Abstract
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide. In the past decade, there have been improvements in non-drug therapies and drug therapies for HCC treatment. Non-drug therapies include hepatic resection, liver transplantation, transarterial chemoembolization (TACE) and ablation. The former two surgical treatments are beneficial for patients with early and mid-stage HCC. As the first choice for non-surgical treatment, different TACE methods has been developed and widely used in combination therapy. Ablation has become an important alternative therapy for the treatment of small HCC or cases of unresectable surgery. Meanwhile, the drugs including small molecule targeted drugs like sorafenib and lenvatinib, monoclonal antibodies such as nivolumab are mainly used for the systematic treatment of advanced HCC. Besides strategies described above are recommended as first-line therapies due to their significant increase in mean overall survival, there are also potential drugs in clinical trials or under preclinical development. In addition, a number of potential preclinical surgical or adjuvant therapies are being studied, such as oncolytic virus, mesenchymal stem cells, biological clock, gut microbiome composition and peptide vaccine, all of which have shown different degrees of inhibition on HCC. With some potential anti-HCC drugs being reported, many promising therapeutic targets in related taxonomic signaling pathways including cell cycle, epigenetics, tyrosine kinase and so on that affect the progression of HCC have also been found. Together, the rational application of existing therapies and drugs as well as the new strategies will bring a bright future for the global cure of HCC in the coming decades.
Keywords: HCC, clinical, preclinical, non-drug therapies, drug therapies
Introduction
Hepatocellular carcinoma (HCC), one of the most common solid malignancies, is a leading cause of cancer-related death worldwide. According to the World Health Organization’s statistics in 2018, HCC was ranked sixth in incidence and fourth in mortality, causing approximately 840 000 new cases and over 780 000 deaths per year. The incidence of HCC varies geographically, and most HCC cases occur in less developed regions, such as Eastern Asia (comprising 54.8% of cases) and South-Eastern Asia (comprising 10.8% of cases) [1-3]. The tumorigenesis of HCC is a complex process involving multiple risk factors. The prevalence of risk factors varies with the distribution of HCC worldwide. Chronic Hepatitis B virus (HBV) infection and Aflatoxin B1 (AFB1) are the major risk factors in developing regions like China and India [4,5]. In the developed areas, HCC primarily develops from cirrhosis caused by Hepatitis C virus (HCV) [6] and Nonalcoholic fatty liver disease (NAFLD) [7].
Together with the recognition of its severity and harmfulness, great progress has been made in HCC treatment. Treatment options largely depend on the tumor stage of HCC. Up to now, researchers have proposed more than 10 cancer staging systems to predict the prognosis of HCC or to select the optimal treatment regimen, such as Barcelona Clinic Liver Cancer (BCLC) staging system and Hong Kong Liver Cancer (HKLC) staging system [8]. Of these staging systems, the most widely recognized and clinically used is BCLC, which classified HCC as stage 0, A, B, C and D. Based on these five categories, HCC stage can be further subdivided, such as stage A1, A2, A3, and A4. Since the assessment and classification of BCLC are based on tumor burden, patient performance status (PS) and liver dysfunction, it has a strong ability to classify and predict prognosis [9]. Through the monitoring of high-risk groups, early stage HCC patients can be identified for diagnosis and treatment, and finally, different treatment methods can be proposed for different patients, which is incomparable to other staging systems (Figure 1). Under the guidance of BCLC, the early stage (BCLC 0/A) HCC patients are mostly treated with Liver transplantation (LT) and other curative therapies. For the intermediate HCC (BCLC B), locoregional treatments like transarterial chemoembolization (TACE) are mainly. For patients with advanced HCC (aHCC), systemic pharmacological treatment is currently the most effective. Although various therapies are available for HCC, the overall survival of patients is still far from satisfactory and there is a great unmet need for more efficient therapies.
Figure 1.
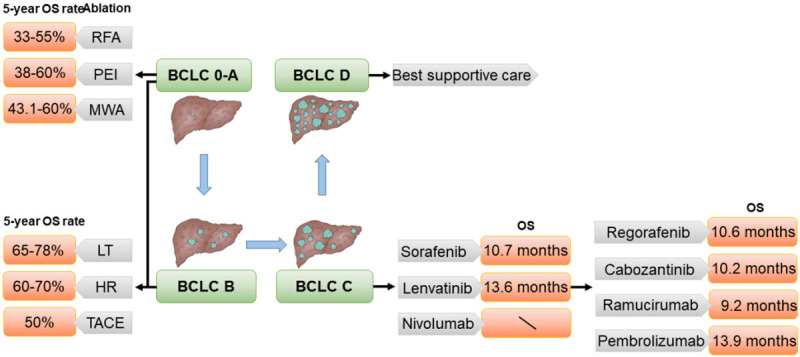
Treatment and prognosis of hepatocellular carcinoma in different stages. BCLC (Barcelona Clinic Liver Cancer), RFA (radiofrequency ablation), PEI (percutaneous ethanol injection), MWA (microwave ablation), LT (liver transplantation), HR (hepatic resection), TACE (transarterial chemoembolization), OS (overall survival).
Herein, we review various clinical therapies for patients with HCC. Meanwhile, we discuss multiple recent preclinical drugs and targets that promise to provide more efficient therapies.
Clinical non-drug therapies
Hepatic resection
Hepatic resection (HR) is a recommended treatment option in patients with good liver function and HCC satisfying the Milan criteria, which involves up to 3 lesions < 3 cm or a single lesion < 5 cm and no extra hepatic manifestations or vascular invasion [10]. It had a 5-year overall survival (OS) rate of 60-70% [11]. Recent reports have shown that HR is an appropriate treatment for many patients with advanced disease or portal hypertension, as long as normal liver function is maintained. Data from 3286 HCC patients treated with LT (n = 1218) or HR (n = 2068) showed considering disease-free survival (DFS), the cure fraction was 74.1% after LT and was 24.1% after HR (effect size > 0.8) [12]. Unfortunately, more than half of patients with primary HCC are diagnosed when their disease has reached the intermediate or advanced stages. Most of these patients developed multinodular tumors or macrovascular invasion. Besides, because HCC often occurs at the same time as liver cirrhosis, portal hypertension occurs in a significant proportion of patients. None of these patients should receive liver resection as a first-line treatment according to official western guidelines [13,14]. However, recent studies [15-18] have demonstrated that HR is still safe and effective for HCC patients with large polytuberous HCC or HCC patients involving macrovascular invasion or portal hypertension, and the standard for the use of hepatectomy should be amplified. At present, laparoscopic, hand-assisted laparoscopic, hybrid approach with laparoscopic mobilization and open parenchymal transection, and robotic approaches have been applied in HR [19]. The safety and effectiveness of robotic approaches for minimally invasive surgery in HR have been demonstrated [20].
Liver transplantation
Liver Transplantation is an ideal treatment for selected patients with HCC, as it removes both the tumor and potential cirrhosis, with 5-year survival rate exceeding 70% [21]. LT is suitable for patients with BCLC stage A cancer meeting the Milan criterion (single HCC nodules < 5 cm or less than 3 nodules, all with a diameter of less than 3 cm). Patients with HCC meeting the Milan criterion had a 5-year OS rate of 65-78% after LT [22]. LT is the only potentially curative treatment for selected patients with cirrhosis and HCC who are not candidates for resection. The Milan criteria do not include objective measures of tumor biology. Thus, they neither correlate well with a post transplant histological study of the liver explant nor accurately predict HCC recurrence after LT [23]. Different studies [24-27] have shown that combination of one or several biomarkers integrated into prognostic models predict the risk of HCC recurrence after LT more accurately than Milan criteria alone. Therefore, many expanded criteria are now incorporating different biologic markers, such as alpha-fetoprotein. Among the various therapeutic options, surgery remains the preferable option. However, the scarcity of donors for LT has causes surgeons to adopt alternative therapy that may bridge patients until an eventual transplant becomes available. LT removes both tumors and cirrhosis, though transplanted patients frequently develop into metabolic, cardiovascular, renal, and even other oncological diseases [12]. Because the lack of donors has limited the use of LT, many studies have been conducted to replace donor organs, including the liver support system. The liver support system is mainly divided into artificial liver support system and biological artificial liver support system [28]. Recently, with experimental work and early clinical applications reported, the ability of the artificial liver support system to partially replace the detoxification function of the liver and to correct various biochemical parameters has been demonstrated. However, the biological artificial system still faces high production cost and various development problems. In addition, the survival advantage of artificial liver still needs further clinical data proof [29].
Transarterial chemoembolization
Transarterial chemoembolization is an effective treatment for HCC patients in intermediate stage [30], which effectively prolongs the survival time of HCC patients and improves the 2-year survival rate. Thus, it has become the first choice of non-surgical treatment for liver cancer [31]. TACE injects cytotoxic chemotherapeutic drugs and embolization particles into the tumor-feeding artery through the arteries, resulting in ischemic necrosis of the tumor [32].
Conventional TACE (cTACE) is to inject the artery of tumor nodule with the emulsion of lipiodol chemotherapy drug, and then use the same vascular embolization to obtain the synergistic effect of cytotoxic activity of drug and ischemia. Decompensated cirrhosis is generally considered an absolute contraindication for TACE [33]. Other absolute contraindications include a high tumor burden, large replacement of the liver lobe, and tumor nodules ≥ 10 cm, bile duct obstruction, and untreated high-risk varicose veins are relative contraindications, not absolute contraindications [34]. Although the effectiveness and superiority of cTACE are fully documented, there are still some unresolved issues. For example, drugs such as doxorubicin may release into the systemic circulation in the interval between injection and embolization and result in systemic toxicity [35,36].
To overcome the drawback of cTACE including systemic toxicity, lack of standardization and unpredictability of outcomes, non-absorbable embolized cell microspheres with cytotoxic drugs (drug-eluting bead transarterial chemoembolization, DEB-TACE) have been developed to avoid systemic toxicity. The risk of systemic drug release is minimal due to both high-affinity carrier activity of DEB-TACE and the absence of time interval between injection and embolization [37]. DEB-TACE, especially the adsorbed DEB known as DEB-DOXTM, has been widely used in the treatment of liver cancer in Europe and the United States [38]. Burrel et al. reported the one-year, three-year and five-year survival rate of DEB-DOXTM is 89.9%, 66.3% and 38.3%, respectively, with the median survival time of 48.6 months [39]. A meta-analysis of seven studies (693 patients) compared DEB-TACE with cTACE. The results indicated no significant difference in the pooled estimates of tumor response between DEB-TACE and cTACE. Therefore, DEB-TACE plays the same role in tumor response as cTACE. Interestingly, Zou et al. concluded that DEB-TACE was superior to cTACE in higher complete response rates and OS rates in HCC patients [40].
Transarterial radioembolization (TARE) is safe and effective in the treatment of unresectable HCC. TARE includes selective intra-arterial administration through the percutaneous pathway of microspheres containing radioactive compounds (such as yttrium-90 or Lipiodol labeled with iodine-131 or rhenium-188) [41]. About 90% of blood supply for HCC comes from hepatic arteries, while 70% of blood supply for normal liver parenchyma comes from the portal venous system. TARE utilizes this concept to provide targeted therapy for HCC with minimal substantial damage. Although TACE is a standard treatment paradigm for patients with BCLC-B HCC, studies have shown an increasing role for TARE. While imaging response rates and median overall survival (mOS) from day of treatment appear comparable between TACE and TARE, most patients treated with TARE have the more advanced disease than those with TACE and the treatment is less selective. Nevertheless, TARE outperformed TACE in time to disease progression (TTP), toxic side effects, and quality of life after treatment. TARE has been shown to be effective in patients with portal vein thrombosis (PVT), a relative to TACE. TARE can also be used as an alternative to ablation and assisted resection of BCLC stage A HCC [42].
A multi-center study showed that TACE combined with sorafenib (TACE-S) prolonged median TTP by nearly two months, however, the difference was not statistically significant [31]. Several mechanisms may underlie the complementary action of TACE and sorafenib. TACE embolization of the hepatic artery reduced the blood supply of HCC and achieved a therapeutic purpose. However, the side effects of TACE include increased expression of vascular endothelial growth factor (VEGF), impaired liver function, and an increased likelihood of liver cancer recurrence. Sorafenib can block the angiogenesis and growth of liver cancer and significantly improve OS and TTP in patients with advanced liver cancer. Therefore, sorafenib can reduce the side effects of TACE and improve the positive effects of TACE [43].
Apatinib, a highly selective vascular endothelial growth factor receptor (VEGFR)-blocking agent, has affinity 10 folds that of Sorafenib [44,45]. For intermediate and advanced HCC, the long-term curative effect of TACE combined with apatinib is better than that of TACE alone. The former can obviously prolong the progression-free survival (PFS) of patients and has confirmed safety [46].
In recent years, TACE followed by radiofrequency ablation (RFA) [47] has been widely used. After TACE, the necrotizing effect of RFA treatment on tumor level is enhanced by reducing hepatic arterial blood flow and reducing blood heat absorption. In addition, during the treatment of RFA, tumor necrosis is expected due to ischemia and inflammation caused by oedematous changes after TACE. Current clinical data reveal that TACE combined with RFA is superior to the single use of RFA or TACE alone in inducing higher complete necrosis and increasing OS rates [48]. A meta-analysis by Ni et al. showed significantly higher OS rate and relapse-free survival rate for RFA and TACE combined than for RFA alone [40]. Although it is thought to be a safe and effective choice for treating patients with the treatment of patients, TACE combined with RFA has no advantage for the small disease less than 3 cm, perhaps because RFA alone can achieve complete necrosis, making TACE a redundant method for RFA [48].
The combination of TACE and microwave ablation (MWA) [47] is another popular choice for interventional therapy and is proven to be effective. Chen et al. [40] analyzed the data from 244 patients with HCC treated by TACE-MWA or TACE alone, and found that the total loss rate of the TACE-MWA group was 92.1%, and the TACE group was only 46.3% (P < 0.001), suggesting that TACE-MWA had a better reaction for HCC patients with tumors ≤ 5 cm compared with TACE.
Ablation
With the development of ultrasound guidance and other imaging technologies, percutaneous ablation has become an important alternative therapy for the treatment of small liver cancer and cases of unresectable surgery [37]. Many different ablation methods have been proposed and accepted which include RFA, MWA, percutaneous ethanol injection (PEI), laser ablation (LSA), cryoablation (CRA), high intensity focused ultrasound (HIFU) and their combinations. Ablation techniques lead to tumor tissue necrosis through various mechanisms, such as thermal coagulation, rapid freezing and chemical cell dehydration, with different post-ablation effects [49-52]. Ablative therapy has the characteristics of good curative effect, minimally invasive and easy to relapse. Ablative therapy is generally applicable to patients with small liver cancer, and for patients with Child-Pugh class A (CP-A) or B (CP-B) liver dysfunction, up to three tumors each 3 cm or smaller in diameter [53,54].
In the treatment of early-stage HCC, image-guided percutaneous ablation is considered the best. PEI was once the standard of ablative therapy as a well-tolerated, low-cost and fairly safe treatment. The five-year survival rate of patients who received ethanol injections was reported to be 38-60% [55-58]. Currently, PEI is the preferred treatment only if biliary reflux, tumor adhesion to the gastrointestinal tract, or other causes prevents RFA from being administered [53].
As the most popular ablation method in recent years, RFA has been applied to patients with poor liver function with a limited tumor size of 5 cm, with similar results in OS and DFS compared with HR [59]. The 5-year OS rate of HCC patients after RFA was 39.9-68.5% [60]. Although treatment modality does not affect DFS in patients with tumors ≤ 2 cm in size, underlying substantial status is more important for long-term survival. As the preferred treatment of HR without the advantage of survival, RFA can provide better post-treatment health related quality of life than HR [61]. Although RFA can be successful as first line treatment with size cut off of 2 cm, 3 cm and 5 cm, the maximum tumor size for which and whether RFA is safe and effective remains highly controversial [62]. American Hepato-Pancreato-Biliary Association and BCLC staging system algorithm recommended a cut-off of 3 cm. The effect of RFA technology decreases when the tumor is larger than 2-3 cm in diameter or when the tumor is located near the main blood vessel (so-called “heat sink effect”) [63-65]. With the increase of the target area, the effect of RFA treatment decreased. In addition, some organizational properties, such as conductivity, thermal conductivity, dielectric constant, heat capacity, and blood perfusion rate, have a large effect on the growth of the ablation zone. As the progress of the ablation, the organization becomes dehydrated and charred, thereby increasing the impedance. Therefore, RFA is limited by increasing in impedance and an excessive local temperature [66-69]. Several technical devices can be used to avoid this effect, such as monitoring temperature or impedance during the process or injecting brine into the tissue around the RF needle [70].
Besides RFA, MWA is also one of the most common methods of percutaneous local ablation, which causes tumor necrosis by transferring heat directly. The 5-years OS rate was 43.1-60% for patients after MWA [71]. MWA is based on dielectric heating, which has a higher thermal efficiency than RFA [72]. For MWA, less time is required for ablation than for RFA, providing higher temperatures in the ablative zone. The shorter time and higher thermal efficiency of MWA lead to more predictable ablation zones [73]. Several studies have found that MWA produces significantly larger regions than RFA [74-76]. Thus, the apparent superiority of MWA over RFA has made it an alternative method to RFA. However, recent meta-analysis found that the main complication rates of MWA were higher than RFA although the results did not reach statistical significance [72]. Due to insufficient clinical data, it is difficult to compare the incidence of blood vessels and biliary complications between MWA and RFA, and high-quality evidence is needed. In addition, the current meta-analysis shows that percutaneous MWA provides competitive if not superior results with respect to RFA in terms of CR and recurrence rate, particularly in larger tumours [77]. Compared with RFA, MWA is more invasive and has advantages in large tumors, in locations around large vessels and in highly perfused areas, where RF energy is limited. Therefore, MWA is an alternative to RFA, but more clinical data is needed to prove its superiority.
Irreversible electroporation (IRE) is a non-thermal ablation technique which can induce the formation irreversible nanopores in the cell membrane by placing electrodes in/around the target tumor and applying high-voltage current, thereby altering cell permeability and ultimately leading to apoptosis [78,79]. Different from heat-based ablative technologies like RFA and MWA, IRE is not effective for tissues that lack normal cell membranes [80]. With this method, the skeleton of connective tissue, vessels, and bile ducts are largely preserved [81]. For patients who cannot undergo surgery, thermal ablation, or have tumors adjacent to important structures, IRE is a good alternative for treating HCC [82]. The study by Sebastian Mafeld et al. represented patient survival at 36 months was 52% (95% credible interval [CI], 22-75%) [80]. However, IRE is more invasive and troublesome because general anesthesia requires muscle blockade. In addition, IRE also produces a certain degree of thermal effect, which can damage the bile duct and other structures [60]. Further research is necessary, especially in terms of long-term outcomes.
HIFU is a non-invasive method for treating localized tumors. In vitro, the beam of ultrasonic beam can focus on the tumor target by focusing and directivity. Through thermal effect, caving effect and mechanical effect, HIFU can result in high temperature (65-100°C) in the focal area in seconds, and cause tumor necrosis in the absence of injury intervention tissue [83]. HIFU has been widely used in the treatment of multiple primary solid tumors and even metastatic tumors. It is safe and effective for HCC patients with severe cirrhosis who are not suitable for surgical treatment. The clinic trial by Tan To Cheung et al. shown 3-year OS rate of patients after HIFU was 81.2% [84]. Compared with the typical tumor treatment scheme, such as TACE, HIFU features distinctive advantages including non-invasive operation, small focus, non-radioactive and no damage to normal tissues in the acoustic-propagating path [85]. However, the energy dispersion increases with the increase of the distance the ultrasound travel through the tissue and the blood flow in the lesion [86]. Therefore, in the treatment of deep lesions or high vascular supply lesions, there should be more HIFU energy deposition in the target area. Changing the acoustic environment of tissues is a feasible and important strategy to improve the efficacy of HIFU [87]. Several studies have focused on changing the acoustic environment and changing the acoustic properties of the target area (ethanol injection) by introducing high-volume impedance material (iodized oil), changing the blood supply in the target area, and introducing cavitation nuclei to enhance HIFU cavitation (microbubble contrast) [88,89]. Previous studies have demonstrated that TACE + HIFU combination therapy is more effective than HIFU or TACE alone for treating HCC, and has a better OS rate [90,91].
In contrast to RFA and MWA, CRA uses extremely low temperatures to induce cell dehydration and rupture and damages blood vessels leading to ischemic hypoxia to kill tumors [60]. CRA can control the treatment effect during the procedure by various imaging modalities, such as CT, MRI, or ultrasound [92,93]. Data from a clinical trial involving 1163 patients shown the 5-years OS rate was 59.5% [94]. A meta-analysis concluded that radiofrequency ablation was superior to cryoablation in terms of complications, patient local recurrence, and tumor local recurrence, although there was no significant difference in mortality [95].
Chimeric antigen receptor-engineered T cell (CAR-T) immunotherapy
Adoptive T-cell transfer has been successfully used in the treatment of cancer, such as melanoma and hematologic malignancies [96]. CAR-T cells recognize tumor cell surface antigens by using single-chain variable regions (scFv) composed of variable heavy and light chains of a tumor associated antigen (TAA)-specific monoclonal antibody as the extracellular antigen recognition domain, allowing them to target specific tumor cells. This is connected by the spacer to the transmembrane and intracellular signaling domains for signal transduction and T cell activation [97,98]. At least 10 phase I/II clinical trials in China are currently investigating the use of CAR-T cells in advanced liver cancer [99]. Compared with traditional adoptive T cell transfer therapy, CAR-T has some advantages, such as higher specificity to antigens and higher major histocompatibility complex (MHC) dependence on tumor cells. CAR-T therapy is considered as a potential therapeutic agent for malignant tumors [100,101]. Above clinical non-drug treatments for HCC are summarised in Table 1.
Table 1.
Clinical non-drug treatment for HCC
| Treatment | Application scope | First author/s | Year | Ref. |
|---|---|---|---|---|
| HR | HCC patients with good liver function and HCC satisfying the Milan criteria | Zhong JH | 2016 | [10] |
| LT | HCC patients with BCLC stage A cancer meeting the Milan criterion | Song P | 2017 | [22] |
| TACE | ||||
| cTACE | intermediate stage HCC without portal vein invasion | Raoul JL | 2011 | [34] |
| DEB-TACE | HCC patients with BCLC stage B | Tsurusaki M | 2015 | [38] |
| TARE | HCC patients with unresectable, intermediate stage HCC | Kallini JR | 2016 | [41] |
| TACE-A | HCC patients with BCLC stage C | Verslype C | 2012 | [45] |
| TACE-S | HCC patients with BCLC stage C | Cabibbo G | 2014 | [42] |
| TACE-RFA | HCC patients with BCLC stage A | Han K | 2015 | [46] |
| TACE-MWA | HCC patients with BCLC stage A | Han K | 2015 | [46] |
| Ablation | ||||
| PEI | HCC patients unable to apply RFA | Omata M | 2017 | [54] |
| RFA | HCC patients with poor liver function with limited tumor size of 5 cm | Ahn KS | 2019 | [62] |
| MWA | HCC patients with poor liver function with limited tumor size of 5 cm | Tan W | 2019 | [73] |
| IRE | HCC patients who cannot undergo surgery, thermal ablative procedures or tumor lying close to vital structures | Zimmerman A | 2017 | [82] |
| HIFU | HCC patients with multiple primary solid tumors and even metastatic tumors | You Y | 2016 | [85] |
| CRA | primary HCCs (> 2 cm) | Hu J | 2019 | [93] |
| CAR-T | Patients with advanced liver cancer | Chen Y | 2018 | [99] |
HCC (hepatocellular carcinoma), HR (hepatic resection), LT (liver transplantation), TACE (transarterial chemoembolization), cTACE (conventional TACE), DEB-TACE (drug-eluting bead TACE), TARE (transarterial radioembolization), TACE-A (TACE plus lapatinib), TACE-S (TACE plus sorafenib), PEI (percutaneous ethanol injection), RFA (radiofrequency ablation), MWA (microwave ablation), IRE (irreversible electroporation), HIFU (high intensity focused ultrasound), LITT (laser induced hyperthermia), CRA (cryoablation), CAR-T (chimeric antigen receptor-engineered T cell immunotherapy).
Clinical systemic pharmacological treatments
First-line systemic therapy
Sorafenib
Sorafenib (BAY-43-9006), marketed by Bayer as Nexavar® (USA), is an oral multikinase inhibitor approved by food and drug administration (FDA) for the treatment of unresectable HCC since 2007, which was based on the results from the SHARP and ORIENTAL trials and advanced renal cell carcinoma since 2006 [102,103]. Sorafenib at dose of 800 mg/day is proven to provide consistent survival benefit and is the first-line systemic therapy for patients with progressive or aHCC [102,104].
It inhibits the activity of several tyrosine kinases, which is involved in tumor angiogenesis and progression, including VEGFR-2/3, platelet-derived growth factor receptor (PDGFR), Flt3 and c-Kit, and also targets Raf kinases involved in the MAPK/ERK pathway [105]. Recently, shugoshin-like 1 (SGOL1) is found as an indicator of prognosis and a druggable target for HCC treated with sorafenib by CRISPR screen [106].
The result that sorafenib significantly increased survival of aHCC patients with different territories was based on two phase III randomized, multicenter, double-blind, placebo-controlled trials, the SHARP trial (in Europe and the USA) [102] and ORIENTAL trial (in Asia-Pacific regions) [104] in 2007 (a dose of 400 mg twice daily). In the SHARP trial, 602 aHCC patients in northern America and western Europe were enrolled, and the results that the mOS was 10.7 months in the sorafenib group and 7.9 months in the placebo group, which demonstrated that the survival benefits from sorafenib were superior to placebo. In the ORIENTAL trial, 271 aHCC patients from the Asia-Pacific region were enrolled and the result that the mOS was 6.5 months in the sorafenib group and 4.2 months in the placebo group, which was similar to that of the SHARP trial. However, the SHARP and ORIENTAL trials both showed that sorafenib only prolongs the OS period by approximately 3 months in patients with aHCC.
It was showed that sorafenib treatment provides a survival benefit in all subgroups of patients with HCC. However, heterogeneity in response to sorafenib is increasingly being recognized after analyzing two phase III studies. The magnitude of benefit is greater in patients with disease confined to the liver (without extrahepatic spread), or in these with HCV, or a lower neutrophil-to-lymphocyte ratio, an indicator of inflammation status. These helps inform the prognosis of patients receiving sorafenib therapy and provide further refinements for the design of trials testing new agents vs. sorafenib [107]. The utility of sorafenib in patients with aHCC and CP-B liver function remains a subject of debate. However, recently it was found that CP-B liver function (versus CP-A) is associated with worse OS by a meta-analysis [108]. Hepatic arterial infusion chemotherapy plus sorafenib in phase II trials has shown favorable tumor control and a manageable safety profile, though in phase III trials did not [109]. However, sorafenib was found to be ineffective as an adjuvant treatment after curative resection or as a concurrent treatment with TACE [110-113]. The addition of hepatic arterial infusion chemotherapy to sorafenib did not significantly improve overall survival in patients with aHCC [109].
During Sorafenib treatment, associated toxicities were observed, including gastrointestinal upset, anorexia, hand-foot skin reactions, and fatigue with an overall 30% occurrence of grade 3-4 severity events requiring permanent discontinuation in approximately 28% of treated patients [114].
Lenvatinib
Lenvatinib, an oral multikinase inhibitor, is a potent inhibitor of VEGFR1-3 and other prooncogenic and prooncogenic receptor tyrosine kinases, including fibroblast growth factor receptors (FGFR1-4), PDGFRα, KIT, and RET, in which blockade of activated FGF signaling pathways and potent anti-angiogenic activity underlie antitumor activities [115,116].
The recent open-label, phase III, multicenter, non-inferiority trial (REFLECT) [117] demonstrated lenvatinib was non-inferior to sorafenib in OS in unresectable aHCC. In the non-inferiority trial, 954 aHCC patients in the Asia-Pacific, European, and North American regions were enrolled, and the results that the mOS was 13.6 months (95% CI, 12.1-14.9) in the lenvatinib group and 12.3 months (10.4-13.9; hazard ratio [HR], 0.92; 95% CI, 0.79-1.06) in the sorafenib group, meeting the criteria for non-inferiority and suggesting that the survival benefits from lenvatinib were non-inferior to sorafenib. A post-hoc exploratory analysis [118] of the REFLECT trial reported longer survival among patients with an objective response, which suggested that objective response may serve as a better surrogate endpoint for prolonged overall survival in patients with unresectable aHCC. A multicenter analysis [119] reported lenvatinib can be used safely and efficaciously regardless of age in patients with HCC. It was reported that there was a clear trend in favor of lenvatinib over sorafenib (HR, 0.82; 95% CI, 0.60-1.15) for HBV-positive patient however no differences for HCV-positive, showing that lenvatinib could be the best drug for HBV-positive patients in 59% of cases compared to only 1% of patients treated with sorafenib [120]. Cost-Utility Analysis showed that lenvatinib offered similar clinical effectiveness at a lower cost than sorafenib, suggesting that lenvatinib would be a cost-saving alternative in treating unresectable HCC [121,122].
In the randomized phase III non-inferiority trial, the overall incidence of adverse events (AEs) was similar between the two treatment groups and any-grade adverse events, hypertension (42%), diarrhea (39%), decreased appetite (34%), and decreased weight (31%) for lenvatinib, and palmar-plantar erythrodysaesthesia (52%), diarrhea (46%), hypertension (30%), and decreased appetite (27%) for sorafenib were the most common [117].
Nivolumab
Nivolumab is a programmed cell death protein-1 (PD-1) immune checkpoint inhibitor. In phase I/II, open-label, non-comparative, dose-escalation and expansion trial (CheckMate 040) [123], the safety and efficacy of nivolumab in patients with aHCC with or without chronic viral hepatitis were demonstrated. A dose-escalation phase was conducted in four countries or territories (USA, Spain, Hong Kong, and Singapore), in which the overall objective response rate (ORR) was 15%, with a disease control rate (DCR) of 58% and an OS of 15 months and a dose-expansion phase was conducted in 11 countries (Canada, UK, Germany, Italy, Japan, South Korea, Taiwan et al.), in which more than 200 patients who were treated with nivolumab had a six-month survival rate of 83% and a nine-month survival rate of 74%.
On June 24, 2019, a randomized, multi-center phase III study [124] of nivolumab vs. sorafenib as first-line treatment in patients with aHCC (CheckMate-459) was published for the efficacy of nivolumab in first-line to show superiority over sorafenib, in which 643 patients were randomly assigned to either treatment with a minimum follow-up of 22.8 months. However, according to the predefined threshold of statistical significance (HR, 0.84; P = 0.0419), statistical significance for its primary endpoint of OS was not achieved, and the specific data have not been published. Regardless, the trial CheckMate-459 revealed a clear trend of improvement mOS for patients treated with nivolumab or sorafenib. However, this trial failed to show a superiority of immunotherapy over sorafenib indicating that either better patient selection or combination therapies are required to significantly improve mOS with immunotherapy in aHCC. The exploration of nivolumab in HCC will continue.
Second-line systemic therapy
Regorafenib
Regorafenib, an oral multi-kinase inhibitor, targets angiogenic (VEGFR1-3, TIE2), stromal (PDGFR-β, FGFR), and oncogenic receptor tyrosine kinases (KIT, RET, and RAF) [125].
In 2013, a multicenter, open-label and phase II clinical trial [126] revealed that regorafenib had acceptable tolerability and evidence of antitumor activity in patients with intermediate or aHCC. In the RESORCE study [127], a randomized, double-blind, parallel-group, phase III trial, 573 aHCC patients who tolerated sorafenib (≥ 400 mg/day for ≥ 20 of last 28 days of treatment) in 21 countries were enrolled, and randomly assigned in a 2:1 ratio to receive regorafenib (oral dose 160 mg daily during weeks 1-3 of each 4-week cycle) or matching placebo (once daily during weeks 1-3 of each 4-week cycle). The results that OS with a hazard ratio of 0.63 (95% CI, 0.50-0.79; one-sided P < 0.0001) was improved and median survival was 10.6 months (95% CI, 9.1-12.1) for regorafenib versus 7.8 months for placebo that the survival benefits from regorafenib were superior to placebo. The most common clinically relevant grade 3 or 4 treatment-emergent events were hypertension (15% vs. 5%), hand-foot skin reaction (3% vs. 1%), fatigue (9% vs. 5%), and diarrhea (3% vs. 0%). The exploratory analyses [128] from the phase III RESORCE trial showed that a clinical benefit was conferred regardless of the last sorafenib dose or tumour progression on prior sorafenib, and rates of adverse events were generally similar regardless of the last sorafenib dose.
Besides, regorafenib as a second-line agent in the treatment of HCC is not cost-effective at commonly accepted willingness to pay thresholds because the modest incremental therapeutic benefit is at a relatively high incremental cost of its treatment [129].
Cabozantinib
Cabozantinib is an oral multiple tyrosine kinase receptor inhibitor with activity against VEGFR1-3, MET, AXL, and inhibition of c-MET and VEGFR decrease resistance of VEGFR inhibitor via c-MET axis [130].
In a randomized, double-blind, phase III trial (CELESTIAL) [131], a total of 707 patients with previously treated aHCC were randomly assigned in a 2:1 ratio to receive cabozantinib (60 mg once daily) or matching placebo, and the results showed the mOS was 10.2 months in the cabozantinib group and 8.0 months in the placebo group (HR for death, 0.76; 95% CI, 0.63 to 0.92; P = 0.005), demonstrating that the survival benefits from cabozantinib were superior to placebo. Median PFS was 5.2 months in cabozantinib group and 1.9 months in placebo (HR for disease progression or death, 0.44; 95% CI, 0.36 to 0.52; P < 0.001), and the ORRs were 4% and less than 1%, respectively (P = 0.009), demonstrating that PFS from cabozantinib were superior to placebo. However, grade 3 or 4 adverse events occurred in 68% in the cabozantinib group, which was approximately twice to placebo group.
Based on the exposure-response (ER) analyses [132] in HCC patients from the CELESTIAL trial, cabozantinib exposure at the approved 60 mg daily dose is predicted to provide longer OS, decreased rate of cancer progression or death, but increase of adverse events compared with 40 mg or 20 mg starting doses. Subsequent dose reduction appeared to decrease these risks of adverse events.
A cost-effectiveness analysis [133] reported that cabozantinib at its current cost would not be cost-effective for patients with sorafenib-resistant HCC from the payer’s perspective in the USA, UK or China.
Ramucirumab
Ramucirumab is a recombinant IgG1 monoclonal antibody and VEGFR-2 antagonist.
In a randomized, placebo-controlled, double-blind, multicenter, phase III trial (REACH) [134], 565 patients were enrolled from 154 centers in 27 countries (283 were assigned to ramucirumab with 8 mg/kg every 2 weeks and 282 were assigned to placebo). The result that mOS was 9.2 months (95% CI, 8.0-10.6) in the ramucirumab group and 7.6 months (HR, 0.87; 95% CI, 0.72-1.05; P = 0.14) in the ramucirumab group and 7.6 months (HR, 0.87; 95% CI, 0.72-1.05; P = 0.14) in the placebo group showed that second-line treatment with ramucirumab did not significantly improve survival over placebo in patients with aHCC. Although the OS between the two groups were not statistically significant, subgroup analysis showed that patients with elevated serum alpha fetoprotein (> 400 ng/mL) achieved a better OS benefit from ramucirumab treatment compared with placebo. The mOS in ramucirumab group was 7.8 months, which was significantly greater than 4.2 months in placebo group.
Accordingly, a randomized, double-blind, placebo-controlled, phase 3 trial (REACH-2) was initiated in these patients with advanced HCC and increased α-fetoprotein concentrations [135]. In REACH-2 study [136], 292 patients were enrolled in 20 countries and randomly assigned (197 to the ramucirumab group and 95 to the placebo group). The result that the mOS was 8.5 months (95% CI, 7.0-10.6) in the ramucirumab group and 7.3 months in the placebo group (HR, 0.710; 95% CI, 0.53-0.95; P = 0.019) and PFS was 2.8 months vs. 1.6 months (0.452; P < 0.0001). This study suggested that second-line treatment with ramucirumab significantly improved overall survival in HCC patients with higher α-fetoprotein level of at least 400 ng/mL. Besides, ramucirumab was well tolerated with a manageable safety profile and a low incidence of adverse events. Moreover, ramucirumab is not a cost-effective treatment from a United States payer perspective [137].
Pembrolizumab
Pembrolizumab, a PD-1 monoclonal antibody is IgG4 antibody specific for the human. Aimed to assess the efficacy and safety of pembrolizumab in patient’s population with aHCC previously treated with sorafenib, a non-randomized, multicenter, open-label, phase II trial (KEYNOTE-224) was led. In KEYNOTE-224 [138], 104 eligible patients in 47 medical centers and hospitals across ten countries were enrolled and treated. The results showed that pembrolizumab was effective and tolerable in patients with HCC who had previously been treated with sorafenib.
Accordingly, further assessment in randomized, double-blind, phase III study in patients with HCC (KEYNOTE-240) was conducted to evaluate the efficacy and safety of pembrolizumab in this population. In KEYNOTE-240 [139], 413 patients at 119 medical centers in 27 countries were enrolled and randomly assigned at a two-to-one ratio to receive pembrolizumab plus best supportive care (BSC) or placebo plus BSC. The mOS was 13.9 months (95% CI, 11.6-16.0) in pembrolizumab group versus 10.6 months (95% CI, 8.3-13.5) in placebo group (HR, 0.781; 95% CI, 0.611-0.998; P = 0.0238), and median follow-up was 13.8 months versus 10.6 months. The median PFS for pembrolizumab was 3.0 months versus 2.8 months for placebo at the first interim analysis (HR, 0.775; 95% CI, 0.609-0.987; P = 0.0186) and 3.0 months versus 2.8 months at final analysis (HR, 0.718; 95% CI, 0.570-0.904; P = 0.0022). It was showed that these differences did not reach statistical significance, which is consistent with those of KEYNOTE-224, supporting a favorable risk-to-benefit ratio for pembrolizumab in this population.
In the phase III KEYNOTE-394 trial in Asia as second-line therapy for patients with aHCC pembrolizumab continues to be assessed (NCT03062358). Above small-molecule inhibitors for first-line and second-line systemic therapy are shown in Figure 2 and summarised in Table 2.
Figure 2.
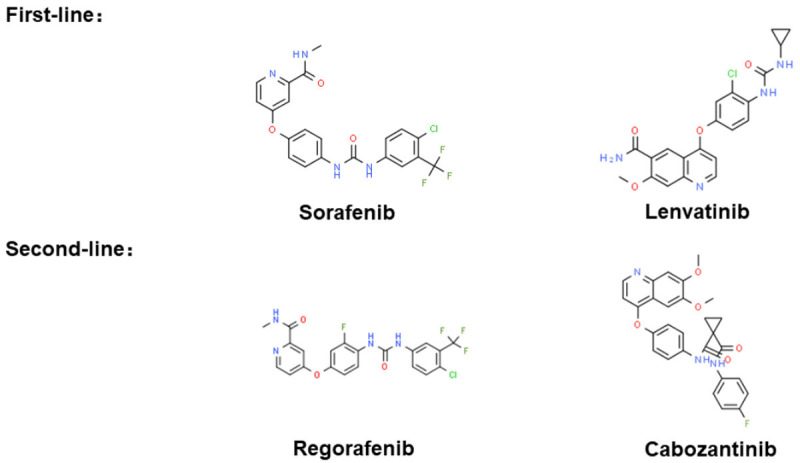
Small-molecule inhibitors for first-line and second-line systemic therapy. The chemical structure is from “chemspider.com”.
Table 2.
Clinical research on molecular targeted drugs for HCC
| Drug | Targets | Phase III study | Versus | Primaryend point | First author/s | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Multikinase inhibitor | |||||||
| Sorafenib | VEGFR2/3, PDGF-R, Flt3, c-Kit, Raf | SHARP (n = 602) | placebo | OS, 10.7 months (7.9) | Llovet JM | 2008 | [102] |
| ORIENTAL (n = 271) | placebo | OS, 6.5 months (4.2) | Cheng AL | 2009 | [104] | ||
| Lenvatinib | VEGFR1-3, FGFR1-4, PDGFRα, KIT, RET | REFLECT (n = 954) | sorafenib | OS, 13.6 months (12.3) | Kudo M | 2018 | [117] |
| Regorafenib | VEGFR1-3, TIE2 PDGFR-β, FGFR, KIT, RET, RAF | RESORCE (n = 573) | placebo | OS, 10.6 months (7.8) | Bruix J | 2017 | [126] |
| Cabozantinib | VEGFR1-3, C-MET, AXL | CELESTIAL (n = 707) | placebo | OS, 10.2 months (8.0) | Abou-Alfa GK | 2018 | [131] |
| Monoclonal antibody | |||||||
| Nivolumab | PD-1 | CheckMate-459 (n = 643) | sorafenib | Not achieved | Yau T | 2019 | [124] |
| Ramucirumab | VEGFR2 | REACH (n = 565) | placebo | OS, 9.2 months (7.6) | Zhu AX | 2015 | [134] |
| REACH-2 (n = 292) | placebo | OS, 8.5 months (7.3) | Zhu AX | 2019 | [136] | ||
| Pembrolizumab | PD-1 | KEYNOTE-240 (n = 413) | placebo | OS, 13.9 months (10.6) | Finn RS | 2019 | [139] |
HCC (hepatocellular carcinoma), OS (overall survival), VEGFR (vascular endothelial growth factor receptor), PDGFR (platelet-derived growth factor receptor), FGFR (fibroblast growth factor receptor), RET (glial cell-derived neurotrophic factor receptor), C-MET (hepatocyte growth factor receptor), KIT (stem cell factor receptor).
Investigational drugs in phase II clinical trials
Capmatinib (INC280)
Capmatinib, a highly potent and selective MET inhibitor in biochemical and cellular assays, causes regression of MET-dependent tumor models in animals at well tolerated doses. It was showed that MET-amplified experimental HCC tumors were highly sensitive to capmatinib. In the phase II clinical trial [140], 38 patients received treatment and it was found that single agent capmatinib at the recommended dose for expansion was tolerable with a manageable safety profile. In subset of patients with MET-dysregulated (MET-high) HCC, antitumor activity existed. The most common causality adverse events were nausea (42%), vomiting (37%), and diarrhea (34%) in the 38 patients.
Nintedanib
Nintedanib, an oral small-molecule, triple angiokinase inhibitor of VEGFR1-3, PDGFRα and β, FGFR1-3, Flt-3, Lck, Lyn and Src, has anti-tumour and anti-angiogenic activity in preclinical models of HCC. In the phase II clinical trial [141], 93 patients were randomized to nintedanib (n = 62) or sorafenib (n = 31). The result that the median TTP was 5.5 vs. 4.6 months (HR, 1.44; 95% CI, 0.81-2.57), the mOS was 11.9 vs. 11.4 months (HR, 0.88; 95% CI, 0.52-1.47), the median PFS was 5.3 vs. 3.9 months (HR, 1.35; 95% CI, 0.78-2.34) showed that nintedanib may have similar efficacy to sorafenib in aHCC.
Axitinib
Axitinib, a selective potent TKI of VEGFR1-3, deserves to be explored in HCC. In a phase II clinical trial [142], axitinib with BSC did not improve OS versus placebo with BSC in the overall population or in stratification subgroups. However, it resulted in significantly longer PFS and TTP and higher clinical benefit rate, with acceptable toxicity in patients with aHCC.
The other phase II clinical trial [143], in which eligible patients were Child-Pugh A/B7, with measurable progressive disease after TKIs/antiangiogenic drugs, found that axitinib showed encouraging tolerable clinical activity in VEGF-pretreated HCC patients. However, further study should be in a selected population incorporating potential biomarkers of response. Besides, the combination of axitinib and TACE was found potentially efficacious for patients with inoperable HCC with a high radiologic response rate [144].
Dovitinib
Dovitinib, a potent inhibitor of FGFRs, VEGFRs, and PDGFRβ, has antitumor activity mediated by antiproliferative and antiangiogenic effects. In phase II clinical trial [145], a total of 165 patients were randomized 1:1 to dovitinib or sorafenib, aim to compare dovitinib versus sorafenib. The mOS (95% CI) was 8.0 months for dovitinib versus 8.4 months for sorafenib, and the median TTP (95% CI) per investigator assessment was 4.1 months versus 4.1 months. The result showed that dovitinib was well tolerated. However, activity was not greater than sorafenib as a frontline systemic therapy for HCC. Based on these data, subsequent phase III study has been not planned.
Decitabine
Decitabine (5-Aza-2’-deoxycytidine), an epigenetic drug inhibiting DNA methylation, has been approved by FDA for treatment of myelodysplastic syndrome and acute myelogenous leukemia. Aimed to determine the safety and efficacy of lower-dose decitabine based therapy in pretreated patients with aHCC, the phase I/II clinical trial was conducted. In phase I/II clinical trial [146], 15 patients were enrolled, and the favorable adverse events and liver function profiles were observed. The median PFS was 4 months (95% CI, 1.7-7), comparing favorably with existing therapeutic options and expression decrement of DNA (cytosine-5-)-methyltransferase 1 (DNMT1) and gobal DNA hypomethylation were observed in peripheral blood mononuclear cells (PBMCs). The result showed the lower-dose decitabine based treatment resulted in beneficial clinical response and favorable toxicity profiles in patients with aHCC.
Codrituzumab
Codrituzumab, a humanized monoclonal antibody against Glypican-3 (GPC3) expressed in HCC, interacts with CD16/FcγRIIIa and triggers antibody-dependent cytotoxicity. In the phase II clinical trial [147], 185 patients with aHCC who had failed prior systemic therapy (125 received codrituzumab and 60 placebo) were enrolled. The result that the median PFS was 2.6 vs. 1.5 (HR, 0.97; P = 0.87) and the mOS was 8.7 vs. 10 (HR, 0.96; P = 0.82), suggesting that codrituzumab did not show clinical benefit in this previously treated HCC population.
Bevacizumab + Erlotinib
Bevacizumab is a monoclonal antibody directed against VEGF. Erlotinib is TKI of EGFR. In the phase II clinical trial [148], 90 patients with aHCC, Child-Pugh class A-B7 cirrhosis and no prior systemic therapy were enrolled and were randomly assigned (1:1) to receive bevacizumab + erlotinib and sorafenib. The results showed that there was no difference in efficacy between the bevacizumab + erlotinib and sorafenib arms, although the safety and tolerability profile of bevacizumab + erlotinib was over sorafenib based on competing risk analysis.
Temozolomide + Veliparib (ABT-888)
Temozolomide (TMZ), a well-studied DNA-methylating agent crossing the blood-brain barrier, is licensed for the treatment of gliomas and frequently used off-label for malignant melanoma and HCC. Veliparib (ABT-888), an orally bioavailable PARP-1/2 inhibitor, possesses an excellent efficacy and pharmacokinetic profile. In the phase II clinical trial [149], 16 patients were enrolled in the first phase of the trial, but the study was discontinued due to a poor ORR. The combination of TMZ and ABT-888 is well tolerated in patients with aHCC. However, the phase II clinical trial failed to show survival benefit. Above investigational small-molecule inhibitors in phase II clinical trials in the last five years are shown in Figure 3.
Figure 3.
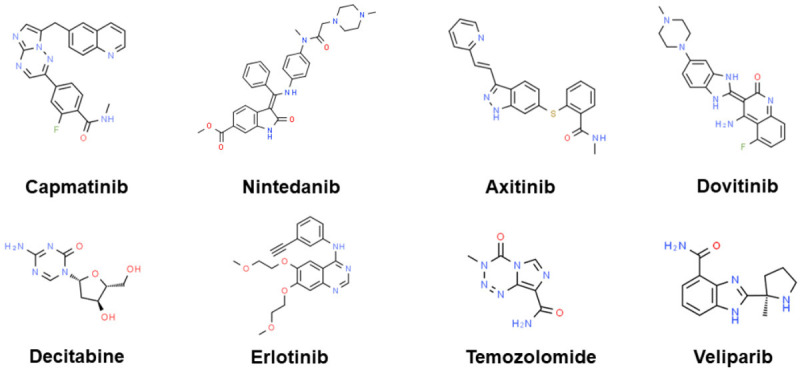
Investigational small-molecule inhibitors in phase II clinical trials in the last five years. The chemical structure is from “chemspider.com”.
Drugs combined with sorafenib
In the phase II clinical trial, no evidence was found that sorafenib with mapatumumab [150], everolimus [151], bevacizumab [152], trebananib [153], resminostat [154], gemcitabine and oxaliplatin (GEMOX) [155], bavituximab [156] improved the efficacy compared with sorafenib alone.
Resminostat
The result of the phase II clinical trial [157] showed the combination of sorafenib and resminostat in HCC patients was safe and had early signs of efficacy.
AEG35156
The result of the phase II clinical trial [158] showed AEG35156 with sorafenib had additional activity in terms of ORR and was well tolerated. AEG35156 with sorafenib had moderate benefit on PFS, especially in the dose-reduced subgroups.
Capecitabine
The result of the phase II clinical trial [159] showed that the combination of sorafenib and capecitabine at tolerable doses may be an active and safe palliative treatment for HCC in Child-Pugh class A-B7 patients with cirrhosis. However, comparison with single-agent sorafenib was not allowed, due to the small sample size.
Refametinib
In the phase II clinical trial [160], a mOS of 12.7 months with refametinib plus sorafenib in a small population of RAS-mutant patients may indicate a synergistic effect between sorafenib and refametinib.
Modified FOLFOX
FOLFOX4 consisted of bolus 5-fluorouracil (5-FU) 400 mg/m2, infusional 5-FU 600 mg/m2 over 22 hours on day 1 and 2, bolus leucovorin 100-200 mg/m2, and oxaliplatin 85 mg/m2 repeated every 2 weeks. In the phase II clinical trial [161] of sorafenib with modified FOLFOX, the pre-specified endpoint with encouraging efficacy was met but moderate hepatotoxicity, which showed sorafenib with modified FOLFOX may be effective in select patients with adequate liver reserve. Above investigational small-molecule inhibitors combined with sorafenib in phase II clinical trials in the last five years are shown in Figure 4.
Figure 4.
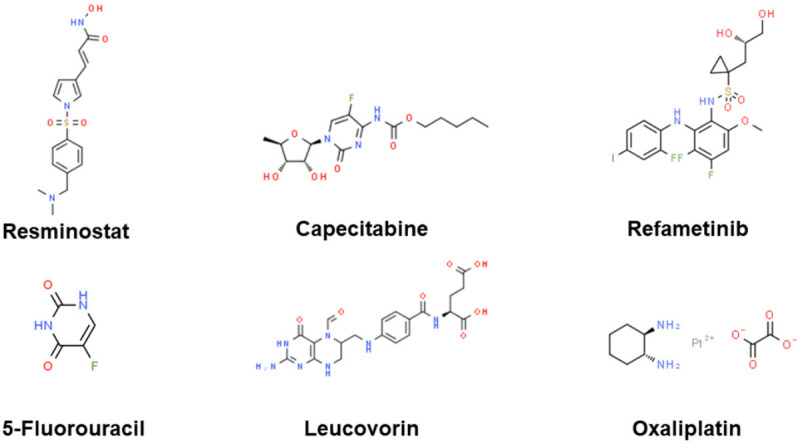
Investigational small-molecule inhibitors combined with sorafenib in phase II clinical trials in the last five years. The chemical structure is from “chemspider.com”.
Preclinical non-drug therapies
Oncolytic virus
Oncolytic viruses have shown the possibility for treating HCC. Recently, research has reported a hereditary virus type 1-based oncolytic vector, LDO-GFP, which is a novel killer against HCC and other types of cancer cells [162]. Intratumoral and intravenous injections of LDO-GFP have shown strong anti-HCC ability with low toxicity. LDO-GFP virus therapy can provide a potentially less toxic and more effective option for local and systemic treatment of HCC. This approach also provides new insights into the efforts to develop the best oncolytic carriers for cancer treatment.
At the same time, some researchers integrated the GP73 promoter and SphK1-shRNA into the Ad5 adenoviral vector to construct GP73-SphK1sR-Ad5, which exhibits a strong inhibitory effect in HCC cell line Huh7 and promote apoptosis [163]. In addition, intratumoral injection of GP73-SphK1sR-Ad5 significantly inhibits tumor growth and prolong the survival time of mice, though its toxicity remains unclear.
Cytokine-armed vaccinia virus also showed a good inhibitory effect against HCC [164]. For example, the recombinant VV-IL-37 which was constructed by inserting IL-37 gene into the poxvirus genome significantly inhibit HCC growth, which may be related to the abnormal regulation of the JAK/STAT3 signaling pathway.
Mesenchymal stem cells
In recent years, mesenchymal stem cells (MSCs) have the potential for cell therapy of HCC. Bone marrow MSCs have an inhibitory effect against HCC, suggesting that bone marrow MSCs have the potential as a new therapeutic agent [165,166]. It has also been reported that co-treatment of bone marrow MSCs with melatonin enhanced the anti-HCC effect of MSCs by inducing apoptosis and preventing inflammation [167].
In terms of adjuvant therapy, some researchers have found that through direct co-culture and indirect separation and culture experiments, adipose tissue mesenchymal cells (AT-MSCs) can enhance the inhibition effect of growth, migration, and invasion in HCC cells both in vitro and in vivo. The up-regulation of p53 and caspases expression, down-regulation of transcriptional activation factor 3 and matrix metallopeptidases might be responsible for this effect [168]. The above studies provided new ideas and understanding for stem cell treatment of HCC.
Biological clock
The progression of HCC depends on a variety of mechanisms. They may cause genetic mutations in proto-oncogenes and tumor suppressor genes, as well as dysfunctions in key signaling pathways, such as Wnt/β-catenin, PI3K/Akt/mTOR, Hedgehog and MAPK signal pathways which are closely related to the biological clock circuit. Disorders in these pathways may lead to the development of HCC [169].
Recently, the biological clock was used for HCC treatment, including time-dependent changes in diet and lifestyle. The molecular clock and circadian system that are still being evaluated [170]. A more comprehensive understanding of the biological clock-related non-alcoholic fatty liver disease signaling pathway in the process of cirrhosis and HCC can provide new strategies for HCC treatment.
Gut microbiome composition
NAFLD includes a variety of liver pathological conditions, such as simple steatosis to non-alcoholic steatohepatitis (NASH), which causes cirrhosis and HCC. In fact, there have been reports that qualitative and quantitative changes in gut microbiome composition (referred to as ‘dysbiosis’) and derangement in the gut-liver axis have been shown to be independently related to the development of NAFLD and its progression to NASH and HCC [171]. Therefore, the regulation of microbial flora in specific species may become a method in personalized NAFLD treatment, and then a potential treatment for HCC.
Intestinal biological disorders are also causally related to the development of HCC [172]. Depletion of the host flora after gut sterilization can significantly inhibit tumorigenesis in HCC induced by diethylnitrosamine [173]. Consistent with these findings, there have been reports that mice grown under sterile conditions have smaller and fewer liver tumors [174]. These studies have shown that intestinal microflora is related to promoting tumorigenesis, mediating proliferation and apoptosis [173,174]. Regulation of the intestinal flora will also be a potential means of treating liver cancer.
Bioartificial liver devices
At present, organ transplantation and hepatectomy are still efficient HCC treatments. However, the surgical risk leads to liver failure or abnormal liver function, The emergence of extracorporeal bioartificial liver (BAL) devices has made a breakthrough in the postoperative treatment of HCC [175].
BAL devices connect to the patient’s venous circulation with the possibility of plasma separation. Plasma flows through the bioreactor containing liver cells to complete normal metabolic exchange then flow back, thus simulating liver metabolism in vitro. The extracorporeal liver assists device (ELAD), which cultured human hepatoblastoma cell line HepG2 C3A in a hollow fiber dialysis box to simulate normal liver metabolism [176]. Another BAL system is HepatAssist, which uses pig liver cells [175].
The bioreactor and cultured liver cells are vital. How to improve the bioreactor to be adapted to the cultivation of different types of liver cells and to screen the liver cells with vitality and function [177] would be the real problem. The bioreactor with liver-like structure and microenvironment for the growth of liver cells, and the liver cells excluding immunoreactivity and tumorigenesis will be the main reform direction of this technology.
GPC3 peptide vaccine
GPC3 was first discovered by Nobuhiro et al. and Nakano et al. [178] almost at the same time in 2001. GPC3 is a membrane protein specifically up-regulated for HCC. In the absence of disease, GPC3 is rarely expressed in the normal organs of adults [179]. However, the expression of GPC3 in HCC tissues caused by Hepatitis B or Hepatitis C is about 80% higher than in surrounding normal tissues [178].
Currently, GPC3 peptides developed as tumor vaccines are limited to HLA-A24 and HLA-A2. According to Nobuhiro et al., GPC3 peptide can induce the immune response of mice to produce specific cytotoxic T lymphocytes (CTL) and anti-tumor activity without autoimmune phenomenon [180]. Subsequently, results of a phase I clinical trial shows that 11 patients with HCC who received GPC3 peptide vaccine produced GPC3 peptid-specific CTL [180]. However, the immunological mechanism of GPC3 peptide remains unknown. Above preclinical non-drug therapies for HCC are summarised in Table 3.
Table 3.
Preclinical non-drug therapies for HCC
| Therapy | Mechanism or pathway | First author/s | Year | Ref. |
|---|---|---|---|---|
| Virus or vector | ||||
| LDO-GFP | A HSV-1-based oncolytic vector with strong anti-hepatocellular carcinoma ability | Luo Y | 2019 | [162] |
| Ad5 | Integrat the GP73 promoter and SphK1-shRNA into the Ad5 adenoviral vector to construct GP73-SphK1sR-Ad5 | Bai Y | 2019 | [163] |
| Poxvirus | Insert the IL-37 gene into the poxvirus genome and successfully constructed recombinant VV-IL37 | Zhang ZH | 2019 | [164] |
| Mesenchymal stem cells | ||||
| Bone marrow MSCs | Co-treatment of bone marrow mesenchymal stem cells with melatonin | Mohamed Y | 2019 | [167] |
| Adipose tissue MSCs | Enhance the inhibition of RT, might related to the up-regulation of p53 and caspases expression, signal transduction and down-regulation of STAT3 and MMPs | Wu LY | 2019 | [168] |
| Biological clock | Wnt/β-catenin, PI3K/akt/mTor, Hedgehog and MAPK signal pathways | Mazzoccoli | 2018 | [169] |
| Gut microbiome composition | qualitative and quantitative changes in gut microbiome composition and derangement in the gut-liver axis | Meroni M | 2019 | [171] |
| Bioartificial liver devices | Mimics liver metabolism and provides liver function support after liver resection or transplantation | Leikin J | 2018 | [175] |
| GPC3 peptide vaccine | High anti-hepatocellular carcinoma activity | Tsuchiya N | 2017 | [180] |
HCC (hepatocellular carcinoma), HSV-1 (hereditary virus type 1), MSCs (mesenchymal stem cells), STAT3 (transcriptional activation factor 3), MMPs (matrix metallopeptidases), GPC3 (Glypican-3).
Preclinical drugs and targets
Potential drugs
Recently, it was reported that Actinidia Chinensis Planch root extract (acRoots) had an inhibition role on HCC. High TAR (HIV-1) RNA binding protein 2 (TARBP2) expression associated with advanced HCC was downregulated with the treatment of it. acRoots could inhibited the malignant behavior of HCC by reducing TARBP2 expression which was impacted by suppressing the significantly positively correlated transcription factor distal-less homeobox 2 (DLX2), leading to a reduction in JNK/AKT signaling pathway activation [181]. Another traditional Chinese medicine Licorice is rich in many natural flavonoids, including isoliquiritigenin (ISL) which has great anticancer potential. Recently, one study first demonstrated that ISL restrains the cell cycle transition and suppresses the proliferation and migration of Hep3B cells by suppressing cyclin D1 and PI3K/AKT pathway. These results suggested that ISL could be a promising agent for preventing HCC [182]. Hydroxygenkwanin (HGK) is also a flavonoid extracted from flower buds of Daphne genkwa, a Chinese medicinal herb. A study by Chou et al. validated that HGK could remarkably suppress the proliferation, migration and invasion of liver cancer cells. HGK induced miR-320a expression, which suppressed the expression of the transcription factor FOXM1 and downstream FOXM1-regulated genes that correlate with epithelial-mesenchymal transition (EMT) in turn. It demonstrated that HGK is a potential therapeutic agent against HCC [183]. Sanguinarine, a natural benzo phenanthridine alkaloid, restrains tumor growth and HIF-1α signaling, suppresses the expression changes of EMT markers as well as Smad and PI3K/AKT pathway proteins. It could also suppress TGF-β-induced cell migration in HCC cells. These findings support sanguinarine is a promising candidate for HCC treatment [184]. Extracts of XS-5 and XS-6 could efficiently induce apoptosis and restrain cell proliferation, migration, and invasion of HCC cells by blocking the PI3K/AKT/mTOR pathway, suggesting XS-5 and XS-6 as novel natural anti-HCC agents [185].
As a medication for treating an immune deficiency, Yu ping feng san (YPFS) was combined with chemotherapy drugs to treat cancer, including HCC. Yuan et al. reported that YPFS inhibited the immune-related factor-TSLP to weaken the activation of the TSLP-STAT3 signaling pathway, thus restraining the formation of hepatic microvessels and inhibiting HCC [186]. Well-known Metformin (MET) owns multiple biological effects such as anticancer and hepatoprotective activity. Sun et al. proved that the joint treatment with aloin and MET enhances the antitumor effect by inducing apoptosis and autophagy in HCC via activating PI3K/AKT/mTOR pathway. Therefore, aloin combined with MET is prospective to be a novel medication for HCC treatment [187]. Meanwhile, cobimetinib, a MEK inhibitor, which has been approved to treat melanomas with a BRAF mutation, was discovered to dose-dependently inhibit tumor angiogenesis. It could suppress endothelial cell proliferation and the formation of capillary network, as well as negatively influence the apoptosis pathways by increasing pro-apoptotic protein Bim and decreasing anti-apoptotic proteins Mcl-1 and Bcl-2 in HCC. It also could restrain ERK signaling. These results supported clinical trials of cobimetinib for HCC therapy [188].
Generally, there are frequently genetic changes in HCC like the overexpression of the MDM2 oncogene and mutations in the p53 tumor suppressor. Wang et al. invented a novel MDM2 inhibitor (named SP141) that acts directly on MDM2 and exhibits anti-HCC activity independent of p53 status. SP141 directly binds the MDM2 protein and promotes MDM2 degradation to inhibit cell growth and prevents cell migration and invasion. These support the development of SP141 as a lead candidate for HCC therapy [189]. Gnocchi et al. identified lysophosphatidic acid (LPA) has a simulative effect on the pathogenesis of HCC and LPA receptor 6 (LPAR6) supports HCC tumorigenicity. They screened out two novel LPAR6 antagonists, C75 and XAA, which actively suppress HCC growth without exerting toxic effects. Furthermore, these compounds induced G1-phase cell cycle arrest [190]. The chemokine system has an important effect on mediating a proinflammatory microenvironment for tumor growth in HCC. CXCR6 receptor and its natural ligand CXCL16 are highly expressed in HCC. Peddibhotla et al. reported the lead compound 81 is an effective (EC50 = 40 nM) and selective orally bioavailable small molecule antagonist of human CXCR6 receptor. Compound 81 significantly reduced tumor growth in a 30-day mouse xenograft model of HCC [191]. Antibody-based c-mesenchymal-epithelial transition factor (c-Met) inhibition is a promising strategy for HCC treatment. Yin et al. reported the one-armed anti-c-Met antibody blocked the interaction of hepatocyte growth factor (HGF) and c-Met as well as the subsequent signal transduction. The HGF-induced HCC cell migration and autocrine stimulation of HepG2 cell proliferation were also strongly inhibited [192].
Potential targets
Effective targeting therapies are limited in HCC clinic treatment. Recently, signaling pathways have been reported to be major targets that could be used in the management of HCC. Elucidating inside signaling cascades could shed light on new targets and strategies for developing targeting therapies for HCC [193,194]. The following is a list of potential therapeutic targets for HCC in related taxonomic signaling pathways (Table 4).
Table 4.
Preclinical targets for the treatment of HCC
| Components | Expression in HCC | Mechanism of action | Functions | First author/s | Year | Ref. |
|---|---|---|---|---|---|---|
| Cell cycle targets | ||||||
| SPAG5 | Up | Related to p53 and cell cycle signal pathway | Indexe of tumor proliferation | Chen W | 2019 | [195] |
| CCT6A | Up | Affect the G1-To-S phase transition | Promote HCC cell proliferation | Zeng G | 2019 | [196] |
| TRIM59 | Up | Degradation of PPM1B | Promote tumor growth in HCC | Ying H | 2019 | [197] |
| CENP-E | Down | Terminate cell cycle at the G1-S phase and accelerate cell apoptosis | Suppress the proliferation of HCC cells | He P | 2019 | [198] |
| DHX9-NONO-SFPQ/BIN1/PLK1 axis | Up | Splicing regulator NONO through oncogenic isoform switch of BIN1 | Enhance carcinogenesis in HCC | Hu Z | 2019 | [199] |
| Apoptosis targets | ||||||
| NQO1 | Up | Inhibit proteasome-mediated degradation SIRT6 | Sustain HCC cell proliferation | Zhou HZ | 2019 | [200] |
| IFITM3-ERK1/2-c-myc path | Up | Upregulate c-myc expression via the ERK1/2 signalling pathway | Promote HCC proliferation | Min J | 2019 | [201] |
| Epigenetic targets | ||||||
| KIAA1429 | Up | m6A-dependent post-transcriptional modification of GATA3 | Contribute to liver cancer progression | Lan T | 2019 | [202] |
| MTF2 | Up | Activate Snail transcription | Induce EMT and progression of HCC | Wu TT | 2019 | [203] |
| miRNAs | ||||||
| miR-1203 | Up | Direct bind and inhibit SOCS3 | Involved in HCC metastases promoted invasion | Shi J | 2019 | [204] |
| miR-16 | Down | Target FEAT through NF-κB signaling pathway | Inhibit HCC progression | Su XF | 2019 | [205] |
| miR-3150b | Down | Target GOLPH3 | Inhibit HCC cell proliferation, migration and invasion | Zhang Y | 2019 | [206] |
| miR-4319 | Down | Target FOXQ1 | Induce an inhibition of EMT and prevented cancer stemness of HCC | Han S | 2019 | [207] |
| miR-541-3p | Down | Directly target and inhibit TMPRSS4 protein expression | Suppress the invasion and migration of HCC cells | Xia YH | 2019 | [208] |
| miR-29a | Down | Via Bcl-2 pathway | Regulate liver tumor-initiating cells expansion | Song S | 2019 | [209] |
| miR-449a | Down | Targeting Notch1 | Inhibit migration and invasion | Han B | 2019 | [210] |
| miR-28-5p | Down | Via IGF-1 Pathway | Regulate liver cancer stem cell expansion | Xia Q | 2019 | [211] |
| miR-181c | Up | Target SPP1 | SPP1 enhances cell growth in HCC | Wang J | 2019 | [212] |
| miR-144 | Down | Target CLK3 | CLK3 contributes to HCC aggressive progression | Li H | 2019 | [213] |
| miR-139 and -378a | Down | Inhibit PIK3CA expression | Inhibitory effects on the proliferation of HCC in vitro | Dong W | 2019 | [214] |
| lncRNAs | ||||||
| GATA3-AS1 | Up | Suppression of PTEN, CDKN1A, and TP53 | Promote cell proliferation and metastasis in HCC | Luo X | 2019 | [219] |
| BZRAP1-AS1 | Up | Mediate THBS1 methylation | Silencing suppresses tumor angiogenesis in HCC | Wang W | 2019 | [217] |
| lncRNA-GMAN | Up | Interact with eIF4B | Promote HCC progression | Xu J | 2019 | [218] |
| RP5-833A20.1 | Down | Through Akt/ERK pathway by targeting miR-18a-5p | Suppress tumorigenesis in HCC | Chen Z | 2019 | [220] |
| AURKAPS1 | Up | Regulate miR-142, miR-155 and miR-182 | Potentiates malignant HCC progression | Li J | 2019 | [221] |
| lncRNA NEAT1 | Up | Regulation of Let-7b-IGF-1R Axis | Promote HCC cell proliferation and reduce apoptosis | Liu Q | 2019 | [222] |
| Regulation of miR-296-5p/CNN2 Axis | Promote proliferation, migration and invasion in HCC cells | Li Y | 2019 | [223] | ||
| ASB16-AS1 | Up | Regulate miR-1827/FZD4 Axis and activate Wnt/β-Catenin Pathway | Promote growth and invasion of HCC | Yao X | 2019 | [224] |
| lncRNA HCG11 | Up | Via miR-26a-5p/ATG12 axis | Accelerate the progression of HCC | Li ML | 2019 | [225] |
| lncRNA TINCR | Up | Sponge miR-214-5p to upregulate ROCK1 | Lead to an increased rate of HCC proliferation | Hu M | 2020 | [226] |
| RP11-81H3.2 | Up | Inhibit microRNA-490-3p and up-regulate Consequential Tankyrase 2 | Act as an oncogene in HCC | Chen W | 2019 | [227] |
| circRNAs | ||||||
| circ_0001955 | Up | Sponge miR-516a-5p to release TRAF6 and MAPK11 | Facilitate HCC tumorigenesis | Yao Z | 2019 | [228] |
| hsa-circ-0046600 | Up | Target the miR-640/HIF-1α signalling pathway | Emerging roles in the progression of HCC | Zhai Z | 2019 | [230] |
| circ_0091579 | Up | Regulate microRNA-490-3p | Promote proliferative ability and metastasis of HCC | Niu WY | 2019 | [231] |
| circ_0003418 | Down | Through Wnt/β-Catenin pathway | Inhibit tumorigenesis in HCC | Chen H | 2019 | [232] |
| circABCB10 | Up | Increase HMG20A expression by sponging miR-670-3p | Promote HCC progression | Fu Y | 2019 | [233] |
| circASAP1 | Up | Regulate miR-326/miR-532-5p-MAPK1 pathway | Promote HCC cell proliferation and invasion | Hu ZQ | 2019 | [229] |
| Metabolic Enzyme/Protease | ||||||
| SOAT1 | Up | Alter the distribution of cellular cholesterol | Promote proliferation and migration of HCC | Jiang Y | 2019 | [234] |
| TGM3 | Up | Depletion inhibiting AKT, ERK, p65 and GSK3β/β-catenin activation | Promote EMT and hepatocellular carcinogenesis | Hu JW | 2019 | [235] |
| OGDHL | Down | Reprogram glutamine metabolism | Silencing promotes HCC | Dai WQ | 2019 | [236] |
| HIF-2α and NEDDylation | Up | SB3 increases HIF-2α stabilization through direct/selective NEDDylation | Favor HCC progression | Cannito S | 2019 | [237] |
| GSTA1 | Down | Regulate LKB1/AMPK/Mtor directly or not | Suppress tumor progression | Liu X | 2020 | [238 |
| Tyrosine kinase pathway targets | ||||||
| Protein Tyrosine Kinase/RTK | ||||||
| SNX5 | Up | Modulate the EGFR-ERK1/2 signaling pathway | Promote HCC progression | Zhou Q | 2019 | [239] |
| HB-EGF | Up | Enhance the expression and proteolytic cleavage by TMPRSS4 | Promote angiogenesis and HCC progression | Dong ZR | 2019 | [240] |
| PlGF | Up | Associated with angiogenesis markers (CD31, CD34, and CD105) | Facilitate neoangiogenesis in HCC | Liu Z | 2019 | [241] |
| MAPK/ERK pathway | ||||||
| p38γ | Up | Promote the phosphorylation of protein at CDK target residues | Induce proliferation after partial hepatectomy | Tomás-Loba A | 2019 | [242] |
| UBE2Z | Up | Target ERK and stat3 signaling pathway | Accelerate HCC progression | Shi X | 2019 | [243] |
| NOD1 | Down | Suppress SRC-MAPK pathway | Inhibit proliferation in HCC | Ma X | 2019 | [244] |
| Immune and inflammatory pathway targets | ||||||
| JAK/STAT pathway | ||||||
| TMUB1 | Down | Regulate Signal Transducer and Activator of Transcription 1 (STAT1) | Negatively regulate HCC proliferation | Chen Y | 2019 | [245 |
| NF-κB signaling pathway | ||||||
| TNFAIP1 | Down | Block NF-κB activation by downregulation of CSNK2B | Tumor suppressor | Xiao Y | 2020 | [246] |
| IGFBP2 | Up | Regulate NF-κB signaling pathway | Promote HCC progression | Guo Q | 2019 | [247] |
| Developmental biology signal transduction targets | ||||||
| OCT4 | Up | Regulate multiple novel HCC signature genes transcription | HCC tumorigenesis and progression | Ye C | 2019 | [248] |
| Wnt/β-catenin pathway | ||||||
| TNKS/β-catenin pathway | Up | Mediate EMT marker expression | An anti-proliferation and anti-metastatic target in HCC cell lines | Huang J | 2020 | [249] |
| UBQLN4 | Up | Activate wnt-β-catenin pathway | Promote progression of HCC | Yu Y | 2020 | [250] |
| MSI1 | Up | Via the Wnt/β-catenin signaling pathway | Increase malignancy of HCC | Liu Q | 2019 | [251] |
| Positively regulate growth and proliferation of hepatoma cells | Li J | 2019 | [252] | |||
| TM4SF1 | Up | Positively regulate β-catenin/TCF signalling | Promote the growth and motility of HCC cells | Zhu C | 2019 | [253] |
| DKK1 | Up | Activate the Wnt/β-catenin signaling pathway | Contribute to HCC tumorigenesis | Zhang R | 2019 | [254] |
| Notch signaling pathway | ||||||
| MSI2 | Up | Via notch1 signaling pathway | Contribute to the maintenance of CD44v6+ liver cancer stem cells | Wang X | 2019 | [255] |
| ZBP-89 | Down | Suppression of Notch1 signaling pathway | Negatively regulate self-renewal of liver cancer stem cells | Wang N | 2020 | [256] |
| Other targets | ||||||
| SEC14L2 | Down | Putative transcriptional activatory activity | Inhibit cancer cells and tumor growth | Li Z | 2019 | [258] |
| Sec62 | Up | Activate integrinα/CAV1 signalling | Promoted migration and invasion of HCC cells | Du J | 2019 | [259] |
| ALOX12-12-HETE-GPR31 axis | Up | 12-HETE upregulated GPR31 and activated PI3K/AKT/NF-κB pathway | Promote recurrence of HCC in fatty liver | Yang F | 2019 | [260] |
HCC (hepatocellular carcinoma), miRNAs (microRNAs), lncRNAs (long non-coding RNAs), circRNAs (circular RNAs).
Cell cycle targets
There are quite a few upregulated indexes of tumor proliferation, one of which is overexpression of sperm-associated antigen 5 (SPAG5) related to p53 and cell cycle signal pathway. In HCC patients with high SPAG5 expression, the OS, PFS, relapse free survival (RFS), and disease-specific survival (DSS) were remarkably decreased (P < 0.01). SPAG5 is not only a marker of poor prognosis but also a potential therapeutic target for HCC [195]. Another index is chaperonin-containing tailless complex polypeptide 1 (CCT) which can fold actins and tubulins indicating that it would be involved in cancer cell progression. In HCC tissues, both mRNA and protein levels of CCT6A were increased, which significantly correlated with reduced overall survival (P = 0.023). Overexpression of CCT6A is oncogenic while its depletion inhibited HCC cell proliferation by hindering the G1-to-S phase transition as it downregulates cyclin D [196]. In addition, Tripartite motif 59 (TRIM59), one of Tripartite motif protein family, is commonly up-regulated in many human cancers which knockdown also attenuated proliferation, induced cells stagnation at G1/S phase and decreased tumor growth in the mouse xenograft model. TRIM59 overexpression in HCC patients was associated with reduced expression of PPM1B and increased CDKs phosphorylation and cell cycle proteins. TRIM59 may serve as a new prognostic biomarker candidate and a potential anti-HCC target through PPM1B/CDKs signaling pathway [197]. Human kinesin centromere-associated protein E (CENP-E), one of spindle checkpoint proteins, has been identified as a tumor inhibitor in several kinds of cancer. Further studies found that it suppressed the proliferation of HCC cells by terminating cell cycle progression at the G1-S phase and accelerating cell apoptosis. Thus, CENP-E could also be a novel target for new treatments and a useful prognostic biomarker for HCC patients [198]. In a study by Hu et al, they found that non-POU domain-containing octamer-binding protein (NONO) was highly expressed and played an oncogenic role to promote tumorigenesis in liver cancer cells by modulating the splicing switch of bridging integrator 1-S (BIN1-S) to generate BIN1 long isoform contains exon 12a (BIN1-L) associated with polo-like kinase 1 (PLK1) to enhance its protein stability, which is dependent on the DExH-box helicase 9 (DHX9)-NONO-SFPQ complex. So the newly identified DHX9-NONO-SFPQ/BIN1/PLK1 axis is also a novel, potential therapeutic target for HCC [199].
Apoptosis targets
In Zhou et al’s study, NAD (P) H: quinone oxidoreductase 1 (NQO1) is considerably increased as well as potentiating the apoptosis evasion of the liver cancer cells. An oncogenic function of NQO1 in sustaining HCC cell proliferation depended on the SIRT6/AKT/XIAP signaling pathway that its overexpression enhanced SIRT6 protein stability by suppressing ubiquitin-mediated 26S proteasome degradation which could increase phosphorylation and activity of AKT, then activated AKT phosphorylated anti-apoptotic protein XIAP at Ser87 which determined its protein stability [200]. Thus, NQO1 might serve as novel therapeutic target for HCC. On the contrary, the down-regulation of interferon-induced transmembrane protein 3 (IFITM3) which is described to be associated with cancer development significantly reduced proto-oncogene c-myc expression via the ERK1/2 signaling pathway, inhibiting the proliferation of HCC in vitro and in vivo. Thus, a novel IFITM3-ERK1/2-c-myc regulatory path was identified and their dysfunction may result in HCC tumorigenesis [201].
Epigenetic targets
At present, epigenetic targets have become important targets for anti-tumor research, including HCC. KIAA1429, the largest known component in the m6A methyltransferase complex, is highly involved in m6A methylation. A study by Lan et al. found it was remarkably upregulated in HCC tissues and had the direct downstream target GATA3. KIAA1429-mediated m6A modification acted on the 3’UTR of GATA3 pre-mRNA, which leads to degradation of the latter and the separation of the RNA-binding protein HuR. Silencing it could have the effect of anti-HCC [202]. Another one is metal regulatory transcription factor 2 (MTF2), a protein binding to the metal response element of the mouse metallothionein promoter, which plays a role in chromosome inactivation and pluripotency. The expression of MTF2 was also upregulated in HCC which strongly correlated with the clinical characteristics and prognosis. It was reported that MTF2 promotes the proliferation, migration, and invasion of HCC cells by regulating Snail transcription, providing a potential therapeutic candidate for HCC patients [203]. In addition to these, here are some kinds of emerging epigenetic targets.
MicroRNAs
MicroRNAs (miRNAs) have been shown to be important regulators in multiple human diseases, including malignant tumors. Studies have confirmed that abnormally expressed miRNAs take part in the tumorigenesis, progression, recurrence and drug resistance of HCC [204-206]. In a study, through the miRNA microarray screening, miR-1203 was validated to be the most significant miRNA and highly correlated with HCC metastases. Up-regulated miR-1203 in HCC promoted invasion by direct binding and inhibiting suppressor of cytokine signaling 3 (SOCS3). Thus, miR-1203 might serve as a potential therapeutic target for the prevention of HCC [204]. However, there are also lots of miRNAs that were decreased in HCC. It has been proved that miR-16 expression takes part in the initiation and development of several cancers, including HCC. Recently, it was verified that miR-16 negatively regulated HCC cell progression via EMT and NF-κB signaling pathway by direct targeting FEAT (an unrecognized protein, identified as a promoter of tumorigenesis), which indicated that miR-16 could be a potential candidate for HCC [207]. Coincidentally, miR-3150b was also downregulated and could inhibit HCC cell proliferation, migration, and invasion by directly targeting and negatively regulating Golgi phosphoprotein-3 (GOLPH3) [208]. The level of miR-4319, a posttranscriptional regulator, was remarkably decreased as well and repressed malignant progression of HCC by targeting FOXQ1 (Forkhead box Q1), which highlights the miR-4319/FOXQ1 cascade as a new target [205]. As for miR-541-3p, Xia et al. found it was downregulated in HCC and suppressed the invasion and migration of HCC cells through directly targeting and inhibiting transmembrane protease serines 4 (TMPRSS4) protein expression. So the newly identified miR-541-3p/TMPRSS4 axis might serve as a novel potential anti-HCC target for HCC [209].
The following three miRNAs have a similar functional mechanism. A study by Song et al. reports that miR-29a is downregulated in liver tumor-initiating cells (T-ICs) while a forced miR-29a expression plays an inhibitory role in liver T-ICs self-renewal and tumorigenesis, mechanistically miR-29a downregulating Bcl-2 via binding its mRNA 3’-UTR in liver T-ICs [206]. miR-449a, a short-term recurrence-related miRNA, was also decreased in HCC cell lines while the expression of Notch1 increased. It was reported that miR-449a inhibited the translation of Notch1 protein by binding to 3’-UTR of its mRNA directly to suppress the invasiveness in vitro by regulating EMT [210]. Another study’s data described that miR-28-5p was lowly expressed in sorted EpCAM-and CD24-positive liver CSCs while its overexpression inhibited liver CSC expansion. Mechanistically, miR-28-5p downregulates the expression of its direct target insulin-like growth factor-1 (IGF-1) via binding to its 3’-UTR [211]. These findings support miR-29a, miR-449a, and miR-28-5p as optimal therapeutic targets for HCC.
Secreted phosphoprotein 1 (SPP1) was noticed as one of the signature genes up-regulated in HCC tissues. With SPP1 depletion, the cell proliferation was impaired, along cell apoptosis resistance by down-regulating SPP1. Wang et al. also noticed miR-181c as an upstreaming inhibitor of SPP1. These results suppose SPP1 as an enhancer of HCC growth targeted by miR-181c and probably to be an innovational target for HCC prevention and treatment [212]. Another markedly upregulated substance in HCC tissues was CLK3 (CDC Like Kinase 3) whose knockdown could suppress HCC development in vitro and in vivo. Moreover, CLK3 was demonstrated as a direct target of tumor suppressor miR-144 and their expression was negatively correlated in HCC. miR-144 inhibits HCC development and metastasis by suppressing CLK3 and Wnt/β-catenin signaling while the latter inhibition could be partly abolished by overexpression of CLK3, indicating that miR-144/CLK3 could be used for HCC diagnosis and treatment [213]. Mechanism of above miRNAs as emerging epigenetic targets for HCC therapy is shown in Figure 5.
Figure 5.
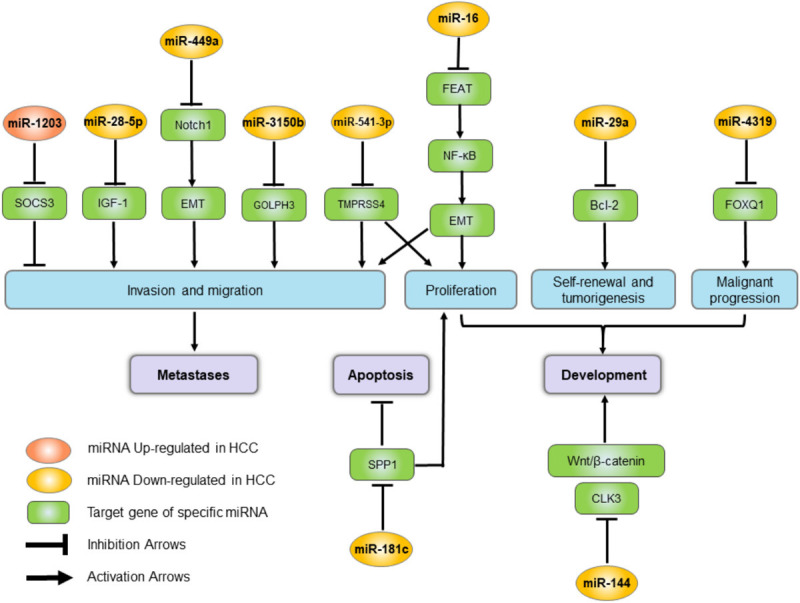
Mechanism of miRNAs as emerging epigenetic targets for HCC therapy. miRNAs (microRNAs), CLK3 (CDC Like Kinase 3), SOCS3 (suppressor of cytokine signaling 3), EMT (epithelial-mesenchymal transition), GOLPH3 (Golgi phosphoprotein-3), TMPRSS4 (transmembrane protease serines 4), SPP1 (secreted phosphoprotein 1), FOXQ1 (Forkhead box Q1), IGF-1 (insulin-like growth factor-1).
Furthermore, PIK3CA gene is one of the most important oncogenes in HCC, which mainly encodes the p110a protein subunit. Because miRNAs are involved in the inhibition of PIK3CA expression in post-transcriptional regulation, overexpression of miRNAs is a promising treatment for liver cancer. In the recent study [214], submicron acoustic phase-shifted nanodroplets were used to transport pre-miRNA plasmids for liver cancer treatment under focused ultrasound (US) activation, which is proved to be efficient in vitro and in vivo. Two miRNAs (miR-139 and -378a) that inhibit PIK3CA and have better inhibitory effects on the proliferation of liver cancer cells in vitro were obtained. The constructed pre-miRNA plasmid was loaded onto NDS and delivered to liver cancer lesions by ultrasound irradiation. Under ultrasound irradiation, the tumor suppression rate of Pre-miR-139 and -378a PLNDs is several times that of the control group [214].
At the same time, some studies have developed an ultrasonic-triggered phase change size cationic nanodroplet for microscale size and duration of ultrasound contrast agents [215]. As an ultrasonic-sensitive core, perfluoropentane (PFP) realizes micron-scale ultrasonic-triggered phase transitions and dimensional changes. Positively charged C9F17-PAsp (DET) guarantees miRNA load. PGA-g-MPEG, as the shell of nanodroplets, modifies the nanodroplets, improves stability, and protects the loaded miRNA from degradation, which may be a potential treatment strategy for liver cancer.
Long non-coding RNAs
Long non-coding RNAs (lncRNAs) have recently been known to play important roles in the development and in the progression of diverse human cancers and could regulate growth and metastasis of HCC, which therefore represents an opportunity to uncover new targets for HCC therapy [216-218]. LncRNA GATA3-AS1 was significantly upregulated in HCC tissues. A study showed that it enhances HCC cell proliferation and metastasis by inhibiting PTEN, CDKN1A, and TP53 which contribute to providing a promising lncRNA-based targeted approach [219]. It was identified that lncRNA BZRAP1-AS1 was also highly expressed in HCC tissues and cells. Silencing of it inhibited the angiogenesis and tumor growth of HCC in vivo by up-regulating THBS1. BZRAP1-AS1 may be a promising marker for HCC treatment [217]. Highly-expressed gastric cancer metastasis-associated lncRNA (GMAN) inhibits apoptosis and promotes metastasis in HCC by stabilizing the phosphorylation of eIF4B. Furthermore, it prevents the dephosphorization of eIF4B via the protein phosphatase 2A subunit B (PPP2R2A). The lncRNA-GMAN/p-eIF4B pathway may be an attractive target for the treatment of HCC [218]. Differently, lncRNA RP5-833A20.1 was downregulated in HCC while it could obviously inhibit the tumorigenesis in HCC via suppressing Akt/ERK pathway (that decreasing the levels of Bcl-2, p-Akt, and p-ERK) by functioning as a competing endogenous RNA for miR-18a-5p. Therefore, it might be a valuable and potential therapeutic target [220].
LncRNA AURKAPS1, the pseudo-gene of AURKA, is highly involved in cancer. A study identified higher levels of AURKAPS1 in HCC and AURKAPS1 enhances the invasion and metastasis of HCC cells by regulating miRNAs like competitively binding with miR-182, miR-155, and miR-142 to increase the protein expression of RAC1, promote the activation of ERK and potentiate the formation of membrane ruffles. So the combination of AURKAPS1/miRNAs may be a new theoretical basis for the treatment of HCC [221]. As for lncRNA nuclear-enriched abundant transcript 1 (NEAT1), emerging evidence has validated that it’s associated with various carcinogenesis. Its overexpression in HCC could promote the proliferation of HCC cells by inhibiting the expression of the miR-let-7b, a miRNA with the effect of a tumor suppressor gene, regulated by IGF-1R. What’s more, it could remarkably reduce miR-296-5p level in HCC cells as a sponge and its silence decreased the expression of Calponin 2 (CNN2), a direct target of miR-296-5p. So lncRNA NEAT1 contributes to HCC progression by regulating miR-Let-7b/IGF-1R axis and miR-296-5p/CNN2 axis, which is conducive to the diagnosis and treatment of HCC [222,223]. ASB16-AS1 was also identified to be overexpressed in HCC tissues. It directly interacted with and sponged miR-1827 to promote FZD4 expression, which eventually activated Wnt/β-catenin pathway and contributed to HCC progression. A novel mechanism of lncRNA ASB16-AS1/miR-1827/FZD4/Wnt/β-catenin signaling was uncovered [224].
It has reported that lncRNA HLA complex group 11 (HCG11) functions as an oncogenic role in multiple cancers, whose enrichment was enhanced in HCC. Li et al. found it promoted the proliferation, metastasis, and autophagy while impeded the apoptosis of HCC cells through HCG11/miR-26a-5p/ATG12 axis. MiR-26a-5p bound to the other two. The depletion of miR-26a-5p or the accumulation of ATG12 could alleviate HCG11 interference suppressive effects. HCG11 might be a potential anti-HCC target [225]. Recently, lncRNA TINCR and ROCK1 were found to be upregulated in HCC as well leading to an increased rate of cancer cell proliferation, while the downregulated miR-214-5p played an opposite role. Mechanically, as a sponge TINCR absorbs miR-214-5p to upregulate ROCK1 targeted by miR-214-5p in HCC, which contributes to the development of novel therapeutic targets [226]. A study by Chen et al. has identified lncRNA RP11-81H3.2 as an enriched one in HCC that promotes proliferation, migration, and invasion both in vitro and in vivo. RP11-81H3.2 binds to and suppresses miR-490-3p expression, thus, further enhancing the expression of itself, which eventually target TNKS2 to work. These have proved RP11-81H3.2 is another novel target for the diagnosis and treatment of HCC [227].
Circular RNAs
Circular RNAs (circRNAs) have been increasingly validated to function as novel promising therapeutic RNA molecules for various types of human diseases, including HCC [228,229]. There are many circRNAs identified to be differentially expressed between HCC and normal samples. It was revealed that circ_0001955, TRAF6, and MAPK11 levels were up-regulated, while miR-516a-5p level was decreased in HCC tumor tissues. Circ_0001955 was demonstrated as a sponge for miR-516a-5p whose two target genes were TRAF6 and MAPK11. By sponging miR-516a-5p to release the two genes expression Circ_0001955 facilitated HCC tumorigenesis [228]. The expression of hsa-circ-0046600 was significantly high in HCC tissue affecting the HCC malignant biological behavior while downregulating it suppressed the migration of HepG2 and SK-HEP-1 cells. Mechanically, it promotes the expression of proteins such as HIF-1α by competitively binding to miR-640 in HCC. Thus, hsa-circ-0046600 can serve as a potential target for the diagnosis and treatment of HCC [230]. Circ_0091579 is also highly expressed in HCC and its down-regulation can inhibit the proliferation and metastasis of HCC cells by promoting miR-490-3p expression [231]. While the level of circ_0003418 was decreased in HCC tissues and cell lines, the study revealed that it inhibited HCC proliferation, migration and invasion by suppressing the Wnt/β-catenin pathway. Therefore, circ-0003418 may be a biomarker and therapeutic target [232].
Highly expressed circABCB10 was also found in HCC tissues and cell lines with direct relation to low survival in HCC patients. The silencing of circABCB10 inhibited proliferation and invasion of HCC. Further study revealed that circABCB10 sponged miR-670-3p to upregulate HMG20A expression which accelerated HCC progression [233]. Recently, Hu et al. identified overexpressed circASAP1 in high metastatic (potential) HCC cell lines as a key regulator in HCC metastasis that promotes HCC cell proliferation and invasion by regulating miR-326/miR-532-5p-MAPK1 pathway and mediates tumor-associated-macrophage infiltration by regulating the miR-326/miR-532-5p-CSF-1 signaling. Hence, circABCB10 and circASAP1 may be two potential targets for HCC therapy [229].
Metabolic enzyme/protease
Metabolic enzyme or protease plays an important role in the tumorigenesis and development of HCC. Jiang et al. identified the proteomic landscape in early HCC. According to the heterogeneity in this stage, they stratify the cohort into the subtypes S-I, S-II, and S-III which is characterized by disrupted cholesterol homeostasis. A specific feature of S-III subtype is the high expression of sterol O-acyltransferase 1 (SOAT1) whose knockdown alters the distribution of cellular cholesterol and effectively suppresses the proliferation and migration of HCC [234]. As for transglutaminase 3 (TGM3) that plays a momentous role in several human cancers, it was identified to be overexpressed in HCC. Its depletion, on the one hand, inhibited AKT, extracellular signal-regulated kinase (ERK), p65 and glycogen synthase kinase 3β (GSK3β)/β-catenin activation but promoted levels of cleaved caspase 3, on the other hand, increased E-cadherin levels and decreased vimentin levels, suggesting that TGM3 enhanced HCC cells proliferation, EMT and potentially promoted HCC metastasis [235]. Thus, SOAT1 and TGM3 might serve as novel therapeutic targets for HCC.
Besides, mitochondrial dysfunction and subsequent metabolic deregulation are frequently observed in cancers including HCC. A study established that silencing of oxoglutarate dehydrogenase-like (OGDHL), one of the rate-limiting components of the key mitochondrial multi-enzyme OGDH complex, activated the mTORC1 signaling pathway in an α-KG-dependent manner, which in turn induced transcriptional expression of SCD1 and FASN, so promoting de novo lipogenesis, resulting in HCC development and survival by modulating glutamine metabolic pathways. These suggest OGDHL as a promising prognostic biomarker and therapeutic target for HCC [236]. SerpinB3 (SB3), hypoxia and hypoxia-inducible factor (HIF)-2α-dependent cysteine-protease inhibitor, was up-regulated in HCC whose release in cancer cells was able to stimulate proliferation and EMT. It could favor HCC progression by increasing HIF-2α stabilization through direct/selective NEDDylation. These all identify HIF-2α and NEDDylation as two novel putative therapeutic targets to interfere with the procarcinogenesis of SerpinB3 in the development of HCC [237]. Additionally, Glutathione S-transferase (GST) family members have a momentous effect on detoxification, metabolism and carcinogenesis. Liu et al. found that HCC patients with better pathological differentiation had higher GSTA1 abundance. GSTA1 overexpression could inhibit proliferation and metastasis via regulating LKB1/AMPK/Mtor directly or not [238]. Therefore, another promising therapeutic target for HCC is indicated.
Tyrosine kinase pathway targets
Protein tyrosine kinase/RTK
Epidermal growth factor receptor (EGFR) is a well-studied tyrosine kinase. Several related targets are illustrated to contribute to the probable treatment of HCC. Sorting nexin (SNX) family members are implicated in tumor progression. A study showed that SNX5 was elevated in tumors compared with APTs in HCC patients. The overexpression of SNX5 enhanced HCC cell proliferation, migration, invasion, and metastasis via the activation of the EGFR-ERK1/2 pathway by blocking EGF-mediated EGFR internalization. It suggests that SNX5 is a potential therapeutic target for HCC [239]. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is highly involved in the development of several malignancies. It’s reported that the level of HB-EGF was positively correlated with the expression of transmembrane protease serine 4 (TMPRSS4) and the microvessel density (MVD) in clinical samples of serum or HCC tissues from HCC patients. TMPRSS4 promoted angiogenesis and HCC progression by significantly enhancing the expression and proteolytic cleavage of HB-EGF, which suggests that HB-EGF can serve as a potential therapeutic target for HCC as well [240]. Moreover, cancer-associated fibroblasts (CAFs) play pivotal roles in the progression of HCC by secreting cytokines and angiogenic factors. In Liu et al’s study, results demonstrated that CAFs overexpressed the placental growth factor (PlGF), which is specifically associated with the neoangiogenesis of HCC. Thus, PlGF may be a corresponding effective therapeutic target for HCC, too [241].
MAPK/ERK pathway
A study by Tomás-Loba et al. revealed that p38 MAPK gamma (p38γ) just acts as a CDK-like kinase and cooperates with CDKs to regulate entry into the cell cycle. In mouse hepatocytes, by promoting the phosphorylation of retinoblastoma tumor suppressor protein at known CDK target residues p38γ induces proliferation after partial hepatectomy. In human HCC, p38γ is highly expressed, suggesting the potential of p38γ as a therapeutic target in HCC [242]. As a member of ubiquitin-conjugating enzymes, UBE2Z was also significantly overexpressed in HCC tissues. Using siRNA to knockdown UBE2Z could inhibit tumor cell proliferation, migration, and invasion via repressing the expression of MMP2 and MMP9. The expression of p-ERK, p-p38, p-JNK, p-Stat3, and p-JAK2 were effectively reduced simultaneously, suggesting that UBE2Z might accelerate HCC progression by targeting ERK and stat3 signaling pathway [243]. As for nucleotide-binding oligomerization (NOD) 1, a pattern recognition receptor, it was responsible for sensing pathogenic peptide in innate immunity, which is conducive to fight against infection. Recently, it was verified that NOD1 as a new tumor suppressor in HCC cells inhibited HCC by inducing cell cycle arrest via directly inhibiting SRC-MAPK axis. Thus, it provided a novel strategy for HCC treatment by regulation of NOD1/SRC/MAPK axis [244].
Immune and inflammatory pathway targets
JAK/STAT pathway
Transmembrane and ubiquitin-like domain-containing 1 protein (TMUB1) was negatively associated with HCC pathological malignancy. Chen et al. described that TMUB1 promoted the expression of STAT1 and suppressed the expression of CCND1 in HCC cells which inhibit HCC proliferation, suggesting TMUB1 as a promising target for the treatment of HCC [245].
NF-κB signaling pathway
Tumor necrosis factor a-induced protein 1 (TNFAIP1) is often downregulated in cancer. One study found overexpression of TNFAIP1 inhibits HCC cell proliferation, metastasis, angiogenesis and promotes cancer cell apoptosis. What’s more, further results support that TNFAIP1 can suppress HCC by modulating TNFAIP1/CSNK2B/NF-κB pathway, which may provide a new target for anti-HCC [246]. Insulin-like growth factor-binding protein 2 (IGFBP2) expression was upregulated and connected with strong metastatic potentials whose depletion significantly suppressed HCC tumorigenesis. The underlying mechanism involved IGFBP2-mediated nuclear localization of p65, which activated nuclear factor kappa B (NF-κB) and zinc finger E-Box binding homeobox 1 (ZEB1) transcription by binding to the gene promoter. For the first time, IGFBP2 was identified as a novel therapeutic target in HCC [247].
Developmental biology signal transduction targets
Many studies have found that the signal transduction pathways in developmental biology involve multiple potential targets for anti-HCC therapy. Using RNA-seq, a study by Ye et al. found that eight top-ranked and validated differentially expressed genes (DEGs) (TK1, CTTN, TRIP13, etc) all contained putative master pluripotency factor OCT4 binding motifs in their promoter regions. The expression levels of TK1, TRIP13 and OCT4 were concurrently higher in HCC tumors than in adjacent peritumor tissues (APTs). Their common transcriptional regulation by OCT4 suggests a pivotal regulatory role of OCT4 in the HCC tumorigenesis and progression, which positions OCT4 as a potential drug target for HCC [248]. In addition, promising therapeutic targets for HCC have also been identified in the following two signaling pathways.
Wnt/β-catenin pathway
The Wnt/β-catenin pathway is involved in the tumorigenesis and development of HCC, including malignant events like the EMT, metastasis, and invasion. Studies have verified the suppression of tankyrases (TNKS) by using a TNKS inhibitor NVP-TNKS656 attenuates Wnt/β-catenin signaling in many cancer cells, which not only abrogated the proliferation of the HCC cell lines but also inhibited metastasis, invasion and EMT phenotypic features. Mechanistically, this probably correlated with the stabilization of AXIN levels and the downregulation of β-catenin, which mediates EMT marker expression. The TNKS/β-catenin signaling pathway is a potential anti-HCC proliferation and metastatic target [249].
What’s more, quite a few targets have been found in this signaling pathway. Ubiquilin-4 (UBQLN4), a member of the ubiquitin-proteasome system, is usually upregulated in many tumor cells, including HCC tissues concerning poor prognosis of HCC patients. A recent study described inhibition of UBQLN4 suppressed tumor formation and progression in vitro and in vivo via regulating Wnt/β-catenin pathway. Moreover, as a downstream target of miR-370, its overexpression reversed tumor suppression of miR-370. These findings may provide a potential target in the development of anti-HCC therapeutic strategies [250]. Musashi1 (MSI1), a member of the RNA-binding protein (RBP) family, specifically binding to mRNA to regulate protein translation, was elevated in HCC than that in APTs. Upregulation of msi1 could decrease the expression of APC, a key inhibitory factor of Wnt pathway, but increase the expression of β-Catenin and CD44, a known cancer stem cell marker, which may contribute to the maintenance of stem-cell-like characteristics of HCC cells and enhance malignant development of HCC cells by activating of the Wnt/β-catenin signaling pathway. These all provide a solid theoretical and experimental basis for the optimization of the future HCC diagnosis and treatment program [251,252]. In a study by Zhu et al, transmembrane 4 L6 family member 1 (TM4SF1) was identified as a binding protein of DVL2 and positively regulated Wnt/β-catenin signaling by strengthening the DVL2-Axin interaction. It was increased in HCC and induced by Kras signaling. The overexpression of TM4SF1 promoted the growth and motility of HCC cells and up-regulated the target genes (Axin2 and cyclin D1), which was impaired by its down-regulation that promoted the ubiquitination of β-catenin. It’s shown that TM4SF1 functions as an oncogenic role in HCC progression and might be a target [253]. The expression of dickkopf-related protein 1 (DKK1) is dysregulated in varieties of human cancers. Zhang et al. reported that DKK1 was upregulated in HCC and contributes to HCC tumorigenesis by activating the Wnt/β-catenin signaling pathway, while genetic depletion of it inhibited the proliferation, colony-forming ability, invasion, and tumor formation of HCC cells [254]. Thus, from the above studies, UBQLN4, Musashi1, TM4SF1 and DKK1 all provide potential therapeutic options for HCC in clinical practice.
Notch signaling pathway
Liver cancer stem cells (LCSCs) contribute to HCC development, metastasis, and drug resistance. Musashi2 (MSI2) and Notch1 signaling participate in the maintenance of CSCs. Both of them were upregulated in sorted CD44v6+ cells than CD44v6- cells and had essential effects on maintaining the stemness of CD44v6+ LCSCs. MSI2 expression was positively correlated with high CD44v6 expression in HCC tissues. The mechanism of MSI2 was to directly bind to Lunatic fringe (LFNG) mRNA and protein, leading to Notch1 activation, which could be a potential molecular target for stem cell-targeted therapy for liver cancer [255]. Besides, ZBP-89 has been demonstrated to function as a candidate tumor suppressor in HCC. Localized with Notch1 intracellular domain (NICD1) in the nucleus, ZBP-89 negatively regulates HCC stemness via inhibiting the Notch1 signaling by competitive binding to NICD1 with MAML1. These support the upregulation of ZBP-89 as a potential therapeutic strategy for HCC treatment [256].
Other targets
Master regulators (MRs) are one or more transcription factors (TFs) that most cancer signaling pathways seem to converge on [257], which direct tumor development, progression, and metastasis through hierarchical control of gene expression patterns. One study predicted the lipid-binding protein SEC14L2 with putative transcriptional activator activity as a conservative MR in HCC and restoration of SEC14L2 expression significantly inhibited tumor growth, which supports MRs to direct targeted therapeutics for HCC [258]. Nevertheless, postsurgical recurrence within 2 years is the major cause of the poor survival of HCC patients. Sec62, an independent prognostic factor for early recurrence in postoperative HCC patients, promoted migration and invasion of HCC cells in vitro. One of the targets of it was integrin α/CAV1 signaling and the overexpression of integrin α partially rescued the Sec62 knockdown-induced suppression of cell migration. Sec62 might be an attractive drug target for anti-HCC postsurgical recurrence [259]. As for ALOX12-12-HETE pathway, it was strongly activated in nonalcoholic fatty liver disease (NAFLD), which induced severe HCC recurrence. In vitro studies by Yang et al. showed that 12-HETE upregulated the expression of GPR31 and induced EMT and matrix metalloprotein (MMPs) by activating PI3K/AKT/NF-κB pathway, while knockdown of GPR31 in cancer cells restrained the HCC recurrence in NAFLD. ALOX12-12-HETE-GPR31 played a crucial role and might be a potential therapeutic target to reduce HCC recurrence after surgery in fatty livers [260]. Mechanism of potential anti-HCC targets in multiple signaling pathways is shown in Figure 6.
Figure 6.
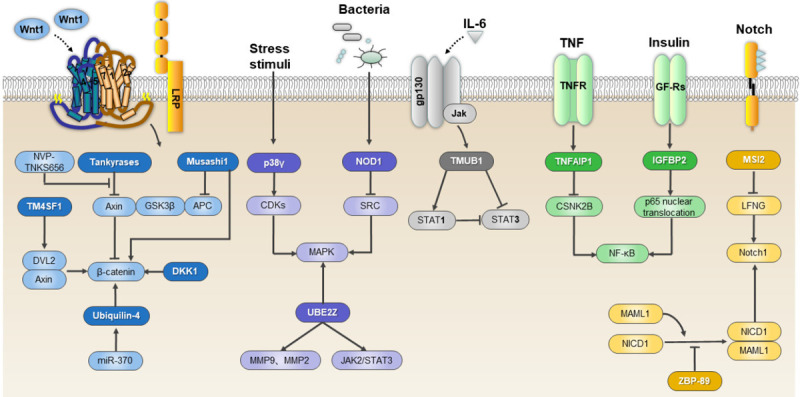
Mechanism of potential anti-HCC targets in multiple signaling pathways. TM4SF1 (transmembrane 4 L6 family member 1), NVP-TNKS656 (a TNKS inhibitor), Axin (scaffold protein), GSK3β (glycogen synthase kinase 3 beta), APC (adenomatous polyposis coli), DKK1 (dickkopf-related protein 1), p38γ (p38 MAPK gamma), NOD1 (nucleotide-binding oligomerization 1), UBE2Z (ubiquitin conjugating enzyme E2Z), TMUB1 (transmembrane and ubiquitin-like domain-containing 1 protein), TNFAIP1 (tumor necrosis factor a-induced protein 1), IGFBP2 (insulin-like growth factor-binding protein 2), MSI2 (Musashi2).
Conclusion
As one of the most common malignant tumors, HCC is closely related to HCV, aflatoxin and other factors, and there is still room for development of its treatments. Although surgical treatment can greatly improve the survival rate of early and mid-stage patients, it is still not applicable for most HCC patients due to the fact like the shortage of donors. TACE, as the first choice for non-surgical treatment, combined with RFA, MWA and sorafenib has shown a significant clinical advantage in the OS rate recently. CAR-T therapy in clinical trials has become a possible treatment for liver cancer for its high specificity to antigens and high MHC dependency on tumor cells. Different from non-drug therapy, the few first-line anti-HCC drugs like Sorafenib are usually used for advanced HCC patients where other therapies have failed, due to their high toxicity. And the use of Nivolumab is more likely to be based on clinical reality or in combination with other drugs. The second-line drugs are often alternatives to patients with intolerance or resistance. Although some drugs are currently in clinical trials, they do not work as well as existing drugs. Thus, the further development of potential preclinical drugs and studies on miRNAs, lncRNAs and many other signaling pathway targets for new drugs research may bring new opportunities for the treatment of HCC. The progress of non-drug therapies such as oncolytic viruses and vectors, peptide vaccine, biological clock for NAFLD conditioning will guide new trends in the treatment of HCC, along with potential HCC drugs to improve the pattern of HCC treatment. The concerted efforts of people all over the world hold the promise of a substantial reduction in the incidence and death from HCC in the coming decades.
Acknowledgements
This work was supported by National S&T Major project (2018ZX09201018), National keypoint research and invention program of China Ministry of Science and Technology (MOST-2016YFC1303200) and Project of the National Natural Sciences Foundation of China (81773198).
Disclosure of conflict of interest
None.
Abbreviations
- HCC
Hepatocellular carcinoma
- TACE
transarterial chemoembolization
- HBV
Chronic Hepatitis B virus
- AFB1
Aflatoxin B1
- NAFLD
Nonalcoholic fatty liver disease
- BCLC
Barcelona Clínic Liver Cancer
- HKLC
Hong Kong Liver Cancer
- PS
performance status
- LT
Liver transplantation
- aHCC
advanced HCC
- HR
Hepatic resection
- OS
overall survival
- DFS
disease-free survival
- cTACE
Conventional TACE
- TARE
Transarterial radioembolization
- mOS
median overall survival
- TACE-S
TACE combined with sorafenib
- TTP
TARE outperformed TACE in time to disease progression
- PVT
portal vein thrombosis
- VEGF
vascular endothelial growth factor
- PFS
progression-free survival
- RFA
radiofrequency ablation
- MWA
microwave ablation
- PEI
ethanol injection
- LSA
laser ablation
- HIFU
high intensity focused ultrasound
- CP-A
Child-Pugh class A
- CP-B
Child-Pugh class B
- CRA
cryoablation
- IRE
Irreversible electroporation
- CAR-T
Chimeric antigen receptor-engineered T cell
- scFv
single-chain variable regions
- TAA
tumor associated antigen
- MHC
major histocompatibility complex
- PDGFR
platelet-derived growth factor receptor
- SGOL1
shugoshin-like 1
- FGFRs
fibroblast growth factor receptors
- PD-1
programmed cell death protein-1
- ORR
objective response rate
- DCR
disease control rate
- DNMT1
decrement of DNA (cytosine-5-)-methyltransferase 1
- PBMCs
peripheral blood mononuclear cells
- GPC3
Glypican-3 (GPC3)
- TMZ
Temozolomide
- GEMOX
gemcitabine and oxaliplatin
- 5-FU
5-fluorouracil
- MSCs
mesenchymal stem cells
- AT-MSCs
adipose tissue mesenchymal cells
- NASH
non-alcoholic steatohepatitis
- BAL
bioartificial liver
- ELAD
extracorporeal liver assist device
- CTL
cytotoxic T lymphocytes
- acRoots
Actinidia Chinensis Planch root extract
- TARBP2
TAR (HIV-1) RNA binding protein 2
- DLX2
distal-less homeobox 2
- ISL
isoliquiritigenin
- HGK
Hydroxygenkwanin
- EMT
epithelial-mesenchymal transition
- YPFS
Yu ping feng san
- MET
Metformin
- LPA
lysophosphatidic acid
- LPAR6
LPA receptor 6
- c-Met
c-mesenchymal-epithelial transition factor
- HGF
hepatocyte growth factor
- SPAG5
sperm-associated antigen 5
- RFS
relapse free survival
- DSS
disease-specific survival
- CCT
complex polypeptide 1
- TRIM59
Tripartite motif 59
- CENP-E
centromere-associated protein E
- BIN1-S
bridging integrator 1-S
- PLK1
polo-like kinase 1
- DHX9
DExH-box helicase 9
- NONO
non-POU domain-containing octamer-binding protein
- BIN1-L
BIN1 long isoform contains exon 12a
- NQO1: NAD(P)H
quinone oxidoreductase 1
- IFITM3
interferon-induced transmembrane protein 3
- MTF2
metal regulatory transcription factor 2
- miRNAs
MicroRNAs
- SOCS3
suppressor of cytokine signaling 3
- GOLPH3
Golgi phosphoprotein-3
- FOXQ1
Forkhead box Q1
- TMPRSS4
transmembrane protease serines 4
- T-ICs
tumor-initiating cells
- IGF-1
insulin-like growth factor-1
- SPP1
Secreted phosphoprotein 1
- CLK3
CDC Like Kinase 3
- US
ultrasound
- PFP
perfluoropentane
- lncRNAs
Long non-coding RNAs
- PPP2R2A
protein phosphatase 2A subunit B
- NEAT1
nuclear-enriched abundant transcript 1
- CNN2
Calponin 2
- HCG11
HLA complex group 11
- circRNAs
Circular RNAs
- SOAT1
sterol O-acyltransferase 1
- TGM3
transglutaminase 3
- ERK
extracellular signal-regulated kinase
- GSK3β
glycogen synthase kinase 3β
- OGDHL
oxoglutarate dehydrogenase-like
- SB3
SerpinB3
- HIF
hypoxia-inducible factor
- GST
Glutathione S-transferase
- EGFR
Epidermal growth factor receptor
- SNX
Sorting nexin
- HB-EGF
Heparin-binding epidermal growth factor-like growth factor
- TMPRSS4
transmembrane protease serine 4
- MVD
microvessel density
- CAFs
cancer-associated fibroblasts
- PlGF
placental growth factor
- p38γ
p38 MAPK gamma
- NOD
nucleotide-binding oligomerization
- TMUB1
Transmembrane and ubiquitin-like domain-containing 1 protein
- TNFAIP1
Tumor necrosis factor a-induced protein 1
- IGFBP2
Insulin-like growth factor-binding protein 2
- NF-κB
nuclear factor kappa B
- ZEB1
zinc finger E-Box binding homeobox 1
- DEGs
differentially expressed genes
- APTs
adjacent peritumor tissues
- TNKS
tankyrases
- UBQLN4
Ubiquilin-4
- MSI1
Musashi1
- RBP
RNA-binding protein
- TM4SF1
transmembrane 4 L6 family member 1
- DKK1
dickkopf-related protein 1
- LCSCs
Liver cancer stem cells
- MSI2
Musashi2
- LFNG
Lunatic fringe
- NICD1
Notch1 intracellular domain
- MRs
Master regulators
- TFs
transcription factors
- MMPs
matrix metalloprotein
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S206–214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlabe S, Rockstroh JK. Advances in the treatment of HIV/HCV coinfection in adults. Expert Opin Pharmacother. 2018;19:49–64. doi: 10.1080/14656566.2017.1419185. [DOI] [PubMed] [Google Scholar]
- 4.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 5.Wild CP, Miller JD, Groopman JD, editors. Mycotoxin control in low-and-middle-income countries. Lyon (FR): International Agency for Research on CancerInternational Agency for Research on Cancer; 2015. [PubMed] [Google Scholar]
- 6.Cheng P, Cheng Y, Su MX, Li D, Zhao GZ, Gao H, Li Y, Zhu JY, Li H, Zhang T. Bicluster and pathway enrichment analysis of HCV-induced cirrhosis and hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:3741–3745. doi: 10.7314/apjcp.2012.13.8.3741. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 8.Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, Lee FY, Lin HC, Huo TI. Prognosis of hepatocellular carcinoma: assessment of eleven staging systems. J Hepatol. 2016;64:601–608. doi: 10.1016/j.jhep.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 10.Zhong JH, Torzilli G, Xing H, Li C, Han J, Liang L, Zhang H, Dai SY, Li LQ, Shen F, Yang T. Controversies and evidence of hepatic resection for hepatocellular carcinoma. BBA Clin. 2016;6:125–130. doi: 10.1016/j.bbacli.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 12.Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma: erratum. Ann Surg. 2019;269:e59. doi: 10.1097/SLA.0000000000003141. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Changchien CS, Hu TH, Lee CM, Kee KM, Lin CY, Chen CL, Chen TY, Huang YJ, Lu SN. The efficacy of treatment schedules according to Barcelona clinic liver cancer staging for hepatocellular carcinoma-survival analysis of 3892 patients. Eur J Cancer. 2008;44:1000–1006. doi: 10.1016/j.ejca.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Zhong JH, Peng NF, You XM, Ma L, Xiang X, Wang YY, Gong WF, Wu FX, Xiang BD, Li LQ. Tumor stage and primary treatment of hepatocellular carcinoma at a large tertiary hospital in China: a real-world study. Oncotarget. 2017;8:18296–18302. doi: 10.18632/oncotarget.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T, Kudo M. Management of hepatocellular carcinoma: report of consensus meeting in the 45th annual meeting of the Japan Society of Hepatology (2009) Hepatol Res. 2010;40:667–685. doi: 10.1111/j.1872-034X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- 16.Ho MC, Hasegawa K, Chen XP, Nagano H, Lee YJ, Chau GY, Zhou J, Wang CC, Choi YR, Poon RT, Kokudo N. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014) Liver Cancer. 2016;5:245–256. doi: 10.1159/000449336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, Shiina S, Cheng AL, Jia JD, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK. Asian Pacific Association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, Khin MW, Koo WH. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 19.Hester CA, Yopp AC. Surgical therapies in hepatocellular carcinoma. In: Hoshida Y, editor. Hepatocellular Carcinoma: Translational Precision Medicine Approaches. Cham (CH): Humana Press Copyright 2019, Springer Nature Switzerland AG; 2019. pp. 145–167. [Google Scholar]
- 20.Levi Sandri GB, de Werra E, Mascianà G, Colasanti M, Santoro R, D’Andrea V, Ettorre GM. Laparoscopic and robotic approach for hepatocellular carcinoma-state of the art. Hepatobiliary Surg Nutr. 2016;5:478–484. doi: 10.21037/hbsn.2016.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich NE, Parikh ND, Singal AG. Hepatocellular carcinoma and liver transplantation: changing patterns and practices. Curr Treat Options Gastroenterol. 2017;15:296–304. doi: 10.1007/s11938-017-0133-3. [DOI] [PubMed] [Google Scholar]
- 22.Song P, Cai Y, Tang H, Li C, Huang J. The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines from 2001 to 2017. Biosci Trends. 2017;11:389–398. doi: 10.5582/bst.2017.01202. [DOI] [PubMed] [Google Scholar]
- 23.Citores MJ, Lucena JL, de la Fuente S, Cuervas-Mons V. Serum biomarkers and risk of hepatocellular carcinoma recurrence after liver transplantation. World J Hepatol. 2019;11:50–64. doi: 10.4254/wjh.v11.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YK, Kim SH, Lee SD, Hong SK, Park SJ. Pretransplant serum levels of C-reactive protein predict prognoses in patients undergoing liver transplantation for hepatocellular carcinoma. Transplant Proc. 2015;47:686–693. doi: 10.1016/j.transproceed.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Cho Y, Kim HY, Cho EJ, Lee DH, Yu SJ, Lee JW, Yi NJ, Lee KW, Kim SH, Kim JM, Joh JW, Teperman LW, Park JS, Kim YJ, Suh KS, Yoon JH. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the milan criteria. Ann Surg. 2016;263:842–850. doi: 10.1097/SLA.0000000000001578. [DOI] [PubMed] [Google Scholar]
- 26.Toso C, Meeberg G, Hernandez-Alejandro R, Dufour JF, Marotta P, Majno P, Kneteman NM. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation. Hepatology. 2015;62:158–165. doi: 10.1002/hep.27787. [DOI] [PubMed] [Google Scholar]
- 27.Xu ZG, Ye CJ, Liu LX, Wu G, Zhao ZX, Wang YZ, Shi BQ, Wang YH. The pretransplant neutrophil-lymphocyte ratio as a new prognostic predictor after liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. Biomark Med. 2018;12:189–199. doi: 10.2217/bmm-2017-0307. [DOI] [PubMed] [Google Scholar]
- 28.Lee JG, Bak SY, Nahm JH, Lee SW, Min SO, Kim KS. Toward angiogenesis of implanted bio-artificial liver using scaffolds with type I collagen and adipose tissue-derived stem cells. Korean J Hepatobiliary Pancreat Surg. 2015;19:47–58. doi: 10.14701/kjhbps.2015.19.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García Martínez JJ, Bendjelid K. Artificial liver support systems: what is new over the last decade? Ann Intensive Care. 2018;8:109. doi: 10.1186/s13613-018-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacco R, Tapete G, Simonetti N, Sellitri R, Natali V, Melissari S, Cabibbo G, Biscaglia L, Bresci G, Giacomelli L. Transarterial chemoembolization for the treatment of hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2017;4:105–110. doi: 10.2147/JHC.S103661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu W, Jin XL, Yang C, Du P, Jiang FQ, Ma JP, Yang J, Xie P, Zhang Z. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther. 2017;18:433–438. doi: 10.1080/15384047.2017.1323589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 33.Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: current state of the art. World J Gastroenterol. 2018;24:161–169. doi: 10.3748/wjg.v24.i2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 35.de Baere T, Arai Y, Lencioni R, Geschwind JF, Rilling W, Salem R, Matsui O, Soulen MC. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc Intervent Radiol. 2016;39:334–343. doi: 10.1007/s00270-015-1208-y. [DOI] [PubMed] [Google Scholar]
- 36.Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009–2019. doi: 10.4254/wjh.v7.i16.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, Zhou X. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126. doi: 10.1186/s12957-017-1196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsurusaki M, Murakami T. Surgical and locoregional therapy of HCC: TACE. Liver Cancer. 2015;4:165–175. doi: 10.1159/000367739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi PS, Huang M, Zhang M, Xu L, Xu MQ. Comparison of transarterial chemoembolization combined with radiofrequency ablation therapy versus surgical resection for early hepatocellular carcinoma. Am Surg. 2018;84:282–288. [PubMed] [Google Scholar]
- 41.Sacco R, Conte C, Tumino E, Parisi G, Marceglia S, Metrangolo S, Eggenhoffner R, Bresci G, Cabibbo G, Giacomelli L. Transarterial radioembolization for hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:25–29. doi: 10.2147/JHC.S50359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial radioembolization with yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther. 2016;33:699–714. doi: 10.1007/s12325-016-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabibbo G, Tremosini S, Galati G, Mazza G, Gadaleta-Caldarola G, Lombardi G, Antonucci M, Sacco R. Transarterial chemoembolization and sorafenib in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2014;14:831–845. doi: 10.1586/14737140.2014.920694. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh MY, Lin ZY, Chuang WL. Serial serum VEGF-A, angiopoietin-2, and endostatin measurements in cirrhotic patients with hepatocellular carcinoma treated by transcatheter arterial chemoembolization. Kaohsiung J Med Sci. 2011;27:314–322. doi: 10.1016/j.kjms.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–529. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 46.Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii41–48. doi: 10.1093/annonc/mds225. [DOI] [PubMed] [Google Scholar]
- 47.Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327–10335. doi: 10.3748/wjg.v21.i36.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng S, Zhang Y, Peng H, Ke Z, Xu L, Su T, Tsung A, Tohme S, Huang H, Zhang Q, Lencioni R, Zeng Z, Peng B, Chen M, Kuang M. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett. 2016;373:193–202. doi: 10.1016/j.canlet.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Chinnaratha MA, Chuang MY, Fraser RJ, Woodman RJ, Wigg AJ. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294–301. doi: 10.1111/jgh.13028. [DOI] [PubMed] [Google Scholar]
- 50.Orlacchio A, Bolacchi F, Chegai F, Bergamini A, Costanzo E, Del Giudice C, Angelico M, Simonetti G. Comparative evaluation of percutaneous laser and radiofrequency ablation in patients with HCC smaller than 4 cm. Radiol Med. 2014;119:298–308. doi: 10.1007/s11547-013-0339-y. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, Bai W, Dong Z, Lu Y, Zeng Z, Lou M, Wang H, Gao X, Chang X, An L, Qu J, Li J, Yang Y. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548. [DOI] [PubMed] [Google Scholar]
- 52.Yang B, Zan RY, Wang SY, Li XL, Wei ML, Guo WH, You X, Li J, Liao ZY. Radiofrequency ablation versus percutaneous ethanol injection for hepatocellular carcinoma: a meta-analysis of randomized controlled trials. World J Surg Oncol. 2015;13:96. doi: 10.1186/s12957-015-0516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. quiz 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 56.Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, Hamada E, Takahashi M, Shiratori Y, Terano A, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023–1028. doi: 10.2214/ajr.160.5.7682378. [DOI] [PubMed] [Google Scholar]
- 57.Shiina S, Tateishi R, Imamura M, Teratani T, Koike Y, Sato S, Obi S, Kanai F, Kato N, Yoshida H, Omata M, Koike K. Percutaneous ethanol injection for hepatocellular carcinoma: 20-year outcome and prognostic factors. Liver Int. 2012;32:1434–1442. doi: 10.1111/j.1478-3231.2012.02838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sung YM, Choi D, Lim HK, Lee WJ, Kim SH, Kim MJ, Paik SW, Yoo BC, Koh KC, Lee JH, Choi MS. Long-term results of percutaneous ethanol injection for the treatment of hepatocellular carcinoma in Korea. Korean J Radiol. 2006;7:187–192. doi: 10.3348/kjr.2006.7.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shiina S, Sato K, Tateishi R, Shimizu M, Ohama H, Hatanaka T, Takawa M, Nagamatsu H, Imai Y. Percutaneous ablation for hepatocellular carcinoma: comparison of various ablation techniques and surgery. Can J Gastroenterol Hepatol. 2018;2018:4756147. doi: 10.1155/2018/4756147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang G, Chen X, Lau WY, Shen F, Wang RY, Yuan SX, Geng WX, Zhou WP. Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br J Surg. 2014;101:1006–1015. doi: 10.1002/bjs.9539. [DOI] [PubMed] [Google Scholar]
- 62.Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: radiofrequency ablation vs. resection vs. transplantation? Clin Mol Hepatol. 2019;25:354–359. doi: 10.3350/cmh.2018.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, Gwak GY, Paik YH, Lim HY, Kim MJ. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015;276:274–285. doi: 10.1148/radiol.15141215. [DOI] [PubMed] [Google Scholar]
- 64.Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 65.Seror O. Ablative therapies: advantages and disadvantages of radiofrequency, cryotherapy, microwave and electroporation methods, or how to choose the right method for an individual patient? Diagn Interv Imaging. 2015;96:617–624. doi: 10.1016/j.diii.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 67.Iannuccilli JD, Dupuy DE. How to set up a successful tumor ablation practice. Tech Vasc Interv Radiol. 2013;16:201–208. doi: 10.1053/j.tvir.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192–200. doi: 10.1053/j.tvir.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10:38–46. doi: 10.1053/j.tvir.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu A, Ishizaka H, Awata S, Shiraishi A, Hirasawa S, Tatezawa T, Kano M, Shimodaira K, Taketomi-Takahashi A, Tsushima Y, Endo K. Expansion of radiofrequency ablation volume by saturated NaCl saline injection in the area of vaporization. Acta Radiol. 2009;50:61–64. doi: 10.1080/02841850802562071. [DOI] [PubMed] [Google Scholar]
- 71.Vogl TJ, Nour-Eldin NA, Hammerstingl RM, Panahi B, Naguib NNN. Microwave Ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms-review article. Rofo. 2017;189:1055–1066. doi: 10.1055/s-0043-117410. [DOI] [PubMed] [Google Scholar]
- 72.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan W, Deng Q, Lin S, Wang Y, Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:264–272. doi: 10.1080/02656736.2018.1562571. [DOI] [PubMed] [Google Scholar]
- 74.Andreano A, Huang Y, Meloni MF, Lee FT Jr, Brace C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37:2967–2973. doi: 10.1118/1.3432569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, Liu GJ, Wu MC. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22:1983–1990. doi: 10.1007/s00330-012-2442-1. [DOI] [PubMed] [Google Scholar]
- 76.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 77.Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344. doi: 10.3109/02656736.2015.1127434. [DOI] [PubMed] [Google Scholar]
- 78.Glybochko PV, Alyaev YG, Amosov AV, Enikeev DV, Chinenov DV, Krupinov GE, Chernov YN, Tivtikyan AS. [Irreversible electroporation to treat prostate cancer (Nanoknife)] . Urologiia. 2016:153–157. [PubMed] [Google Scholar]
- 79.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–1063. doi: 10.4254/wjh.v7.i8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mafeld S, Wong JJ, Kibriya N, Stenberg B, Manas D, Bassett P, Aslam T, Evans J, Littler P. Percutaneous Irreversible Electroporation (IRE) of hepatic malignancy: a bi-institutional analysis of safety and outcomes. Cardiovasc Intervent Radiol. 2019;42:577–583. doi: 10.1007/s00270-018-2120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53:1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 82.Zimmerman A, Grand D, Charpentier KP. Irreversible electroporation of hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017;4:49–58. doi: 10.2147/JHC.S129063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang L, Zhou K, Zhang J, Ma Y, Yang W, Ran L, Jin C, Dimitrov DD, Zhu H. Efficacy and safety of high-intensity focused ultrasound ablation for hepatocellular carcinoma by changing the acoustic environment: microbubble contrast agent (SonoVue) and transcatheter arterial chemoembolization. Int J Hyperthermia. 2019;36:244–252. doi: 10.1080/02656736.2018.1558290. [DOI] [PubMed] [Google Scholar]
- 84.Cheung TT, Fan ST, Chu FS, Jenkins CR, Chok KS, Tsang SH, Dai WC, Chan AC, Chan SC, Yau TC, Poon RT, Lo CM. Survival analysis of high-intensity focused ultrasound ablation in patients with small hepatocellular carcinoma. HPB (Oxford) 2013;15:567–573. doi: 10.1111/hpb.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.You Y, Wang Z, Ran H, Zheng Y, Wang D, Xu J, Wang Z, Chen Y, Li P. Nanoparticle-enhanced synergistic HIFU ablation and transarterial chemoembolization for efficient cancer therapy. Nanoscale. 2016;8:4324–4339. doi: 10.1039/c5nr08292g. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 87.Liu L, Xiao Z, Xiao Y, Wang Z, Li F, Li M, Peng X. Combination of high-intensity focused ultrasound irradiation and hydroxyapatite nanoparticle injection to injure normal goat liver tissue in vivo without costal bone incision. Genet Mol Res. 2014;13:8301–8308. doi: 10.4238/2014.October.20.6. [DOI] [PubMed] [Google Scholar]
- 88.Chen C, Liu Y, Maruvada S, Myers M, Khismatullin D. Effect of ethanol injection on cavitation and heating of tissues exposed to high-intensity focused ultrasound. Phys Med Biol. 2012;57:937–961. doi: 10.1088/0031-9155/57/4/937. [DOI] [PubMed] [Google Scholar]
- 89.Cheng SQ, Zhou XD, Tang ZY, Yu Y, Bao SS, Qian DC. Iodized oil enhances the thermal effect of high-intensity focused ultrasound on ablating experimental liver cancer. J Cancer Res Clin Oncol. 1997;123:639–644. doi: 10.1007/s004320050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim J, Chung DJ, Jung SE, Cho SH, Hahn ST, Lee JM. Therapeutic effect of high-intensity focused ultrasound combined with transarterial chemoembolisation for hepatocellular carcinoma < 5 cm: comparison with transarterial chemoembolisation monotherapy--preliminary observations. Br J Radiol. 2012;85:e940–946. doi: 10.1259/bjr/32750755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Y, Li J, Zhang SJ, Zhao YF, Zhao LS, Ma XX, Feng LS, Fan ZJ. [Clinical observation on high intensity focused ultrasound combined with transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma] . Zhonghua Wai Ke Za Zhi. 2012;50:691–694. [PubMed] [Google Scholar]
- 92.Hu J, Chen S, Wang X, Lin N, Yang J, Wu S. Image-guided percutaneous microwave ablation versus cryoablation for hepatocellular carcinoma in high-risk locations: intermediate-term results. Cancer Manag Res. 2019;11:9801–9811. doi: 10.2147/CMAR.S227961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orlacchio A, Bazzocchi G, Pastorelli D, Bolacchi F, Angelico M, Almerighi C, Masala S, Simonetti G. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: initial experience. Cardiovasc Intervent Radiol. 2008;31:587–594. doi: 10.1007/s00270-008-9293-9. [DOI] [PubMed] [Google Scholar]
- 94.Rong G, Bai W, Dong Z, Wang C, Lu Y, Zeng Z, Qu J, Lou M, Wang H, Gao X, Chang X, An L, Li H, Chen Y, Hu KQ, Yang Y. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One. 2015;10:e0123065. doi: 10.1371/journal.pone.0123065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang YZ, Zhou SC, Zhou H, Tong M. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:1131–1135. doi: 10.5754/hge121142. [DOI] [PubMed] [Google Scholar]
- 96.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srivastava S, Riddell SR. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Y, E CY, Gong ZW, Liu S, Wang ZX, Yang YS, Zhang XW. Chimeric antigen receptor-engineered T-cell therapy for liver cancer. Hepatobiliary Pancreat Dis Int. 2018;17:301–309. doi: 10.1016/j.hbpd.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 100.Schubert ML, Hoffmann JM, Dreger P, Müller-Tidow C, Schmitt M. Chimeric antigen receptor transduced T cells: tuning up for the next generation. Int J Cancer. 2018;142:1738–1747. doi: 10.1002/ijc.31147. [DOI] [PubMed] [Google Scholar]
- 101.Sridhar P, Petrocca F. Regional delivery of Chimeric Antigen Receptor (CAR) T-cells for cancer therapy. Cancers (Basel) 2017;9:92. doi: 10.3390/cancers9070092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 103.Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Sorafenib. Profiles Drug Subst Excip Relat Methodol. 2019;44:239–266. doi: 10.1016/bs.podrm.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 104.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 105.Cervello M, Bachvarov D, Lampiasi N, Cusimano A, Azzolina A, McCubrey JA, Montalto G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle. 2012;11:2843–2855. doi: 10.4161/cc.21193. [DOI] [PubMed] [Google Scholar]
- 106.Sun W, He B, Yang B, Hu W, Cheng S, Xiao H, Yang Z, Wen X, Zhou L, Xie H, Shen X, Wu J, Zheng S. Genome-wide CRISPR screen reveals SGOL1 as a druggable target of sorafenib-treated hepatocellular carcinoma. Lab Invest. 2018;98:734–744. doi: 10.1038/s41374-018-0027-6. [DOI] [PubMed] [Google Scholar]
- 107.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 108.McNamara MG, Slagter AE, Nuttall C, Frizziero M, Pihlak R, Lamarca A, Tariq N, Valle JW, Hubner RA, Knox JJ, Amir E. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis. Eur J Cancer. 2018;105:1–9. doi: 10.1016/j.ejca.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 109.Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, Aikata H, Nagano H, Hatano E, Sasaki Y, Hino K, Kumada T, Yamamoto K, Imai Y, Iwadou S, Ogawa C, Okusaka T, Kanai F, Akazawa K, Yoshimura KI, Johnson P, Arai Y. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 110.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 111.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 112.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, Kwon OS, Yeon JE, Kim BH, Hwang J. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70:684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 114.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C, Takayama T, Yoon SK, Nakajima K, Lehr R, Heldner S, Sanyal AJ. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609–617. doi: 10.1111/ijcp.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, Minoshima Y, Iwata M, Funahashi Y. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. doi: 10.1155/2014/638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641–2653. doi: 10.1002/cam4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 118.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, Piscaglia F, Ueshima K, Aikata H, Vogel A, Lopez C, Pracht M, Meng Z, Daniele B, Park JW, Palmer DH, Dutcus CE, Tamai T, Saito K, Lencioni R. Analysis of survival and objective response (OR) in patients with hepatocellular carcinoma in a phase III study of lenvatinib (REFLECT) J. Clin. Oncol. 2019;37:186. [Google Scholar]
- 119.Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Koizumi Y, Hiasa Y. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: a multicenter analysis with propensity score matching. Hepatol Res. 2020;50:75–83. doi: 10.1111/hepr.13427. [DOI] [PubMed] [Google Scholar]
- 120.Casadei Gardini A, Puzzoni M, Montagnani F, Marisi G, Tamburini E, Cucchetti A, Solaini L, Foschi FG, Conti F, Ercolani G, Cascinu S, Scartozzi M. Profile of lenvatinib in the treatment of hepatocellular carcinoma: design, development, potential place in therapy and network meta-analysis of hepatitis B and hepatitis C in all phase III trials. Onco Targets Ther. 2019;12:2981–2988. doi: 10.2147/OTT.S192572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J, McFarlane T, Tully S, Wong W. Lenvatinib versus sorafenib as first-line treatment of unresectable hepatocellular carcinoma: a cost-utility analysis. Oncologist. 2020;25:e512–e519. doi: 10.1634/theoncologist.2019-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kobayashi M, Kudo M, Izumi N, Kaneko S, Azuma M, Copher R, Meier G, Pan J, Ishii M, Ikeda S. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol. 2019;54:558–570. doi: 10.1007/s00535-019-01554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. LBA38_PRCheckMate 459: a randomized, multi-center phase III study of nivolumab(NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019:3. [Google Scholar]
- 125.Ettrich TJ, Seufferlein T. Regorafenib. Recent Results Cancer Res. 2018;211:45–56. doi: 10.1007/978-3-319-91442-8_3. [DOI] [PubMed] [Google Scholar]
- 126.Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, Mazzaferro V, Wiest R, Reig M, Wagner A, Bolondi L. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412–3419. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 127.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 128.Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello C, Cabrera R, Chang C, Sun W, LeBerre MA, Baumhauer A, Meinhardt G, Bruix J. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353–358. doi: 10.1016/j.jhep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 129.Shlomai A, Leshno M, Goldstein DA. Regorafenib treatment for patients with hepatocellular carcinoma who progressed on sorafenib-A cost-effectiveness analysis. PLoS One. 2018;13:e0207132. doi: 10.1371/journal.pone.0207132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cochin V, Gross-Goupil M, Ravaud A, Godbert Y, Le Moulec S. Cabozantinib: mechanism of action, efficacy and indications. Bull Cancer. 2017;104:393–401. doi: 10.1016/j.bulcan.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 131.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguyen L, Chapel S, Tran BD, Lacy S. Cabozantinib exposure-response analyses of efficacy and safety in patients with advanced hepatocellular carcinoma. J Pharmacokinet Pharmacodyn. 2019;46:577–589. doi: 10.1007/s10928-019-09659-y. [DOI] [PubMed] [Google Scholar]
- 133.Liao W, Huang J, Hutton D, Zhu G, Wu Q, Wen F, Bai L, Li Q. Cost-effectiveness analysis of cabozantinib as second-line therapy in advanced hepatocellular carcinoma. Liver Int. 2019;39:2408–2416. doi: 10.1111/liv.14257. [DOI] [PubMed] [Google Scholar]
- 134.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 135.Zhu AX, Baron AD, Malfertheiner P, Kudo M, Kawazoe S, Pezet D, Weissinger F, Brandi G, Barone CA, Okusaka T, Wada Y, Park JO, Ryoo BY, Cho JY, Chung HC, Li CP, Yen CJ, Lee KD, Chang SC, Yang L, Abada PB, Chau I. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: analysis of REACH trial results by child-pugh score. JAMA Oncol. 2017;3:235–243. doi: 10.1001/jamaoncol.2016.4115. [DOI] [PubMed] [Google Scholar]
- 136.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 137.Zheng H, Qin Z, Qiu X, Zhan M, Wen F, Xu T. Cost-effectiveness analysis of ramucirumab treatment for patients with hepatocellular carcinoma who progressed on sorafenib with α-fetoprotein concentrations of at least 400 ng/ml. J Med Econ. 2020;23:347–352. doi: 10.1080/13696998.2019.1707211. [DOI] [PubMed] [Google Scholar]
- 138.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 139.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 140.Qin S, Chan SL, Sukeepaisarnjaroen W, Han G, Choo SP, Sriuranpong V, Pan H, Yau T, Guo Y, Chen M, Ren Z, Xu J, Yen CJ, Lin ZZ, Manenti L, Gu Y, Sun Y, Tiedt R, Hao L, Song W, Tanwandee T. A phase II study of the efficacy and safety of the MET inhibitor capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919889001. doi: 10.1177/1758835919889001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Palmer DH, Ma YT, Peck-Radosavljevic M, Ross P, Graham J, Fartoux L, Deptala A, Studeny M, Schnell D, Hocke J, Loembé AB, Meyer T. A multicentre, open-label, phase-I/randomised phase-II study to evaluate safety, pharmacokinetics, and efficacy of nintedanib vs. sorafenib in European patients with advanced hepatocellular carcinoma. Br J Cancer. 2018;118:1162–1168. doi: 10.1038/s41416-018-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang YK, Yau T, Park JW, Lim HY, Lee TY, Obi S, Chan SL, Qin S, Kim RD, Casey M, Chen C, Bhattacharyya H, Williams JA, Valota O, Chakrabarti D, Kudo M. Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann Oncol. 2015;26:2457–2463. doi: 10.1093/annonc/mdv388. [DOI] [PubMed] [Google Scholar]
- 143.McNamara MG, Le LW, Horgan AM, Aspinall A, Burak KW, Dhani N, Chen E, Sinaei M, Lo G, Kim TK, Rogalla P, Bathe OF, Knox JJ. A phase II trial of second-line axitinib following prior antiangiogenic therapy in advanced hepatocellular carcinoma. Cancer. 2015;121:1620–1627. doi: 10.1002/cncr.29227. [DOI] [PubMed] [Google Scholar]
- 144.Chan SL, Yeo W, Mo F, Chan AWH, Koh J, Li L, Hui EP, Chong CCN, Lai PBS, Mok TSK, Yu SCH. A phase 2 study of the efficacy and biomarker on the combination of transarterial chemoembolization and axitinib in the treatment of inoperable hepatocellular carcinoma. Cancer. 2017;123:3977–3985. doi: 10.1002/cncr.30825. [DOI] [PubMed] [Google Scholar]
- 145.Cheng AL, Thongprasert S, Lim HY, Sukeepaisarnjaroen W, Yang TS, Wu CC, Chao Y, Chan SL, Kudo M, Ikeda M, Kang YK, Pan H, Numata K, Han G, Balsara B, Zhang Y, Rodriguez AM, Zhang Y, Wang Y, Poon RT. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2016;64:774–784. doi: 10.1002/hep.28600. [DOI] [PubMed] [Google Scholar]
- 146.Mei Q, Chen M, Lu X, Li X, Duan F, Wang M, Luo G, Han W. An open-label, single-arm, phase I/II study of lower-dose decitabine based therapy in patients with advanced hepatocellular carcinoma. Oncotarget. 2015;6:16698–16711. doi: 10.18632/oncotarget.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, Ross P, Peron JM, Ebert O, Chan S, Poon TP, Colombo M, Okusaka T, Ryoo BY, Minguez B, Tanaka T, Ohtomo T, Ukrainskyj S, Boisserie F, Rutman O, Chen YC, Xu C, Shochat E, Jukofsky L, Reis B, Chen G, Di Laurenzio L, Lee R, Yen CJ. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol. 2016;65:289–295. doi: 10.1016/j.jhep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 148.Thomas MB, Garrett-Mayer E, Anis M, Anderton K, Bentz T, Edwards A, Brisendine A, Weiss G, Siegel AB, Bendell J, Baron A, Duddalwar V, El-Khoueiry A. A randomized phase II open-label multi-institution study of the combination of bevacizumab and erlotinib compared to sorafenib in the first-line treatment of patients with advanced hepatocellular carcinoma. Oncology. 2018;94:329–339. doi: 10.1159/000485384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gabrielson A, Tesfaye AA, Marshall JL, Pishvaian MJ, Smaglo B, Jha R, Dorsch-Vogel K, Wang H, He AR. Phase II study of temozolomide and veliparib combination therapy for sorafenib-refractory advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2015;76:1073–1079. doi: 10.1007/s00280-015-2852-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ciuleanu T, Bazin I, Lungulescu D, Miron L, Bondarenko I, Deptala A, Rodriguez-Torres M, Giantonio B, Fox NL, Wissel P, Egger J, Ding M, Kalyani RN, Humphreys R, Gribbin M, Sun W. A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma. Ann Oncol. 2016;27:680–687. doi: 10.1093/annonc/mdw004. [DOI] [PubMed] [Google Scholar]
- 151.Koeberle D, Dufour JF, Demeter G, Li Q, Ribi K, Samaras P, Saletti P, Roth AD, Horber D, Buehlmann M, Wagner AD, Montemurro M, Lakatos G, Feilchenfeldt J, Peck-Radosavljevic M, Rauch D, Tschanz B, Bodoky G. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29) Ann Oncol. 2016;27:856–861. doi: 10.1093/annonc/mdw054. [DOI] [PubMed] [Google Scholar]
- 152.Hubbard JM, Mahoney MR, Loui WS, Roberts LR, Smyrk TC, Gatalica Z, Borad M, Kumar S, Alberts SR. Phase I/II randomized trial of sorafenib and bevacizumab as first-line therapy in patients with locally advanced or metastatic hepatocellular carcinoma: north central cancer treatment group trial N0745 (Alliance) Target Oncol. 2017;12:201–209. doi: 10.1007/s11523-016-0467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abou-Alfa GK, Blanc JF, Miles S, Ganten T, Trojan J, Cebon J, Liem AK, Lipton L, Gupta C, Wu B, Bass M, Hollywood E, Ma J, Bradley M, Litten J, Saltz LB. Phase II study of first-line trebananib plus sorafenib in patients with advanced hepatocellular carcinoma. Oncologist. 2017;22:780–e765. doi: 10.1634/theoncologist.2017-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tak WY, Ryoo BY, Lim HY, Kim DY, Okusaka T, Ikeda M, Hidaka H, Yeon JE, Mizukoshi E, Morimoto M, Lee MA, Yasui K, Kawaguchi Y, Heo J, Morita S, Kim TY, Furuse J, Katayama K, Aramaki T, Hara R, Kimura T, Nakamura O, Kudo M. Phase I/II study of first-line combination therapy with sorafenib plus resminostat, an oral HDAC inhibitor, versus sorafenib monotherapy for advanced hepatocellular carcinoma in east Asian patients. Invest New Drugs. 2018;36:1072–1084. doi: 10.1007/s10637-018-0658-x. [DOI] [PubMed] [Google Scholar]
- 155.Assenat E, Pageaux GP, Thézenas S, Peron JM, Bécouarn Y, Seitz JF, Merle P, Blanc JF, Bouché O, Ramdani M, Poujol S, de Forges H, Ychou M, Boige V. Sorafenib alone vs. sorafenib plus GEMOX as 1(st)-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer. 2019;120:896–902. doi: 10.1038/s41416-019-0443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mokdad AA, Zhu H, Beg MS, Arriaga Y, Dowell JE, Singal AG, Yopp AC. Efficacy and safety of bavituximab in combination with sorafenib in advanced hepatocellular carcinoma: a single-arm, open-label, phase II clinical trial. Target Oncol. 2019;14:541–550. doi: 10.1007/s11523-019-00663-3. [DOI] [PubMed] [Google Scholar]
- 157.Bitzer M, Horger M, Giannini EG, Ganten TM, Wörns MA, Siveke JT, Dollinger MM, Gerken G, Scheulen ME, Wege H, Zagonel V, Cillo U, Trevisani F, Santoro A, Montesarchio V, Malek NP, Holzapfel J, Herz T, Ammendola AS, Pegoraro S, Hauns B, Mais A, Lauer UM, Henning SW, Hentsch B. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma-The SHELTER study. J Hepatol. 2016;65:280–288. doi: 10.1016/j.jhep.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 158.Lee FA, Zee BC, Cheung FY, Kwong P, Chiang CL, Leung KC, Siu SW, Lee C, Lai M, Kwok C, Chong M, Jolivet J, Tung S. Randomized phase II Study of the X-linked Inhibitor of Apoptosis (XIAP) Antisense AEG35156 in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC) Am J Clin Oncol. 2016;39:609–613. doi: 10.1097/COC.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 159.Patt Y, Rojas-Hernandez C, Fekrazad HM, Bansal P, Lee FC. Phase II trial of sorafenib in combination with capecitabine in patients with hepatocellular carcinoma: INST 08-20. Oncologist. 2017;22:1158–e1116. doi: 10.1634/theoncologist.2017-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lim HY, Merle P, Weiss KH, Yau T, Ross P, Mazzaferro V, Blanc JF, Ma YT, Yen CJ, Kocsis J, Choo SP, Sukeepaisarnjaroen W, Gérolami R, Dufour JF, Gane EJ, Ryoo BY, Peck-Radosavljevic M, Dao T, Yeo W, Lamlertthon W, Thongsawat S, Teufel M, Roth K, Reis D, Childs BH, Krissel H, Llovet JM. Phase II studies with refametinib or refametinib plus sorafenib in patients with RAS-mutated hepatocellular carcinoma. Clin Cancer Res. 2018;24:4650–4661. doi: 10.1158/1078-0432.CCR-17-3588. [DOI] [PubMed] [Google Scholar]
- 161.Goyal L, Zheng H, Abrams TA, Miksad R, Bullock AJ, Allen JN, Yurgelun MB, Clark JW, Kambadakone A, Muzikansky A, Knowles M, Galway A, Afflitto AJ, Dinicola CF, Regan E, Hato T, Mamessier E, Shigeta K, Jain RK, Duda DG, Zhu AX. A Phase II and biomarker study of sorafenib combined with modified folfox in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2019;25:80–89. doi: 10.1158/1078-0432.CCR-18-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luo Y, Lin C, Ren W, Ju F, Xu Z, Liu H, Yu Z, Chen J, Zhang J, Liu P, Huang C, Xia N. Intravenous injections of a rationally selected oncolytic herpes virus as a potent virotherapy for hepatocellular carcinoma. Mol Ther Oncolytics. 2019;15:153–165. doi: 10.1016/j.omto.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bai YH, Yun XJ, Xue Y, Zhou T, Sun X, Gao YJ. A novel oncolytic adenovirus inhibits hepatocellular carcinoma growth. J Zhejiang Univ Sci B. 2019;20:1003–1013. doi: 10.1631/jzus.B1900089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang Z, Zhang J, Zhang Y, Xing J, Yu Z. Vaccinia virus expressing IL-37 promotes antitumor immune responses in hepatocellular carcinoma. Cell Biochem Funct. 2019;37:618–624. doi: 10.1002/cbf.3438. [DOI] [PubMed] [Google Scholar]
- 165.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 166.Li T, Song B, Du X, Wei Z, Huo T. Effect of bone-marrow-derived mesenchymal stem cells on high-potential hepatocellular carcinoma in mouse models: an intervention study. Eur J Med Res. 2013;18:34. doi: 10.1186/2047-783X-18-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohamed Y, Basyony MA, El-Desouki NI, Abdo WS, El-Magd MA. The potential therapeutic effect for melatonin and mesenchymal stem cells on hepatocellular carcinoma. Biomedicine (Taipei) 2019;9:24. doi: 10.1051/bmdcn/2019090424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu L, Tang Q, Yin X, Yan D, Tang M, Xin J, Pan Q, Ma C, Yan S. The therapeutic potential of adipose tissue-derived mesenchymal stem cells to enhance radiotherapy effects on hepatocellular carcinoma. Front Cell Dev Biol. 2019;7:267. doi: 10.3389/fcell.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mazzoccoli G, Miele L, Marrone G, Mazza T, Vinciguerra M, Grieco A. A role for the biological clock in liver cancer. Cancers (Basel) 2019;11:1778. doi: 10.3390/cancers11111778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mazzoccoli G, De Cosmo S, Mazza T. The Biological clock: a pivotal hub in non-alcoholic fatty liver disease pathogenesis. Front Physiol. 2018;9:193. doi: 10.3389/fphys.2018.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meroni M, Longo M, Dongiovanni P. The role of probiotics in nonalcoholic fatty liver disease: a new insight into therapeutic strategies. Nutrients. 2019;11:2642. doi: 10.3390/nu11112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 173.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 174.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leikin JB. Current and prospective therapies for acute liver failure. Dis Mon. 2018;64:492. doi: 10.1016/j.disamonth.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 176.Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol. 2017;23:1974–1979. doi: 10.3748/wjg.v23.i11.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Starokozhko V, Groothuis GMM. Challenges on the road to a multicellular bioartificial liver. J Tissue Eng Regen Med. 2018;12:e227–e236. doi: 10.1002/term.2385. [DOI] [PubMed] [Google Scholar]
- 178.Shimizu Y, Suzuki T, Yoshikawa T, Tsuchiya N, Sawada Y, Endo I, Nakatsura T. Cancer immunotherapy-targeted glypican-3 or neoantigens. Cancer Sci. 2018;109:531–541. doi: 10.1111/cas.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H, Ashihara H, Katagiri T, Furukawa Y, Fujiyama S, Ogawa M, Nakamura Y, Nishimura Y. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16–25. doi: 10.1016/s0006-291x(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 180.Tsuchiya N, Yoshikawa T, Fujinami N, Saito K, Mizuno S, Sawada Y, Endo I, Nakatsura T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology. 2017;6:e1346764. doi: 10.1080/2162402X.2017.1346764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fang T, Zhao Z, Yuan F, He M, Sun J, Guo M, Huang P, Yang B, Xia J. Actinidia Chinensis Planch Root extract attenuates proliferation and metastasis of hepatocellular carcinoma by inhibiting the DLX2/TARBP2/JNK/AKT pathway. J Ethnopharmacol. 2020;251:112529. doi: 10.1016/j.jep.2019.112529. [DOI] [PubMed] [Google Scholar]
- 182.Huang Y, Liu C, Zeng WC, Xu GY, Wu JM, Li ZW, Huang XY, Lin RJ, Shi X. Isoliquiritigenin inhibits the proliferation, migration and metastasis of Hep3B cells via suppressing cyclin D1 and PI3K/AKT pathway. Biosci Rep. 2020;40:BSR20192727. doi: 10.1042/BSR20192727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chou LF, Chen CY, Yang WH, Chen CC, Chang JL, Leu YL, Liou MJ, Wang TH. Suppression of hepatocellular carcinoma progression through FOXM1 and EMT inhibition via hydroxygenkwanin-induced miR-320a expression. Biomolecules. 2019;10:20. doi: 10.3390/biom10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Su Q, Fan M, Wang J, Ullah A, Ghauri MA, Dai B, Zhan Y, Zhang D, Zhang Y. Sanguinarine inhibits epithelial-mesenchymal transition via targeting HIF-1α/TGF-β feed-forward loop in hepatocellular carcinoma. Cell Death Dis. 2019;10:939. doi: 10.1038/s41419-019-2173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim J, Jung KH, Ryu HW, Kim DY, Oh SR, Hong SS. Apoptotic effects of xanthium strumarium via PI3K/AKT/mTOR pathway in hepatocellular carcinoma. Evid Based Complement Alternat Med. 2019;2019:2176701. doi: 10.1155/2019/2176701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yuan Q, Yao F, Zhou L, Liang G, Song X, Jiang G, Zhou M, Zhang L. Yu Ping Feng San exert anti-angiogenesis effects through the inhibition of TSLP-STAT3 signaling pathways in hepatocellular carcinoma. Evid Based Complement Alternat Med. 2019;2019:1947156. doi: 10.1155/2019/1947156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun R, Zhai R, Ma C, Miao W. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med. 2020;9:1141–1151. doi: 10.1002/cam4.2723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 188.Tong X, Wang Q, Wu D, Bao L, Yin T, Chen H. MEK inhibition by cobimetinib suppresses hepatocellular carcinoma and angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2020;523:147–152. doi: 10.1016/j.bbrc.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 189.Wang W, Hu B, Qin JJ, Cheng JW, Li X, Rajaei M, Fan J, Yang XR, Zhang R. A novel inhibitor of MDM2 oncogene blocks metastasis of hepatocellular carcinoma and overcomes chemoresistance. Genes Dis. 2019;6:419–430. doi: 10.1016/j.gendis.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gnocchi D, Kapoor S, Nitti P, Cavalluzzi MM, Lentini G, Denora N, Sabbà C, Mazzocca A. Novel lysophosphatidic acid receptor 6 antagonists inhibit hepatocellular carcinoma growth through affecting mitochondrial function. J Mol Med (Berl) 2020;98:179–191. doi: 10.1007/s00109-019-01862-1. [DOI] [PubMed] [Google Scholar]
- 191.Peddibhotla S, Hershberger PM, Jason Kirby R, Sugarman E, Maloney PR, Hampton Sessions E, Divlianska D, Morfa CJ, Terry D, Pinkerton AB, Smith LH, Malany S. Discovery of small molecule antagonists of chemokine receptor CXCR6 that arrest tumor growth in SK-HEP-1 mouse xenografts as a model of hepatocellular carcinoma. Bioorg Med Chem Lett. 2020;30:126899. doi: 10.1016/j.bmcl.2019.126899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yin Y, Guo J, Teng F, Yu L, Jiang Y, Xie K, Jiang M, Fang J. Preparation of a novel one-armed anti-c-met antibody with antitumor activity against hepatocellular carcinoma. Drug Des Devel Ther. 2019;13:4173–4184. doi: 10.2147/DDDT.S224491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu C, Peng X, Li Y, Liu S, Hou R, Zhang Y, Zuo S, Liu Z, Luo R, Li L, Fang W. Positive feedback loop of FAM83A/PI3K/AKT/c-Jun induces migration, invasion and metastasis in hepatocellular carcinoma. Biomed Pharmacother. 2020;123:109780. doi: 10.1016/j.biopha.2019.109780. [DOI] [PubMed] [Google Scholar]
- 194.Kong J, Li D, Zhang S, Zhang H, Fu Y, Qian B, Bei C, Tan S, Zhu X. Okadaic acid promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells by inhibiting protein phosphatase 2A. J Cell Biochem. 2020 doi: 10.1002/jcb.29629. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 195.Chen W, Chen X, Li S, Ren B. Expression, immune infiltration and clinical significance of SPAG5 in hepatocellular carcinoma: a gene expression-based study. J Gene Med. 2020;22:e3155. doi: 10.1002/jgm.3155. [DOI] [PubMed] [Google Scholar]
- 196.Zeng G, Wang J, Huang Y, Lian Y, Chen D, Wei H, Lin C, Huang Y. Overexpressing CCT6A contributes to cancer cell growth by affecting the G1-To-S phase transition and predicts a negative prognosis in hepatocellular carcinoma. Onco Targets Ther. 2019;12:10427–10439. doi: 10.2147/OTT.S229231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ying H, Ji L, Xu Z, Fan X, Tong Y, Liu H, Zhao J, Cai X. TRIM59 promotes tumor growth in hepatocellular carcinoma and regulates the cell cycle by degradation of protein phosphatase 1B. Cancer Lett. 2020;473:13–24. doi: 10.1016/j.canlet.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 198.He P, Hu P, Yang C, He X, Shao M, Lin Y. Reduced expression of CENP-E contributes to the development of hepatocellular carcinoma and is associated with adverse clinical features. Biomed Pharmacother. 2020;123:109795. doi: 10.1016/j.biopha.2019.109795. [DOI] [PubMed] [Google Scholar]
- 199.Hu Z, Dong L, Li S, Li Z, Qiao Y, Li Y, Ding J, Chen Z, Wu Y, Wang Z, Huang S, Gao Q, Zhao Y, He X. Splicing regulator p54(nrb)/Non-POU domain-containing octamer-binding protein enhances carcinogenesis through oncogenic isoform switch of MYC Box-Dependent interacting protein 1 in hepatocellular carcinoma. Hepatology. 2019 doi: 10.1002/hep.31062. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 200.Zhou HZ, Zeng HQ, Yuan D, Ren JH, Cheng ST, Yu HB, Ren F, Wang Q, Qin YP, Huang AL, Chen J. NQO1 potentiates apoptosis evasion and upregulates XIAP via inhibiting proteasome-mediated degradation SIRT6 in hepatocellular carcinoma. Cell Commun Signal. 2019;17:168. doi: 10.1186/s12964-019-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Min J, Hu J, Luo C, Zhu J, Zhao J, Zhu Z, Wu L, Yuan R. IFITM3 upregulates c-myc expression to promote hepatocellular carcinoma proliferation via the ERK1/2 signalling pathway. Biosci Trends. 2020;13:523–529. doi: 10.5582/bst.2019.01289. [DOI] [PubMed] [Google Scholar]
- 202.Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, Yan X, Liao H, Chen X, Xie K, Li J, Liao M, Huang J, Yuan K, Zeng Y, Wu H. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18:186. doi: 10.1186/s12943-019-1106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wu TT, Cai J, Tian YH, Chen JF, Cheng ZL, Pu CS, Shi WZ, Suo XP, Wu XJ, Dou XW, Zhang KM. MTF2 induces epithelial-mesenchymal transition and progression of hepatocellular carcinoma by transcriptionally activating snail. Onco Targets Ther. 2019;12:11207–11220. doi: 10.2147/OTT.S226119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shi J, Li X, Hu Y, Zhang F, Lv X, Zhang X, Chen Q, Hu S. MiR-1203 is involved in hepatocellular carcinoma metastases and indicates a poor prognosis. Neoplasma. 2020;67:267–276. doi: 10.4149/neo_2019_190414N328. [DOI] [PubMed] [Google Scholar]
- 205.Han S, Shi Y, Sun L, Liu Z, Song T, Liu Q. MiR-4319 induced an inhibition of epithelial-mesenchymal transition and prevented cancer stemness of HCC through targeting FOXQ1. Int J Biol Sci. 2019;15:2936–2947. doi: 10.7150/ijbs.38000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Song S, Sun K, Dong J, Zhao Y, Liu F, Liu H, Sha Z, Mao J, Ding G, Guo W, Fu Z. microRNA-29a regulates liver tumor-initiating cells expansion via Bcl-2 pathway. Exp Cell Res. 2020;387:111781. doi: 10.1016/j.yexcr.2019.111781. [DOI] [PubMed] [Google Scholar]
- 207.Su XF, Li N, Meng FL, Chu YL, Li T, Gao XZ. MiR-16 inhibits hepatocellular carcinoma progression by targeting FEAT through NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:10274–10282. doi: 10.26355/eurrev_201912_19665. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Y, Wang J, Su H. MiR-3150b inhibits hepatocellular carcinoma cell proliferation, migration and invasion by targeting GOLPH3. J Investig Med. 2020;68:770–775. doi: 10.1136/jim-2019-001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xia YH, Ren L, Li JZ, Gao F. Role of miR-541-3p/TMPRSS4 in the metastasis and EMT of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2019;23:10721–10728. doi: 10.26355/eurrev_201912_19773. [DOI] [PubMed] [Google Scholar]
- 210.Han B, Huang J, Yang Z, Zhang J, Wang X, Xu N, Meng H, Wu J, Huang Q, Yang X, Shen R, Sun C. miR-449a is related to short-term recurrence of hepatocellular carcinoma and inhibits migration and invasion by targeting Notch1. Onco Targets Ther. 2019;12:10975–10987. doi: 10.2147/OTT.S216997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xia Q, Han T, Yang P, Wang R, Li H, Zhang J, Zhou X. MicroRNA-28-5p regulates liver cancer stem cell expansion via IGF-1 pathway. Stem Cells Int. 2019;2019:8734362. doi: 10.1155/2019/8734362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang J, Hao F, Fei X, Chen Y. SPP1 functions as an enhancer of cell growth in hepatocellular carcinoma targeted by miR-181c. Am J Transl Res. 2019;11:6924–6937. [PMC free article] [PubMed] [Google Scholar]
- 213.Li H, Cui X, Hu Q, Chen X, Zhou P. CLK3 is a direct target of mir-144 and contributes to aggressive progression in hepatocellular carcinoma. Onco Targets Ther. 2019;12:9201–9213. doi: 10.2147/OTT.S224527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dong W, Wu P, Zhou D, Huang J, Qin M, Yang X, Wan M, Zong Y. Ultrasound-mediated gene therapy of hepatocellular carcinoma using pre-microRNA plasmid-loaded nanodroplets. Ultrasound Med Biol. 2020;46:90–107. doi: 10.1016/j.ultrasmedbio.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 215.Guo H, Xu M, Cao Z, Li W, Chen L, Xie X, Wang W, Liu J. Ultrasound-assisted miR-122-loaded polymeric nanodroplets for hepatocellular carcinoma gene therapy. Mol Pharm. 2020;17:541–553. doi: 10.1021/acs.molpharmaceut.9b00983. [DOI] [PubMed] [Google Scholar]
- 216.Deng Y, Wei Z, Huang M, Xu G, Wei W, Peng B, Nong S, Qin H. Long non-coding RNA F11-AS1 inhibits HBV-related hepatocellular carcinoma progression by regulating NR1I3 via binding to microRNA-211-5p. J Cell Mol Med. 2020;24:1848–1865. doi: 10.1111/jcmm.14881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang W, Chen G, Wang B, Yuan Z, Liu G, Niu B, Chen Y, Zhou S, He J, Xue H. Long non-coding RNA BZRAP1-AS1 silencing suppresses tumor angiogenesis in hepatocellular carcinoma by mediating THBS1 methylation. J Transl Med. 2019;17:421. doi: 10.1186/s12967-019-02145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu J, Lu Y, Liu Q, Xia A, Zhao J, Xu X, Sun Q, Qi F, Sun B. Long noncoding RNA GMAN promotes hepatocellular carcinoma progression by interacting with eIF4B. Cancer Lett. 2020;473:1–12. doi: 10.1016/j.canlet.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 219.Luo X, Zhou N, Wang L, Zeng Q, Tang H. Long noncoding RNA GATA3-AS1 promotes cell proliferation and metastasis in hepatocellular carcinoma by suppression of PTEN, CDKN1A, and TP53. Can J Gastroenterol Hepatol. 2019;2019:1389653. doi: 10.1155/2019/1389653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen Z, Ma Y, Pan Y, Zuo S, Zhu H, Yu C, Zhu C, Sun C. Long noncoding RNA RP5-833A20.1 suppresses tumorigenesis in hepatocellular carcinoma through Akt/ERK pathway by targeting miR-18a-5p. Onco Targets Ther. 2019;12:10717–10726. doi: 10.2147/OTT.S219797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li J, Guo W, Xue W, Xu P, Deng Z, Zhang D, Zheng S, Qiu X. Long noncoding RNA AURKAPS1 potentiates malignant hepatocellular carcinoma progression by regulating miR-142, miR-155 and miR-182. Sci Rep. 2019;9:19645. doi: 10.1038/s41598-019-56036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Q, Shi H, Yang J, Jiang N. Long non-coding RNA NEAT1 promoted hepatocellular carcinoma cell proliferation and reduced apoptosis through the regulation of Let-7b-IGF-1R axis. Onco Targets Ther. 2019;12:10401–10413. doi: 10.2147/OTT.S217763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li Y, Ding X, Xiu S, Du G, Liu Y. LncRNA NEAT1 promotes proliferation, migration and invasion via regulating miR-296-5p/CNN2 Axis in hepatocellular carcinoma cells. Onco Targets Ther. 2019;12:9887–9897. doi: 10.2147/OTT.S228917. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Yao X, You G, Zhou C, Zhang D. LncRNA ASB16-AS1 promotes growth and invasion of hepatocellular carcinoma through regulating miR-1827/FZD4 axis and activating Wnt/β-Catenin pathway. Cancer Manag Res. 2019;11:9371–9378. doi: 10.2147/CMAR.S220434. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 225.Li ML, Zhang Y, Ma LT. LncRNA HCG11 accelerates the progression of hepatocellular carcinoma via miR-26a-5p/ATG12 axis. Eur Rev Med Pharmacol Sci. 2019;23:10708–10720. doi: 10.26355/eurrev_201912_19771. [DOI] [PubMed] [Google Scholar]
- 226.Hu M, Han Y, Zhang Y, Zhou Y, Ye L. lncRNA TINCR sponges miR-214-5p to upregulate ROCK1 in hepatocellular carcinoma. BMC Med Genet. 2020;21:2. doi: 10.1186/s12881-019-0940-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 227.Chen W, Li K, Zhu K, Yan R, Cai QC, Li WH, Dang CX. RP11-81H3.2 acts as an oncogene via microRNA-490-3p inhibition and consequential tankyrase 2 Up-regulation in hepatocellular carcinoma. Dig Dis Sci. 2019 doi: 10.1007/s10620-019-06007-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 228.Yao Z, Xu R, Yuan L, Xu M, Zhuang H, Li Y, Zhang Y, Lin N. Circ_0001955 facilitates hepatocellular carcinoma (HCC) tumorigenesis by sponging miR-516a-5p to release TRAF6 and MAPK11. Cell Death Dis. 2019;10:945. doi: 10.1038/s41419-019-2176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hu ZQ, Zhou SL, Li J, Zhou ZJ, Wang PC, Xin HY, Mao L, Luo CB, Yu SY, Huang XW, Cao Y, Jia F, Zhou J. Circular RNA sequencing identifies CircASAP1 as a key regulator in hepatocellular carcinoma metastasis. Hepatology. 2019 doi: 10.1002/hep.31068. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 230.Zhai Z, Fu Q, Liu C, Zhang X, Jia P, Xia P, Liu P, Liao S, Qin T, Zhang H. Emerging roles of hsa-circ-0046600 targeting the miR-640/HIF-1α signalling pathway in the progression of HCC. Onco Targets Ther. 2019;12:9291–9302. doi: 10.2147/OTT.S229514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Niu WY, Chen L, Zhang P, Zang H, Zhu B, Shao WB. Circ_0091579 promotes proliferative ability and metastasis of liver cancer cells by regulating microRNA-490-3p. Eur Rev Med Pharmacol Sci. 2019;23:10264–10273. doi: 10.26355/eurrev_201912_19664. [DOI] [PubMed] [Google Scholar]
- 232.Chen H, Liu S, Li M, Huang P, Li X. Circ_0003418 inhibits tumorigenesis and cisplatin chemoresistance through Wnt/β-Catenin pathway in hepatocellular carcinoma. Onco Targets Ther. 2019;12:9539–9549. doi: 10.2147/OTT.S229507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fu Y, Cai L, Lei X, Wang D. Circular RNA ABCB10 promotes hepatocellular carcinoma progression by increasing HMG20A expression by sponging miR-670-3p. Cancer Cell Int. 2019;19:338. doi: 10.1186/s12935-019-1055-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 234.Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, Xing B, Sun W, Ren L, Hu B, Li C, Zhang L, Qin G, Zhang M, Chen N, Zhang M, Huang Y, Zhou J, Zhao Y, Liu M, Zhu X, Qiu Y, Sun Y, Huang C, Yan M, Wang M, Liu W, Tian F, Xu H, Zhou J, Wu Z, Shi T, Zhu W, Qin J, Xie L, Fan J, Qian X, He F. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257–261. doi: 10.1038/s41586-019-0987-8. [DOI] [PubMed] [Google Scholar]
- 235.Hu JW, Yang ZF, Li J, Hu B, Luo CB, Zhu K, Dai Z, Cai JB, Zhan H, Hu ZQ, Hu J, Cao Y, Qiu SJ, Zhou J, Fan J, Huang XW. TGM3 promotes epithelial-mesenchymal transition and hepatocellular carcinogenesis and predicts poor prognosis for patients after curative resection. Dig Liver Dis. 2020;52:668–676. doi: 10.1016/j.dld.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 236.Dai W, Xu L, Yu X, Zhang G, Guo H, Liu H, Song G, Weng S, Dong L, Zhu J, Liu T, Guo C, Shen X. OGDHL silencing promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J Hepatol. 2020;72:909–923. doi: 10.1016/j.jhep.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 237.Cannito S, Foglia B, Villano G, Turato C, Delgado TC, Morello E, Pin F, Novo E, Napione L, Quarta S, Ruvoletto M, Fasolato S, Zanus G, Colombatto S, Lopitz-Otsoa F, Fernández-Ramos D, Bussolino F, Sutti S, Albano E, Martínez-Chantar ML, Pontisso P, Parola M. SerpinB3 differently up-regulates hypoxia inducible factors-1α and -2α in hepatocellular carcinoma: mechanisms revealing novel potential therapeutic targets. Cancers (Basel) 2019;11:1933. doi: 10.3390/cancers11121933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu X, Sui X, Zhang C, Wei K, Bao Y, Xiong J, Zhou Z, Chen Z, Wang C, Zhu H, Tang F. Glutathione S-transferase A1 suppresses tumor progression and indicates better prognosis of human primary hepatocellular carcinoma. J Cancer. 2020;11:83–91. doi: 10.7150/jca.36495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhou Q, Huang T, Jiang Z, Ge C, Chen X, Zhang L, Zhao F, Zhu M, Chen T, Cui Y, Li H, Yao M, Li J, Tian H. Upregulation of SNX5 predicts poor prognosis and promotes hepatocellular carcinoma progression by modulating the EGFR-ERK1/2 signaling pathway. Oncogene. 2020;39:2140–2155. doi: 10.1038/s41388-019-1131-9. [DOI] [PubMed] [Google Scholar]
- 240.Dong ZR, Sun D, Yang YF, Zhou W, Wu R, Wang XW, Shi K, Yan YC, Yan LJ, Yao CY, Chen ZQ, Zhi XT, Li T. TMPRSS4 drives angiogenesis in hepatocellular carcinoma by promoting HB-EGF expression and proteolytic cleavage. Hepatology. 2019 doi: 10.1002/hep.31076. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 241.Liu Z, Chen M, Zhao R, Huang Y, Liu F, Li B, Qin Y. CAF-induced placental growth factor facilitates neoangiogenesis in hepatocellular carcinoma. Acta Biochim Biophys Sin (Shanghai) 2020;52:18–25. doi: 10.1093/abbs/gmz134. [DOI] [PubMed] [Google Scholar]
- 242.Tomás-Loba A, Manieri E, González-Terán B, Mora A, Leiva-Vega L, Santamans AM, Romero-Becerra R, Rodríguez E, Pintor-Chocano A, Feixas F, López JA, Caballero B, Trakala M, Blanco Ó, Torres JL, Hernández-Cosido L, Montalvo-Romeral V, Matesanz N, Roche-Molina M, Bernal JA, Mischo H, León M, Caballero A, Miranda-Saavedra D, Ruiz-Cabello J, Nevzorova YA, Cubero FJ, Bravo J, Vázquez J, Malumbres M, Marcos M, Osuna S, Sabio G. p38γ is essential for cell cycle progression and liver tumorigenesis. Nature. 2019;568:557–560. doi: 10.1038/s41586-019-1112-8. [DOI] [PubMed] [Google Scholar]
- 243.Shi X, Wang B, Chen X, Zheng Y, Ding Y, Wang C. Upregulation of ubiquitin-conjugating enzyme E2Z is associated with human hepatocellular carcinoma. Biochem Biophys Res Commun. 2020;523:25–32. doi: 10.1016/j.bbrc.2019.11.170. [DOI] [PubMed] [Google Scholar]
- 244.Ma X, Qiu Y, Zhu L, Zhao Y, Lin Y, Ma D, Qin Z, Sun C, Shen X, Li T, Han L. NOD1 inhibits proliferation and enhances response to chemotherapy via suppressing SRC-MAPK pathway in hepatocellular carcinoma. J Mol Med (Berl) 2020;98:221–232. doi: 10.1007/s00109-019-01868-9. [DOI] [PubMed] [Google Scholar]
- 245.Chen Y, Fu H, Zhang Y, Chen P. Transmembrane and ubiquitin-like domain containing 1 protein (TMUB1) negatively regulates hepatocellular carcinoma proliferation via regulating signal transducer and activator of transcription 1 (STAT1) Med Sci Monit. 2019;25:9471–9482. doi: 10.12659/MSM.920319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xiao Y, Huang S, Qiu F, Ding X, Sun Y, Wei C, Hu X, Wei K, Long S, Xie L, Xun Y, Chen W, Zhang Z, Liu N, Xiang S. Tumor necrosis factor α-induced protein 1 as a novel tumor suppressor through selective downregulation of CSNK2B blocks nuclear factor-κB activation in hepatocellular carcinoma. EBioMedicine. 2020;51:102603. doi: 10.1016/j.ebiom.2019.102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Guo Q, Yu DY, Yang ZF, Liu DY, Cao HQ, Liao XW. IGFBP2 upregulates ZEB1 expression and promotes hepatocellular carcinoma progression through NF-κB signaling pathway. Dig Liver Dis. 2020;52:573–581. doi: 10.1016/j.dld.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 248.Ye C, Zhang X, Chen X, Cao Q, Zhang X, Zhou Y, Li W, Hong L, Xie H, Liu X, Cao H, Wang YJ, Kang B. Multiple novel hepatocellular carcinoma signature genes are commonly controlled by the master pluripotency factor OCT4. Cell Oncol (Dordr) 2020;43:279–295. doi: 10.1007/s13402-019-00487-3. [DOI] [PubMed] [Google Scholar]
- 249.Huang J, Qu Q, Guo Y, Xiang Y, Feng D. Tankyrases/β-catenin signaling pathway as an anti-proliferation and anti-metastatic target in hepatocarcinoma cell lines. J Cancer. 2020;11:432–440. doi: 10.7150/jca.30976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yu Y, Xu P, Cui G, Xu X, Li K, Chen X, Bao J. UBQLN4 promotes progression of HCC via activating wnt-β-catenin pathway and is regulated by miR-370. Cancer Cell Int. 2020;20:3. doi: 10.1186/s12935-019-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu Q, Zhou C, Zhang B. Upregulation of musashi1 increases malignancy of hepatocellular carcinoma via the Wnt/β-catenin signaling pathway and predicts a poor prognosis. BMC Gastroenterol. 2019;19:230. doi: 10.1186/s12876-019-1150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Li J, Yan K, Yang Y, Li H, Wang Z, Xu X. Musashi-1 positively regulates growth and proliferation of hepatoma cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:1436–1442. doi: 10.12122/j.issn.1673-4254.2019.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhu C, Luo X, Wu J, Liu Y, Liu L, Ma S, Xie R, Wang S, Ji W. TM4SF1, a binding protein of DVL2 in hepatocellular carcinoma, positively regulates beta-catenin/TCF signalling. J Cell Mol Med. 2019 doi: 10.1111/jcmm.14787. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang R, Lin HM, Broering R, Shi XD, Yu XH, Xu LB, Wu WR, Liu C. Dickkopf-1 contributes to hepatocellular carcinoma tumorigenesis by activating the Wnt/β-catenin signaling pathway. Signal Transduct Target Ther. 2019;4:54. doi: 10.1038/s41392-019-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 255.Wang X, Wang R, Bai S, Xiong S, Li Y, Liu M, Zhao Z, Wang Y, Zhao Y, Chen W, Billiar TR, Cheng B. Musashi2 contributes to the maintenance of CD44v6+ liver cancer stem cells via notch1 signaling pathway. J Exp Clin Cancer Res. 2019;38:505. doi: 10.1186/s13046-019-1508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang N, Li MY, Liu Y, Yu J, Ren J, Zheng Z, Wang S, Yang S, Yang SL, Liu LP, Hu BG, Chong CC, Merchant JL, Lai PB, Chen GG. ZBP-89 negatively regulates self-renewal of liver cancer stem cells via suppression of Notch1 signaling pathway. Cancer Lett. 2020;472:70–80. doi: 10.1016/j.canlet.2019.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Moran B, Rahman A, Palonen K, Lanigan FT, Gallagher WM. Master transcriptional regulators in cancer: discovery via reverse engineering approaches and subsequent validation. Cancer Res. 2017;77:2186–2190. doi: 10.1158/0008-5472.CAN-16-1813. [DOI] [PubMed] [Google Scholar]
- 258.Li Z, Lou Y, Tian G, Wu J, Lu A, Chen J, Xu B, Shi J, Yang J. Discovering master regulators in hepatocellular carcinoma: one novel MR, SEC14L2 inhibits cancer cells. Aging (Albany NY) 2019;11:12375–12411. doi: 10.18632/aging.102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Du J, Zhao Z, Zhao H, Liu D, Liu H, Chen J, Cheng B, Zhai X, Yin Z, Zhang Y, Ling C. Sec62 promotes early recurrence of hepatocellular carcinoma through activating integrinα/CAV1 signalling. Oncogenesis. 2019;8:74. doi: 10.1038/s41389-019-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yang F, Zhang Y, Ren H, Wang J, Shang L, Liu Y, Zhu W, Shi X. Ischemia reperfusion injury promotes recurrence of hepatocellular carcinoma in fatty liver via ALOX12-12HETE-GPR31 signaling axis. J Exp Clin Cancer Res. 2019;38:489. doi: 10.1186/s13046-019-1480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


