Abstract
Gastric cancer (GC) is the second most common cancer in China. The ToGA study showed that trastuzumab in combination with fluoropyrimidine plus cisplatin prolonged overall survival (OS) in patients with human epidermal growth factor receptor 2 (HER2)-positive advanced GC (AGC). However, some patients may not be able to receive this regimen. We conducted a clinical study to evaluate the efficacy and safety of trastuzumab in combination with docetaxel+capecitabine (DX) in patients with HER2-positive AGC. This phase II, multi-center, open-label, single arm study enrolled patients with HER2-positive AGC who had not received prior treatment for metastatic disease. Patients were treated with a regimen of trastuzumab (8 mg/kg loading dose followed by 6 mg/kg, day 1), capecitabine (1000 mg/m2 twice daily, days 1-14) and docetaxel (60 mg/m2, day 1 for 6 cycles) every 3 weeks. The primary endpoint was progression-free survival (PFS) and the secondary endpoints were objective response rate (ORR), OS and safety profiles. Sixty-seven patients with AGC were enrolled from 14 centers. 64 were included in the full analysis set (FAS). The median PFS was 8.1 months (95% confidence interval [CI]: 5.6-12.8) and the median OS was 20.9 months (95% CI: 15.1-33.0). Response was evaluated in 59 patients. The ORR was 67.8%. The most common adverse events of Grade ≥3 were neutropenia, leukopenia, hand-foot syndrome, febrile neutropenia and anemia. We concluded that combination treatment with trastuzumab and DX was well-tolerated and highly effective in patients with HER2-positive AGC, and may offer an alternative to current treatments.
Keywords: Gastric cancer, trastuzumab, HER2, docetaxel, capecitabine
Introduction
Gastric or gastroesophageal junction (GEJ) cancer is both the second most common and second most lethal cancer in China [1]. China has one of the highest age standardized-incidence rates per 100,000 individuals in the world [2]. Eighty percent of patients diagnosed with gastric cancer (GC) in China are in an advanced stage [3]. The 5-year survival rate for Chinese patients with GC was 35.9% between 2010 and 2014, much lower than that observed in Korea (68.9%) and Japan (60.3%) [4]. Although studies suggest a modest survival advantage can be gained from chemotherapy [5-9], more effective systemic regimens need to be identified for treatment of patients with advanced gastric cancer (AGC).
Some GC tumors are human epidermal growth factor receptor 2 (HER2)-positive and the rate of HER2 overexpression on GC tumors in Chinese patients is 7-12% [10,11]. Trastuzumab (Herceptin®, Roche, Shanghai Roche Pharmaceuticals Ltd., China) is a humanized antibody targeting HER2 that binds and inhibits tumor proliferation and survival [12]. The ToGA trial demonstrated that trastuzumab in combination with capecitabine or 5-fluorouracil (5-FU) plus cisplatin improved progression-free survival (PFS) and overall survival (OS) in patients with HER2-positive AGC [13]. Trastuzumab is the only targeted therapy currently approved for a first-line treatment of patients with HER2+ AGC [14]. First-line trastuzumab used in combination with cisplatin and capecitabine or 5-FU is the current standard treatment for HER2-positive metastatic GC (mGC) [9,15]. While the ToGA trial showed the benefits of trastuzumab in combination with capecitabine or 5-FU plus cisplatin, data demonstrating the benefit of other chemotherapy regimens combined with trastuzumab for the treatment of AGC is limited [16].
Despite the survival benefit achieved with trastuzumab, some patients develop resistance to the treatment [12]. While the potential molecular mechanisms behind this resistance are under investigation [17-19], further work is needed to reach a definitive conclusion [20].
Other chemotherapy regimens that may show benefit in combination with trastuzumab include docetaxel plus capecitabine (DX), which has shown a synergistic effect in AGC animal models [21] and activity in patients with AGC or advanced esophageal cancer (AEC) [22,23]. However, the combination of trastuzumab plus DX for AGC has not yet been studied. We evaluated the efficacy and safety of trastuzumab in combination with DX as a first-line therapy in Chinese patients with HER2-positive AGC.
Methods
Patients
This study was a phase II, multicenter, open label, single-arm clinical study designed to evaluate the efficacy and safety of trastuzumab in combination with DX as a first-line treatment in Chinese patients with inoperable, locally advanced or recurrent and/or HER2-positive metastatic GC or GEJ adenocarcinoma.
Additional eligibility criteria included >18 years of age, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0-1, and an expected survival of ≥3 months. Major exclusion criteria included previous chemotherapy for advanced/metastatic disease, previous or current use of any epidermal growth factor receptor-targeted drugs, loss of upper gastrointestinal tract physical integrity or malabsorption syndrome, active (significant or uncontrolled) gastrointestinal bleeding, history or evidence of brain metastases, residual relevant toxicity from previous therapy (except alopecia), other malignancy within the last 5 years (exceptions: carcinoma in situ of the cervix or basal cell carcinoma), congestive heart failure, baseline left ventricular ejection fraction (LVEF) less than 50%, angina pectoris requiring medication, evidence of transmural myocardial infarction on electrocardiogram (ECG), poorly controlled hypertension (systolic blood pressure [BP] >180 mmHg or diastolic BP >100 mmHg), clinically significant valvular heart disease or high risk uncontrollable arrhythmias.
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the institutional review board at each participating center. All patients provided written informed consent before enrollment and were capable of protocol compliance. This study was registered at ClinicalTrials.gov (NCT02004769).
Tumor specimens and HER2 status confirmation
All tumor specimens collected from patients who received tumor resection during surgery or biopsy at our hospital were processed as formalin-fixed paraffin-embedded (FFPE) tissues. The HER2 status of tumors was evaluated using immunohistochemistry (IHC) with anti-HER2/neu (4B5) monoclonal antibody (Ventana Medical Systems, AZ, USA) [24]. If the IHC score was 2+, fluorescence in situ hybridization (FISH) was performed using the PathVysion HER-2 DNA Probe Kit (Abbott Molecular, IL, USA). A tumor was considered HER2-positive if it scored IHC2+ and had a confirmed FISH+ result (HER2: chromosome enumeration probe 17 ratio ≥2), or if it scored IHC3+. FISH analyses for HER2 status were carried out according to the manufacturer’s protocol (Vysis, Inc., IL, USA). HER2 testing was performed at either Sun Yat-Sen University Cancer Center or a local hospital laboratory.
Treatment plan and tumor assessment
Patients received one cycle of treatment with trastuzumab in combination with DX every 3 weeks. All patients were treated until either disease progression, occurrence of unacceptable toxicity or withdrawal from the study for other reasons. Each cycle of treatment included trastuzumab plus DX administered as follows: trastuzumab was administered by intravenous (IV) infusion at a loading dose of 8 mg/kg on treatment Cycle 1 Day 1, followed by a 6 mg/kg dose every 3 weeks (i.e., per cycle); capecitabine was administered for the first 14 days of each cycle (evening of Day 1 to morning of Day 15) and given orally at a dose of 1000 mg/m2 twice daily (total daily dosage, 2000 mg/m2); and docetaxel was administered at a dose of 60 mg/m2 as an IV infusion (infusion time: 1 h) on Day 1 of each cycle (maximally 6 cycles), starting after completion of the trastuzumab infusion.
The primary end point was progression-free survival (PFS). The secondary end points were objective response rate (ORR), overall survival (OS) and safety. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, using only measurable target lesions [25]. To be considered measurable by computerized tomography (CT) or magnetic resonance imaging (MRI), tumor lesions had to be at ≥10 m in at least one dimension. For a lymph node to be considered pathologically enlarged and measurable, it must be ≥15 mm in the shorter axis when assessed by CT scan.
Complete tumor assessment was performed ≤21 days before the first dose of study treatment; follow-up tumor assessments were performed at 6-week intervals from the start of study treatment. The duration of follow-up was either when 80% of patients died or 18 months after the last patient was enrolled, whichever occurred first.
Performance status and safety assessments
ECOG PS was assessed at baseline and every 21 days during the scheduled visits. The National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.0) was used for safety evaluations, with the exception of cardiac failure which was graded according to the New York Heart Association classification system. Complete medical histories, including demographics, were recorded during the screening visits. Physical examinations were performed at baseline and at each study visit, or as clinically indicated. 12-lead ECG was performed on each patient at baseline, before initiating each treatment cycle and at any time during the study if clinically indicated. LVEF was measured using a multiple-uptake gated acquisition scan, cardiac MRI or ECG. Baseline LVEF assessments were performed ≤21 days prior to the start of study treatment and then every 12 weeks (more often if clinically indicated) during the study and in the 6 months following the end of treatment/withdrawal from the study.
Statistical analysis
The sample size was determined to be 65 patients assuming an improvement in PFS from 5.5 months to 6.7 months with the addition of trastuzumab [13] and allowing for a 5% drop out rate. The full analysis set (FAS) consisted of all intention-to-treat patients who received at least one dose of any drug as part of the study treatment and was the main population for efficacy evaluation. The safety analysis set (SAF) consisted of all patients who had received at least one dose of any drug as part of the study treatment and completed at least one safety evaluation.
The primary efficacy parameter, duration of PFS, was defined as the time from the initiation of treatment to the date of progressive disease (PD) or death. OS was measured from the initiation of treatment to the date of the last follow-up or death by any cause. Overall tumor response was defined as the occurrence rate of a confirmed complete response (CR) or a partial response (PR) determined by confirmed radiographic evaluations of target and non-target lesions. PFS and OS were analyzed using the Kaplan-Meier method and 95% confidence intervals (CI) were calculated using the Clopper-Pearson Exact method [26]. The change in tumor size from baseline was analyzed using a waterfall plot. The ORR and PFS analyses and the safety evaluation were conducted after 80% of PFS events were observed. OS and safety analyses were conducted when 80% of deaths were observed, or 18 months after the last patient was enrolled, whichever occurred first. Descriptive statistics were calculated for safety analyses.
All statistical analyses were performed using SPSS software (SPSS 11.0, Chicago, Ill., USA). All p values were two sided and a p value ≤0.05 was considered statistically significant.
Whole exome sequencing
All FFPE tumor specimens of twenty seven patients were sent to HaploX (Shenzhen, Guangdong, China) for next generation high throughput sequencing. The resulting tumor DNA libraries were sequenced using the Illumina HiSeq Genome Analyzer (Illumina, San Diego, CA, USA), which yielded approximately 150 bp of paired end sequence. FASTQ files were then generated.
Gene variant calling and filtering
Variant calling was carried out using a series of published software in accordance with official instructions as follows: we used Burrows-Wheeler Aligner (version 0.7.10-r789) [27] and Sambamba (version 0.5.9) [28] to process paired-end FASTQ sequences, then generated duplicate-marked, sorted, base-recalibrated, indexed Binary Alignment/Map (BAM) files; SpeedSeq (version 0.1.2) [29] and GATK4 HaplotypeCaller (version 4.0.12.0) were used to call germline single nucleotide polymorphisms and insertions/deletions simultaneously. Variants identified by both methods were considered as high confidence mutations. Bcftools software (version 1.9-94-g9589876) was used to create intersections of variant call format files. Next, we used ANNOVAR with online databases (version hg38) to annotate the variant calls. The online databases used included COSMIC v76, refGene, avsnp147, ALL.sites.2015_08_edit, EAS.sites.2015_08_edit, esp6500siv2_all, exac03 and clinvar_20170905. Mutations with single nucleotide variants (SNVs) in the ALL.sites.2015_08_edit database that were detected at a frequency of ≤0.1% were analyzed. SNVs annotated with “intergenic” or “intronic” in the Func.refGene database and “synonymous SNV” in the ExonicFunc.refGene database were removed. The R package maftools was used to process and visualize the filtered SNVs [30].
Results
Patient characteristics
Between November 2013 and June 2016, a total of 67 patients were enrolled from 14 hospitals in China. Fifty-eight patients completed follow-up and nine discontinued or withdrew from the study (patient withdrew consent, n=5; death or lost to follow-up, n=3; other, n=1). The FAS comprised 64 patients; three patients were not included in the FAS because they did not receive any drugs related to the study treatment. Patient demographics and baseline disease characteristics are shown in Table 1. The median age at the time of enrollment was 58 years (range: 19-76). 31.3% of patients had a history of GC surgery. The number of HER2-positive tumors based on IHC3+ and IHC2+ plus FISH+ were 51 (79.7%) and 13 (20.3%), respectively. All patients had differentiated adenocarcinoma; 11 (17.2%) patients had GC and 53 (82.8%) had GEJ cancer. The most frequent site of metastasis was the lymph node (71.9%), followed by the liver (42.2%), lung (25.0%) and ovary (3.1%).
Table 1.
Baseline patient characteristics (FAS)
| Parameter | FAS (N=64) |
|---|---|
| Median age (range), years | 58 (19-76) |
| Sex, n (%) | |
| Male | 47 (73.4) |
| Female | 17 (26.6) |
| ECOG PS, n (%) | |
| 0 | 14 (21.9) |
| 1 | 50 (78.1) |
| History of gastric cancer surgery, n (%) | |
| No | 44 (68.8) |
| Yes | 20 (31.3) |
| HER2 status, n (%) | |
| HER2++ plus FISH+ | 13 (20.3) |
| HER2+++ | 51 (79.7) |
| Location of primary lesion, n (%) | |
| Gastric | 53 (82.8) |
| Gastroesophageal junction | 11 (17.2) |
| Lauren classification, n (%) | |
| Intestinal | 15 (23.4) |
| Diffuse | 7 (10.9) |
| Mixed | 5 (7.8) |
| Unknown | 37 (57.8) |
| Differentiation, n (%) | |
| G1 | 3 (4.7) |
| G2 | 21 (32.8) |
| G3 | 22 (34.4) |
| Gx | 5 (7.8) |
| Unknown | 13 (20.3) |
| Metastasis, n (%) | |
| Liver | 27 (42.2) |
| Lung | 16 (25.0) |
| Lymph node | 46 (71.9) |
| Ovary | 2 (3.1) |
| Peritoneum | 8 (12.5) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; FAS, full analysis set; HER2, human epidermal growth factor 2.
Efficacy
The median duration of follow-up at the time of analysis (FAS; n=64) was 14.5 months. The median PFS was 8.1 months (95% CI: 5.6-12.8 and the median OS was 20.9 months (95% CI: 15.1-33.0) (Figures 1 and 2). The ORR was evaluated in 59 patients (5 patients were missing) using the RECIST (version 1.1) criteria. Five patients achieved CR and 35 achieved PR; the ORR was 67.8% (95% CI 0.54-0.79) (Table 2). The median time to response was 1.77 months (95% CI: 1.44-4.3) and among the 40 patients who achieved CR or PR, the median duration of response was 11.5 months (95% CI: 6.8-13.2). Figure 3 shows a waterfall plot of tumor size reduction over time, demonstrating that most patients experienced a reduction of tumor size compared to baseline.
Figure 1.
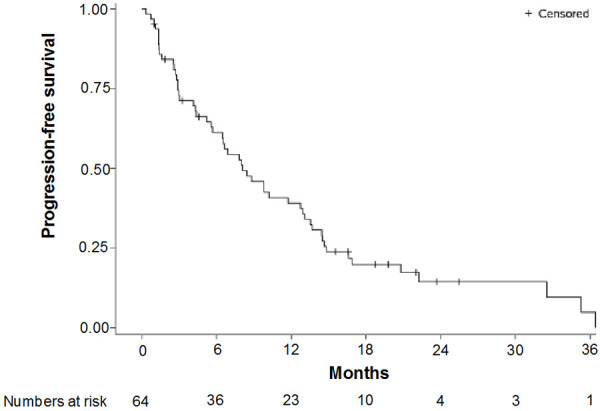
Progression-free survival, Kaplan-Meier.
Figure 2.
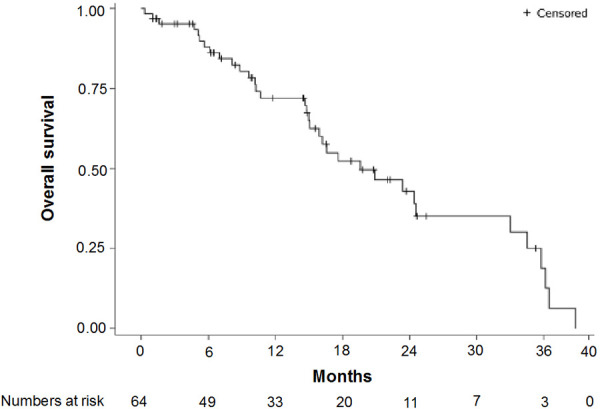
Overall survival, Kaplan-Meier.
Table 2.
Overall objective response rate based on RECIST
| Response | n (%) |
|---|---|
| Total | 59 (100) |
| CR | 5 (8.5) |
| PR | 35 (59.3) |
| SD | 11 (18.6) |
| PD | 8 (13.6) |
| ORR | 40 (67.8) |
Abbreviations: CR, complete response; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria In Solid Tumors; SD, stable disease.
Figure 3.
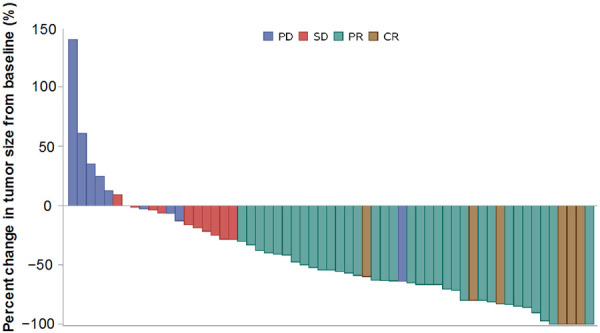
Waterfall plot of percent change in tumor size from baseline. Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Safety
Adverse events (AEs) are listed in Table 3. The median number of trastuzumab treatment cycles was 8.0 (1.0-51.0). Adverse events were observed in 63 of the 67 patients who received at least one dose of the study treatment. The most common Grade ≥3 AEs were neutropenia (22.4%), followed by leukopenia (19.4%), hand-foot syndrome (9.0%), febrile neutropenia (4.5%) and anemia (3.0%) (Table 3). We observed acceptable cardiac safety in this study. An LVEF decrease by ≥15% was reported for one patient. One AE (febrile neutropenia) leading to death was reported in this study.
Table 3.
Adverse events (SAF)
| SAF (N=67), n % | ||
|
| ||
| Any AEs | 63 (94.0) | |
| Any SAEs | 16 (23.9) | |
| Treatment-related SAEs | 14 (20.9) | |
| AEs leading to treatment withdrawal | 7 (10.5) | |
| AEs leading to death | 1 (1.5) | |
|
| ||
| All cause AEs (≥10% incidence rate) | All grade, n (%) | Grade ≥3, n (%) |
|
| ||
| Metabolic and nutritional diseases | ||
| Hypoalbuminemia | 11 (16.4) | 0 |
| Hypocalcemia | 7 (10.45) | 0 |
| Laboratory test | ||
| ALT increase | 13 (19.4) | 1 (1.5) |
| AST increase | 17 (25.4) | 1 (1.5) |
| TBIL increase | 11 (16.4) | 1 (1.5) |
| Blood pressure increase | 8 (11.9) | 0 |
| Neutropenia | 29 (43.3) | 15 (22.4) |
| Platelet count decrease | 13 (19.4) | 0 |
| Leukopenia | 37 (55.2) | 13 (19.4) |
| Skin and subcutaneous tissue diseases | ||
| Hand-foot syndrome | 17 (25.4) | 6 (9.0) |
| Gastrointestinal diseases | ||
| Diarrhea | 8 (11.9) | 0 |
| Nausea | 7 (10.45) | 0 |
| Vomiting | 7 (10.45) | 1 (1.49) |
| Blood and lymphatic diseases | ||
| Anemia | 26 (38.8) | 2 (3.0) |
Abbreviations: AE, adverse event; ALT, alanine transaminase; AST, aspartate aminotransferase; SAE, serious adverse event; SAF, safety analysis set; TBIL, total bilirubin.
Genomic analysis
To explore gene candidates suitable for the prediction of patients with GC most likely to respond to trastuzumab/DX treatment, we applied a batch of tests for each gene mutation status and the study treatment response in seventeen patients’ tumor samples. We found seven genes that were statistically significantly related to treatment response (Figure S1). These seven genes were CTAGE15, RIMBP3, BRSK2, AP3B1, IQCE, MGA and NPBWR2, in which 76%, 76%, 71%, 53%, 53%, 24% and 6% of patients had mutations, respectively. Among the seven mutated genes, two were previously reported to be related to GC: BRSK2 and AP3B1. The response rates for mutant and wild-type AP3B1 in this study were 33.3% and 87.5%, respectively (P=0.0498); response rates for mutant and wild-type BRSK2 were 41.7% and 100%, respectively (P=0.0441). Patients with wild-type AP3B1 or BRSK2 genes had a better response to the study treatment.
Discussion
This phase II, multi-center, open-label, single arm study examined trastuzumab with DX as a treatment regimen for first-line therapy in patients with HER2-positive advanced gastric or GEJ cancer. The results of this study showed a favorable ORR of 67.8% (95% CI: 0.54-0.79), median PFS of 8.1 months (95% CI: 5.6-12.8) and median OS of 20.9 months (95% CI: 15.1-33.0). Treatment outcomes were comparable to previously investigated treatment regimens for this patient population, including the ToGA trial, which reported an ORR of 47%, median PFS of 6.7 months (95% CI: 6-8) and median OS of 13.8 months (95% CI: 12-16) for trastuzumab plus cisplatin and capecitabine/5-FU treated patients [13]. This treatment regimen demonstrated an acceptable safety profile with only 23.9% of patients experiencing an SAE. This drug combination appears to be an effective and safe alternative to the standard cisplatin and 5-FU treatment regimens currently recommended for AGC; however, further clinical studies using trastuzumab plus DX in AGC patients are warranted.
Our results are in line with previous reports from phase II studies evaluating similar regimens of DX in patients with AGC or AEC [22,23]. These studies reported an ORR of 39-46%, median PFS of 4.2-6.1 months and median OS of 9.4-15.8 months [22,23]. Our study used this chemotherapy regimen with the addition of trastuzumab and demonstrated for the first time the clinical efficacy of trastuzumab in combination with DX in HER2-positive AGC. While there are promising options available for a first-line treatment of metastatic AGC that are reported to have good response rates and survival benefits, there is still a lack of effective treatment options for patients who have relapsed after treatment with a cisplatin-containing regimen especially those who recur in a short term. HER2-positive AGC patients have a higher rate of disease recurrence after resection. There is currently no standard second-line therapy for patients with recurrent HER2-positive AGC [9,31] and while several clinical studies have investigated potential second-line treatments in these patients, none have been successful to date [32]. The combination of DX might be an option for these patients if they have maintained a suitable ECOG PS. Patients unable to tolerate platinum-based therapies may also be candidates for this treatment regimen. Thirty-three (51.6%) patients received second line chemotherapy after disease progression. Two patients received surgery after enrolling into the trial and two patients received radiotherapy.
The overall incidence of Grade ≥3 AEs in our study was 49.3% (95% CI: 37.3-61.2). The relative absence of Grade ≥3 AEs other than neutropenia (n=15, 22.4%), leukopenia (n=13, 19.4%) and hand-foot syndrome (n=6, 9.0%) observed in this study was remarkable and is lower than that reported in the two phase II studies evaluating the DX treatment regimen in patients with AGC [22,23]. The ToGA study reported an overall incidence rate of Grade ≥3 AEs of 68%, which is higher than that reported in the present study; incidence rates of 30% and 2% were reported for neutropenia and hand-foot syndrome Grade ≥3, respectively. The incidences of all grades of diarrhea, nausea and vomiting were lower in the present study than that reported for the ToGA trial; the incidence of all grades anemia was higher in this trial (38.8%) than in the ToGA trial (28%) but the occurrence of Grade ≥3 anemia was lower (3.0% and 12%, respectively) [13]. One treatment-related death was reported in this study. This rate is similar to that observed in the ToGA trial (3%) [13] and two phase II trials evaluating DX treatment, which reported 1 (2.2%) and 0 deaths [22,23].
In addition, we found that a lack of response to this treatment regimen was significantly related to missense mutations for two genes, BRSK2 and AP3B1. BRSK2 encodes a serine/threonine-protein kinase of the AMPK family that acts as a checkpoint kinase [33] and was previously found to be amplified in cases of GC [34]. The clinical implications of BRSK2 expression in pancreatic ductal adenocarcinoma have been published; BRSK2 was found to be induced by nutrient deprivation in pancreatic ductal adenocarcinoma cells and to suppress mTORC1 activity [35]. The AP3B1 gene encodes the large B1 subunit of the adaptor-related protein complex-3 and is involved in protein trafficking to lysosomes or specialized endosomal-lysosomal organelles [36]. A previously published expression profile analysis of premalignant lesions of GC revealed that the AP3B1 gene was upregulated [37]. Though some reports demonstrate an association between BRSK2 and AP3B1, the potential roles of these two genes in GC have not been well studied. Additional studies are needed to determine the underlying functions of these two genes in AGC and to confirm the clinical implications of the present findings.
This study was limited in that it was a relatively small, single-arm study so it could not be directly compared to other treatment regimens, and tumor samples were not available from all patients for testing correlations between gene mutations and treatment response.
Conclusions
The combination of trastuzumab plus DX was an effective and safe first-line treatment regimen for AGC. Further studies with larger numbers of patients are warranted to directly compare trastuzumab in combination with DX versus in combination with other platinum-based agents plus fluorouridine regimens and to confirm the results of the present study.
Acknowledgements
This study was funded by Shanghai Roche Pharmaceuticals Ltd. and was supported, in part, by grants from the National Key Research and Development Program of China (2017YFC1308900), the National Natural Science Foundation of China (81872011), the Natural Science Foundation of Guangdong Province (2014A030312015), Science and Technology Program of Guangdong (2015B020232008), and the Science and Technology Program of Guangzhou (15570006). Feng Wang is a recipient of the Young Physician Scientist Program of Sun Yat-sen University Cancer Center (16zxqk03). We thank Sarah Bubeck, PhD of Edanz Medical Writing for providing medical writing support, which was funded by Shanghai Roche Pharmaceuticals Ltd.
All patients provided written informed consent before enrollment and were capable of complying with the protocol.
Disclosure of conflict of interest
None.
Abbreviations
- 5-FU
5-fluorouracil
- AE
adverse event
- AEC
advanced esophageal cancer
- AGC
advanced gastric cancer
- BP
blood pressure
- CI
confidence interval
- CR
complete response
- CT
computerized tomography
- DX
docetaxel plus capecitabine
- ECG
electrocardiogram
- ECOG
Eastern Cooperative Oncology Group
- FAS
full analysis set
- FFPE
formalin-fixed paraffin-embedded
- FISH
fluorescence in situ hybridization
- GC
gastric cancer
- GEJ
gastroesophageal junction
- HER2
human epidermal growth factor receptor 2
- IHC
immunohistochemistry
- IV
intravenous
- LVEF
left ventricular ejection fraction
- mAb:
monoclonal antibody
- mGC
metastatic gastric cancer
- MRI
magnetic resonance imaging
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- PS
performance status
- RECIST
Response Evaluation Criteria in Solid Tumors
- SNV
single nucleotide variant
Supporting Information
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621–8. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388:2606. doi: 10.1016/S0140-6736(16)32226-7. [DOI] [PubMed] [Google Scholar]
- 4.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (London, England) 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T, Chen L. [Efficacy and safety of SOX regimen as neoadjuvant chemotherapy for advanced gastric cancer] . Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:104–106. [PubMed] [Google Scholar]
- 6.Li ZY, Koh CE, Bu ZD, Wu AW, Zhang LH, Wu XJ, Wu Q, Zong XL, Ren H, Tang L, Zhang XP, Li JY, Hu Y, Shen L, Ji JF. Neoadjuvant chemotherapy with FOLFOX: improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol. 2012;105:793–799. doi: 10.1002/jso.23009. [DOI] [PubMed] [Google Scholar]
- 7.Kim YW, Kim MJ, Ryu KW, Lim HS, Lee JH, Kong SY, Lee JS, Choi IJ, Kim CG, Lee JY, Cho SJ, Kook MC, Park YI, Kim SK, Park SR. A phase II study of perioperative S-1 combined with weekly docetaxel in patients with locally advanced gastric carcinoma: clinical outcomes and clinicopathological and pharmacogenetic predictors for survival. Gastric Cancer. 2016;19:586–596. doi: 10.1007/s10120-015-0490-3. [DOI] [PubMed] [Google Scholar]
- 8.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 9.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. doi: 10.1186/s40880-019-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu MZ, Li Q, Wang ZQ, Liu TS, Liu Q, Wei XL, Jin Y, Wang DS, Ren C, Bai L, Zhang DS, Wang FH, Li YH, Xu RH. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer. 2014;134:2468–2477. doi: 10.1002/ijc.28559. [DOI] [PubMed] [Google Scholar]
- 11.Zhou F, Li N, Jiang W, Hua Z, Xia L, Wei Q, Wang L. Prognosis significance of HER-2/neu overexpression/amplification in Chinese patients with curatively resected gastric cancer after the ToGA clinical trial. World J Surg Oncol. 2012;10:274. doi: 10.1186/1477-7819-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 13.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 14.Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, Narita Y, Satake H, Matsuda Y, Sonoda H, Shimokawa M, Maehara Y Kyushu Study Group of Clinical Cancer (KSCC) Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604) Eur J Cancer. 2018;105:41–49. doi: 10.1016/j.ejca.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi57–63. doi: 10.1093/annonc/mdt344. [DOI] [PubMed] [Google Scholar]
- 16.Soularue É, Cohen R, Tournigand C, Zaanan A, Louvet C, Bachet JB, Hentic O, Samalin E, Chibaudel B, de Gramont A, André T for GERCOR. Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: a retrospective study. Bull Cancer. 2015;102:324–331. doi: 10.1016/j.bulcan.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng ZL, Liu Z, Zhao Q, He CY, Lu JH, Wang ZQ, Qiu MZ, Wang F, Wang FH, Li YH, Wang XN, Xie D, Jia WH, Shao YW, Xu RH. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68:1152–1161. doi: 10.1136/gutjnl-2018-316522. [DOI] [PubMed] [Google Scholar]
- 18.Jin MH, Nam AR, Park JE, Bang JH, Bang YJ, Oh DY. Resistance mechanism against trastuzumab in HER2-positive cancer cells and its negation by Src inhibition. Mol Cancer Ther. 2017;16:1145–1154. doi: 10.1158/1535-7163.MCT-16-0669. [DOI] [PubMed] [Google Scholar]
- 19.Pietrantonio F, Fucà G, Morano F, Gloghini A, Corso S, Aprile G, Perrone F, De Vita F, Tamborini E, Tomasello G, Gualeni AV, Ongaro E, Busico A, Giommoni E, Volpi CC, Laterza MM, Corallo S, Prisciandaro M, Antista M, Pellegrinelli A, Castagnoli L, Pupa SM, Pruneri G, de Braud F, Giordano S, Cremolini C, Di Bartolomeo M. Biomarkers of primary resistance to trastuzumab in HER2-positive metastatic gastric cancer patients: the AMNESIA case-control study. Clin Cancer Res. 2018;24:1082–1089. doi: 10.1158/1078-0432.CCR-17-2781. [DOI] [PubMed] [Google Scholar]
- 20.Gerson JN, Skariah S, Denlinger CS, Astsaturov I. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin Investig Drugs. 2017;26:531–540. doi: 10.1080/13543784.2017.1315406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodera Y, Fujiwara M, Yokoyama H, Ohashi N, Miura S, Ito Y, Koike M, Ito K, Nakao A. Combination of oral fluoropyrimidine and docetaxel: reappraisal of synergistic effect against gastric carcinoma xenografts. In Vivo. 2005;19:861–866. [PubMed] [Google Scholar]
- 22.Giordano KF, Jatoi A, Stella PJ, Foster N, Tschetter LK, Alberts SR, Dakhil SR, Mailliard JA, Flynn PJ, Nikcevich DA North Central Cancer Treatment Group. Docetaxel and capecitabine in patients with metastatic adenocarcinoma of the stomach and gastroesophageal junction: a phase II study from the North Central Cancer Treatment Group. Ann Oncol. 2006;17:652–656. doi: 10.1093/annonc/mdl005. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzen S, Duyster J, Lersch C, von Delius S, Hennig M, Bredenkamp R, Peschel C, Lordick F. Capecitabine plus docetaxel every 3 weeks in first- and second-line metastatic oesophageal cancer: final results of a phase II trial. Br J Cancer. 2005;92:2129–2133. doi: 10.1038/sj.bjc.6602645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22:4619–4625. doi: 10.3748/wjg.v22.i19.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JR, Bernstein L, Pike MC. Approximate confidence intervals for probabilities of survival and quantiles in life-table analysis. Biometrics. 1982;38:407–416. [PubMed] [Google Scholar]
- 27.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics (Oxford, England) 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics (Oxford, England) 2015;31:2032–2034. doi: 10.1093/bioinformatics/btv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang C, Layer RM, Faust GG, Lindberg MR, Rose DB, Garrison EP, Marth GT, Quinlan AR, Hall IM. SpeedSeq: ultra-fast personal genome analysis and interpretation. Nat Methods. 2015;12:966–968. doi: 10.1038/nmeth.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network (NCCN) NCCN Guidelines Version 2.2019 Gastric Cancer. [https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf]
- 32.Zhao D, Klempner SJ, Chao J. Progress and challenges in HER2-positive gastroesophageal adenocarcinoma. J Hematol Oncol. 2019;12:50. doi: 10.1186/s13045-019-0737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bright NJ, Thornton C, Carling D. The regulation and function of mammalian AMPK-related kinases. Acta physiologica (Oxford, England) 2009;196:15–26. doi: 10.1111/j.1748-1716.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu YY, Chen HY, Zhang ML, Tian D, Li S, Lee JY. Loss of fragile histidine triad and amplification of 1p36. 22 and 11p15. 5 in primary gastric adenocarcinomas. World J Gastroenterol. 2012;18:4522. doi: 10.3748/wjg.v18.i33.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saiyin H, Na N, Han X, Fang Y, Wu Y, Lou W, Yang X. BRSK2 induced by nutrient deprivation promotes Akt activity in pancreatic cancer via downregulation of mTOR activity. Oncotarget. 2017;8:44669–44681. doi: 10.18632/oncotarget.17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapuy B, Tikkanen R, Muhlhausen C, Wenzel D, von Figura K, Honing S. AP-1 and AP-3 mediate sorting of melanosomal and lysosomal membrane proteins into distinct post-Golgi trafficking pathways. Traffic (Copenhagen, Denmark) 2008;9:1157–1172. doi: 10.1111/j.1600-0854.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee CH, Bang SH, Lee SK, Song KY, Lee IC. Gene expression profiling reveals sequential changes in gastric tubular adenoma and carcinoma in situ. World J Gastroenterol. 2005;11:1937–1945. doi: 10.3748/wjg.v11.i13.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


