Abstract
Doxorubicin (DOX)-induced cardiotoxicity is a major limitation to its clinical application. Cardiotoxicity of DOX is dose-dependent that begins with the first dose. Oxidative stress and inflammation are involved in DOX-related cardiotoxicity. This study aimed to determine whether multiple markers of inflammation, hypercoagulability and endothelial injury correlate with the risk of early DOX-induced cardiotoxicity in breast cancer patients. Blood samples of 51 breast cancer patients treated with DOX-based chemotherapy were collected before (baseline) and after the first cycle of chemotherapy. The risk of cardiotoxicity was defined as an asymptomatic reduction of cardiac left ventricle ejection fraction (LVEF) >10% at completion of chemotherapy versus baseline. Plasma samples were examined for multiple biomarkers of inflammation, hypercoagulability and endothelial dysfunction, including C-reactive protein (CRP), thrombomodulin (TM), thrombin-antithrombin complex (TAT), myeloperoxidase (MPO), von Willebrand factor (vWF) and P-selectin. Surrogate markers of neutrophil extracellular traps (NETs) nucleosomes and double stranded DNA (dsDNA) were also measured. Patients with abnormal decline of LVEF >10% (n=21) had significantly elevated levels of MPO and TM both at baseline, and after the first dose of DOX-based chemotherapy relative to patients with normal LVEF (n=30) after adjusting for race, age, BMI and type of breast cancer. The first dose of DOX also induced significantly higher circulating levels of TAT complex and nucleosomes in patients at risk of cardiotoxicity in comparison with patients without. The comparison between the means of the biomarkers in after-before DOX-based chemotherapy of the two groups of patients showed significant differences for MPO, TAT complex and CRP. The results from this study suggest that the risk of DOX-induced cardiotoxicity in breast cancer is associated with endothelial dysfunction, inflammation and prothrombotic state before and after the first dose of chemotherapy.
Keywords: Chemotherapy, doxorubicin, cardiotoxicity, biomarkers, inflammation, endothelial dysfunction, thrombosis
Introduction
Doxorubicin (DOX), a commonly used anticancer agent is known for the unpredictable cardiotoxicity that limits its dosing and impacts patient’s survival and quality of life independently of the oncological prognosis [1,2]. DOX-induced cardiotoxicity is cumulative dose-dependent that begins with the first dose with asymptomatic myocardial injury and may progress to irreversible symptomatic heart failure (HF) years after treatment [3,4]. The damaging effects of DOX on the heart often are not detected until years after cessation of the chemotherapy, therefore it is important to identify patients at risk before or at the early doses of chemotherapy [5,6]. Because the mechanism of DOX-induced cardiotoxicity is not completely uncovered, to date there are no tools to predict it or prevent it. Current routine methods of quantifying LVEF such as multigated acquisition (MUGA) scan and echocardiography lack sensitivity to detect the early subclinical cardiac damage [7,8]. Blood cardiac biomarkers, such as cardiac troponins and B-type natriuretic peptide (BNP) have been used in the diagnostics of cardiac injury and heart failure [9-11], but several studies [12,13], including our previous study [14], failed to detect the subclinical DOX-induced cardiotoxicity.
The formation of free radicals with oxidative stress is considered a primary mechanism of DOX cardiotoxicity along with multiple others (i.e. binding to topoisomerases, dysregulation of Ca2+ homeostasis, activation of the ubiquitin-proteasome system, release of vasoactive amines, impaired cardiac repair) [15]. Traditionally, DOX-induced cardiotoxicity is focused on the effects on cardiomyocytes that lead to contractile dysfunction, however recent studies have focused on DOX-induced systemic inflammation and endothelial injury, which can possibly trigger the development and progression of cardiomyopathy [16]. Previous data indicate that the early subclinical DOX-induced cardiotoxicity in breast cancer patients is associated with increased expression of neutrophil-specific genes (i.e. DEFA 1-4, MPO, BPI, CAMP, CTSG, PGLYRP1, CD177, S100A12) [17] and circulating chemokines implicated in inflammatory response and immune trafficking (i.e. CCL23, CCL27 and MIF) [14]. Neutrophils are early responders to stimuli that lead to tissue injury, including inflammation, infection, trauma, cancer, and thrombosis [18,19]. Activated neutrophils release the components of the neutrophil granules, including heme-enzyme myeloperoxidase (MPO), serine proteases, alpha-defensins and bactericidal proteins, which in addition to their antimicrobial activity are also involved in several inflammation-associated diseases [20,21]. Neutrophils have been implicated in the progression of cardiovascular diseases including atherosclerosis, thrombosis and acute coronary syndrome [22]. Neutrophil granule proteins predictive of infarct size, LVEF, new cardiovascular events, and death after acute myocardial infarction [23,24]. Recent study showed increased infiltration of neutrophils in the heart tissue of mice with DOX-induced cardiotoxicity, and incubation of cardiac fibroblasts with DOX in vitro resulted in upregulation of several inflammatory cytokines [25,26]. More recently, neutrophil extracellular traps (NETs) have been recognized as an additional mechanism of defense through a process called NETosis [27,28]. NETs can activate endothelial cells and platelets, resulting in endothelial dysfunction, proinflammatory immune response, and thrombotic lesions [29]. Furthermore, endothelial dysfunction has been associated with cardiovascular disease, such as hypertension, coronary artery disease, chronic heart failure, peripheral artery disease, diabetes [16,30,31]. Evidence indicates that NETs play important role in thrombosis [32]. In cancer, including breast cancer, NETs have been shown to sequester circulating tumor cells and to promote metastasis and the inhibition of components of NETs has been tested in various studies to reduce metastasis in cancer [33,34]. DOX increased significantly the cell-free DNA, considered a surrogate marker of NETs, in a dose-dependent manner with a corresponding elevation of TAT complex in animal studies [35].
Cancer patients are at increased risk of deep vein thrombosis and pulmonary embolism [36,37]. Clinically detectable venous thromboembolism is present in 15% of all cancer patients, and the number is likely to be even higher when subclinical thromboembolism is taken into account [38]. In patients with breast cancer, the baseline risk for thrombosis is <1% [39], but increases significantly following chemotherapy treatment up to 5-17% [40,41]. Furthermore, cancer chemotherapy increases the risk of cancer-related thrombosis, which is a major risk factor for cardiovascular diseases [42,43].
A number of reports indicate that plasma levels of inflammatory markers increased in chronic heart failure (HF) and could also be subclinical indicators of future HF [44]. Inflammation is strictly correlated with clotting activation and prognosis in heart failure [45]. DOX induces severe inflammatory responses in various organs including liver, kidney, intestine and blood vessels, in addition to its major adverse effect of cardiac toxicity [46]. The association between inflammation, hypercoagulability and the risk of DOX-induced cardiotoxicity has not been previously reported. Therefore, the aim of this study was to determine whether the risk of DOX-induced cardiotoxicity after the first cycle of chemotherapy in breast cancer patients correlate with circulating biomarkers of hypercoagulability, inflammation and endothelial dysfunction.
Materials and methods
Study population
Patients with early breast cancer eligible for DOX-based chemotherapy were enrolled at the Winthrop Rockefeller Cancer Institute, UAMS. This study was approved by the Institutional Review Board (IRB) of UAMS (Protocol #130212) and from IRB of the Central Veterans Healthcare system (CAVHS) (Protocol #1423976-2), where the samples were processed and stored. The study was performed on a total of 51 subjects enrolled in the study between 2012 and 2019, and plasma assays were performed whenever there were sufficient blood samples collected from each subject both prior to (T0) and after the first cycle (T1) of chemotherapy. All participants signed an IRB approved informed consent where they were informed for the use of their blood samples and medical records for research purposes. The inclusion criteria included early ER+/PR+/Her2-, ER+/PR-/Her2- or triple negative, stage I to III breast cancers within 18-99 years of age. Participants were ineligible if they were pregnant or breast feeding, and had no prior history of chemotherapy or radiotherapy. All patients were treated with a predefined protocol which included a combination of DOX (60 mg/m2) with cyclophosphamide (600 mg/m2) in each cycle for 4 cycles every 2 weeks. Of the 51 enrolled patients, 15 patients were treated with DOX-chemotherapy after a surgical removal of the tumor (adjuvant chemotherapy) and 36 patients were treated with neoadjuvant chemotherapy, before surgery. None of the patients had implants or tissue expanders. Patients with hypertension who were taking antihypertensive medications (β-blockers and ACE inhibitors) prior to chemotherapy were prescribed to continue with this treatment concomitant with the DOX-based chemotherapy. Patients with diabetes also continued to be treated with insulin or metformin concomitant with the chemotherapy.
Assessment of LVEF as a measure of cardiac function
Cardiac toxicity was evaluated by clinical assessment of LVEF with MUGA scan and/or ECHO before initiation of chemotherapy and after the 4th cycle of DOX-based chemotherapy. A decline of LVEF by >10% or below 50% in comparison with the baseline (before the start of chemotherapy) was considered abnormal [87,88].
Plasma isolation
Blood samples were collected in EDTA collection tubes before the start of DOX-based chemotherapy and 2 weeks after the first cycle. Plasma was isolated by centrifugation at 2000 × g for 20 min and was stored at -80°C until analysis.
Enzyme-linked immunosorbent assays (ELISA)
Stored blood plasma samples were thawed on ice and analyzed using commercially available ELISA kits. Assays were performed on three different occasions between 2016 and 2020 on 10-15 patients with asymptomatic LVEF decline >10% and 10-16 patients with stable LVEF at the end of DOX-based chemotherapy, depending on the availability of plasma samples at both T0 and T1. Nucleosome plasma levels were determined using Cell Death Detection ELISA Plus (Roche Diagnostics, Indianapolis, USA) following manufacturer’s instructions. The method is based on two monoclonal mice antibodies against DNA and histones. Optical density values in the nucleosome assay were normalized to an internal positive control and expressed as arbitrary units of nucleosomes per milliliter (AU/mL); coefficient of variation (CV) was 26.5%. As an indirect way to assess NETs, dsDNA in plasma was quantified using Quant-iT PicoGreen dsDNA Reagent (Fisher Sci) according to the manufacturer’s instructions, with the exception that 50 μl of patient plasma was added to 50 μl of the PicoGreen reagent solution and the samples were excited at 480 nm and emission 520 nm on a fluorescence microplate reader. ELISA kits used, dilutions and inter-assay CVs were: TAT complex (Abcam, 1:10, 7.5%); MPO (Abcam, 1:10, 12.5%); vWF (Abcam, 1:50, 3.8%); TM (R&D Systems, 1:10, 5.1%), P-selectin (R&D Systems, 1:20, 12.3%), C-reactive protein (Fisher Sci, 1:1000, 12.5%).
Statistical analysis
The early changes in the plasma biomarkers between baseline (T0) and after the first cycle (T1) of DOX-based chemotherapy were determined in association with the risk of asymptomatic decline of LVEF. The concentration of each biomarker in both groups of patients (normal and abnormal LVEF) at each time-point (T0 and T1) was presented as means ± SD, and P<0.05 was considered statistically significant. Two sample t-tests and chi-square (X2) tests were performed to evaluate differences in patients’ characteristics between groups. Paired t-tests, as well as analysis of covariance (ANCOVA) with adjustments for race, age, body weight mass (BMI) and type of breast cancer were conducted to determined group differences (ABN vs. NORM) or paired-wise differences for the patients between different time points (T0-T1). Pearson correlation were performed to identify the correlation between markers. Analyses were performed using JMP (SAS, Cary, NC) and Partek Genomics SuiteTM 6.6 (Partek Inc., St. Louis, MO).
Results
Demographic characteristics of the study participants
The characteristics of the patients are presented in Table 1. Of the 51 patients enrolled, 21 had asymptomatic LVEF >10% decrease in comparison with the baseline (ABN group) and 30 patients had LVEF ≤10% (NORM group). The median change of LVEF among the ABN group e was 10.9%, while in the NORM group the median change in LVEF was 0.3%. LVEF was assessed with mixed multigated acquisition (MUGA) scan and/or transthoracic echocardiography (ECHO) in 4 of the patients, all of whom had an abnormal LVEF decrease >10%. In the ABN group of patients 9 patients had hypertension and 4 patients had diabetes, versus 12 with hypertension and 2 with diabetes in the NORM group. There were no significant differences between the two groups of patients (NORM and ABN) with respect to the age, BMI, race, history on hypertension, diabetes, and type of breast cancer. Patients in the ABN group had significantly higher baseline LVEF, and lower LVEF after 4 cycles of DOX-based chemotherapy.
Table 1.
Demographic characteristics of the study participants
| aNORM (n=30) | ABN (n=21) | bP | |
|---|---|---|---|
| Age | 52.7 ± 9.8 | 51.4 ± 13.8 | 0.70 |
| BMI | 31.4 ± 7.8 | 35.4 ± 8.0 | 0.10 |
| Race | 0.14 | ||
| EA | 23 | 12 | |
| AA | 7 | 9 | |
| Asian | 0 | 0 | |
| Breast cancer | 0.66 | ||
| ER+/PR+/Her2- | 19 | 15 | |
| ER+/PR-/Her2- | 3 | 1 | |
| ER-/PR-/Her2- | 8 | 5 | |
| ER | 0.42 | ||
| Positive | 22 | 16 | |
| Negative | 8 | 5 | |
| PR | 0.33 | ||
| Positive | 19 | 15 | |
| Negative | 11 | 6 | |
| Triple negative | 0.42 | ||
| Yes | 8 | 5 | |
| No | 22 | 16 | |
| Hypertension | 0.84 | ||
| Yes | 12 | 9 | |
| No | 18 | 12 | |
| Diabetes | 0.18 | ||
| Yes | 2 | 4 | |
| No | 28 | 17 | |
| LVEF baseline (%) | 62.3 ± 6.7 | 67.6 ± 6.4 | 0.008 |
| LVEF after 4 cycles (%) | 62.0 ± 7.0 | 56.7 ± 8.7 | 0.025 |
| LVEF (1-6 months) post DOX (%) | N/A | 57.1 ± 9.3 | N/A |
All biomarker data are geometric means ± standard deviation (SD).
p-values represent differences in NORM vs. ABN patients for each characteristic.
Continuous variables were evaluated by two-sample t-tests (Italic), and chi square (X2) tests were used to investigate the differences in distributions of categorical variables from the groups. Data significant at P<0.05.
Baseline biomarkers levels
The comparison of the baseline levels (T0) of the examined biomarkers between the two groups of patients using two sample t-test showed higher, but not significant difference (Model 1 in Table 2 and Figure 1). However, after applying ANCOVA modeling with adjustment for race, age, BMI, and type of breast cancer in Model 3, MPO and TM became statistically signifcant in ABN at baseline compared to NORM patients (Table 2).
Table 2.
Differences of the circulating biomarkers among patients with normal and abnormal LVEF at baseline and after the first cycle of DOX-based chemotherapy
| ABN | NORM | Mean Difference | aModel 1 | bModel 2 | cModel 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Mean ± SD | (ABN-NORM) | p | FC | Trend | p | FC | p | FC | ||
| Baseline | ||||||||||
| MPO (ng/ml) | 169.9 ± 50.7 | 132.6 ± 45.6 | 37.3 | 0.10 | 1.28 | ABN ↑ | 0.02 | 1.24 | 0.01 | 1.31 |
| TM (pg/ml) | 4.0 ± 1.1 | 3.6 ± 0.9 | 0.4 | 0.29 | 1.11 | ABN ↑ | 0.03 | 1.30 | 0.07 | 1.28 |
| Nucleosomes (AU) | 144.3 ± 78.9 | 113.4 ± 73.3 | 30.9 | 0.28 | 1.27 | ABN ↑ | 0.40 | 1.24 | 0.17 | 1.50 |
| vWF (IU/ML) | 5.4 ± 2.4 | 4.9 ± 1.7 | 0.5 | 0.52 | 1.10 | ABN ↑ | 0.48 | 1.14 | 0.58 | 1.11 |
| TAT complex (ng/ml) | 14.3 ± 5.7 | 12.4 ± 4.6 | 1.8 | 0.40 | 1.15 | ABN ↑ | 0.57 | 1.10 | 0.82 | 1.04 |
| dsDNA (ng/ml) | 135.1 ± 39.2 | 131.2 ± 26.9 | 3.9 | 0.80 | 1.03 | ABN ↑ | 0.75 | 1.04 | 0.52 | 1.12 |
| P-Selectin (ug/ml) | 0.104 ± 0.07 | 0.099 ± 0.08 | 0.01 | 0.85 | 1.05 | ABN ↑ | 0.79 | 1.12 | 0.69 | 1.09 |
| CRP (mg/L) | 6.8 ± 2.0 | 5.5 ± 2.5 | 1.3 | 0.29 | 1.23 | ABN ↑ | 0.82 | 1.04 | 0.79 | 1.06 |
| After 1st cycle | ||||||||||
| TAT complex (ng/ml) | 23.6 ± 10.5 | 12.1 ± 3.5 | 11.6 | 0.001 | 1.96 | ABN ↑ | 0.005 | 1.89 | 0.02 | 1.89 |
| Nucleosomes (AU) | 156.6 ± 84.2 | 94.9 ± 35.5 | 61.7 | 0.02 | 1.65 | ABN ↑ | 0.04 | 1.66 | 0.04 | 1.77 |
| TM (pg/ml) | 4.3 ± 1.0 | 3.6 ± 0.6 | 0.7 | 0.02 | 1.20 | ABN ↑ | 0.018 | 1.26 | 0.10 | 1.18 |
| MPO (ng/ml) | 269.6 ± 112.5 | 174.5 ± 76.0 | 95.1 | 0.04 | 1.54 | ABN ↑ | 0.007 | 1.73 | 0.04 | 1.89 |
| CRP (mg/L) | 8.1 ± 1.5 | 6.0 ± 2.3 | 2.1 | 0.047 | 1.35 | ABN ↑ | 0.15 | 1.25 | 0.30 | 1.23 |
| dsDNA (ng/ml) | 163.1 ± 54.6 | 128.8 ± 17.7 | 34.3 | 0.07 | 1.27 | ABN ↑ | 0.21 | 1.19 | 0.46 | 1.11 |
| P-Selectin (ug/ml) | 0.12 ± 0.08 | 0.09 ± 0.06 | 0.03 | 0.24 | 1.34 | ABN ↑ | 0.53 | 1.26 | 0.94 | -1.04 |
| vWF (IU/ML) | 5.8 ± 2.2 | 4.9 ± 2.0 | 0.83 | 0.29 | 1.17 | ABN ↑ | 0.27 | 1.25 | 0.46 | 1.24 |
ABN: Patients with abnormal LVEF decline; NORM: Patients with normal heart function. Mean ± SD, FC: fold change of ABN/NORM.
Two sample t-tests comparing ABN and NORM.
ANCOVA comparing ABN and NORM adjusted for race, age, and BMI.
ANCOVA comparing ABN and NORM adjusted for race, age, BMI, hypertension, diabetes, and type of breast cancer.
Data significant at P<0.05.
Figure 1.
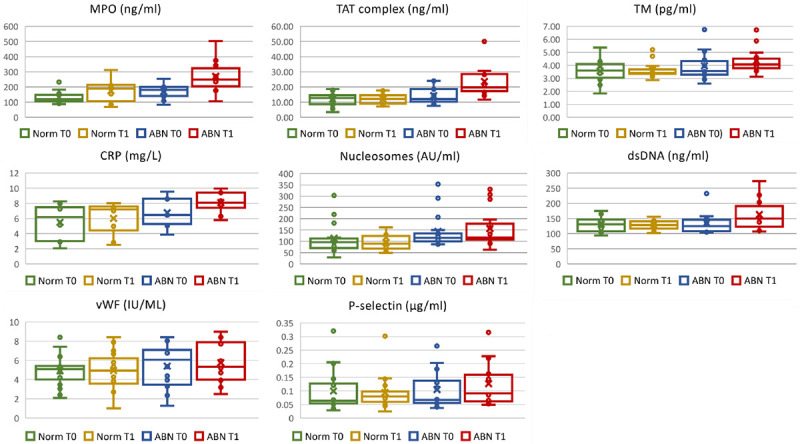
Box and whisker plots showing plasma markers in breast cancer patients with normal LVEF (NORM) and abnormal LVEF decline (ABN) before (T0) and 14 days after the first cycle (T1) of DOX-based chemotherapy. MPO, myeloperoxidase; TAT complex, thrombin-anti-thrombin complex; TM, thrombomodulin; CRP, c-reactive protein; dsDNA, double stranded DNA; vWF, von Willebrand factor.
Levels of biomarkers after the first cycle of DOX-based chemotherapy
Two sample t-test were also used to compare the levels of biomarkers after 1st cycle of DOX-based chemotherapy (T1), and significant elevation of TAT complex, nucleosomes, TM, MPO, and CRP were observed among patients with LVEF decline >10% (Model 1 in Table 2 and Figure 1). After adjusting for age, race, BMI, diabetes, hypertension and type of breast cancer in Model 2 and Model 3, plasma levels of TAT complex, nucleosomes, and MPO remained significantly elevated among ABN group of patients with LVEF >10% decrease versus NORM group patients with LVEF ≤10%.
Paired-wise analyses were conducted to determine the changes of biomarkers before and after the first of DOX-based chemotherapy. In the group of patients with normal LVEF, the plasma biomarkers after the 1st cycle of chemotherapy did not differ significantly from the baseline levels (Table 3). Changes of plasma biomarkers before and after the 1st cycle of DOX-based chemotherapy among ABN group (T1-T0) were determined using pair-t-tests and the levels of TAT complex, MPO and CRP in the ABN group increased significantly after chemotherapy. After controlling for variables age, race, BMI, status on diabetes, hypertension, and type of breast cancer, TAT complex, MPO and CRP remained significantly elevated in the ABN group (Table 3).
Table 3.
Comparison between the means of after-before (T1-T0) DOX-based chemotherapy differences of the two groups of breast cancer patients, group with normal LVEF (NORM) and group with abnormally declined LVEF (ABN)
| NORM | ABN | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Mean | MeanDiff | ap | FC | Mean | MeanDiff | ap | FC | bp | FC | |||
|
|
|
|
|
|||||||||
| T0 | T1 | T1-T0 | T0 | T1 | T1-T0 | Model 1 | Model 2 | |||||
| TAT complex | 12.4 | 12.1 | -0.4 | 0.83 | -1.03 | 14.3 | 23.6 | 9.4 | 0.006 | 1.66 | 0.01 | 1.59 |
| MPO | 132.6 | 174.5 | 42.0 | 0.20 | 1.32 | 169.9 | 269.6 | 99.8 | 0.026 | 1.59 | 0.03 | 1.68 |
| CRP | 5.5 | 6.0 | 0.5 | 0.26 | 1.10 | 6.8 | 8.1 | 1.3 | 0.031 | 1.20 | 0.03 | 1.20 |
| TM | 3.57 | 3.61 | 0.0 | 0.79 | 1.01 | 3.95 | 4.33 | 0.4 | 0.09 | 1.10 | 0.18 | 1.10 |
| dsDNA | 131.2 | 128.8 | -2.4 | 0.64 | -1.02 | 135.1 | 163.1 | 28.0 | 0.20 | 1.21 | 0.20 | 1.21 |
| P-Selectin | 0.10 | 0.09 | -0.01 | 0.82 | -1.06 | 0.10 | 0.13 | 0.0 | 0.24 | 1.21 | 0.30 | 1.23 |
| Nucleosomes | 113.4 | 94.9 | -18.5 | 0.22 | -1.20 | 144.3 | 156.6 | 12.3 | 0.43 | 1.09 | 0.19 | 1.13 |
| vWF | 4.91 | 4.93 | 0.0 | 0.97 | 1.01 | 5.4 | 5.8 | 0.4 | 0.64 | 1.07 | 0.75 | 1.04 |
Paired t-tests comparing T0 and T1.
ANCOVA comparing T0 and T1 adjusted for age, race, BMI, diabetes, hypertension, and type of breast cancer.
dsDNA, double stranded DNA; MPO, myeloperoxidase; TAT complex, thrombin-anti-thrombin complex; TM, thrombomodulin; CRP, c-reactive protein; vWF, von Willebrandt factor. NORM, patients with normal LVEF; ABN, patients with abnormally decline LVEF; MeanDiff, differences between the means of T1-T0 of the biomarkers in the two groups of patients; FC, fold change.
Correlation matrix of markers
Figure 2 depicts the pairwise Pearson correlations matrix between the plasma markers in the two groups of breast cancer patients (ABN and NORM) at different time points (T0 and T1). From the correlation matrix among NORM patients at both T0 and T1, nucleosomes, CRP, TAT complex, dsDNA, and MPO, were positively correlated while inverse correlated with TM, P-selectin, and vWF, reflecting similar modulating pathways for those markers. After 1st cycle of DOX-based chemotherapy, most markers were positively correlated except for nucleosomes vs. vWF, CRP vs. TM, and TAT complex vs. vWF. On the other hand, most markers of inflammation, hypercoagulability and thrombosis among ABN patients were positively correlated at baseline except nucleosomes, suggesting a pre-thrombosis state that might be an early sign of an asymptomatic myocardial injury.
Figure 2.
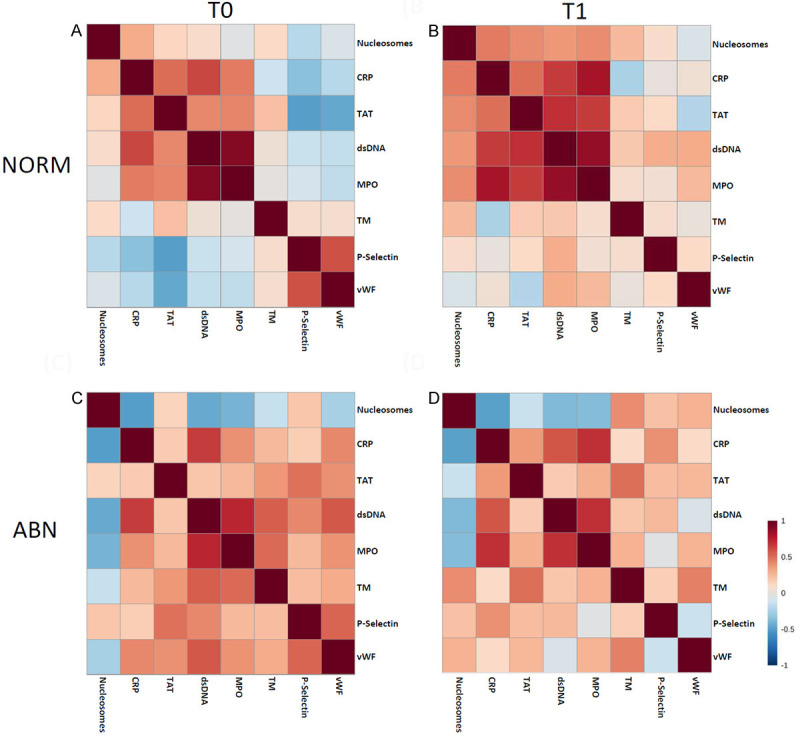
The pairwise Pearson correlations between plasma markers among the study sample by LVEF and by time points. A. NORM at baseline; B. NORM after 4 cycles of DOX chemotherapy; C. ABN at baseline; D. ABN after 4 cycles of DOX chemotherapy.
Discussion
The results from this exploratory hypothesis-driven study showed, for the first time, to our knowledge, that: (1) elevated circulating levels of MPO and TM before and after the first dose of DOX chemotherapy were associated with increased risk for cardiotoxicity in breast cancer; and (2) higher levels of circulating markers of hypercoagulability, inflammation, endothelial dysfunction and NETosis after the first dose of DOX chemotherapy correlated with the risk of subsequent cardiotoxicity in cancer.
Breast cancer is the most common neoplasm in women and the second leading cause of cancer-related mortality in females worldwide [47]. Despite the lower incidence of thrombotic complications in breast cancer compared with other malignancies, breast cancer patients are at higher risk compared with the general population. The number of patients who have complications is considerable given the high incidence of breast malignancies in the female population worldwide [48]. A population-based study of breast cancer survivors showed that women who received anthracyclines, including DOX, daunorubicin, or epirubicin had higher rates of heart failure than did women who received non-anthracycline or no chemotherapy [49].
Cancer cells produce and release procoagulant and fibrinolytic proteins, inflammatory cytokines, and procoagulant microparticles [50]. They also express adhesion molecules binding to the receptors of host vascular cells (i.e., endothelial cells, platelets, and leukocytes), thereby stimulating the prothrombotic properties of these normal cells, including the shed of NETs and cell-specific microparticles [33,51]. The results from the present study showed significantly elevated circulating levels of MPO and TM at the baseline. MPO is a well-known neutrophil granule protein and a component of NETs [27,52]. The extracellular release of MPO triggers a process of immune recognition that generates anti-neutrophil antibodies, which are associated with chronic inflammation [53], autoimmune diseases [54], cardiovascular diseases [55,56] and recently with cancer [57,58]. Because oxidative stress has been suggested as a major mechanism of DOX-induced cardiotoxicity [15], the elevated MPO after DOX exposure could be considered a potential biomarker of the risk for a subsequent cardiac dysfunction in cancer patients [59]. Our findings showing increased circulating levels of MPO before and after the first cycle of DOX-based chemotherapy suggest that MPO may predict the risk of cardiotoxicity both before and after the initial dose of DOX. TM, a transmembrane endothelial protein facilitates the thrombin-mediated activation of protein C and plays role in coagulation, fibrinolysis and inflammation [60,61]. In the presence of inflammation, neutrophil proteases increase the release of TM from the cell surface and thereby increase its circulating levels [62]. Experimental and clinical studies have identified TM as a marker of generalized endothelial injury [63]. Elevated circulating levels of TM have been reported in intravascular coagulation [64], venous thrombosis [65] and cancer [66]. Yang and co-authors [67] found that TM regulates sensitivity to DOX in non-small cell lung cancer through epithelial-mesenchymal transition. Our data suggest that the higher circulating levels of TM prior to and after the first dose of DOX-based chemotherapy may potentially predict the risk of cardiotoxicity in breast cancer.
The observed elevated circulating levels of nucleosomes after one dose of DOX-based chemotherapy correlate with previously detected enhanced activation of neutrophils associated with increased expression of genes encoding neutrophil-specific proteins [17], which may be associated with the subsequent release of nuclear content. Neutrophil activity represents one of the earliest responses to injury and current evidence support the role of NETs in acute and chronic inflammation [68]. The correlation between the higher circulating levels of surrogate NET markers after the 1st cycle of chemotherapy and the increased risk for DOX-induced cardiotoxicity in the group of patients with abnormal LVEF in this study, suggest that NETosis may be an initiator of the early inflammatory response induced by chemotherapy with DOX. Given the prothrombotic and inflammatory properties of NETs [69], we were interested to measure the levels of markers of hypercoagulability and inflammation. TAT levels reflect the functional state of the coagulation system and represent a diagnostic tool for the detection of hypercoagulability [70]. TAT measurement has been used in humans for diagnosis and assessment of treatment-induced intravascular coagulation, deep vein thrombosis, and pulmonary thromboembolism [71,72]. In this study plasma levels of TAT complex after 1st cycle of DOX-based chemotherapy were significantly higher in the group of patients with abnormal LVEF decline relative to the group of patients with normal LVEF and therefore were able to predict the subsequent cardiotoxicity after the 1st cycle of DOX-base chemotherapy, but not at the baseline. CRP is a marker of inflammation [73], atherothrombosis [74], endothelial dysfunction [75] and cardiovascular diseases [76]. Plasma levels of CRP after 1st cycle of DOX-based chemotherapy were significantly higher among patients with abnormal LVEF decline compared to patients with normal LVEF. From the paired-wise analysis, CRP also increased significantly after the therapy. However, precautions must be made for patients with hypertensions and diabetes since the significance disappeared after considering those conditions in the analysis (Table 2). Endothelium modulates thrombosis through the release of vasoactive factors such as TM, P-selectin and vWF, which modulate platelet activity, coagulation and vascular contractility, all of which contribute to thrombotic formation [77,78]. Platelet activator and pro-coagulant [79] marker, P-selectin has been implicated in the adverse cardiovascular events [80]. Elevated plasma levels of vWF have been associated with myocardial infarction [81], coronary artery disease [82] and ischemic stroke [83]. In our study however, P-selectin and vWF were not significantly altered between the two groups of patients.
DOX and other chemotherapy agents may disrupt the protective function of endothelial lining of coronary blood vessels, which leads to the development of severe chronic vascular diseases [31,84]. ROS generation has a central role in DOX-induced endothelial dysfunction, similar to the effects on cardiomyocytes [85]. DOX is introduced into the systemic circulation where the first cellular contact causing injury to the vascular endothelium before it travels into other tissues such as the heart [16,86]. Moreover, the contribution of vascular endothelial dysfunction to the development of cardiovascular diseases is well established [87,88]. The initial asymptomatic endothelial cell insult by DOX therapy carries a risk for the later onset of vascular disorders and their negative effects on the cardiomyocyte health and function [16,86]. For example, children who received DOX therapy often develop severe vascular disease pathology as adults [86].
The limitations of this study include the small number of patients examined, which resulted in a great variation in the resulting data and a weaker correlation with cardiotoxicity. The use of different imaging tools for LVEF although in a small number of the patients might have affected the results [87]. Further studies with a larger group of patients, along with the dynamic profile of the suggested markers of hypercoagulability and endothelial dysfunction during the course of DOX chemotherapy in correlation with the risk of cardiotoxicity are needed.
In conclusion, the collective results from this study suggest that the risk of DOX-induced cardiotoxicity in breast cancer is associated with endothelial dysfunction, inflammation and prothrombotic state before and after the first dose of chemotherapy. Inhibition of components of NETs, thromboprophylaxis and/or maintaining endothelial function during treatment without affecting DOX anti-cancer efficacy would be essential for preserving cardiovascular homeostasis. Early identification of patients at risk for DOX-induced cardiac damage might reduce the incidents of cardiotoxicity-associated morbidity and mortality by implementation of other treatment modalities or cardioprotective treatment.
Acknowledgements
We thank all the patients and the personnel of the Cancer Clinical Trials and Regulatory Affairs Office at UAMS. We thank Mr. Eric Siegel for the insightful discussion and Ms. Yingni Che for the technical assistance. This study was supported by grants from Arkansas Breast Cancer Research Program (VKT), NIH/NIA Claude Pepper Center grant P30 AG028718 (JYW), and by funds from the Laura F. Hutchins, M.D. Distinguished Chair for Hematology and Oncology (IM).
Disclosure of conflict of interest
None.
References
- 1.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kero AE, Jarvela LS, Arola M, Malila N, Madanat-Harjuoja LM, Matomaki J, Lähteenmäki PM. Cardiovascular morbidity in long-term survivors of early-onset cancer: a population-based study. Int J Cancer. 2014;134:664–73. doi: 10.1002/ijc.28385. [DOI] [PubMed] [Google Scholar]
- 3.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J. Clin. Oncol. 2008;26:3777–84. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groarke JD, Nohria A. Anthracycline cardiotoxicity: a new paradigm for an old classic. Circulation. 2015;131:1946–9. doi: 10.1161/CIRCULATIONAHA.115.016704. [DOI] [PubMed] [Google Scholar]
- 5.Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008;130:688–95. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- 6.Jain D, Russell RR, Schwartz RG, Panjrath GS, Aronow W. Cardiac complications of cancer therapy: pathophysiology, identification, prevention, treatment, and future directions. Curr Cardiol Rep. 2017;19:36. doi: 10.1007/s11886-017-0846-x. [DOI] [PubMed] [Google Scholar]
- 7.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–93. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mookadam F, Sharma A, Lee HR, Northfelt DW. Intersection of cardiology and oncology clinical practices. Front Oncol. 2014;4:259. doi: 10.3389/fonc.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal N, Wentworth B, Choudhary R, Landa Ade L, Kipper B, Fard A, Maisel AS. Cardiac biomarkers: new tools for heart failure management. Cardiovasc Diagn Ther. 2012;2:147–64. doi: 10.3978/j.issn.2223-3652.2012.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato Y, Taniguchi R, Makiyama T, Nagai K, Okada H, Yamada T, Matsumori A, Takatsu Y. Serum cardiac troponin T and plasma brain natriuretic peptide in patients with cardiac decompensation. Heart. 2002;88:647–648. doi: 10.1136/heart.88.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wettersten N, Maisel AS. Biomarkers for heart failure: an update for practitioners of internal medicine. Am J Med. 2016;129:560–567. doi: 10.1016/j.amjmed.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2008;97:318–26. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 13.Jungandreas K, Vogt A, Voigt W, Jordan K, Strauß HG. Natriuretic peptides and troponin I do not predict chemotherapy-induced cardiac toxicity. J Cardiovasc Dis Diagn. 2014;2:140. [Google Scholar]
- 14.Yu LR, Cao Z, Makhoul I, Daniels JR, Klimberg S, Wei JY, Bai JP, Li J, Lathrop JT, Beger RD, Todorova VK. Immune response proteins as predictive biomarkers of doxorubicin-induced cardiotoxicity in breast cancer patients. Exp Biol Med (Maywood) 2018;243:248–55. doi: 10.1177/1535370217746383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Moon M, Dawood S, McManus B, Liu PP. Mechanisms and management of doxorubicin cardiotoxicity. Herz. 2011;36:296–305. doi: 10.1007/s00059-011-3470-3. [DOI] [PubMed] [Google Scholar]
- 16.Luu AZ, Chowdhury B, Al-Omran M, Teoh H, Hess DA, Verma S. Role of endothelium in doxorubicin-induced cardiomyopathy. JACC Basic Transl Sci. 2018;3:861–870. doi: 10.1016/j.jacbts.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todorova VK, Makhoul I, Siegel ER, Wei J, Stone A, Carter W, Klimberg VS. Biomarkers for presymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. PLoS One. 2016;11:e0160224. doi: 10.1371/journal.pone.0160224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 19.Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273:329–343. doi: 10.1111/imr.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair. 2013;6:11. doi: 10.1186/1755-1536-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaul DS, Stein S, Matter CM. Neutrophils in cardiovascular disease. Eur Heart J. 2017;38:1702–1704. doi: 10.1093/eurheartj/ehx244. [DOI] [PubMed] [Google Scholar]
- 23.Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, Wackers FJ, Jang IK. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103:333–7. doi: 10.1016/j.amjcard.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 24.Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, Cosentino M, Venco A. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost. 2011;106:591–599. doi: 10.1160/TH11-02-0096. [DOI] [PubMed] [Google Scholar]
- 25.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res. 2012;110:126–44. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 26.Bhagat A, Kleinerman ES. The role of the innate immune system in doxorubicin-induced cardiotoxicity. J Immunol. 2019;202(Suppl):187. [Google Scholar]
- 27.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi H, Yang S, Zhang L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front Immunol. 2017;8:928–42. doi: 10.3389/fimmu.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130–138. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morbidelli L, Donnini S, Ziche M. Targeting endothelial cell metabolism for cardio-protection from the toxicity of antitumor agents. Cardio Oncology. 2016;2:3–10. doi: 10.1186/s40959-016-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snoderly HT, Boone BA, Bennewitz MF. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019;21:145–51. doi: 10.1186/s13058-019-1237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers (Basel) 2018;10:380. doi: 10.3390/cancers10100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9:2313–21. doi: 10.1111/j.1538-7836.2011.04465.x. [DOI] [PubMed] [Google Scholar]
- 36.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011–8. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 37.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson MJ, Sproule MW, Paul J. The prevalence and associated variables of deep venous thrombosis in patients with advanced cancer. Clin Oncol (R Coll Radiol) 1999;11:105–110. doi: 10.1053/clon.1999.9023. [DOI] [PubMed] [Google Scholar]
- 39.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J. Clin. Oncol. 1991;9:286–94. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]
- 40.Levine MN, Gent M, Hirsh J, Arnold A, Goodyear MD, Hryniuk W, De PS. The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer. N Engl J Med. 1988;318:404–7. doi: 10.1056/NEJM198802183180703. [DOI] [PubMed] [Google Scholar]
- 41.Walker AJ, West J, Card TR, Crooks C, Kirwan CC, Grainge MJ. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood. 2016;127:849–57. doi: 10.1182/blood-2015-01-625582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thrombo Res. 2006;118:555–568. doi: 10.1016/j.thromres.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Horsted F, West J, Grainge MJ. Risk of thromboembolismin patients with cancer: a systemic review and meta-analysis. PLoS Med. 2012;9:e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcucci R, Gori AM, Giannotti F, Baldi M, Verdiani V, Del Pace S, Nozzoli C, Abbate R. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. J Thromb Haemost. 2006;4:1017–22. doi: 10.1111/j.1538-7836.2006.01916.x. [DOI] [PubMed] [Google Scholar]
- 45.Levi M, Van Der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;22:2698–704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Chen Q, Qi H, Wang C, Wang C, Zhang J, Dong L. Doxorubicin-induced systemic inflammation is driven by upregulation of toll-like receptor TLR4 and endotoxin leakage. Cancer Res. 2016;76:6631–6642. doi: 10.1158/0008-5472.CAN-15-3034. [DOI] [PubMed] [Google Scholar]
- 47.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 48.Kyriazi V. Breast cancer as an acquired thrombophilic state. J Breast Cancer. 2012;15:148–156. doi: 10.4048/jbc.2012.15.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinder MC, Zhigang D, Goodwin JS, Hortobagyi GN, Sharon H, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J. Clin. Oncol. 2007;25:3808–15. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 50.Falanga A, Russo L, Milesi V, Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Crit Rev Oncol Hematol. 2017;118:79–83. doi: 10.1016/j.critrevonc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. 1984;3:99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- 52.Khan AA, Alsahli MA, Rahmani AH. Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci (Basel) 2018;6:33. doi: 10.3390/medsci6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Strzepa A, Pritchard KA, Dittel BN. Myeloperoxidase: a new player in autoimmunity. Cell Immunol. 2017;317:1–8. doi: 10.1016/j.cellimm.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 56.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Münzel T, Simoons ML, Hamm CW CAPTURE Investigators. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–5. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 57.Scandolara TB, Panis C. Neutrophil traps, anti-myeloperoxidase antibodies and cancer: are they linked? Immunol Lett. 2020;221:33–38. doi: 10.1016/j.imlet.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Mariani F, Boarino V, Bertani A, Merighi A, Pedroni M, Rossi G, Mancini S, Sena P, Benatti P, Roncucci L. Myeloperoxidase-positive cell infiltration of normal colorectal mucosa is related to body fatness and is predictive of adenoma occurrence. Int J Obes. 2017;41:982–985. doi: 10.1038/ijo.2017.80. [DOI] [PubMed] [Google Scholar]
- 59.Ky B, Putt M, Sawaya H, French B, Januzzi JL, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–16. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34:107–25. doi: 10.1007/s00281-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 61.Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304:H1585–97. doi: 10.1152/ajpheart.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boehme MW, Deng Y, Raeth U, Bierhaus A, Ziegler R, Stremmel W, Nawroth PP. Release of thrombomodulin from endothelial cells by concerted action of TNF-alpha and neutrophils: in vivo and in vitro studies. Immunology. 1996;87:134–40. [PMC free article] [PubMed] [Google Scholar]
- 63.Strijbos MH, Rao C, Schmitz PI, Kraan J, Lamers CH, Sleijfer S, Terstappen LW, Gratama JW. Correlation between circulating endothelial cell counts and plasma thrombomodulin levels as markers for endothelial damage. Thromb Haemost. 2008;100:642–7. [PubMed] [Google Scholar]
- 64.Lin SM, Wang YM, Lin HC, Lee KY, Huang CD, Liu CY, Wang CH, Kuo HP. Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis. Crit Care Med. 2008;36:683–9. doi: 10.1097/CCM.0B013E31816537D8. [DOI] [PubMed] [Google Scholar]
- 65.Bouman AC, Cheung YW, Spronk HM, Schalkwijk CG, Ten Cate H, Ten Wolde M, Ten Cate-Hoek AJ. Biomarkers for post thrombotic syndrome: a case-control study. Thromb Res. 2014;134:369–75. doi: 10.1016/j.thromres.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Asanuma K, Nakamura T, Asanuma Y, Kakimoto T, Yada Y, Hagi T, Kita K, Matsumine A, Sudo A. Serum thrombomodulin as a metastatic and prognostic marker in soft tissue sarcomas. Cancer Biomark. 2019;26:163–170. doi: 10.3233/CBM-182075. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Cheng BJ, Lu S. Thrombomodulin regulates doxorubicin sensitivity through epithelial-mesenchymal transition in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21:95–101. [PubMed] [Google Scholar]
- 68.Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol. 2017;8:81. doi: 10.3389/fimmu.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2019;39:1724–1738. doi: 10.1161/ATVBAHA.119.312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harenberg J. Lab tech in thrombosis- a manual. Springer, Dordrecht; 1999. Thrombin-antithrombin (TAT) complex. [Google Scholar]
- 71.Wada H, Sakuragawa N, Mori Y, Takagi M, Nakasaki T, Shimura M, Hiyoyama K, Nisikawa M, Gabazza EC, Deguchi K, Kazama M, Shiku H. Hemostatic molecular markers before the onset of disseminated intravascular coagulation. Am J Hematol. 1999;60:273–8. doi: 10.1002/(sici)1096-8652(199904)60:4<273::aid-ajh4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 72.Asakura H, Wada H, Okamoto K, Iba T, Uchiyama T, Eguchi Y, Kawasugi K, Koga S, Mayumi T, Koike K, Gando S. Evaluation of haemostatic molecular markers for diagnosis of disseminated intravascular coagulation in patients with infections. Thromb Haemost. 2006;95:282–7. doi: 10.1160/TH05-04-0286. [DOI] [PubMed] [Google Scholar]
- 73.Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–93. [PubMed] [Google Scholar]
- 74.Devaraj S, Singh U, Jialal I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem. 2009;55:229–38. doi: 10.1373/clinchem.2008.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hein TW, Singh U, Vasquez-Vivar J, Devaraj S, Kuo L, Jialal I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. 2009;206:61–8. doi: 10.1016/j.atherosclerosis.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cozlea DL, Farcas DM, Nagy A, Keresztesi AA, Tifrea R, Cozlea L, Carazsca E. The impact of C reactive protein on global cardiovascular risk on patients with coronary artery disease. Curr Health Sci J. 2013;39:225–231. [PMC free article] [PubMed] [Google Scholar]
- 77.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. Von willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myers DD Jr, Schaub R, Wrobleski SK, Londy FJ 3rd, Fex BA, Chapman AM, Greenfield LJ, Wakefield TW. P-selectin antagonism causes dose-dependent venous thrombosis inhibition. Thromb Haemost. 2001;85:423–429. [PubMed] [Google Scholar]
- 79.Ferroni P, Martini F, Riondino S, La Farina F, Magnapera A, Ciatti F, Guadani F. Soluble P-selectin as a marker of in vivo platelet activation. Clinica Chimica Acta. 2009;399:88–91. doi: 10.1016/j.cca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 80.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–79. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Zhao J, Zhang Y, Xue X, Yin J, Liao L, Xu C, Hou Y, Yan S, Liu J. Kinetics of plasma von Willebrand factor in acute myocardial infarction patients: a meta-analysis. Oncotarget. 2017;8:90371–9. doi: 10.18632/oncotarget.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whincup PH, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, Rumley A, Lowe GD. von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J. 2002;23:1764–70. doi: 10.1053/euhj.2001.3237. [DOI] [PubMed] [Google Scholar]
- 83.Dhanesha N, Prakash P, Doddapattar P, Khanna I, Pollpeter MJ, Nayak MK, Staber JM, Chauhan AK. Endothelial cell-derived von willebrand factor is the major determinant that mediates von willebrand factor-dependent acute ischemic stroke by promoting postischemic thrombo-inflammation. Arterioscler Thromb Vasc Biol. 2016;36:1829–37. doi: 10.1161/ATVBAHA.116.307660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bar-Joseph H, Ben-Aharon I, Tzabari M, Tsarfaty G, Stemmer SM, Shalgi R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS One. 2011;6:e23492. doi: 10.1371/journal.pone.0023492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. J Biol Chem. 2000;275:33585–92. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- 86.Mitry MA, Edwards JG. Doxorubicin induced heart failure: phenotype and molecular mechanisms. Int J Cardiol Heart Vasc. 2016;10:17–24. doi: 10.1016/j.ijcha.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.López-Candales A. Cardio-oncology: in search of the right balance. Postgrad Med. 2019;131:79–81. doi: 10.1080/00325481.2019.1568020. [DOI] [PubMed] [Google Scholar]
- 88.Mookadam F, Sharma A, Lee HR, Northfelt DW. Intersection of cardiology and oncology clinical practices. Front Oncol. 2014;4:259. doi: 10.3389/fonc.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]


