Abstract
The anti-diabetes drug metformin has emerged as a promising antitumor agent in pancreatic ductal adenocarcinoma (PDAC) among other cancers by promoting the infiltration of immune cells in the tumor microenvironment (TME). However, the mechanisms underlying the antitumor effects of metformin in PDAC remain unclear. In this study, we revealed that metformin induced stimulator of interferon genes (STING) expression in pancreatic cancer cells in a dose- and time-dependent manner. Metformin also activated the STING/IRF3/IFN-β pathway by inhibiting AKT signaling in PDAC cells. Importantly, the combination of metformin with the STING agonist 2’3’-cGAMP exerted synergistic effects in activating the STING/IRF3/IFN-β pathway in pancreatic cancer cells. Additionally, metformin augmented the antitumor effects of 2’3’-cGAMP in mouse models by enhancing the infiltration of T cells in the TME. These findings unveiled a previously unknown mechanism contributing to the antitumor effects of metformin in PDAC, and provide a rationale for its use in combination with existing or novel immunotherapies.
Keywords: Metformin, STING, PDAC, AKT pathway
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related deaths worldwide; the 5-year overall survival rate of PDAC patients is only 8%, the lowest among all cancers [1,2]. According to the Global Burden of Diseases (GBD) study, pancreatic cancer was the eighth most common cause of cancer-related mortality in 2016 [3]. Despite the advances in surgical techniques and chemotherapy, the prognosis of PDAC patients remains poor. Recently, immune checkpoint blockade (ICB) has emerged as a promising treatment approach for patients that do not respond well to radiotherapy and chemotherapy; however, ICB has proven ineffective in treating PDAC patients, which may reflect the immunosuppressive tumor microenvironment (TME) of pancreatic cancer [4]. Thus, exploring novel therapeutic strategies for pancreatic cancer remains of high clinical importance.
The biguanide metformin is one of the most widely used anti-diabetes medications [5]. Mounting evidence suggests that metformin can reduce tumor burden in various cancer types, including lung, prostate, and colon [6-10]. It has also become evident that the antitumor effects of metformin are mediated through diverse mechanisms, including the inhibition of AMPK/mTOR [11] and insulin/insulin-like growth factor-1 (IGF-1) pathways [12]. Metformin has also been shown to suppress tumor growth by modulating antitumor immune responses, such as inducing immune cell infiltration in tumors [13], inhibiting the infiltration of tumor-associated macrophages (TAMs) [14], and promoting antitumor CD8+ T cell immune responses [15]. However, the mechanisms underlying the antitumor effects of metformin in PDAC remains unclear.
The stimulator of interferon genes (STING) is a transmembrane protein activated by cyclic dinucleotides (CDNs) generated by cyclic GMP-AMP synthase (cGAS) [16]. STING regulates the TME of solid tumors by promoting CD8+ T cell recruitment and activation [17]. STING activation results in the activation of TANK-binding kinase-1 (TBK1), phosphorylation of IRF-3, and production of type I interferons (IFNs) [18], which trigger tumor antigen cross-presentation and antitumor T cell immune responses [19]. Although interventions enhancing cGAS-cGAMP-STING signaling have been shown to exert potent anti-cancer effects [20], the therapeutic potential of STING pathway modulation in pancreatic cancer remains unclear.
Herein, we show that metformin represses tumor growth by promoting STING expression and activation, subsequently increasing T cells infiltration in the TME. Our findings unveil a previously unknown mechanism involved in the antitumor effects of metformin and support the potential therapeutic benefit of the combination of metformin with existing immunotherapies to treat pancreatic cancer.
Materials and methods
Cell culture and antibodies
The human PDAC cell lines PANC-1, BxPC-3, and SW1990 were purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Panc02 cells were obtained from Tong Pai Technology (Shanghai, China). PANC-1 cells were cultured in Dulbecco’s Modified Eagle Medium (HyClone Laboratories, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, USA) and 1% penicillin/streptomycin. BxPC-3 cells were cultured in RMPI-1640 (Gibco, USA) supplemented with 10% FBS, while SW1990 cells were maintained in L-15 medium (HyClone Laboratories, Logan, UT, USA) containing 10% FBS. All cell lines were maintained at 37°C in a humidified 5% CO2 atmosphere.
Antibodies against STING (19851-1-AP, 1:1000), GAPDH (10494-1-AP, 1:3000), AKT (10176-2-AP, 1:1000), and phospho-AKT (66444-1-Ig, 1:1000) were purchased from Proteintech, while those against IRF3 (4302S, 1:1000) and phospho-IRF3 (37829S, 1:1000) were obtained from Cell Signaling Technology.
Western blot
Cell lysates were prepared in RIPA lysis buffer (Beyotime Institute of Biotechnology, Guangzhou, China) supplemented with 1% protease and phosphatase inhibitors according to the manufacturer’s instructions. Protein concentrations were determined using a BCA protein assay kit (Pierce Biotechnology, USA) Subsequently, equal amounts of proteins were separated using 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Pierce Biotechnology, USA). Membranes were blocked with 5% non-fat milk in TBST (10 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.05% Tween-20) for 1 h at room temperature, followed by overnight incubation with primary antibodies at 4°C. After washing with TBST, membranes were incubated with HRP-conjugated secondary antibodies for 1 h. Finally, the membranes were incubated with ECL detection reagents and imaged using the ChemiDoc XRS imaging system (Bio-Rad Laboratories, Inc. Hercules, CA, USA). Protein band intensities were determined using Image Lab software (Bio-Rad Laboratories, Inc. Hercules, CA, USA); GAPDH was used as a loading control.
Quantitative real-time PCT (qRT-PCR)
TRIzol reagent (Thermo Fisher Scientific, USA) was used to extract total RNA from cells, and cDNA was synthesized using 2 μg of RNA and the cDNA Reverse Transcription kit (PrimeScript™ RT reagent Kit, Code No. RR037A). The qRT-PCR analysis was conducted using a PCR kit (TB Green™ Fast qPCR Mix, Code No. RR430A) according to the manufacturer’s instructions. Relative mRNA levels were calculated using the 2-ΔΔCt method were normalized to the respective GAPDH mRNA levels.
Enzyme-linked immunosorbent assay (ELISA)
IFN-β levels were determined by ELISA. Cells were lysed, and the supernate was collected after centrifugation. Standards and samples were added to the wells, which were pre-incubated with an anti-IFN-β monoclonal antibody. After a 2 h incubation, samples were incubated for 1 h with a detection antibody. Samples were then incubated with the HRP conjugate for 30 min, and the chromogenic substrate was added. After development in the dark for 30 min, stop solution was added, and absorbance (OD) at 450 nm was measured on a microplate reader. The curve-fitting method was used to plot a four-parameter logistic curve based on the standards and calculate IFN-β levels in the test samples.
Syngeneic tumor model and treatments
Six-week-old C57BL/6 mice were purchased from Charles River Laboratories (Wuhan, China). Panc02 cells (5 × 106 cells in 100 μL 1 × PBS) were injected subcutaneously into the right flanks. The tumor volume was measured every other day. The mass of tumors was also measured. Metformin was administered at 200 mg/kg daily, starting on the day of tumor cell transplantation. According to the Reagan-Shaw method for dose transformation from animal to human studies [21], the human equivalent of a murine dose of 200 mg/kg is 972 mg for an average-sized (60 kg) adult human. Therefore, this dose is clinically relevant and within the safe therapeutic range for humans. Five and ten days after implantation, 10 μg of 2’3’-cGAMP in Lipofectamine 2000 was injected into transplanted tumors. All experimental procedures involving animals were approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology.
The resecting tumors were dissociated in Cell Lytic M (#C2978; Sigma) supplemented with a protease inhibitor cocktail (S8820; Sigma). Following a 10 min incubation at room temperature, samples were centrifuged at 12,000 × g. Proteins from tumors were extracted for Western blot and ELISA [22].
Flow cytometry analysis
Mouse tumors were used to prepare singlecell suspensions. Samples were stained with the following antibodies: APC-conjugated anti-CD45 (#103112; Biolegend, USA); FITC-conjugated anti-CD4 (#100510; Biolegend, USA), and PE-conjugated anti-CD8 (#100708; Biolegend, USA). After 15 min incubation at room temperature, cells were washed three times with PBS, resuspended in PBS, and analyzed by flow cytometry. Data were analyzed with FlowJo.
Bioinformatics mining
The analytical tool Tumor IMmune Estimation Resource TIMER; (https://cistrome.shinyapps.io/timer/) [23] was used to estimate the correlation between STING or IFNB1 mRNA levels and the immune infiltration levels in pancreatic adenocarcinoma (PAAD). The correlation between the mRNA levels of STING and IFNB1 in PAAD was assessed using GEPIA (http://gepia.cancer-pku.cn/).
Statistical analysis
We used GraphPad Prism 6 software (GraphPad Software, Inc) for all statistical analyses. Statistical analyses were performed using one-sided or two-sided paired Student’s t-test for comparisons between two groups and one-way or two-way ANOVA with a post hoc test for multiple comparisons. P-values < 0.05 were considered statistically significant. All values were expressed as means ± standard deviation (SD).
Results
Metformin up-regulates STING in pancreatic cancer cells
It has been reported previously that STING signaling plays an important role in the regulation of immune cell infiltration in the TME [24]. To assess the relationship between STING and immune cell infiltration in PDAC, we used TIMER to analyze the association between STING mRNA levels and the infiltration levels of different T cell subsets. Interestingly, we found a significant association between STING expression levels and the infiltration levels of CD8+ and CD4+ T cells in PAAD (Figure 1A).
Figure 1.
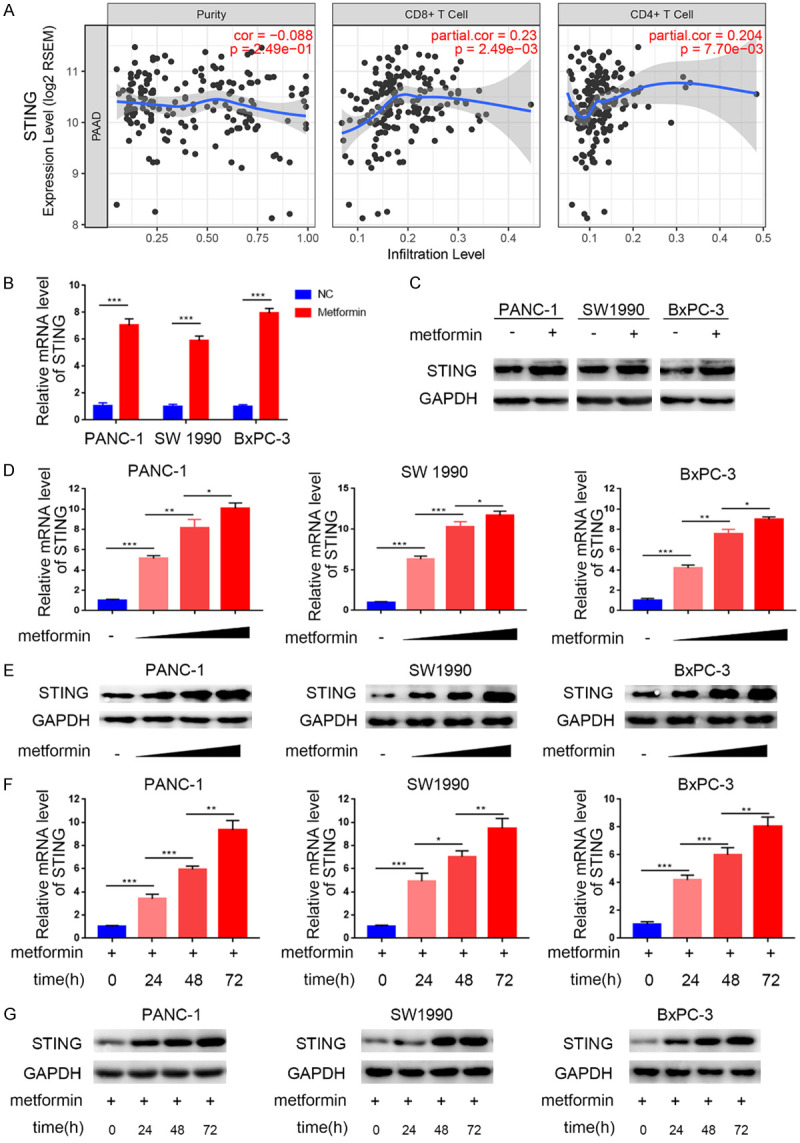
STING is up-regulated by metformin treatment in pancreatic cancer. (A) TIMER web tool was used to search the relationship of the mRNA expression level of STING and the infiltration of CD4+/CD8+ T cells. Purity was meanwhile measured as blank control. (B and C) RT-PCR (B) analysis to show the mRNA expression level of STING and Western blot analysis (C) to show the protein level of STING in PANC-1, SW1990, and BxPC-3 cell lines with/without treatment with metformin (5 mM) for 24 h. Data were shown as the mean ± SD of three independent experiments. GAPDH served as an internal reference. ***, P < 0.001. (D and E) RT-PCR analysis (D) to show the mRNA expression level and Western blot analysis (E) to show the protein expression level of STING in PANC-1, SW1990 and BxPC-3 cell lines with the treatment of metformin (0 mM, 1.25 mM, 2.5 mM, or 5 mM, respectively) for 24 h. Data were shown as the mean ± SD of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (F and G) RT-PCR analysis (F) to show the mRNA expression level and Western blot analysis (G) to show the protein expression level of STING in PANC-1, SW1990 and BxPC-3 cell lines with treatment of metformin (5 mM) for 0 h, 24 h, 48 h, or 72 h respectively. Data presented as the mean ± SD of three independent experiments. GAPDH served as an internal reference. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Previous studies have shown that metformin modulated antitumor immune responses by regulating T cell infiltration in the TME [13,25]. Hence, we investigated the effects of metformin on the STING pathway in three pancreatic cell lines. We found that metformin-treated pancreatic cells had higher STING mRNA and protein levels than control cells (Figure 1B, 1C). We also found that the metformin-induced STING up-regulation at the mRNA and protein levels was dose-dependent (Figure 1D-G). These results suggest that metformin induces the expression of STING in pancreatic cancer cells.
Metformin activates the STING/IRF3/IFN-β pathway in pancreatic cancer cells
STING pathway activation has been shown to induce immune cell infiltration by activating the IRF3/IFN-β pathway [17]. Here, we explored the ability of metformin to activate the STING/IRF3 pathway in pancreatic cancer cells. We found that metformin-treated cells had higher phosphorylation levels of IRF3 than control cells, indicating STING/IRF3 pathway activation (Figure 2A). We also found that STING mRNA levels were associated with those of IFNB1 (Figure 2B) and with CD4+/CD8+ T cell infiltration levels (Figure 2C) in pancreatic cancer. Furthermore, metformin treatment in pancreatic cancer cells induced IFN-β expression at the mRNA and protein level in a dose-dependent manner (Figure 2D-G), suggesting that metformin activated the STING/IRF3/IFN-β pathway in pancreatic cancer cells.
Figure 2.
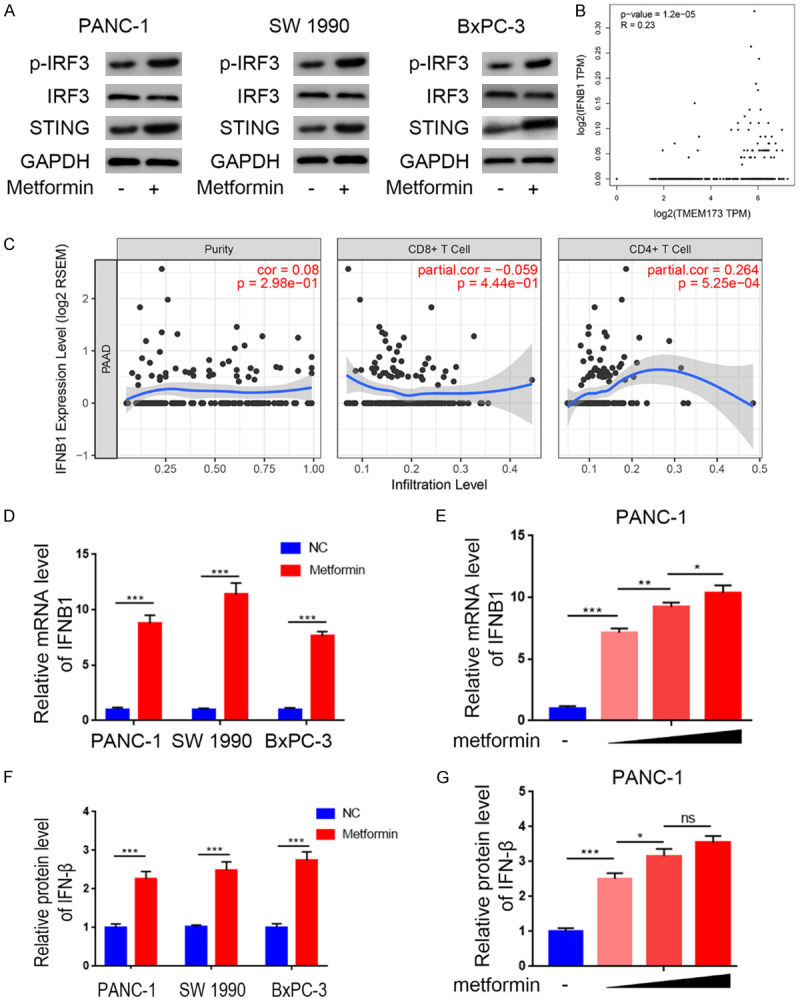
Metformin activated STING/IRF3/IFN-β pathway in pancreatic cancer cells. A. Western blot analysis to show the protein expression level of STING, IRF3, and p-IRF3 in PANC-1, SW1990 and BxPC-3 cell lines with or without treatment with metformin (5 mM) for 24 h. GAPDH served as an internal reference. B. GEPIA web tool was searched for the relationship between the of mRNA expression level of IFN-β and STING (TMEM173) in PAAD patients. C. TIMER web tool was searched for the relationship between the expression level of IFN-β and the infiltration of CD4+/CD8+ T cells, purity was meanwhile measured as blank control. D. RT-PCR analysis to show the mRNA expression level of IFN-β in PANC-1, SW1990, and BxPC-3 cell lines with the treatment of metformin (5 mM) for 24 h. Data presented as the mean ± SD of three independent experiments. ***, P < 0.001. E. RT-PCR analysis to show the mRNA expression level of IFN-β in PANC-1, SW1990, and BxPC-3 cell lines with the treatment with metformin(0 mM, 1.25 mM, 2.5 mM, or 5 mM, respectively) for 24 h. Data presented as the mean ± SD of three independent experiments.*, P < 0.05; **, P < 0.01; ***, P < 0.001. F. ELISA analysis of the protein expression level of IFN-β in PANC-1, SW1990, and BxPC-3 cell lines with the treatment of metformin(5 mM) for 24 h. Data presented as the mean ± SD of three independent experiments. ***, P < 0.001. G. ELISA analysis of the protein expression level of IFN-β in PANC-1, SW1990, and BxPC-3 cell lines with the treatment with metformin (0 mM, 1.25 mM, 2.5 mM, or 5 mM, respectively) for 24 h. Data presented as the mean ± SD of three independent experiments.ns, not significant; *, P < 0.05; ***, P < 0.001.
Metformin enhances STING expression by inhibiting the AKT pathway in pancreatic cancer cells
Previous studies have shown a negative correlation between AKT phosphorylation levels and STING pathway activation [26] and that metformin negatively regulates the AKT pathway in cancer cells [27,28]. Therefore, we examined whether the effects of metformin on STING expression levels in pancreatic cancer cells are mediated through the AKT signaling pathway. We found that metformin treatment in three different pancreatic cancer cell lines inhibited AKT phosphorylation (Figure 3A) while increasing STING expression. However, STING induction in response to metformin was concealed or abrogated by the AKT inhibitor MK2206 (Figure 3B, 3C). Conversely, the metformin-induced STING up-regulated was rescued when cells were treated with the AKT agonist SC79 (Figure 3D, 3E). These results suggest that the metformin-mediated STING up-regulation was dependent on AKT pathway inhibition.
Figure 3.
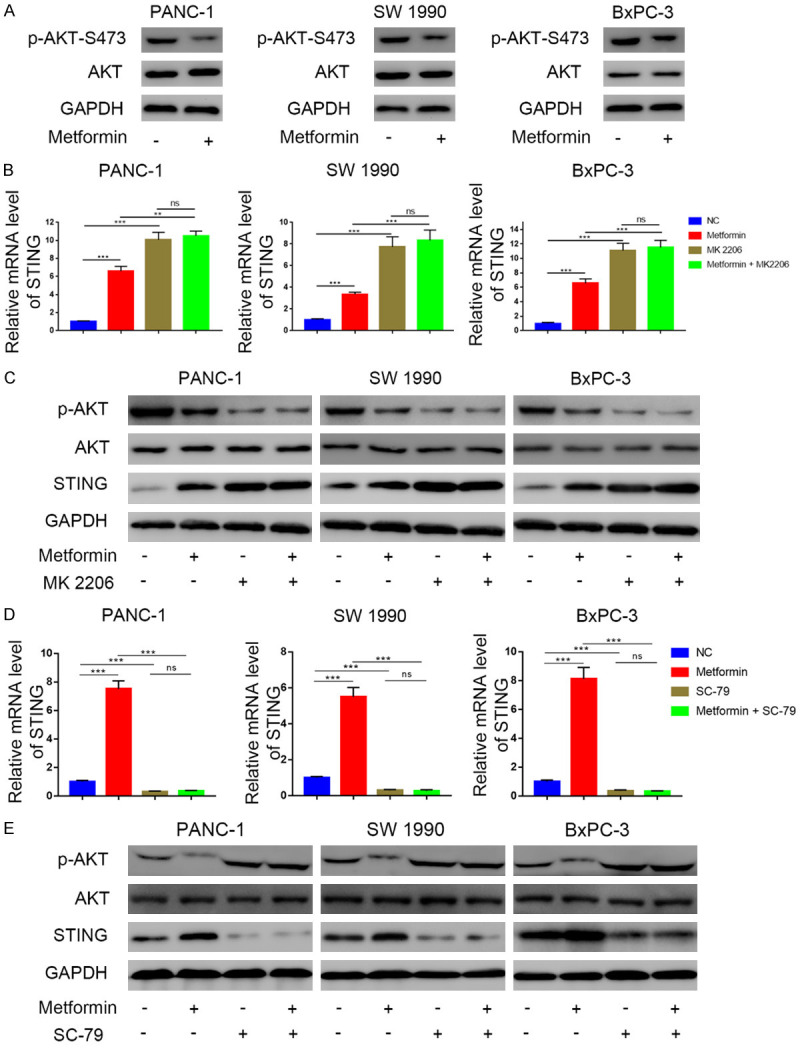
The promotion of metformin towards STING is driven by the suppression of phosphorylation level of AKT in pancreatic cancer. A. Western blot analysis to show the protein expression level of AKT and p-AKT in PANC-1, SW1990 and BxPC-3 cell lines with or without treatment with metformin (5 mM) for 24 h. GAPDH served as an internal reference. B. RT-PCR analysis to show the mRNA expression level of STING in PANC-1, SW1990 and BxPC-3 cell lines treated with metformin (5 mM), MK2206 (10 μM), or the combination of metformin (5 mM) and MK2206 (10 μM). Data presented as the mean ± SD of three independent experiments. ns, not significant; **, P < 0.01; ***, P < 0.001. C. Western blot analysis to show the protein expression level of p-AKT, STING in PANC-1, SW1990 and BxPC-3 cell lines treated with metformin (5 mM), MK2206 (10 μM), or the combination of metformin (5 mM) and MK2206 (10 μM). GAPDH served as an internal reference. D. RT-PCR analysis to show the mRNA expression level of STING in PANC-1, SW1990 and BxPC-3 cell lines treated with metformin (5 mM), SC79 (20 μM), or the combination of metformin (5 mM) and SC79 (20 μM). Data presented as the mean ± SD of three independent experiments. ns, not significant; **, P < 0.01; ***, P < 0.001. E. Western blot analysis to show the protein level of p-AKT, STING in PANC-1, SW1990 and BxPC-3 cell lines treated with metformin (5 mM), SC79 (20 μM), or the combination of metformin (5 mM) and SC79 (20 μM). GAPDH served as an internal reference.
Metformin and cGAMP treatment synergistically activate the STING/IRF3/IFN-β pathway in pancreatic cancer cells
Having established that metformin activates the STING/IRF3/IFN-β pathway in pancreatic cancer cells, we investigated the potential synergistic effects of the STING pathway agonist 2’3’-cGAMP in pancreatic cancer when combined with metformin. We found that 2’3’-cGAMP increased p-IRF3 and IFN-β levels in pancreatic cancer cells (Figure 4A, 4B); this increase was potentiated when cells were simultaneously treated with metformin and 2’3’-cGAMP (Figure 4C, 4D). These results demonstrated the synergistic effects of metformin and 2’3’-cGAMP on STING/IRF3/IFN-β pathway activation (Figure 6).
Figure 4.
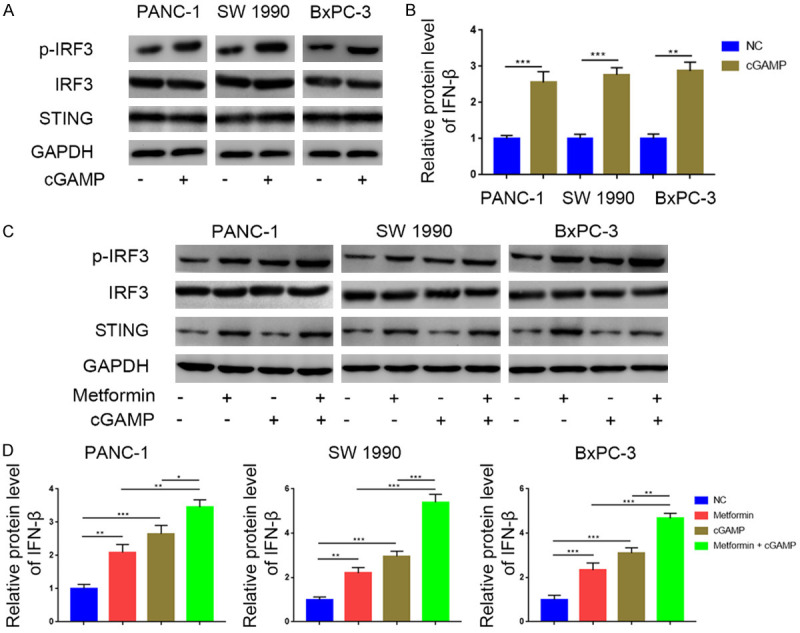
Metformin and cGAMP treatment synergistically activated the STING/IRF3/IFN-β pathway in pancreatic cancer. A. Western blot analysis to show the protein expression level of STING, IRF3, and p-IRF3 in PANC-1, SW1990 and BxPC-3 cell lines treated with or without 2’3’-cGAMP (150 nM). GAPDH served as an internal reference. B. ELISA analysis to show the protein expression level of IFN-β in PANC-1, SW1990 and BxPC-3 cell lines treated with or without 2’3’-cGAMP (150 nM). Data presented as the mean ± SD of three independent experiments. **, P < 0.01; ***, P < 0.001. C. Western blot analysis to show the protein expression level of STING, IRF3, and p-IRF3 in PANC-1, SW1990 and BxPC-3 cell lines treated with metformin (5 mM), 2’3’-cGAMP (150 nM) or combination of metformin (5 mM) and 2’3’-cGAMP (150 nM). D. ELISA analysis to show the protein expression level of IFN-β in PANC-1, SW1990 and BxPC-3 cell lines treated with metformin (5 mM), 2’3’-cGAMP (150 nM), or combination of metformin (5 mM) and 2’3’-cGAMP (150 nM). GAPDH served as an internal reference. **, P < 0.01; ***, P < 0.001.
Figure 6.
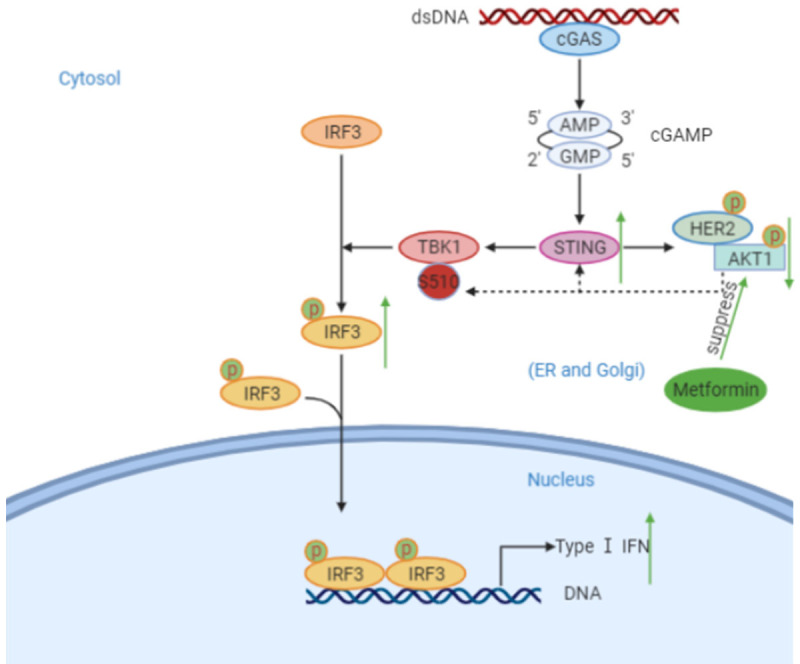
Scheme showing the activation of the STING pathway by cytosolic DNA and regulation by metformin. Cytosolic dsDNA is recognized by cGAS, which leads to the generation of cGAMP. cGAMP binds and activates STING, which then translocate from the ER to perinuclear sites. This translocation results in the recruitment and activation of TBK1 by autophosphorylation. The activation of TBK1 phosphorylates the transcription factor IRF3, which translocates to the nucleus to induce transcription of type I IFN genes. Metformin can suppress the phosphorylation of AKT and promotion the expression of STING, which then further leads to the up-regulation of phosphorylation of IRF3 and the generation of IFN-β.
Metformin suppresses tumor growth and promotes T cell infiltration in vivo
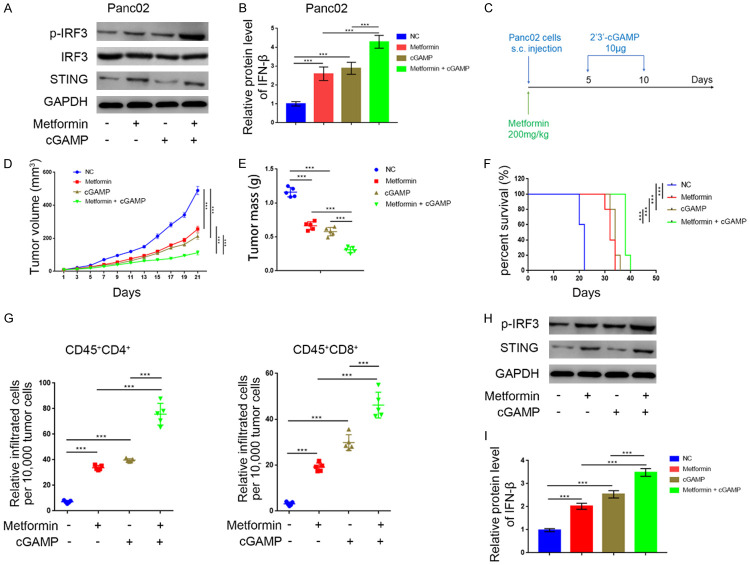
Next, we investigated the effects of metformin in T cell infiltration in vivo. We confirmed that metformin activated the STING/IRF3/IFN-β pathway in murine Panc02 tumor cells, and the combination of metformin and 2’3’-cGAMP further increased p-IRF3 and IFN-β levels compared with metformin or 2’3’-cGAMP alone (Figure 5A, 5B). After subcutaneously injecting Panc02 cells into the flanks of C57BL immune-proficient mice, we treated mice with metformin and 2’3’-cGAMP (Figure 5C). STING pathway activation after treatment with metformin or 2’3’-cGAMP significantly reduced tumor volume and mass. Importantly, the combination of metformin and 2’3’-cGAMP showed a synergistic effect in inhibiting tumor growth (Figure 5D, 5E) and prolonging the survival of tumor-bearing mice (Figure 5F). Moreover, treatment with metformin and 2’3’-cGAMP alone or in combination promoted tumor infiltration by CD45+CD4+ and CD45+CD8+ T cells (Figure 5G), which may explain their synergistic effect in suppressing tumor growth. Additionally, we confirmed that the combination of metformin and 2’3’-cGAMP had a synergistic effect on STING/IRF3/IFN-β pathway activation in vivo (Figure 5H, 5I). These results suggest that metformin suppressed tumor growth and promoted T cell infiltration in vivo, especially when mice were also treated with 2’3’-cGAMP.
Figure 5.
Metformin suppressed the tumor growth and promoted the infiltration level of T cells in vivo. A. Western blot analysis to show the protein expression level of STING, IRF3, and p-IRF3 in Panc02 cell line treated with metformin (5 mM), 2’3’-cGAMP (150 nM), or the combination of metformin (5 mM) and 2’3’-cGAMP (150 nM). GAPDH served as an internal reference. B. ELISA analysis to show the protein expression level of IFN-β in Panc02 cell line treated with metformin, 2’3’-cGAMP (150 nM), or combination of metformin (5 mM) and 2’3’-cGAMP (150 nM). GAPDH served as an internal reference. ***, P < 0.001. C. Schematic diagram of the procedure of in vivo experiments, cGAMP and metformin doses were indicated above. D. The Panc02 tumors growth curves (n = 5/group). Groups were compared with each other. ***, P < 0.001. E. The Panc02 tumors mass (n = 5/group). Groups were compared with each other. ***, P < 0.001. F. Kaplan-Meier percent survival curves for each group with different treatments. ***, P < 0.001. G. Flow cytometry analysis to show the percentage of CD45+CD4+ and CD45+CD8+ T cells infiltrated per 10,000 tumor cells. Data presented as the mean ± SD of five independent experiments. ***, P < 0.001. H. Western blot analysis to show the protein expression level of STING, IRF3, and p-IRF3 in the collected tumors. I. ELISA analysis to show the protein expression level of IFN-β in in the collected tumors. GAPDH served as an internal reference. ***, P < 0.001.
Discussion
Tumors cells can escape the host’s immune responses through multifarious tumor cell-intrinsic and extrinsic mechanisms [19], contributing to immunotherapy resistance. The particularly low immunogenicity of pancreatic cancer, possibly due to the presence of an extensive fibroinflammatory and desmoplastic stroma with a rich extracellular matrix, is largely responsible for the absence of infiltrating CD8+ T cells and immunotherapy failure [29]. Therefore, improved immunotherapies are of utmost necessity.
The anti-diabetes drug metformin has recently emerged as a promising antitumor agent in various cancers, including prostate cancer [30], ovarian cancer [31], and non-small cell lung cancer (NSCLC) [8]. The discovery of the potent antitumor effects of metformin has provided hope for cancer treatment, especially pancreatic cancer, which shows little to no response to surgery, radiotherapy, and chemotherapy. Notably, preliminary studies have shown that metformin improved outcomes in pancreatic cancer patients [32]. In this study, we confirmed metformin’s ability to inhibit tumor growth and prolong survival of tumor-bearing mice, further supporting its potential use to treat pancreatic cancer.
The mechanisms underlying metformin’s antitumor effects remain unclear, especially in regard to immune regulation. Previous studies have shown that metformin downregulates CD39+/CD73+ T cells infiltration [33], inhibits the infiltration of TAMs [14], and induces tumor infiltration by inflammatory cells [13]. In this study, we demonstrated that metformin enhanced the infiltration of CD4+ and CD8+ T cells in the TME, which could explain the ability of metformin to inhibit pancreatic cancer growth and provides a rationale for its use in combination with existing or novel immunotherapies.
The STING pathway plays an essential role in antitumor immune responses [34] by inducing the production of type I interferons (IFNs) and other pro-inflammatory cytokines [35]. STING/TBK1/IRF3 also promotes immune cell infiltration [36]. In this study, we confirmed that STING levels positively correlated with the extent of CD4+/CD8+ T cell infiltration in pancreatic tumors. We also identified STING/IRF3/IFN-β pathway activation as a previously unknown mechanism contributing to the antitumor effects of metformin.
HER2/AKT signaling has been previously shown to negatively regulate the STING pathway [27], and metformin has been demonstrated to improve clinical outcomes in HER2+ breast cancer [37]. In this study, we confirmed the link between metformin, AKT, and STING in pancreatic cancer. We also showed that metformin enhanced STING expression by suppressing AKT phosphorylation and subsequent activation, unveiling a previously unknown mechanism involved in the metformin-mediated STING pathway activation (Figure 6).
The STING agonist 2’3’-cGAMP has been shown to modulate the TME and reduce the tumor burden in pancreatic cancer [38]. In this study, we showed that 2’3’-cGAMP suppressed tumor growth and prolonged the survival of tumor-bearing mice. Importantly, 2’3’-cGAMP promoted the infiltration of CD4+ and CD8+ T cells in the TME; all these effects were potentiated by the combined treatment with metformin and 2’3’-cGAMP. 2’3’-cGAMP is a second messenger of the STING pathway [39] directly binding to and activating STING [40,41]. Our findings provide evidence that the combined treatment with metformin and 2’3’-cGAMP exerts synergistic antitumor effects because they activate the STING/IRF3/IFN-β via different mechanisms.
Conclusion
In this study, we established the ability of metformin to suppress tumor growth in pancreatic cancer. Importantly, metformin inhibited AKT phosphorylation and induced STING expression, activating the STING/IRF3/IFN-β pathway and promoting T cells infiltration in the TME. We also demonstrated that metformin augmented the antitumor effects of 2’3’-cGAMP in pancreatic cancer, providing a rationale for its use in combination with cGAMP or other STING-activating agents (Figure 6). Future clinical studies are required to further investigate the clinical benefits of metformin combined with existing or novel immunotherapies.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81702374 (X.J.)), Hepatobiliary and pancreatic cancer research foundation of Xiaoping Chen science and technology development foundation (Grant No. CXPJJH11900001-2019219 and No. CXPJJH11900001-2019335).
Disclosure of conflict of interest
None.
Abbreviations
- PDAC
Pancreatic ductal adenocarcinoma
- STING
stimulator of interferon genes
- GEPIA
Gene Expression Profiling Interaction Analysis
- IRF3
Interferon regulatory factor 3
- IFN-β
interferon-beta
- HER2
human epidermal growth factor receptor 2
- AKT
protein kinase B
- 2’3’-cGAMP
2’3’-cyclic GMP-AMP
- CDNs
cyclic dinucleotides
- cGAS
cyclic GMP-AMP synthase
- dsDNA
double-stranded DNA
- TBK1
TANK-binding kinase-1
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, Aremu O, Artaman A, Asgedom SW, Assadi R, Atey TM, Avila-Burgos L, Awasthi A, Ba Saleem HO, Barac A, Bennett JR, Bensenor IM, Bhakta N, Brenner H, Cahuana-Hurtado L, Castaneda-Orjuela CA, Catala-Lopez F, Choi JJ, Christopher DJ, Chung SC, Curado MP, Dandona L, Dandona R, das Neves J, Dey S, Dharmaratne SD, Doku DT, Driscoll TR, Dubey M, Ebrahimi H, Edessa D, El-Khatib Z, Endries AY, Fischer F, Force LM, Foreman KJ, Gebrehiwot SW, Gopalani SV, Grosso G, Gupta R, Gyawali B, Hamadeh RR, Hamidi S, Harvey J, Hassen HY, Hay RJ, Hay SI, Heibati B, Hiluf MK, Horita N, Hosgood HD, Ilesanmi OS, Innos K, Islami F, Jakovljevic MB, Johnson SC, Jonas JB, Kasaeian A, Kassa TD, Khader YS, Khan EA, Khan G, Khang YH, Khosravi MH, Khubchandani J, Kopec JA, Kumar GA, Kutz M, Lad DP, Lafranconi A, Lan Q, Legesse Y, Leigh J, Linn S, Lunevicius R, Majeed A, Malekzadeh R, Malta DC, Mantovani LG, McMahon BJ, Meier T, Melaku YA, Melku M, Memiah P, Mendoza W, Meretoja TJ, Mezgebe HB, Miller TR, Mohammed S, Mokdad AH, Moosazadeh M, Moraga P, Mousavi SM, Nangia V, Nguyen CT, Nong VM, Ogbo FA, Olagunju AT, Pa M, Park EK, Patel T, Pereira DM, Pishgar F, Postma MJ, Pourmalek F, Qorbani M, Rafay A, Rawaf S, Rawaf DL, Roshandel G, Safiri S, Salimzadeh H, Sanabria JR, Santric Milicevic MM, Sartorius B, Satpathy M, Sepanlou SG, Shackelford KA, Shaikh MA, Sharif-Alhoseini M, She J, Shin MJ, Shiue I, Shrime MG, Sinke AH, Sisay M, Sligar A, Sufiyan MB, Sykes BL, Tabares-Seisdedos R, Tessema GA, Topor-Madry R, Tran TT, Tran BX, Ukwaja KN, Vlassov VV, Vollset SE, Weiderpass E, Williams HC, Yimer NB, Yonemoto N, Younis MZ, Murray CJL, Naghavi M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Bosch N, Vinaixa J, Navarro P. Immune evasion in pancreatic cancer: from mechanisms to therapy. Cancers (Basel) 2018;10:6. doi: 10.3390/cancers10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 6.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 8.Lin JJ, Gallagher EJ, Sigel K, Mhango G, Galsky MD, Smith CB, LeRoith D, Wisnivesky JP. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med. 2015;191:448–454. doi: 10.1164/rccm.201407-1395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 11.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 12.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haikala HM, Anttila JM, Marques E, Raatikainen T, Ilander M, Hakanen H, Ala-Hongisto H, Savelius M, Balboa D, Von Eyss B, Eskelinen V, Munne P, Nieminen AI, Otonkoski T, Schuler J, Laajala TD, Aittokallio T, Sihto H, Mattson J, Heikkila P, Leidenius M, Joensuu H, Mustjoki S, Kovanen P, Eilers M, Leverson JD, Klefstrom J. Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti-PD-1 immunotherapy. Nat Commun. 2019;10:620. doi: 10.1038/s41467-019-08541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Tong D, Liu G, Gao J, Wang LA, Xu J, Yang X, Xie Q, Huang Y, Pang J, Wang L, He Y, Zhang D, Ma Q, Lan W, Jiang J. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin Cancer Res. 2018;24:5622–5634. doi: 10.1158/1078-0432.CCR-18-0420. [DOI] [PubMed] [Google Scholar]
- 15.McCloskey CW, Cook DP, Kelly BS, Azzi F, Allen CH, Forsyth A, Upham J, Rayner KJ, Gray DA, Boyd RW, Murugkar S, Lo B, Trudel D, Senterman MK, Vanderhyden BC. Metformin abrogates age-associated ovarian fibrosis. Clin Cancer Res. 2020;26:632–642. doi: 10.1158/1078-0432.CCR-19-0603. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantelidou C, Sonzogni O, De Oliveria Taveira M, Mehta AK, Kothari A, Wang D, Visal T, Li MK, Pinto J, Castrillon JA, Cheney EM, Bouwman P, Jonkers J, Rottenberg S, Guerriero JL, Wulf GM, Shapiro GI. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9:722–737. doi: 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrales L, McWhirter SM, Dubensky TW Jr, Gajewski TF. The host STING pathway at the interface of cancer and immunity. J Clin Invest. 2016;126:2404–2411. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, Ascano M, Kelley M, Johnson DB, Balko JM, Wilson JT. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14:269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 22.Francica BJ, Ghasemzadeh A, Desbien AL, Theodros D, Sivick KE, Reiner GL, Hix Glickman L, Marciscano AE, Sharabi AB, Leong ML, McWhirter SM, Dubensky TW Jr, Pardoll DM, Drake CG. TNFalpha and radioresistant stromal cells are essential for therapeutic efficacy of cyclic dinucleotide sting agonists in nonimmunogenic tumors. Cancer Immunol Res. 2018;6:422–433. doi: 10.1158/2326-6066.CIR-17-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, Huttenlocher A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70:710–721. doi: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Zhang Q, Zhang F, Meng F, Liu S, Zhou R, Wu Q, Li X, Shen L, Huang J, Qin J, Ouyang S, Xia Z, Song H, Feng XH, Zou J, Xu P. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nature Cell Biology. 2019;21:1027–1040. doi: 10.1038/s41556-019-0352-z. [DOI] [PubMed] [Google Scholar]
- 27.Odell ID, Flavell RA. HER2 joins AKT to inhibit STING immunity. Nat Cell Biol. 2019;21:917–918. doi: 10.1038/s41556-019-0368-4. [DOI] [PubMed] [Google Scholar]
- 28.Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Velez-Delgado A, Mathew E, Li D, Mendez FM, Flannagan K, Rhim AD, Simeone DM, Beatty GL, Pasca di Magliano M. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 2017;66:124–136. doi: 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campi R, Brookman-May SD, Subiela Henriquez JD, Akdogan B, Brausi M, Klatte T, Langenhuijsen JF, Linares-Espinos E, Marszalek M, Roupret M, Stief CG, Volpe A, Minervini A, Rodriguez-Faba O. Impact of metabolic diseases, drugs, and dietary factors on prostate cancer risk, recurrence, and survival: a systematic review by the European association of urology section of oncological urology. Eur Urol Focus. 2019;5:1029–1057. doi: 10.1016/j.euf.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol. 2011;123:200–204. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 32.Pearson RK. Antidiabetic therapies affect risk of pancreatic cancer. Yearb Med. 2010;2010:430–431. [Google Scholar]
- 33.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, Zhang C, Yue D, Qin G, Zhang T, Li F, Chen X, Ping Y, Wang D, Gao Q, He Q, Huang L, Li H, Huang J, Zhao X, Xue W, Sun Z, Lu J, Yu JJ, Zhao J, Zhang B, Zhang Y. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018;78:1779–1791. doi: 10.1158/0008-5472.CAN-17-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 2020;10:26–39. doi: 10.1158/2159-8290.CD-19-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q, Manohar M, Wei Y, Pandol SJ, Habtezion A. STING signalling protects against chronic pancreatitis by modulating Th17 response. Gut. 2019;68:1827–1837. doi: 10.1136/gutjnl-2018-317098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, Cristea S, Nguyen T, Diao L, Li L, Fan Y, Yang Y, Wang J, Glisson BS, Wistuba II, Sage J, Heymach JV, Gibbons DL, Byers LA. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9:646–661. doi: 10.1158/2159-8290.CD-18-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J, Johnson BD, Dwinell MB. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;7:115. doi: 10.1186/s40425-019-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, Gravekamp C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2:901–910. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Škrnjug I, Guzmán CA, Ruecker C. Cyclic GMP-AMP displays mucosal adjuvant activity in mice. PLoS One. 2014;9:e110150. doi: 10.1371/journal.pone.0110150. [DOI] [PMC free article] [PubMed] [Google Scholar]