemmerer D, Peter L, Lupp A, et al. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:1659–1668. [DOI] [PubMed] [Google Scholar]
- 31.Brunner P, Jorg AC, Glatz K, et al. The prognostic and predictive value of sstr2-immunohistochemistry and sstr2-targeted imaging in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2017;44:468–475. [DOI] [PubMed] [Google Scholar]
- 32.Qian ZR, Li T, Ter-Minassian M, et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas. 2016;45:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen K, Binderup T, Langer SW, et al. P53, somatostatin receptor 2a and chromogranin A immunostaining as prognostic markers in high grade gastroenteropancreatic neuroendocrine neoplasms. BMC Cancer. 2020;20:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toriihara A, Baratto L, Nobashi T, et al. Prognostic value of somatostatin receptor expressing tumor volume calculated from 68Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2019;46:2244–2251. [DOI] [PubMed] [Google Scholar]