Abstract
In the REGONIVO study, regorafenib combined with nivolumab was effective in the treatment of microsatellite stable (MSS) metastatic colorectal cancer (mCRC), which indicated anti-angiogenic drugs may enhance the efficacy of immune checkpoint inhibitors. Therefore, we designed a single-arm, single-center, open-label, phase II trial to determine the toxicity and efficacy of SHR-1210 (an anti-PD-1 antibody) plus apatinib in MSS mCRC. The sample size was estimated using a Simon Optimum two-stage design. 10 patients were included at the first stage and if one effective patient observed, an additional 19 patients would be added. Patients with MSS mCRC who refractory to second-line treatment or intolerant to standard treatment were given SHR-1210 200 mg every 2 weeks and apatinib 250-375 mg once daily until unacceptable toxicity or disease progression occurred. In our study, the objective response rate was 0% and the disease control rate was 22.2%. The median progression-free survival was 1.83 months (95% confidence interval (CI) 1.80-1.86 months), and the median overall survival was 7.80 months (95% CI 0-17.07). Treatment-related adverse events (AEs) occurred in all patients (100%). The most common treatment-related AEs were hypertension and proteinuria (70% each). Grade 3 AEs were observed in nine patients (9/10, 90%), and the commonest was hypertension (30%). In conclusion, SHR-1210 combined with apatinib has failed to improve the efficacy of treatment of MSS mCRC, and the intolerable toxicity may be the leading cause.
Keywords: Apatinib, immunotherapy, metastatic colorectal cancer, microsatellite stable, SHR-1210
Introduction
In 2018, there were estimated to be more than 1.8 million new colorectal cancer cases (CRC) and 881 thousand deaths caused by CRC [1]. In China, CRC ranks fifth in cancer-related mortality, and the estimated number of colorectal cancer-related deaths in 2015 is 191 thousand [2].
At present, metastatic colorectal cancer patients are recommended to treat with oxaliplatin followed by irinotecan-containing therapy [3], or given fluoropyrimidine-based therapy with anti-vascular endothelial growth factor or with anti-epidermal growth factor receptor antibodies. For whom refractory or intolerant to above therapies, regorafenib or trifuridine/tipiracil is recommended [4]. But the prognosis remains poor and the 5-year survival rate is approximately 11% [5]. It is worth mentioning that tumor immunotherapy has made a breakthrough in recent years.
The use of immune checkpoint inhibitors (ICI) has brought new hope for improving the therapeutic effect of mCRC. Pembrolizumab and nivolumab were approved by the US Food and Drug Administration for treatment of mCRC with microsatellite instability-high (MSI-H). In a clinical trial that patients with mCRC were treated with pembrolizumab, the immune-related objective response rate was 40% in patients with MSI-H mCRC, and 0% in MSS mCRC patients [6]. However, only approximately 15% of all CRCs and 5% of mCRCs were MSI-H [7], which means that most of CRC with MSS are difficult to benefit from single-agent ICI. Therefore, it is urgent to find a new treatment model, and the combination therapy of ICI may bring new hope.
Previous studies had shown that antiangiogenesis agents combined with immune checkpoint blockade could significantly improve the validity of treatment for malignant tumors, such as metastatic melanoma [8]. Regorafenib is an orally administered multikinase inhibitor with survival benefits in mCRC patients who were refractory to all standard therapies [9]. A phase Ib trial from Japan, REGONIVO, was conducted to evaluate the safety and efficacy of regorafenib combined with nivolumab in the treatment of advanced gastric cancer and CRC. The preliminary results were encouraging. The objective response rate (ORR) of advanced gastric cancer was 44% and MSS CRC was 36%. This study showed that regorafenib combined with nivolumab was safe and controllable, and had good anti-tumor activity in advanced gastric cancer and CRC with MSS [10].
Apatinib, another antiangiogenic tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor (VEGFR)-2, is approved for the treatment of advanced or metastatic gastric cancer in China [11]. Previous studies had shown that apatinib monotherapy was effective in the treatment of mCRC [12,13]. The median progression-free survival (PFS) was 3.717 months (95% CI 3.198-4.235), and the median overall survival (OS) was 7.335 months (95% CI, 6.738-7.932) [13]. SHR-1210, an anti-programmed death-1 (PD-1) antibody, which was approved in China for the treatment of recurrent/refractory classical Hodgkin’s lymphoma [14], had shown anti-tumor effects and tolerable AEs in the treatment of various other malignancies [15-17]. In addition, some studies had shown that SHR-1210 combined with apatinib was safe and effective in the treatment of advanced solid tumors [18].
Antiangiogenesis has been considered as a possible way to reverse the resistance of immunotherapy, but it needs more researches to verify. Therefore, we designed a phase II clinical trial to explore the feasibility of SHR-1210 (an anti-PD-1 antibody) combined with apatinib in the treatment of patients with proficient mismatch repair (pMMR)/MSS mCRC who refractory to at least the second-line treatment or intolerant to standard treatment.
Patients and methods
Patient selection
The study enrolled patients with pMMR/MSS mCRC who refractory to at least second-line systemic therapy or intolerant to standard therapy. The eligibility criteria included age 18-75 years old, histologically confirmed colorectal cancer, metastatic stage, MSS, refractory to at least second-line systemic therapy or intolerant to standard treatment, ECOG performance status of 0 or 1, adequate organ function, acceptable hematologic function, a life expectancy greater than 3 months, at least one measurable disease based on RECIST v1.1.
The exclusion criteria were as follows: long-term application of hormone therapy; uncontrolled blood pressure (blood pressure ≥140/90 mmHg) in drug-therapy-receiving patients; untreated brain metastases; severe and active infection or autoimmune disease; innate or acquired immune deficiency; bleeding tendency; serious cardiopathy, respiratory, hepatopathy, nephropathy disease; had other malignant tumors in the past 5 years, history of any other antiangiogenic treatment (except for Avastin) or anti-PD-1/programmed death-ligand 1 (PD-L1) antibody treatment.
Study design and treatment
The study was a single-arm, single-center, open-label, phase II clinical trial. This study aimed to assess the safety and efficacy of SHR-1210 combined with apatinib in the treatment of patients with pMMR/MSS mCRC refractory to at least the second-line treatment or intolerant to standard treatment. Patients who met the inclusion criteria were enrolled in the study. Eligible participants discontinued combination treatment if one of the following events occurred: withdrawal of consent, death, intolerant of toxicity, disease progression (PD), or discontinuation owing to the investigator’s decision. Apatinib was given orally at 375 mg once daily for patients. For those who could not tolerate the toxicity, the dose of apatinib could be reduced to 250 mg. SHR-1210 was given intravenously at a dose of 200 mg once every two weeks. Tumors were evaluated at baseline and every 8 weeks until 6 months and every 12 weeks after that. The efficacy evaluation was performed according to RECIST version 1.1. Patients with PD could continue to receive treatment, while still receiving a clinical benefit (as determined by the investigator), were re-evaluated after 4 weeks.
Outcomes
The primary endpoint was ORR. The secondary endpoints included PFS, OS, DCR and safety. Toxicity was assessed throughout the whole course of treatment until 90 days after the final cycle. The AEs were evaluated using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver.5.0. An efficacy evaluation was assessed by computed tomography; magnetic resonance imaging according to RECIST version 1.1 every 4 weeks until PD or the participants were lost to follow-up.
Statistical analysis
The sample size of the study was estimated using a Simon optimum two-stage design. This study aimed to rule out an unacceptably low ORR of 5% (p0=0.05) in favor of an improved ORR of 20% (p1=0.20). The study design was two-sided, with α=0.05 and 80% power. The sample size for the first stage was 10 patients, and if at least one response was noted, an additional 19 patients could be enrolled. And if four (14.8%) or more responses were noted, further study could be considered.
Patients underwent follow-up until February 13, 2020. All patients were included in the safety analysis. Statistical analyses were performed using SPSS 22.0 or GraphPad Prism 7.0 software. Kaplan-Meier analysis was used to calculate PFS and OS with associated 95% CIs in the study. AEs were evaluated using NCI-CTCAE ver.5.0.
Study oversight
This study was approved by the Institutional Review Board, and all patients provided written informed consent following the Declaration of Helsinki principles. Patients were enrolled at Sun Yat-sen University Cancer Center, China.
Results
Patient characteristics
Based on the inclusion and exclusion criteria, a total of 10 patients were enrolled in the first stage at the time of the data cutoff (February 13, 2020). The median age was 54 years old (range 40 to 66), and 70% of patients were female. Ten patients (10/10, 100%) had distant metastasis at study entry, and five patients (5/10, 50%) had disease involvement at more than two sites. Seven patients (7/10, 70%) were observed with lung metastases, six patients (6/10, 60%) with liver metastases, seven patients (7/10, 70%) with peritoneum metastases, five patients (5/10, 50%) with lymph node metastases, and four patients (4/10, 40%) with other sites of metastases. RAS status was wild type in one patient (1/10, 10%), mutated in seven patients (7/10, 70%), whereas it was not tested in two patients (2/10, 20%). BRAF status was wild type in eight patients (8/10, 80%), mutated in one patient (1/10, 10%), whereas it was not tested in one patient (1/10, 10%). All patients had received surgery and previous systemic treatment. The previous systemic treatment, including FOLFOX, FOLFIRI, FOLFOXIRI, XELOX, and XELIRI, with four patients (4/10, 40.0%) had failed second-line or subsequent chemotherapy and eight patients (8/10, 80%) received bevacizumab. Patient’s clinical and tumor genomic characteristics are shown in Table 1.
Table 1.
Patient’s characteristics
| Characteristics | No. of patients (n=10) n (%) |
|---|---|
| Age, y, median (range) | 54 (40-66) |
| Sex | |
| Men | 3 (30%) |
| Women | 7 (70%) |
| ECOG | |
| 0-1 | 10 (100%) |
| 2 | 0 (0%) |
| Diagnosis | |
| Colon Cancer | 8 (80%) |
| Rectal Cancer | 2 (20%) |
| Tumor site | |
| Left | 4 (40%) |
| Right | 6 (60%) |
| Differentiation | |
| Medium-high | 7 (70%) |
| Low | 3 (30%) |
| No. of metastatic sites | |
| ≤2 | 5 (50%) |
| ≥3 | 5 (50%) |
| Metastatic organ | |
| Lung | 7 (70%) |
| Liver | 6 (60%) |
| Peritoneum | 7 (70%) |
| Lymph node | 5 (50%) |
| Other sites | 4 (40%) |
| RAS | |
| Wild type | 1 (10%) |
| Mutation | 7 (70%) |
| Unknow | 2 (20%) |
| BRAF | |
| Wild type | 8 (80%) |
| Mutation | 1 (10%) |
| Unknow | 1 (10%) |
| Surgical history | |
| Yes | 10 (100%) |
| No | 0 (0%) |
| No. of previous chemotherapy lines | |
| ≤2 | 6 (60%) |
| ≥3 | 4 (40%) |
| Bevacizumab prior to apatinib | |
| Yes | 8 (80%) |
| No | 2 (20%) |
Treatment toxicity
As of the data cutoff date, ten patients were included in the safety analysis. All toxicities occurring in the patients are shown in Table 2. Treatment-related AEs occurred in ten patients (10/10, 100%). The most common treatment-related AEs were hypertension and proteinuria (n=7 each, 70%). Other AEs included elevated transaminase in five patients (50%); HSF and rash in four patients (40% each); capillary proliferation, diarrhea, nausea, vomiting, neutropenia, anemia and thrombocytopenia in three patients (30% each); fatigue and hoarseness in two patients (20% each); hyperbilirubinemia, fever, pneumonia, mucositis oral, poor appetite and leukopenia in one patient (10% each). Grade 3 AEs were observed in nine patients (9/10, 90%): hypertension in three patients (30%); thrombocytopenia, proteinuria and diarrhea in two patients (20% each); and pneumonia, hyperbilirubinemia, HSF, elevated transaminase, rash and neutropenia in one patient (10% each).
Table 2.
Summary of treatment-related adverse events
| Adverse events (AEs) | Grade 1-2 (%) | Grade 3-4 (%) | Total (%) |
|---|---|---|---|
| SHR-1210-related AEs | |||
| Capillary proliferation | 3 (30%) | 0 (0%) | 3 (30%) |
| Pneumonia | 0 (0%) | 1 (10%) | 1 (10%) |
| Fever | 1 (10%) | 0 (0%) | 1 (10%) |
| Apatinib-related AEs | |||
| Hypertension | 4 (40%) | 3 (30%) | 7 (70%) |
| Proteinuria | 5 (50%) | 2 (20%) | 7 (70%) |
| Hand-foot syndrome | 3 (30%) | 1 (10%) | 4 (40%) |
| Diarrhea | 1 (10%) | 1 (10%) | 2 (20%) |
| Hoarseness | 2 (20%) | 0 (0%) | 2 (20%) |
| Oral mucositis | 1 (10%) | 0 (0%) | 1 (10%) |
| AEs related to both treatments | |||
| Rash | 3 (30%) | 1 (10%) | 4 (40%) |
| Nausea | 3 (30%) | 0 (0%) | 3 (30%) |
| Vomiting | 3 (30%) | 0 (0%) | 3 (30%) |
| Fatigue | 2 (20%) | 0 (0%) | 2 (20%) |
| Diarrhea | 0 (0%) | 10 (10%) | 1 (10%) |
| Poor appetite | 1 (10%) | 0 (0%) | 1 (10%) |
| Leukopenia | 1 (10%) | 0 (0%) | 1(10%) |
| Neutropenia | 2 (20%) | 1 (10%) | 3 (30%) |
| Anemia | 3 (30%) | 0 (0%) | 3 (30%) |
| Thrombocytopenia | 1 (10%) | 2 (20%) | 3 (30%) |
| Elevated transaminase | 4 (40%) | 1 (10%) | 5 (50%) |
| Hyperbilirubinemia | 0 (0%) | 1 (10%) | 1 (10%) |
Abbreviation: AE, adverse event. Severity was graded according to NCI Common Terminology Criteria for Adverse Events, version 5.0.
A total of two patients (2/10, 20%) discontinued SHR-1210 treatment due to grade 3 AEs (pneumonia and elevated transaminase separately). Three patients interrupted SHR-1210 treatment: one patient due to grade 2 fever, grade 3 hypertension and hyperbilirubinemia; besides, grade 3 thrombocytopenia and HSF in one patient each. Three patients (3/10, 30%) discontinued apatinib due to grade 3 AEs: one case of proteinuria, which led to the discontinuation of apatinib for more than 28 days; hyperbilirubinemia and elevated transaminase in another two cases, respectively. Seven patients (7/10, 70%) interrupted apatinib treatment: one patient due to grade 2 fever, grade 3 hypertension and pneumonia; besides, the other six patients suffered from grade 3 hypertension, grade 2 hypertension, grade 3 hypertension and grade 2 HSF, grade 3 diarrhea and grade 2 HSF, grade 3 proteinuria, grade 3 proteinuria and diarrhea separately. Among them, four patients received apatinib at a reduced dose of 250 mg/d (listed in Table S1), and it turned out that two patients no longer had grade 3 AE after reduction, the other two patients had first AE remission but had a new grade 3 AE. There were no grade 4 AEs in our analysis and no treatment-related deaths as of the data cutoff date.
Efficacy
As of February 13, 2020, ten patients were enrolled in the study. One patient withdrew from the trial after one dose of SHR-1210 due to grade 3 elevated transaminase, therefore, was excluded from the final analysis. One patient who was considered to have clinically progressive disease refused to accept radiographic assessment due to poor physical condition. The other eight patients were evaluated by image examination. Thus, among the ten enrolled patients, nine patients were included in the efficacy analysis. The results showed that no patients in this study manifested complete response or partial response, while progressive diseases and stable disease were observed in seven patients (7/9, 77.8%) and two patients (2/9, 22.2%) respectively. In conclusion, the ORR was 0%, and the DCR was 22.2%. Changes in tumor burden from baseline are shown in Figure 1. As shown in Figure 2, the median PFS was 1.83 months (95% CI 1.80-1.86), and the median OS was 7.80 months (95% CI 0-17.07). Overall, the results of the study failed to meet the prespecified primary efficacy endpoint. Notably, as of the data cutoff date, one patient who had stable disease (SD) for 8.0 months remained on treatment, and the characteristics of this patient are shown in Table S2. For this patient, tumors were evaluated by CT from baseline to the last radiographic assessment (Figure S1).
Figure 1.
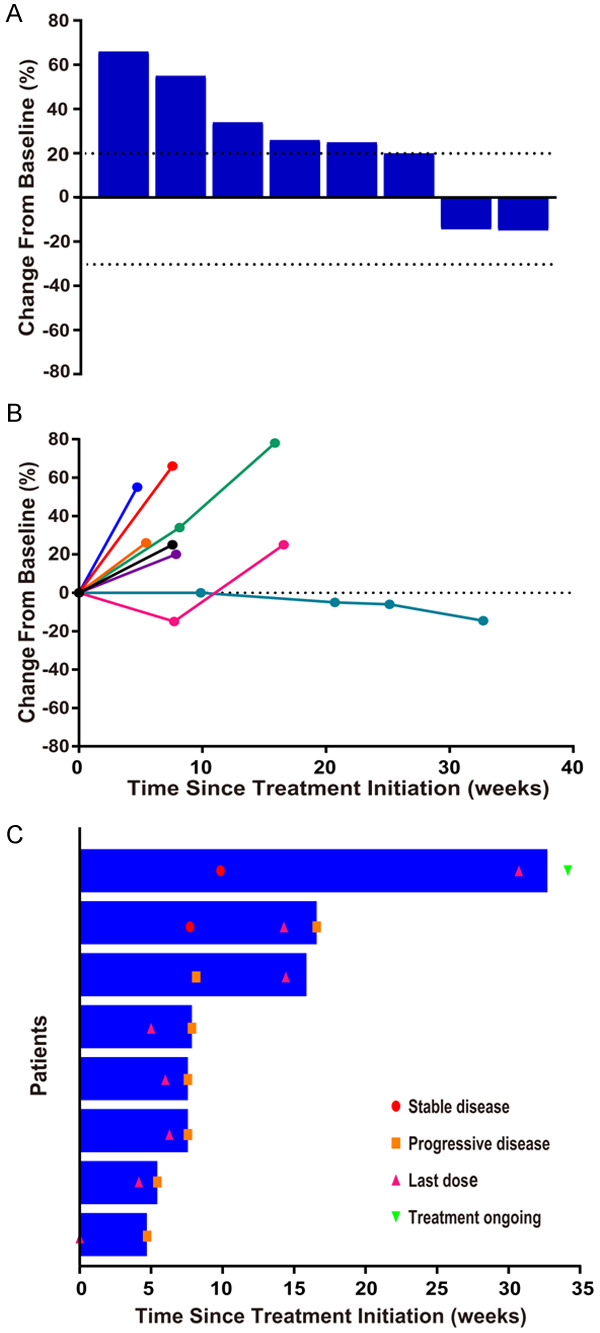
Efficacy of SHR-1210 combined with apatinib. Response was assessed in accordance with the RECIST version 1.1 in 8 patients. A. Best change from baseline in the sum of the longest target lesion diameters per patient. B. Percentage change from baseline in target lesion diameters over time. C. Treatment exposure and response duration. The length of the bar shows the time to the last radiographic assessment.
Figure 2.
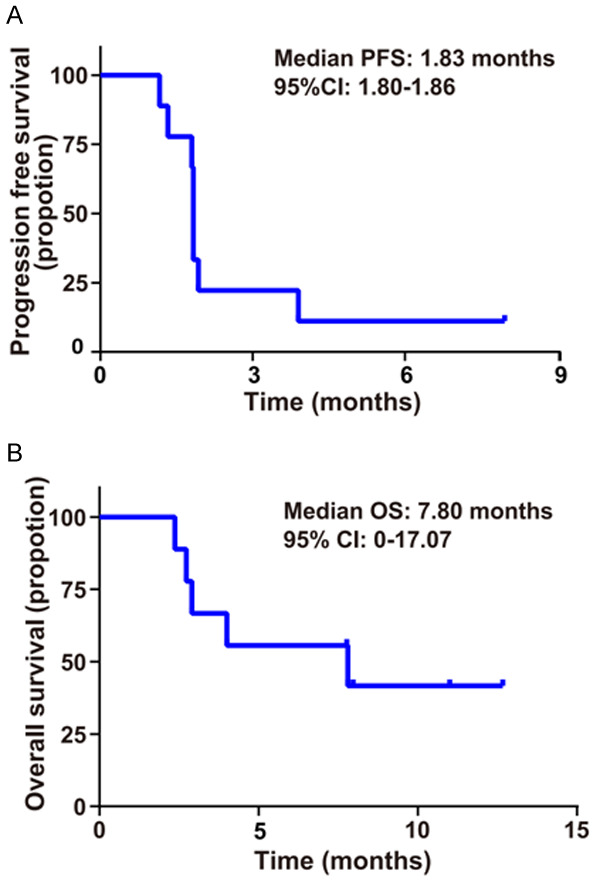
The Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) of patients with SHR-1210 plus apatinib. A. The Kaplan-Meier survival curve of PFS with SHR-1210 plus apatinib. B. Kaplan-Meier survival curve of OS with SHR-1210 plus apatinib.
Discussion
This study aimed to assess the safety and efficacy of SHR-1210 combined with apatinib in the treatment of patients with pMMR/MSS mCRC refractory to at least the second-line treatment or intolerant to standard treatment. However, the results of the study failed to meet the prespecified primary efficacy endpoint, and the AEs were severe.
The treatment of mCRC is still a challenge. Immunotherapy brings new hope for the treatment of mCRC. However, compared with dMMR/MSI-H CRC, PD-1 blockade is ineffective in patients with MSS CRC [6]. According to a study, the density of cytotoxic T cells was higher in MSI CRC samples than in MSS CRC samples [19]. In previous researches, antiangiogenic drugs could significantly enhance the efficacy of PD-1/PD-L1 blockade on tumor growth by inducing tumor vascular normalization, promoting lymphocyte infiltration, and improving the anti-tumor immune effects of CD8+ cytotoxic T lymphocytes in the tumor microenvironment [20,21]. There are also some clinical studies claiming that ICI combined with antiangiogenic drugs could increase the efficacy of immunotherapy for tumors such as melanoma [8], renal cancer [22], and hepatocellular carcinoma [23]. The combination therapy with regorafenib plus nivolumab was reported effective in the treatment of MSS mCRC at the 2019 ASCO Annual Meeting. In the REGONIVO study, ORR of MSS mCRC was 36%, and median PFS of CRC was 7.9 months [10]. In addition, some studies have shown that anti-PD-1 antibody SHR-1210 combined with apatinib, an antiangiogenic drug targeting VEGFR-2, was safe and effective in the treatment of advanced solid tumors [17,18].
However, it is disappointing that SHR-1210 combined with apatinib did not show an advantage in the treatment of MSS mCRC in this study. The potential reasons are as follows: firstly, different antiangiogenic drugs have different targets and mechanisms. In our study, the antiangiogenic drug was apatinib, which potently suppresses the activation of VEGFR-2, c-kit, c-Src and RET, and inhibits cellular phosphorylation of c-kit and PDGFRβ [24,25]. In the REGONIVO study, the antiangiogenic drug was regorafenib, which is able to inhibit activation of VEGFR-1, VEGFR-2, VEGFR-3, FGFR, PDGFR, KIT, RET, TIE2, and BRAF [24,26]. Secondly, in this study, 70% of patients had a mutation in RAS. It was suggested that RAS mutation is predisposed to poor prognosis [27]. Thirdly, in the REGONIVO study, 33% of patients with MSS CRC had PD-L1 positive score (CPS) ≥1, and the median tumor mutational burden (TMB) of patients with MSS CRC was 10.9 mutations per megabase (mut/MB) [10]. Both of them were predictive marker of clinical response to PD-1 blockade [28,29]. But in our study, CPS was unknown, and only 3 patients had tested TMB. Fourthly, in our study, 60% of patients had hepatic metastasis, which was associated with poor response to PD-1 blockade [30]. More importantly, the treatment-related AEs of SHR-1210 combined with apatinib were severe. Treatment-related AEs occurred in ten patients (100%) in our study, and nine patients (90%) experienced grade 3 AEs. Most patients did not receive adequate treatment because of intolerable toxicity. However, the AEs in the other clinical trial of combination therapy with antiangiogenic drugs and ICI were acceptable, and the incidence of grade 3/4 AEs was lower. In the REGONIVO study, the common AEs ≥ grade 3 were rash (12%), proteinuria (12%), and palmar-plantar erythrodysesthesia (10%) [10].
The severe AEs in this study may significantly reduce the efficiency of the combination therapy with SHR-1210 plus apatinib. Undeniably, the high dose of apatinib was the main reason for the severe AEs in this trial. According to a study, which combined SHR-1210 with apatinib to treat advanced hepatocellular carcinoma and gastric or esophagogastric junction cancer, the recommended dose for apatinib was 250 mg daily for phase II trial [18]. However, the initial dose of apatinib in our study was 375 mg. Thus, a reduction dose of apatinib might decrease the occurrence of AEs. Combination therapy was another leading cause of intolerable toxicity. A phase I/II trial had shown that the incidence of treatment-related AEs of all grades was 56.3% in the SHR-1210 treatment and 65.8% in the combination therapy with SHR-1210 plus apatinib group [17]. The incidence of grade 3/4 AE for apatinib was 12.8% [13], and the most common grade 3/4 AEs were as follows: hypertension (14.8%); HFS (11.1%); diarrhea, liver toxicity (3.7% each) [31]. These data show that compared with SHR-1210 alone or apatinib monotherapy, combination therapy with SHR-1210 plus apatinib has an increased incidence of AEs.
Notably, a patient, who had peritoneum metastases, was reported to achieve 8.0 months of SD. Several characteristics of this patient may contribute to the relatively effective response to combined therapy: ECOG performance status 0, absence of hepatic metastasis, number of metastatic sites and previous chemotherapy lines ≤2, high TMB (15.31 mut/MB) [29,30,32]. In the future, it is intriguing to further investigate moleculars like TMB, PD-L1 CPS [28] and POLE/POLD1 [33], to help predict clinical response to immune checkpoint inhibitors in MSS mCRC patients.
In conclusion, apatinib combined with SHR-1210 has failed to improve the efficacy of treatment of MSS mCRC while caused severe adverse effects. Reducing dose of apatinib or combining anti-PD-1/PD-L1 antibody with other well-tolerated antiangiogenic drugs may help in designing new and better treatment strategies.
Acknowledgements
This work was supported by the Medical Scientific Research Foundation of Guangdong Province of China (A2019398); the National Natural Science Foundation of China (81930065); the Science and Technology Program of Guangdong (2019B020227002); the Science and Technology Program of Guangzhou (201904020046, 201803040019, 201704020228); the Sun Yat-sen University Clinical Research 5010 Program (2018014); Feng Wang is the Young Physician Scientist Program of Sun Yat-sen University Cancer Center (16zxqk03). ClinicalTrials.gov PRS Protocol Registration and Results System: NCT03912857.
Disclosure of conflict of interest
None.
Abbreviations
- AE
adverse event
- CI
confidence interval
- CRC
colorectal cancer
- HSF
hand-foot syndrome
- ICI
immune checkpoint inhibitors
- mCRC
metastatic colorectal cancer
- MSI-H
microsatellite instability-high
- MSS
microsatellite stable
- mutations per megabase
mut/Mb
- NCI-CTCAE
National Cancer Institute’s Common Terminology Criteria for Adverse Events
- ORR
objective response rate
- OS
overall survival
- PD
disease progression
- PD-1
programmed death-1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- pMMR
proficient mismatch repair
- SD
stable disease
- TMB
tumor mutational burden
- VEGFR
vascular endothelial growth factor receptor
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D’Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon S. Regorafenib: a review in metastatic colorectal cancer. Drugs. 2018;78:1133–1144. doi: 10.1007/s40265-018-0938-y. [DOI] [PubMed] [Google Scholar]
- 5.Messersmith WA. NCCN guidelines updates: management of metastatic colorectal cancer. J Natl Compr Canc Netw. 2019;17:599–601. doi: 10.6004/jnccn.2019.5014. [DOI] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev. 2016;51:19–26. doi: 10.1016/j.ctrv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, Friedlander P, Flaherty KT, Murphy GF, Rodig S, Velazquez EF, Mihm MC Jr, Russell S, DiPiro PJ, Yap JT, Ramaiya N, Van den Abbeele AD, Gargano M, McDermott D. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603) J. Clin. Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 11.Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78:747–758. doi: 10.1007/s40265-018-0903-9. [DOI] [PubMed] [Google Scholar]
- 12.Li NN, Zhou JF, Zhao L, Ying HY, Jia N. Efficacy and safety of apatinib in treating advanced colorectal cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019;41:170–174. doi: 10.3881/j.issn.1000-503X.10376. [DOI] [PubMed] [Google Scholar]
- 13.Li A, Wang K, Xu A, Wang G, Miao Y, Sun Z, Zhang J. Apatinib as an optional treatment in metastatic colorectal cancer. Medicine (Baltimore) 2019;98:e16919. doi: 10.1097/MD.0000000000016919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, Qu D, Wang X, Lan B, Yang B, Wang P, Zhang H, Yang Q, Jiao Y. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24:1296–1304. doi: 10.1158/1078-0432.CCR-17-2439. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Mo H, Zhang W, Chen X, Qu D, Wang X, Wu D, Wang X, Lan B, Yang B, Wang P, Zhang B, Yang Q, Jiao Y, Xu B. Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer. 2019;125:742–749. doi: 10.1002/cncr.31855. [DOI] [PubMed] [Google Scholar]
- 16.Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, Wang X, Lan B, Wang X, Xu J, Zhang H, Chi Y, Yang Q, Xu B. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119:538–545. doi: 10.1038/s41416-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang L, Wen Y, Hu R, Wang L, Xia Y, Hu C, Qiao Y, Geng X, Chen T, Fei J, Hui K, Jiang X. Safety and efficacy of PD-1 blockade-activated multiple antigen-specific cellular therapy alone or in combination with apatinib in patients with advanced solid tumors: a pooled analysis of two prospective trials. Cancer Immunol Immunother. 2019;68:1467–1477. doi: 10.1007/s00262-019-02375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25:515–523. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 19.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboüe R, Frebourg T, Pagès F, Valge-Archer V, Latouche JB, Galon J. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP, Bergers G. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9:eaak9679. doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH, Laoui D, De Palma M. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9:eaak9670. doi: 10.1126/scitranslmed.aak9670. [DOI] [PubMed] [Google Scholar]
- 22.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, Sznol M, Hainsworth J, Rathmell WK, Stadler WM, Hutson T, Gore ME, Ravaud A, Bracarda S, Suárez C, Danielli R, Gruenwald V, Choueiri TK, Nickles D, Jhunjhunwala S, Piault-Louis E, Thobhani A, Qiu J, Chen DS, Hegde PS, Schiff C, Fine GD, Powles T. Publisher correction: clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:1941. doi: 10.1038/s41591-018-0235-z. [DOI] [PubMed] [Google Scholar]
- 23.Combo Poised to Become Standard in HCC. Cancer Discov. 2020;10:OF3. doi: 10.1158/2159-8290.CD-NB2019-145. [DOI] [PubMed] [Google Scholar]
- 24.Wu JQ, Fan RY, Zhang SR, Li CY, Shen LZ, Wei P, He ZH, He MF. A systematical comparison of anti-angiogenesis and anti-cancer efficacy of ramucirumab, apatinib, regorafenib and cabozantinib in zebrafish model. Life Sci. 2020;247:117402. doi: 10.1016/j.lfs.2020.117402. [DOI] [PubMed] [Google Scholar]
- 25.Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–1380. doi: 10.1111/j.1349-7006.2011.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 27.Bonnot PE, Passot G. RAS mutation: site of disease and recurrence pattern in colorectal cancer. Chin Clin Oncol. 2019;8:55. doi: 10.21037/cco.2019.08.11. [DOI] [PubMed] [Google Scholar]
- 28.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 29.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O’Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, Catenacci D, Cho M, Braiteh F, Klempner SJ, Ali SM, Fakih M. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30:1096–1103. doi: 10.1093/annonc/mdz134. [DOI] [PubMed] [Google Scholar]
- 31.Liao X, Li H, Liu Z, Liao S, Li Q, Liang C, Huang Y, Xie M, Wei J, Li Y. Clinical efficacy and safety of apatinib in patients with advanced colorectal cancer as the late-line treatment. Medicine (Baltimore) 2018;97:e13635. doi: 10.1097/MD.0000000000013635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, Felip E, Zerón-Medina J, Garrido P, Brosseau S, Zalcman G, Mazieres J, Caramela C, Lahmar J, Adam J, Chaput N, Soria JC, Besse B. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu ZX, Xu RH. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019;5:1504–1506. doi: 10.1001/jamaoncol.2019.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


