Abstract
Background:
Some disinfection by-products (DBPs) are reproductive and developmental toxicants in laboratory animals. However, studies of trimester-specific DBP exposure on adverse birth outcomes in humans are inconsistent.
Objective:
We examined whether trimester-specific blood and urinary biomarkers of DBP were associated with small for gestational age (SGA), low birth weight (LBW), and preterm birth.
Methods:
A total of 4,086 blood and 3,951 urine samples were collected across pregnancy trimesters among 1,660 mothers from Xiaogan City, China. Blood samples were quantified for biomarkers of trihalomethanes (THMs): chloroform (TCM), bromodichloromethane, dibromochloromethane, and bromoform. Urine samples were quantified for biomarkers of haloacetic acids (HAA): dichloroacetic acid and trichloroacetic acid. Birth outcomes were abstracted at delivery from medical records. We used Poisson regression models with log link functions to estimate risk ratios (RRs) and 95% confidence intervals (CIs) for SGA, LBW, and preterm birth across tertiles (or categories) of DBP biomarker concentrations measured across pregnancy trimesters. We also examined the relative exposure differences across gestation comparing adverse outcomes with normal births using mixed-effects models.
Results:
Blood TCM concentrations in the second trimester were associated with an elevated risk of SGA comparing middle vs. lowest (RR, 2.34; 95% CI: 1.02, 5.35) and highest vs. lowest (RR, 2.47; 95% CI: 1.09, 5.58) exposure groups. Third-trimester blood TCM concentrations were also associated with an increased risk of SGA comparing the second tertile with the first (RR, 2.61; 95% CI: 1.15, 5.92). We found that maternal blood TCM concentrations were significantly higher for SGA compared with non-SGA births across the period from 23 to 34 wk gestation. Other blood and urinary DBP biomarkers examined were unrelated to SGA, LBW, or preterm birth.
Conclusion:
Blood TCM concentrations in mid to late pregnancy were associated with an increased risk of SGA, whereas other biomarkers of DBPs examined across pregnancy were not associated with birth outcomes. https://doi.org/10.1289/EHP7195
Background
Small for gestational age (SGA), low birth weight (LBW), and preterm birth are major contributors to infant mortality and account for a significant proportion of neonatal deaths in the United States and globally (Behrman and Butler 2007; Lawn et al. 2014). Adverse birth outcomes have also been associated with a greater risk of postneonatal mortality, growth failure, and adult-onset chronic diseases (Abitbol and Rodriguez 2012; Colman et al. 2012; Khashan et al. 2015; Silverberg et al. 2018). The Developmental Origins of Health and Disease paradigm maintains that early-life environments influence health outcomes later in life, and birth weight and gestational age are considered important markers of the intrauterine environment (Aris et al. 2018; Barker 2007; Basso 2008; O’Donnell and Meaney 2017).
Disinfection by-products (DBPs) are inadvertent widespread contaminants formed when oxidizing disinfectants (e.g., chlorine or ozone) react with natural and synthetic organic matter in the treatment of drinking water. Among more than 600 identified DBPs in chlorinated water, trihalomethanes (THMs) and haloacetic acids (HAAs) are the most prevalent species, accounting for 66% and 27%, respectively, of chlorinated DBP compounds in drinking water (Zhang et al. 2009). Exposure to volatile THMs may occur through inhalation and absorption during daily activities; in contrast, ingestion of water is thought to be the main route of exposure to nonvolatile HAAs (Nieuwenhuijsen et al. 2009b).
Toxicological studies have shown that DBP molecules can pass through the placenta (Danielsson et al. 1986), resulting in decreased conception rates, pregnancy loss, reduced birth weight, and dysmorphogenesis in rodents (Hunter et al. 2006; McMaster et al. 2018; Narotsky et al. 2011, 2015). However, epidemiological studies on DBP exposure in relation to adverse birth outcomes have produced inconclusive findings (Cao et al. 2016; Hinckley et al. 2005; Hoffman et al. 2008; Iszatt et al. 2014; Jaakkola et al. 2001; Kogevinas et al. 2016; Levallois et al. 2012; Mashau et al. 2019; Patelarou et al. 2011; Porter et al. 2005; Rivera-Núñez and Wright 2013; Smith et al. 2016; Villanueva et al. 2011; Wright et al. 2004; Yang et al. 2019), largely owing to design and exposure measurement issues. Previous studies mostly applied DBP concentrations measured in water distribution systems as a surrogate metric to assign exposure to individuals or estimated internal exposure dose by combining measures in the water distribution networks and individual water-use activities collected by questionnaires. However, these exposure assessments may result in misclassification due to poor resolution of spatial and temporal variability of monitoring data and inter- and intraindividual physiological differences in absorption, distribution, metabolism, and excretion of DBPs (Grellier et al. 2015).
An important issue is that the critical periods of susceptibility of early-life DBP exposure are understudied, with limited knowledge of the timing of exposure in pregnancy associated with potential adverse birth outcomes. Such data could lead to improved mechanistic insights for disease development and facilitate the establishment of more stringent regulatory drinking water guidelines for the protection of the fetus. Internal exposure biomarkers reflect integrative measures of exposure to DBPs from all routes and sources, providing an accurate exposure assessment for specific exposure windows. Blood THM concentrations are sensitive to low levels of exposure (Weisel et al. 1999). Although the elimination half-life of THMs in blood ranges from minutes to hours, they are believed to reflect steady-state blood concentrations due to the high frequency of daily exposure events and slower partitioning out of adipose tissue (Blount et al. 2011). Urinary dichloroacetic acid (DCAA) and trichloroacetic acid (TCAA), the two most prevalent species of HAAs, are potential biomarkers for ingested DBPs in chlorinated water (Wang et al. 2014; Weisel et al. 1999). Therefore, we aimed to examine whether blood and urinary biomarker concentrations of DBP measured in early, middle, and late pregnancy trimesters were associated with SGA, LBW, and preterm birth using data from a large prospective birth cohort in China.
Methods
Research Design
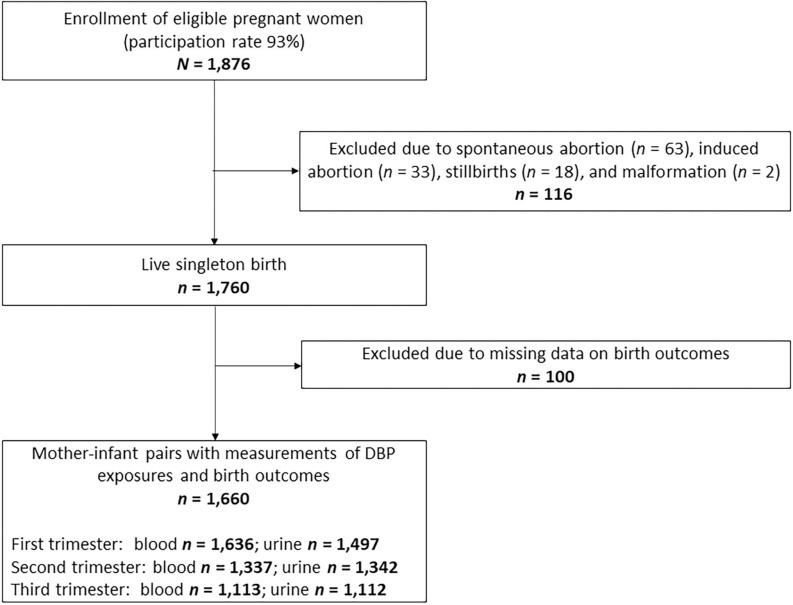
The Xiaogan Disinfection By-Products (XGDBP) Study is a prospective birth cohort conducted in Xiaogan City, China, that investigates the effects of prenatal DBP exposure on fetal growth and development (Chen et al. 2019; Wang et al. 2019). Xiaogan City was served by a single water treatment facility where chlorination was used to disinfect water. The median (arithmetic mean) monthly concentrations of chloroform (TCM), total trichloromethane (TTHMs), DCAA, and TCAA in the water distribution network of Xiaogan City were 4.2 (4.3), 7.1 (7.4), 3.4 (3.5), and , respectively, in measurement years 2015–2016 (Wang et al. 2019). To be included in the study, women had to be between 18 and 40 years of age, carrying a singleton gestation, permanently living in the study area, gestation at the time of study entry, no history of psychiatric illness (to avoid communication barrier), and no self-reported diagnosis of endocrine disease (e.g., diabetes and thyroid disease) that may affect birth outcomes (Billionnet et al. 2017; Sheehan et al. 2015). From 2015 to 2017, 1,876 of 2,021 (participation rate 93%) potentially eligible pregnant women attending the Maternal and Child Health Care Service Center of Xiaonan District were enrolled to participate. All participants completed a questionnaire, underwent physical examination, provided a single urine sample, and had a venous blood drawn at each study visit during early [first trimester, gestational age ; (SD), ], middle (second trimester, gestational age 14–27 wk; mean, ), and late pregnancy (third trimester, gestational age ; mean, ). After excluding 116 women due to stillbirths (), malformation (), spontaneous abortion (), and induced abortion (), and 100 participants due to missing data on birth outcomes, 1,660 women were retained in the current analysis (Figure 1). Our study was approved by the ethics committee of Tongji Medical College. All participants provided written informed consent before enrollment.
Figure 1.
Flowchart for study population.
Covariates
Participant characteristics, including race, height, age, occupation, and socioeconomic status, were collected at recruitment using self-reported baseline questionnaires. Lifestyle factors (e.g., tobacco and alcohol use), secondhand smoke–exposure, dietary habits, medical history, and water use activities (e.g., water sources, water-use frequency, daily tap-water consumption, and the time interval since last bathing/showing) were self-reported and collected at baseline and follow-up visits. Weight was measured by multifunctional anthropometric instruments at prenatal visits during each trimester. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Research staff recorded the date and time of biological specimen collection.
Blood and Urine Sample Collection and Biomarker Quantification
Specimen collection and quantification have been described in detail elsewhere (Wang et al. 2019). In brief, venous blood and urine samples were collected using vacutainers and polypropylene containers, respectively, which were validated to be free of contamination by analyzing a field blank sampling tube/container with boiled spring water (Zeng et al. 2013). We detected blood TCM, bromodichloromethane (BDCM), dibromochloromethane (DBCM), and bromoform (TBM) using a headspace solid-phase microextraction gas–chromatographic method (HS-SPME-GC) and urinary DCAA and TCAA using a liquid–liquid extraction gas–chromatographic method (LLE-GC). For quality control, each analysis run included a blank water sample (boiled spring water) and two pooled controls spiked with target analytes. The blank samples were used to detect possible contamination. The relative SD of repeated pooled controls was ; the spiked recoveries for THMs and HAAs ranged from 82% to 116%. The limits of detection (LODs) for TCM, BDCM, DBCM, TBM, DCAA, and TCAA were , , , , , and , respectively. Values of THMs and HAAs in biological samples below the LODs were replaced by (Hornung et al. 1990). Urinary creatinine and specific gravity (SG) were measured to correct for urine dilution using automated clinical analyzers (Wang et al. 2019).
Birth Outcomes
Infant birth and delivery data including delivery mode, date of birth, sex, gestational age at delivery, and birth weight were abstracted from hospital medical records by trained study staff. Gestational age at delivery was estimated by an obstetrician, by first-trimester ultrasound evaluations and/or self-reported date of last menstrual period. The outcomes of interest in the current study were SGA, LBW, and preterm birth. SGA and appropriate for gestational age (AGA), including both preterm and term-born delivery, were defined as a gender-specific birth weight less than 10th percentile and within 10th–90th percentile for gestational age in a representative Chinese referent population, respectively (Cao et al. 2016; Chen and Jin 2011). Preterm birth was defined as live-singleton births that occurred before 37 completed gestational wk (Spong 2013). LBW was defined as a birth weight of less than (Jin 2015).
Data Analysis
Descriptive statistics were conducted for maternal characteristics and neonatal birth outcomes. One-sample -test and chi-square tests were used to compare the differences in maternal and neonatal characteristics between the subgroups of SGA, LBW, and preterm birth and the total study population. Blood brominated trihalomethanes (Br-THM) were the sum concentrations of BDCM, DBCM, and TBM; TTHMs were the sum concentrations of TCM and Br-THMs. Maternal blood TCM, BDCM, Br-THM, and TTHM and urinary DCAA and TCAA concentrations were divided into tertiles based on all measurements across pregnancy trimesters. Because blood DBCM and TBM concentrations were detected in of the specimens, we constructed a three-level ordinal variable by percentiles: , 60th–80th, and .
Because of the prospective design of this cohort (Zou 2004), we fitted generalized estimating equations with a log link function and Poisson distribution using multiple informant models based on the SAS PROC GENMOD procedure to estimate the risk ratios (RRs) and 95% confidence intervals (CIs) of SGA (vs. AGA), LBW (vs. normal birth weight), and preterm birth (vs. term birth) by tertiles (or categories) of DBP biomarker concentrations during the first, second, and third trimesters of pregnancy (Sánchez et al. 2011). In brief, multiple informant models treat each of the three pregnancy trimesters as informants and simultaneously estimate the association between DBP exposure and study outcomes. This method retains the interpretation of a set of separate multiple regressions for each trimester and also tests the difference in associations between biomarker concentrations and birth outcomes across pregnancy trimesters by jointly estimating the regression models using Type 3 tests (Sánchez et al. 2011). We considered a Type 3 as an indication that associations differed significantly across pregnancy trimesters. Tests for linear trend were conducted by modeling tertiles (or categories) of DBP biomarker concentrations as an ordinal level variable using integer values. To further explore periods of susceptibility across pregnancy trimesters, we used the population exposure pattern approach developed by Sánchez et al. (2011). We applied quadratic mixed-effects models to estimate the relative exposure and 95% CIs of DBP biomarker concentrations across gestational weeks, comparing adverse birth outcomes vs. normal births.
Covariates were selected a priori and were then added in a forward stepwise model selection procedure in multivariable models if their inclusion resulted in a change in the estimated associations between each exposure and birth outcome (Maldonado and Greenland 1993). For consistency, we used the same set of covariates in the final multivariable models: maternal age at recruitment and the gestational week at sample collection were included as continuous variables; active or passive smoking exposure (yes vs. no), delivery mode (spontaneous vs. cesarean), and infant sex (female vs. male) were included as dichotomous variables; prepregnancy BMI (, 18.5-24.9, or ) and sampling time of day (07:00–08:59, 09:00–11:59, or 12:00–17:30) were included as categorical variables using indicator terms (i.e., two dichotomous indicator terms for each 3-category variable); and household income (, 3,000–4,999, or ) were included as an ordinal categorical variable using integer values (i.e., 0, 1, and 2). For the covariates with missing values at a given time point ( for any covariates), a missing indicator was used in the analysis. Urinary creatinine was included as a separate independent variable for associations between HAAs and birth outcomes. This approach allowed for the urinary analyte concentration to be appropriately adjusted for urinary dilution without introducing additional confounding (Barr et al. 2005).
Several sensitivity analyses were conducted. First, we restricted the analysis of associations between urinary HAAs and adverse birth outcomes among women who had a normal range of urinary creatinine (i.e., to ) to assess the influence of highly diluted or concentrated samples. Second, we reanalyzed the aforementioned associations in a subset of participants who had SG measured in urine. We used SG-adjusted concentrations calculated by the formula
where is the SG-corrected concentrations (), is the mean SG of the whole population, and is the unadjusted concentrations. Third, we reanalyzed the associations of blood THMs and urinary HAAs with SGA by excluding women who reported occupational exposures to organic/chlorinated solvents (), which might represent a different source of exposure to DBPs. Fourth, we included both prepregnancy BMI and trimester-specific weight gain as additional covariates in the multivariable models. Statistical analyses were performed using SAS (version 9.4; SAS Institute Inc.).
Results
Characteristics of Mother–Infant Pairs
The incidence of SGA, LBW, and preterm birth was 3.8% (), 2.0% (), and 3.6% (), respectively. The arithmetic mean () gestational age and birth weight of 1,660 infants were and , respectively (Table 1). Slightly fewer than half of the infants were boys (47.6%), and 60% were born by cesarean. Most mothers were of a Han ethnic background (99.4%), with a mean age of at enrollment. Although only 0.7% of mothers reported active smoking, 27.2% reported exposure to secondhand smoke. Only 3.7% reported alcohol consumption. More than 83% of the mothers used chlorinated tap water as their main water source. Women with an SGA birth tended to be thinner (lower BMI) and were more likely to have a spontaneous delivery compared to the overall study sample (Table 1).
Table 1.
Characteristics of mother–infant pairs in the overall study sample and by birth outcomes [ (%) or ].
| Characteristic | Overall ()a | SGA () | LBW () | PTB () |
|---|---|---|---|---|
| Birth outcomes | ||||
| Gestational age (wk) | ||||
| Birth weight (g) | ||||
| Gender | ||||
| Male | 790 (47.6) | 25 (39.7) | 20 (58.8) | 37 (62.7) |
| Female | 870 (52.4) | 38 (60.3) | 14 (41.2) | 22 (37.3) |
| Delivery mode | ||||
| Spontaneous | 664 (40.0) | 31 (49.2) | 10 (29.4) | 23 (39.0) |
| Caesarean | 994 (60.0) | 32 (50.8) | 24 (70.6) | 36 (61.0) |
| Maternal baseline characteristics | ||||
| Age (y) | ||||
| BMI at recruitment () | ||||
| 352 (21.4) | 20 (31.7) | 10 (29.4) | 14 (23.7) | |
| 18.5–24.9 | 1,153 (70.2) | 40 (63.5) | 23 (67.6) | 41 (69.5) |
| 137 (8.3) | 3 (4.8) | 1 (2.9) | 4 (6.8) | |
| Marital status | ||||
| Married | 1,579 (95.6) | 63 (100) | 32 (93.9) | 55 (93.2) |
| Other | 73 (4.4) | 0 (0) | 2 (5.9) | 4 (6.8) |
| Household income (yuan/month) | ||||
| 678 (41.0) | 23 (36.5) | 16 (47.1) | 26 (44.1) | |
| 3,000–4,999 | 756 (45.8) | 29 (46.0) | 13 (38.2) | 26 (44.1) |
| 218 (13.2) | 11 (17.5) | 5 (14.7) | 7 (11.9) | |
| Ethnicity | ||||
| Han | 1,641 (99.4) | 63 (100) | 34 (100.0) | 59 (100.0) |
| Other | 10 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Gravidity (including current pregnancy) | ||||
| 1 | 765 (46.1) | 35 (55.6) | 12 (35.3) | 28 (47.5) |
| 894 (53.9) | 28 (44.4) | 22 (64.7) | 31 (52.5) | |
| Education level | ||||
| Less than high school | 1,039 (62.9) | 37 (58.7) | 20 (58.8) | 37 (62.7) |
| High school and above | 612 (37.1) | 26 (41.3%) | 14 (41.2) | 22 (37.3) |
| Cigarette smoking status | ||||
| Never | 1,649 (99.3) | 62 (98.4) | 34 (100.0) | 59 (100.0) |
| Current/former | 11 (0.7) | 1 (1.6) | 0 (0) | 0 (0) |
| Secondhand smoke | ||||
| Never | 1,208 (72.8) | 43 (68.3) | 26 (76.5) | 44 (74.6) |
| Current/former | 452 (27.2) | 20 (31.7) | 8 (23.5) | 15 (25.4) |
| Alcohol use | ||||
| Never | 1,599 (96.3) | 63 (100) | 34 (100) | 58 (98.3) |
| Ever | 61 (3.7) | 0 (0) | 0 (0) | 1 (1.7) |
| Use of chlorinated tap water | ||||
| No (well or spring water) | 274 (16.5) | 12 (19.0) | 6 (17.6) | 15 (25.4) |
| Yes | 1,386 (83.5) | 51 (81.0) | 28 (82.4) | 44 (74.6) |
| Occupational exposures to organic/chlorinated solvents | ||||
| Ever | 48 (2.9) | 1 (1.6) | 1 (2.9) | 0 (0) |
| Never | 1,612 (97.1) | 62 (98.4) | 33 (97.1) | 59 (100) |
| Time-varying characteristics | ||||
| Sample collection time (h) | ||||
| 0700–0859 | 491 (12) | 24 (15.2) | 12 (14.1) | 21 (14.7) |
| 0900–1159 | 2,904 (70.8) | 104 (65.8) | 56 (65.9) | 95 (66.4) |
| 1200–1730 | 704 (17.2) | 30 (19) | 17 (20) | 27 (18.9) |
| Time interval since last bath/shower (h) | ||||
| 434 (10.5) | 17 (10.7) | 11 (12.4) | 19 (12.8) | |
| 12.1–24 | 2,496 (60.5) | 96 (60.4) | 60 (67.4) | 93 (62.8) |
| 1,193 (29.0) | 46 (28.9) | 18 (20.2) | 36 (24.4) | |
| Gestational age at sample collection (wk) | ||||
| First trimester | ||||
| Second trimester | ||||
| Third trimester | ||||
Note: BMI, body mass index; LBW, low birth weight; PTB, preterm birth; SD, standard deviation; SGA, small for gestational age.
A total of 18 women had missing information on BMI at recruitment, 8 on household income, 9 on ethnicity, 1 on smoking status, 3 on alcohol use, 4 on secondhand-smoke exposure, 2 on delivery way, 8 on marriage status, 1 on gravidity, 52 on sample collection time, 55 on time interval since last bath/shower, and 36 on gestational age at sample collection.
Distribution of Blood THMs and Urinary HAAs
Among 1,660 participants, there were 1,636 (98.6%), 1,337 (80.5%), and 1,113 (67.0%) women with blood drawn and quantified for THMs during the first, second, and third trimesters of pregnancy, respectively. There were 1,497 (90.2%), 1,342 (80.8%), and 1,112 (66.9%) women with urine samples quantified for HAA, during the first, second, and third trimesters of pregnancy, respectively (Table 2). Biomarkers TCM, BDCM, DCAA, and TCAA were detectable in of the samples collected across all pregnancy trimesters, whereas DBCM and TBM were detected in of the specimens (Table 2). Median blood THMs and urinary DCAA (creatinine-adjusted) concentrations were highest in the third trimester, and creatinine-adjusted urinary TCAA concentrations were highest in the first trimester (Table 2).
Table 2.
Distribution of blood THM and urinary HAA concentrations measured across pregnancy trimesters.
| Biomarkers | First trimester | Second trimester | Third trimester | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Median | IQR | % | Median | IQR | % | Median | IQR | ||||
| Blood THMs (ng/L) | ||||||||||||
| TCM | 1,636 | 91.1 | 10 | 5.7–15.8 | 1,337 | 91.7 | 9.6 | 5.7–14.7 | 1,113 | 94.6 | 11.2 | 7.0–16.7 |
| BDCM | 1,636 | 76.5 | 0.79 | 0.52–1.1 | 1,337 | 79.8 | 0.80 | 0.56–1.1 | 1,113 | 82.8 | 0.87 | 0.60–1.2 |
| DBCM | 1,636 | 41.5 | 1,337 | 42.6 | 1,113 | 43.9 | ||||||
| TBM | 1,636 | 46.3 | 1,337 | 47.1 | 1,113 | 52.4 | 2.59 | |||||
| Br-THMs | 1,636 | — | 3.8 | 2.7–10.4 | 1,337 | — | 3.7 | 2.8–11.3 | 1,113 | — | 4.5 | 2.8–17.8 |
| TTHMs | 1,636 | — | 16.5 | 10.3–28.2 | 1,337 | — | 16.3 | 10.5–26.8 | 1,113 | — | 19 | 12.2–36.6 |
| Urinary TCAA | ||||||||||||
| Crude () | 1,497 | 84.7 | 1.4 | 0.70–2.6 | 1,342 | 95.6 | 1.9 | 1.2–2.9 | 1,112 | 95.0 | 1.5 | 1.0–2.3 |
| SG-adjusted () | 1,494 | — | 1.73 | 1.16–2.57 | 1,331 | — | 1.79 | 1.22–2.62 | 1,093 | — | 1.59 | 1.13–2.25 |
| Creatinine-adjusted ( creatinine) | 1,458 | — | 2.28 | 1.41–4.34 | 1,327 | — | 1.92 | 1.31–2.98 | 1,105 | — | 2.14 | 1.51–3.07 |
| Urinary DCAA | ||||||||||||
| Crude () | 1,497 | 90.6 | 6.15 | 2.30–9.52 | 1,342 | 99.0 | 8.12 | 5.33–10.43 | 1,112 | 99.3 | 6.89 | 4.76–9.22 |
| SG-adjusted () | 1,494 | — | 6.63 | 4.34–9.40 | 1,331 | — | 7.45 | 5.29–10.23 | 1,093 | — | 7.14 | 5.19–9.48 |
| Creatinine-adjusted ( creatinine) | 1,458 | — | 8.44 | 5.46–14.61 | 1,327 | — | 8.03 | 5.62–11.41 | 1,105 | — | 9.27 | 6.97–12.85 |
Note: First trimester: gestational age ; mean, . Second trimester: gestational age 14–27 wk; mean, . Third trimester: gestational age ; mean, ). —, no data; BDCM, bromodichloromethane; Br-THM, brominated trihalomethanes; DBCM, dibromochloromethane; DCAA, dichloroacetic acid; HAA, haloacetic acid; IQR, interquartile range; LOD, limit of detection; TBM, bromoform; TCAA, trichloroacetic acid; TCM, chloroform; THM, trihalomethane; TTHMs, total trihalomethanes.
DBP Exposures and Risks of Adverse Birth Outcomes
After adjusting for confounders, blood TCM concentrations in the second trimester of pregnancy were associated with an elevated risk of SGA, comparing middle vs. lowest (RR, 2.34; 95% CI: 1.02, 5.35) and highest vs. lowest (RR, 2.47; 95% CI: 1.09, 5.58) exposure groups (Table 3). The risk of SGA was also higher among women with third-trimester blood TCM concentrations in the medium tertile (RR, 2.61; 95% CI: 1.15, 5.92), compared with women in the lowest tertile. The Type 3 tests revealed that the associations between TCM exposure and SGA differed across pregnancy trimesters (Type 3 , Table 3). The above-mentioned associations did not change substantially when we excluded women reporting occupational exposures to organic/chlorinated solvents, and those associations did not change when both prepregnancy BMI and trimester-specific weight gain were added as covariates (see Tables S1 and S2).
Table 3.
Associations between blood THM and urinary (HAA) concentrations (tertiles or categories) and risk of small for gestational age by pregnancy trimestersa
| Biomarkers | First trimester | Second trimester | Third trimester | Type 3 p-valuec |
|||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |||||
| THMs | |||||||
| TCM | — | — | — | — | — | — | 0.07 |
| T1 () | 1.0 | 24/425 | 1.0 | 8/341 | 1.0 | 8/293 | — |
| T2 () | 0.80 (0.43, 1.48) | 18/422 | 2.34 (1.02, 5.35) | 19/340 | 2.61 (1.15, 5.92) | 21/279 | — |
| T3 () | 0.94 (0.52, 1.69) | 21/410 | 2.47 (1.09, 5.58) | 22/350 | 2.08 (0.89, 4.83) | 17/287 | — |
| p-trend | 0.81 | — | 0.04 | — | 0.12 | — | — |
| BDCM | — | — | — | — | — | — | 0.12 |
| T1 () | 1.0 | 27/433 | 1.0 | 20/347 | 1.0 | 19/285 | — |
| T2 () | 0.84 (0.48, 1.48) | 22/417 | 0.89 (0.47, 1.71) | 17/350 | 0.69 (0.34, 1.40) | 13/287 | — |
| T3 () | 0.57 (0.30, 1.09) | 14/408 | 0.62 (0.30, 1.29) | 12/334 | 0.73 (0.36, 1.47) | 14/287 | — |
| p-trend | 0.09 | — | 0.21 | — | 0.37 | — | — |
| DBCM | — | — | — | — | — | — | 0.45 |
| () | 1.0 | 31/765 | 1.0 | 31/621 | 1.0 | 28/531 | — |
| 60–80th () | 1.74 (0.96, 3.16) | 17/247 | 0.75 (0.34, 1.64) | 8/200 | 1.00 (0.47, 2.13) | 9/162 | — |
| () | 1.50 (0.81, 2.79) | 15/245 | 0.88 (0.43, 1.81) | 10/210 | 1.08 (0.50, 2.29) | 9/166 | — |
| -trend | 0.11 | — | 0.62 | — | 0.87 | — | — |
| TBM | — | — | — | — | — | — | 0.73 |
| () | 1.0 | 36/750 | 1.0 | 30/624 | 1.0 | 27/518 | — |
| 60–80th () | 1.18 (0.64, 2.20) | 14/254 | 1.12 (0.56, 2.25) | 11/201 | 1.01 (0.47, 2.16) | 9/170 | — |
| () | 1.07 (0.57, 2.03) | 13/253 | 0.75 (0.34, 1.64) | 8/206 | 1.17 (0.56, 2.43) | 10/171 | — |
| p-trend | 0.74 | — | 0.57 | — | 0.70 | — | — |
| Br-THMs | — | — | — | — | — | — | 0.57 |
| T1 () | 1.0 | 19/436 | 1.0 | 20/339 | 1.0 | 14/299 | — |
| T2 () | 1.23 (0.66, 2.30) | 21/404 | 0.72 (0.36, 1.43) | 14/345 | 1.32 (0.64, 2.69) | 17/273 | — |
| T3 () | 1.29 (0.70, 2.37) | 23/417 | 0.71 (0.36, 1.39) | 15/347 | 1.15 (0.55, 2.38) | 15/287 | — |
| p-trend | 0.42 | — | 0.30 | — | 0.71 | — | — |
| TTHMs | — | — | — | — | — | — | 0.96 |
| T1 () | 1.0 | 21/433 | 1.0 | 12/339 | 1.0 | 15/283 | — |
| T2 () | 1.27 (0.71, 2.28) | 25/403 | 1.55 (0.75, 3.20) | 20/348 | 0.96 (0.47, 1.97) | 15/290 | — |
| T3 () | 0.85 (0.45, 1.61) | 17/421 | 1.29 (0.61, 2.71) | 17/344 | 1.08 (0.53, 2.19) | 16/286 | — |
| p-trend | 0.65 | — | 0.55 | — | 0.84 | — | — |
| HAAsb | |||||||
| TCAA | — | — | — | — | — | — | 0.69 |
| T1 () | 1.0 | 17/388 | 1.0 | 17/345 | 1.0 | 13/296 | — |
| T2 () | 1.66 (0.84, 3.29) | 25/367 | 1.18 (0.58, 2.37) | 19/336 | 1.64 (0.78, 3.42) | 21/274 | — |
| T3 () | 1.25 (0.51, 3.05) | 16/382 | 0.94 (0.43, 2.06) | 15/350 | 1.04 (0.46, 2.36) | 14/284 | — |
| p-trend | 0.58 | — | 0.87 | — | 1.00 | — | — |
| DCAA | — | — | — | — | — | — | 0.91 |
| T1 () | 1.0 | 17/385 | 1.0 | 18/352 | 1.0 | 18/296 | — |
| T2 () | 1.68 (0.86, 3.25) | 25/367 | 0.91 (0.45, 1.82) | 16/338 | 1.04 (0.52, 2.07) | 18/276 | — |
| T3 () | 1.18 (0.48, 2.87) | 16/385 | 1.02 (0.47, 2.23) | 17/341 | 0.60 (0.28, 1.30) | 12/282 | — |
| p-trend | 0.59 | — | 0.97 | — | 0.19 | — | — |
Note: First trimester: gestational age ; mean, . Second trimester: gestational age 14–27 wk; mean, . Third trimester: gestational age ; mean, ). —, no data; BDCM, bromodichloromethane; BMI, body mass index; Br-THM, brominated trihalomethanes; DBCM, dibromochloromethane; DCAA, dichloroacetic acid; HAA, haloacetic acid; n, numerator for cases of study outcome; N, total number of participants per subgroup; RR, risk ratio; TBM, bromoform; TCAA, trichloroacetic acid; TCM, chloroform; THM, trihalomethane; TTHMs, total trihalomethanes.
aAdjusted for maternal age, BMI at recruitment, household income, active/passive smoking status, gestational age at sampling, the time of day of sample collection, infant sex, and delivery mode.
bModels were additionally adjusted for creatinine by including the concentrations as a continuous covariate.
cType 3 tests were conducted based on multiple informant models by fitting generalized estimating equations with a log link function and Poisson distribution; a Type 3 indicated that the associations differed significantly across pregnancy trimesters.
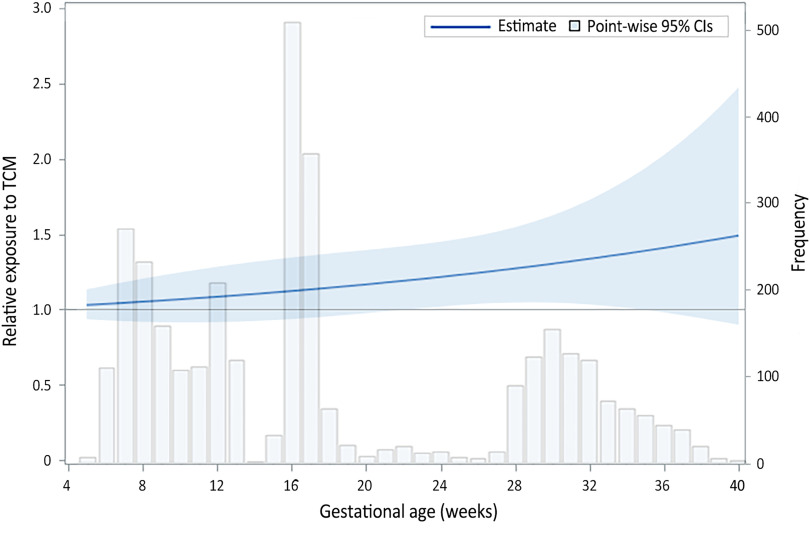
Relative blood TCM concentrations were higher in SGA births compared with AGA births during middle and late pregnancy (Figure 2). More specifically, lower pointwise CI crossed above the null at 23 wk (; 95% CI: 1.02, 1.43) and persisted until 34 wk gestation (; 95% CI: 1.01, 1.86), indicating significantly higher exposure across the period from 23 to 34 wk gestation in SGA compared with AGA births (Figure 2).
Figure 2.
Relative exposure to TCM concentrations across gestational weeks based on quadratic mixed-effects models, comparing SGA () with non-SGA births (). Model was adjusted for maternal age, BMI at the time of study recruitment, household income, active/passive smoking status, gestational age at sampling, the time of day of sample collection, infant sex, and delivery mode. SGA was defined as birth weight less than 10th percentile and non-SGA as within 10th–90th percentiles for gestational age in a representative Chinese referent population. Lower pointwise CI crossed above the null at 23 wk (; 95% CI: 1.02, 1.43) and persisted until 34 wk gestation (; 95% CI: 1.01, 1.86). Note: BMI, body mass index; CIs, confidence interval; SGA, small for gestational age; TCM, chloroform.
Other blood DBP biomarkers examined were not associated with SGA, LBW, or preterm birth (see Table 3 and Tables S3 and S4). Moreover, urinary DCAA and TCAA concentrations were not associated with any tested birth outcomes in this cohort (see Table 3 and Tables S3 and S4). The associations between urinary HAAs and adverse birth outcomes did not change materially when we restricted the analysis to women who had a normal range of urinary creatinine (i.e., to ) and when SG-corrected concentrations were used to correct for urine dilution (see Tables S5 and S6, respectively).
Discussion
The incidence of SGA, LBW, and preterm birth in this cohort was 3.8%, 2.0%, and 3.6%, respectively. Our study population is similar to that of a larger earlier study (1993–1998) that enrolled 200,589 infants born in 21 counties of 2 southern Chinese province (Zhejiang and Jiangsu) and a northern province (Hubei), reporting an incidence of SGA of 5.8% (Li et al. 2017). Similarly, in the Healthy Baby Cohort that enrolled 5,364 pregnant women between 2012 and 2014 in Wuhan, China, the estimated proportion of LBW and preterm birth was 2.2% and 3.3%, respectively (Yang et al. 2016). Results from our prospective birth cohort showed a positive association between maternal concentrations of blood TCM during the second and third trimesters of pregnancy and risk of SGA, defined as birth weight less than 10th percentile for gestational age in a representative Chinese referent population. When the population exposure pattern approach was applied, we found that maternal blood TCM concentrations were significantly higher for SGA compared with non-SGA births across the period from 23 to 34 wk gestation. There was no evidence of any associations between other THM biomarker concentrations and LBW or preterm birth. Maternal urinary DCAA and TCAA were also unrelated to any of the tested birth outcomes in our cohort. Our prospective birth cohort is the first study, to our knowledge, that measured internal biomarkers of DBP exposure across pregnancy in relation to adverse birth outcomes, providing new evidence of a potentially susceptible period of risk for SGA with exposure in middle and late pregnancy to TCM concentrations.
Several prior epidemiological studies have explored the association between trimester-specific DBP exposures and growth-related birth outcomes. In support of our findings, Summerhayes et al. (2012) reported a higher risk of SGA among women with high TCM concentrations during the third trimester by geocoding 314,982 births to water distribution systems in New South Wales, Australia. Similarly, Wright et al. (2004) reported exposure–response effects of elevated third-trimester TCM concentration on a greater risk of SGA among 196,000 infants in Boston, Massachusetts, USA. In a cohort study of 4,161 pregnant women in Lithuania, Grazuleviciene et al. (2011) reported that the estimated internal dose of TCM during second and third trimesters was positively associated with the risk of SGA. In contrast, several studies indicated a lack of association between THM exposure and SGA (Jaakkola et al. 2001; Kogevinas et al. 2016; Patelarou et al. 2011; Rivera-Núñez and Wright 2013; Villanueva et al. 2011). Our findings were also consistent with numerous previous studies showing no association between THMs and LBW or preterm birth (Hinckley et al. 2005; Kogevinas et al. 2016; Patelarou et al. 2011; Villanueva et al. 2011), and between HAAs and SGA, LBW, or preterm birth (Hoffman et al. 2008; Smith et al. 2016), though several studies support an association (Hinckley et al. 2005; Iszatt et al. 2014; Levallois et al. 2012; Porter et al. 2005; Rivera-Núñez and Wright 2013). These previous studies mostly used measures in water distribution networks to assign exposure to individuals or used indirect personal exposure measures by combining monitoring data and individual water-use activities to estimate internal exposure doses, both of which contribute to measurement error. Additionally, the discrepancy between studies could also be explained partly by regional variability in DBP concentrations in water systems. For instance, the water concentrations of TTHMs (mean: ; median: ) in Xiaogan City were among the lowest environmental exposure levels in previous studies from England (mean: ) (Smith et al. 2016), Spain (median range: ) (Villanueva et al. 2011), and United States (mean range: ) (Hinckley et al. 2005).
The determination of individual THMs and HAAs in biological specimens reflects integrative exposures from multiple routes and sources. To date, however, data on internal biomarkers of DBP exposure in relation to SGA, LBW, or preterm birth are lacking. In a previous study, we measured blood THMs and urinary TCAA concentrations in more than 1,100 pregnant women at the time of delivery (Cao et al. 2016; Yang et al. 2019). Consistent with our present study, there was no evidence of any association between urinary HAAs and SGA (Yang et al. 2019). However, our previous study also found a suggestive dose–response relationship between elevated blood Br-THM and TTHM concentrations and a higher incidence of SGA (p for and 0.08, respectively) (Cao et al. 2016). Our findings were also in contrast with a small study of 205 pregnant women from South Africa that reported a positive association between elevated third-trimester TCAA concentrations and the risk of SGA, premature birth, and low birth weight (Mashau et al. 2019). Differences between studies may be explained by the variability in exposure concentrations in different study populations. Most participants (64%) in our previous study lived in Wuhan City (Cao et al. 2016); their median blood concentrations of Br-THMs and TTHMs were uniformly higher than the concentrations of our present cohort population (5.6 vs. ; and 57.7 vs. , respectively) as a result of the difference in water DBP levels between cities. Similarly, the crude urinary concentrations of TCAA for South African women were 28 times higher than those in our present study population (201 vs. ) (Mashau et al. 2019). Discrepancies in the timing of exposure measurement may also contribute to the differences in the reported associations. For instance, our previous study recruited women at the time of delivery (Cao et al. 2016), whereas the present study is a prospective cohort with exposure measured throughout gestation, starting in the first trimester and followed to delivery.
Identifying windows of vulnerability during pregnancy when exposures may have particularly harmful effects on the fetus is crucial for targeting public health interventions (Sánchez et al. 2011). Although several studies have explored the associations of estimated DBP exposures from multiple routes (using aggregate data from municipal water sources) in different trimesters with birth outcomes (Grazuleviciene et al. 2011; Summerhayes et al. 2012), the statistical approaches used in those studies were based on multiple regression models, did not perform formal tests for the differences across the a priori–defined windows (i.e., periods of pregnancy corresponding to the three trimesters), and were limited in the variability of the timing of exposure measurement among participants (i.e., did not measure exposure across gestational weeks) (Sánchez et al. 2011). By applying the population exposure pattern approach, we identified that maternal blood TCM concentrations were significantly higher for SGA than for normal births across the period from 23 to 34 wk of gestation. This finding was consistent with our results based on multiple informant models, suggesting that the middle to late pregnancy is the critical window of susceptibility to TCM exposure. This result is biologically plausible, because fetal weight gain begins to show notable variation in the population after early second trimester (Kiserud et al. 2018), and thus disruption to the mechanisms needed for optimal growth during these time windows could have an important impact.
A substantial body of literature, mainly from animal studies, demonstrates the important role that oxidative stress plays in the relationship between THM exposure and fetal growth and development (Nieuwenhuijsen et al. 2009a). Both in vitro and in vivo studies have shown that TCM and a mixture of THMs induce oxidative stress via the depletion of glutathione and other antioxidant defenses, which, in turn, leads to DNA strand breakage and epigenetic changes (Beddowes et al. 2003; Pereira et al. 2001), and ultimately to abnormal fetal growth (Gluckman et al. 2008). In support of this hypothesis, our previous study using data from the same cohort showed monotonously positive dose–response relationships between blood TCM, Br-THM, and TTHM concentrations and urinary oxidative stress biomarkers (i.e., 8-hydroxy-2-deoxyguanosine and 4-hydroxy-2-nonenal-mercapturic acid) (Liu et al. 2020). Moreover, altered DNA methylation may also represent an intermediate biological mechanism linking THM exposures and adverse fetal development (Salas et al. 2019). In another smaller study, we reported an inverse association between maternal blood THM concentrations and cord blood levels of Alu and LINE-1 methylation among 115 pregnant women (Yang et al. 2017).
The strengths of this study include the prospective design, a comprehensive group of potential confounders, and most importantly, direct measurements of internal exposure biomarkers for two leading DBP species (i.e., THMs and HAAs). As showering and bathing strongly influence short-term blood THM concentrations (Nuckols et al. 2005), we collected blood samples at least 2 h after the last bath or shower to obtain steady-state concentrations of internal exposures. In addition, the wide variability in the timing of exposure assessments across participants enabled us to identify periods of susceptibility to DBP exposure across pregnancy. Our study also had some limitations. First, THMs and HAAs were measured at a single time point during each trimester, which may have attenuated some associations of moderate magnitude toward the null due to the high within-subject variability in DBP concentrations (Wang et al. 2014). Second, nearly 30% of participants did not measure DBP exposures in the third pregnancy, which may have resulted in selection bias. However, the proportion of adverse birth outcomes was similar between included and excluded women due to incomplete THM exposure data (see Table S7). In this case, the missing data in third trimester is likely nondifferential with respect to birth outcomes, likely resulting in associations biased toward the null. Third, some important polymorphisms in genes such as CYP2E1, CYP2D6, and GSTT1, which may affect DBP metabolisms, were not accounted for in this study. Fourth, a significant proportion (62.9%) of the study population reported their educational background as junior school and lower, which potentially limits the generalizability of our findings to women with lower socioeconomic or educational status. Fifth, our findings may have been confounded by other unidentified potential exposure sources and the changing glomerular filtration rate (GFR), blood protein binding, fat accumulation, and plasma volume during pregnancy, which may influence exposure biomarker concentrations (Anderson 2005). In this cohort, the median blood THM and creatinine-adjusted urinary DCAA concentrations were the highest in the third trimester, whereas creatinine-adjusted urinary TCAA concentrations were highest in the first trimester, which might be related to changes in external exposure levels, water-use activity, and the physiological and metabolic status during pregnancy (He et al. 2005). Sixth, the timing of exposure measurement during the second trimester was clustered around 16–17 wk of gestation, which may have generated imprecise vulnerability windows in the second trimester. Finally, we conducted many different comparisons, and some of our findings may be related to multiple tests. Nevertheless, we are reassured by the consistency in results using two different methods showing an increased risk of SGA in relation to TCM exposure in middle to late pregnancy and markedly higher concentrations across pregnancy comparing SGA with non-SGA births. Our results are also consistent with prior studies of predicted TCM concentrations from municipal water monitoring data in relation to the risk of SGA.
Conclusions
In this large prospective birth cohort with low environmental exposure levels, we found that maternal prenatal exposure to TCM, measured as blood biomarker concentrations during pregnancy was positively associated with SGA risk. Additionally, we identified a potentially susceptible period of risk in relation to exposure starting in mid-pregnancy (23 wk gestation) and up to 35 wk gestation. Other blood and urinary DBP biomarkers examined across pregnancy were unrelated to SGA, LBW, or preterm birth. Future studies should validate this period of risk using similar internal biomarkers of exposure measured throughout gestation in a different study population to determine if our findings are consistent and generalizable. Nevertheless, these results should be considered in the establishment of stringent regulatory drinking-water guidelines given their potential impact on fetal growth.
Supplementary Material
Acknowledgments
Y. Sun and Y.X. Wang analyzed and drafted the manuscript. Y.X. Wang and W.Q. Lu designed the study and managed the funding. Y.X. Wang, C. Liu, Y.J. Chen, and Y. Sun participated in participants recruitment, biospecimen sample and epidemiological data collection, clinical data cleaning, and laboratory measurements. C. Messerlian and Y.X. Wang designed the study, directed the analysis, made major revisions to the manuscript. C. Messerlian made major revisions to the manuscript text, and provided scientific direction throughout.
The authors thank all study participants, students, and research staff who participated in this cohort. This work was supported by the National Natural Science Foundation of China (No. 81673123 and 81903281).
References
- Abitbol CL, Rodriguez MM. 2012. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol 8(5):265–274, PMID: 22371245, 10.1038/nrneph.2012.38. [DOI] [PubMed] [Google Scholar]
- Anderson GD. 2005. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44(10):989–1008, PMID: 16176115, 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- Aris IM, Fleisch AF, Oken E. 2018. Developmental origins of disease: emerging prenatal risk factors and future disease risk. Curr Epidemiol Rep 5(3):293–302, PMID: 30687591, 10.1007/s40471-018-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. 2007. The origins of the developmental origins theory. J Intern Med 261(5):412–417, PMID: 17444880, 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200, PMID: 15687057, 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso O. 2008. Birth weight is forever. Epidemiology 19 (2):204–205, PMID: 18277158, 10.1097/EDE.0b013e31816379d9. [DOI] [PubMed] [Google Scholar]
- Beddowes EJ, Faux SP, Chipman JK. 2003. Chloroform, carbon tetrachloride and glutathione depletion induce secondary genotoxicity in liver cells via oxidative stress. Toxicology 187(2–3):101–115, PMID: 12699900, 10.1016/s0300-483x(03)00058-1. [DOI] [PubMed] [Google Scholar]
- Behrman R, Butler A. 2007. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, et al. 2017. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 60(4):636–644, PMID: 28197657, 10.1007/s00125-017-4206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount B, Backer L, Aylward L, Hays S, LaKind J. 2011. Human exposure assessment for DBPs: factors influencing blood trihalomethane levels. In: Encyclopedia of Environmental Health. Nriagu JO, ed. 1st ed. London, UK: Elsevier Science, 100–107, 10.1016/B978-0-444-52272-6.00103-3. [DOI] [Google Scholar]
- Cao WC, Zeng Q, Luo Y, Chen HX, Miao DY, Li L, et al. 2016. Blood biomarkers of late pregnancy exposure to trihalomethanes in drinking water and fetal growth measures and gestational age in a Chinese cohort. Environ Health Perspect 124(4):536–541, PMID: 26340795, 10.1289/ehp.1409234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Jin H. 2011. Neonatal classification. In: Practice of Neonatology. Shao XM, Ye HM, Qiu XS, eds. [in Chinese]. Beijing, China: People’s Medical Publishing House, 46–47. [Google Scholar]
- Chen YJ, Liu C, Huang LL, Ai SH, Sun L, Huang Z, et al. 2019. First-trimester blood concentrations of drinking water trihalomethanes and neonatal neurobehavioral development in a Chinese birth cohort. J Hazard Mater 362:451–457, PMID: 30265976, 10.1016/j.jhazmat.2018.09.040. [DOI] [PubMed] [Google Scholar]
- Colman I, Ataullahjan A, Naicker K, Van Lieshout RJ. 2012. Birth weight, stress, and symptoms of depression in adolescence: evidence of fetal programming in a national Canadian cohort. Can J Psychiatry 57(7):422–428, PMID: 22762297, 10.1177/070674371205700705. [DOI] [PubMed] [Google Scholar]
- Danielsson BR, Ghantous H, Dencker L. 1986. Distribution of chloroform and methyl chloroform and their metabolites in pregnant mice. Biol Res Pregnancy Perinatol 7(2):77–83, PMID: 3730475. [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. 2008. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73, PMID: 18596274, 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazuleviciene R, Nieuwenhuijsen MJ, Vencloviene J, Kostopoulou-Karadanelli M, Krasner SW, Danileviciute A, et al. 2011. Individual exposures to drinking water trihalomethanes, low birth weight and small for gestational age risk: a prospective Kaunas cohort study. Environ Health 10:32, PMID: 21501533, 10.1186/1476-069X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grellier J, Rushton L, Briggs DJ, Nieuwenhuijsen MJ. 2015. Assessing the human health impacts of exposure to disinfection by-products–a critical review of concepts and methods. Environ Int 78:61–81, PMID: 25765762, 10.1016/j.envint.2015.02.003. [DOI] [PubMed] [Google Scholar]
- He XJ, Ejiri N, Nakayama H, Doi K. 2005. Effects of pregnancy on CYPs protein expression in rat liver. Exp Mol Pathol 78(1):64–70, PMID: 15596063, 10.1016/j.yexmp.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hinckley AF, Bachand AM, Reif JS. 2005. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ Health Perspect 113(12):1808–1813, PMID: 16330369, 10.1289/ehp.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, Mendola P, Savitz DA, Herring AH, Loomis D, Hartmann KE. 2008. Drinking water disinfection by-product exposure and fetal growth. Epidemiology 19:729–737, PMID: 18633330, 10.1097/EDE.0b013e3181812bd4. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Hunter ES 3rd, Rogers E, Blanton M, Richard A, Chernoff N. 2006. Bromochloro-haloacetic acids: effects on mouse embryos in vitro and QSAR considerations. Reprod Toxicol 21(3):260–266, PMID: 16293395, 10.1016/j.reprotox.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Iszatt N, Nieuwenhuijsen MJ, Bennett JE, Toledano MB. 2014. Trihalomethanes in public drinking water and stillbirth and low birth weight rates: an intervention study. Environ Int 73:434–439, PMID: 25244706, 10.1016/j.envint.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJ, Magnus P, Skrondal A, Hwang BF, Becher G, Dybing E. 2001. Foetal growth and duration of gestation relative to water chlorination. Occup Environ Med 58(7):437–442, PMID: 11404447, 10.1136/oem.58.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J. 2015. JAMA patient page. Babies with low birth weight. JAMA 313(4):432, PMID: 25626052, 10.1001/jama.2014.3698. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Kenny LC, Lundholm C, Kearney PM, Gong T, McNamee R, et al. 2015. Gestational age and birth weight and the risk of childhood type 1 diabetes: a population-based cohort and sibling design study. Diabetes Care 38(12):2308–2315, PMID: 26519334, 10.2337/dc15-0897. [DOI] [PubMed] [Google Scholar]
- Kiserud T, Benachi A, Hecher K, Perez RG, Carvalho J, Piaggio G, et al. 2018. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol 218(2):S619–S629, PMID: 29422204, 10.1016/j.ajog.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Kogevinas M, Bustamante M, Gracia-Lavedan E, Ballester F, Cordier S, Costet N, et al. 2016. Drinking water disinfection by-products, genetic polymorphisms, and birth outcomes in a European mother-child cohort study. Epidemiology 27:903–911, PMID: 27468006, 10.1097/EDE.0000000000000544. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. 2014. Every newborn: progress, priorities, and potential beyond survival. Lancet 384(9938):189–205, PMID: 24853593, 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- Levallois P, Gingras S, Marcoux S, Legay C, Catto C, Rodriguez M, et al. 2012. Maternal exposure to drinking-water chlorination by-products and small-for-gestational-age neonates. Epidemiology 23:267–276, PMID: 22317810, 10.1097/EDE.0b013e3182468569. [DOI] [PubMed] [Google Scholar]
- Li N, Li Z, Ye R, Liu J, Ren A. 2017. Impact of periconceptional folic acid supplementation on low birth weight and small-for-gestational-age infants in China: a large prospective cohort study. J Pediatr 187:105–110, PMID: 28545876, 10.1016/j.jpeds.2017.04.060. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang YX, Chen YJ, Sun Y, Huang LL, Cheng YH, et al. 2020. Blood and urinary biomarkers of prenatal exposure to disinfection byproducts and oxidative stress: a repeated measurement analysis. Environ Int 137:105518, PMID: 32018134, 10.1016/j.envint.2020.105518. [DOI] [PubMed] [Google Scholar]
- Maldonado G, Greenland S. 1993. Simulation study of confounder-selection strategies. Am J Epidemiol 138(11):923–936, PMID: 8256780, 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- Mashau F, Ncube EJ, Voyi K. 2019. Maternal urinary levels of trichloroacetic acid and association with adverse pregnancy outcomes. J Water Health 17(6):884–895, PMID: 31850896, 10.2166/wh.2019.109. [DOI] [PubMed] [Google Scholar]
- McMaster ME, Ashley-Sing C, Dos Santos Tavares AA, Corral CA, McGill K, McNeil D, et al. 2018. The inhalation effects of by-products from chlorination of heated indoor swimming pools on spinal development in pup mice. Environ Res 166:668–676, PMID: 30015251, 10.1016/j.envres.2018.06.049. [DOI] [PubMed] [Google Scholar]
- Narotsky MG, Best DS, McDonald A, Godin EA, Hunter ES 3rd, Simmons JE. 2011. Pregnancy loss and eye malformations in offspring of F344 rats following gestational exposure to mixtures of regulated trihalomethanes and haloacetic acids. Reprod Toxicol 31(1):59–65, PMID: 20850520, 10.1016/j.reprotox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Narotsky MG, Klinefelter GR, Goldman JM, DeAngelo AB, Best DS, McDonald A, et al. 2015. Reproductive toxicity of a mixture of regulated drinking-water disinfection by-products in a multigenerational rat bioassay. Environ Health Perspect 123(6):564–570, PMID: 25695961, 10.1289/ehp.1408579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Grellier J, Smith R, Iszatt N, Bennett J, Best N, et al. 2009a. The epidemiology and possible mechanisms of disinfection by-products in drinking water. Philos Trans A Math Phys Eng Sci 367(1904):4043–4076, PMID: 19736233, 10.1098/rsta.2009.0116. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Martinez D, Grellier J, Bennett J, Best N, Iszatt N, et al. 2009b. Chlorination disinfection by-products in drinking water and congenital anomalies: review and meta-analyses. Environ Health Perspect 117(10):1486–1493, PMID: 20019896, 10.1289/ehp.0900677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckols JR, Ashley DL, Lyu C, Gordon SM, Hinckley AF, Singer P. 2005. Influence of tap water quality and household water use activities on indoor air and internal dose levels of trihalomethanes. Environ Health Perspect 113(7):863–870, PMID: 16002374, 10.1289/ehp.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KJ, Meaney MJ. 2017. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry 174(4):319–328, PMID: 27838934, 10.1176/appi.ajp.2016.16020138. [DOI] [PubMed] [Google Scholar]
- Patelarou E, Kargaki S, Stephanou EG, Nieuwenhuijsen M, Sourtzi P, Gracia E, et al. 2011. Exposure to brominated trihalomethanes in drinking water and reproductive outcomes. Occup Environ Med 68(6):438–445, PMID: 20952554, 10.1136/oem.2010.056150. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Kramer PM, Conran PB, Tao L. 2001. Effect of chloroform on dichloroacetic acid and trichloroacetic acid-induced hypomethylation and expression of the c-myc gene and on their promotion of liver and kidney tumors in mice. Carcinogenesis 22(9):1511–1519, PMID: 11532874, 10.1093/carcin/22.9.1511. [DOI] [PubMed] [Google Scholar]
- Porter CK, Putnam SD, Hunting KL, Riddle MR. 2005. The effect of trihalomethane and haloacetic acid exposure on fetal growth in a Maryland county. Am J Epidemiol 162(4):334–344, PMID: 16014784, 10.1093/aje/kwi211. [DOI] [PubMed] [Google Scholar]
- Rivera-Núñez Z, Wright JM. 2013. Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J Occup Environ Med 55(10):1125–1134, PMID: 24064786, 10.1097/JOM.0b013e3182a4ffe4. [DOI] [PubMed] [Google Scholar]
- Salas LA, Baker ER, Nieuwenhuijsen MJ, Marsit CJ, Christensen BC, Karagas MR. 2019. Maternal swimming pool exposure during pregnancy in relation to birth outcomes and cord blood DNA methylation among private well users. Environ Int 123:459–466, PMID: 30622071, 10.1016/j.envint.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119(3):409–415, PMID: 21362588, 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan PM, Nankervis A, Araujo Júnior E, Da Silva Costa F. 2015. Maternal thyroid disease and preterm birth: systematic review and meta-analysis. J Clin Endocrinol Metab 100(11):4325–4331, PMID: 26383905, 10.1210/jc.2015-3074. [DOI] [PubMed] [Google Scholar]
- Silverberg O, Park AL, Cohen E, Fell DB, Ray JG. 2018. Premature cardiac disease and death in women whose infant was preterm and small for gestational age: a retrospective cohort study. JAMA Cardiol 3(3):247–251, PMID: 29387888, 10.1001/jamacardio.2017.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RB, Edwards SC, Best N, Wright J, Nieuwenhuijsen MJ, Toledano MB. 2016. Birth weight, ethnicity, and exposure to trihalomethanes and haloacetic acids in drinking water during pregnancy in the Born in Bradford cohort. Environ Health Perspect 124(5):681–689, PMID: 26340797, 10.1289/ehp.1409480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong CY. 2013. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 309(23):2445–2446, PMID: 23645117, 10.1001/jama.2013.6235. [DOI] [PubMed] [Google Scholar]
- Summerhayes RJ, Morgan GG, Edwards HP, Lincoln D, Earnest A, Rahman B, et al. 2012. Exposure to trihalomethanes in drinking water and small-for-gestational-age births. Epidemiology 23:15–22, PMID: 22157301, 10.1097/EDE.0b013e31823b669b. [DOI] [PubMed] [Google Scholar]
- Villanueva CM, Gracia-Lavedán E, Ibarluzea J, Santa Marina L, Ballester F, Llop S, et al. 2011. Exposure to trihalomethanes through different water uses and birth weight, small for gestational age, and preterm delivery in Spain. Environ Health Perspect 119(12):1824–1830, PMID: 21810554, 10.1289/ehp.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Liu C, Chen YJ, Duan P, Wang Q, Chen C, et al. 2019. Profiles, variability and predictors of concentrations of blood trihalomethanes and urinary haloacetic acids along pregnancy among 1760 Chinese women. Environ Res 172:665–674, PMID: 30878738, 10.1016/j.envres.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zeng Q, Wang L, Huang YH, Lu ZW, Wang P, et al. 2014. Temporal variability in urinary levels of drinking water disinfection byproducts dichloroacetic acid and trichloroacetic acid among men. Environ Res 135:126–132, PMID: 25262085, 10.1016/j.envres.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Weisel CP, Kim H, Haltmeier P, Klotz JB. 1999. Exposure estimates to disinfection by-products of chlorinated drinking water. Environ Health Perspect 107(2):103–110, PMID: 9924004, 10.1289/ehp.99107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Schwartz J, Dockery DW. 2004. The effect of disinfection by-products and mutagenic activity on birth weight and gestational duration. Environ Health Perspect 112(8):920–925, PMID: 15175183, 10.1289/ehp.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Cao WC, Zhou B, Zheng TZ, Deng YL, Luo Q, et al. 2019. Urinary biomarker of prenatal exposure to disinfection byproducts, maternal genetic polymorphisms in CYP2E1 and GSTZ1, and birth outcomes. Environ Sci Technol 53(20):12026–12034, PMID: 31525872, 10.1021/acs.est.9b03847. [DOI] [PubMed] [Google Scholar]
- Yang J, Huo W, Zhang B, Zheng T, Li Y, Pan X, et al. 2016. Maternal urinary cadmium concentrations in relation to preterm birth in the Healthy Baby Cohort Study in China. Environ Int 94:300–306, PMID: 27289180, 10.1016/j.envint.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Yang P, Zhou B, Cao WC, Wang YX, Huang Z, Li J, et al. 2017. Prenatal exposure to drinking water disinfection by-products and DNA methylation in cord blood. Sci Total Environ 586:313–318, PMID: 28174046, 10.1016/j.scitotenv.2017.01.224. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Li M, Xie SH, Gu LJ, Yue J, Cao WC, et al. 2013. Baseline blood trihalomethanes, semen parameters and serum total testosterone: a cross-sectional study in China. Environ Int 54:134–140, PMID: 23454109, 10.1016/j.envint.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Zhang W, Gabos S, Schopflocher D, Li X-F, Gati WP, Hrudey SE. 2009. Reliability of using urinary and blood trichloroacetic acid as a biomarker of exposure to chlorinated drinking water disinfection byproducts. Biomarkers 14(6):355–365, PMID: 19583459, 10.1080/13547500903079186. [DOI] [PubMed] [Google Scholar]
- Zou G. 2004. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706, PMID: 15033648, 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.