Enteroendocrine cells, key sensors of gut microbiota and/or microbial metabolites, play crucial roles in mucosal immunity, gut barrier function, visceral hyperalgesia, and gastrointestinal (GI) motility, thereby regulating several GI diseases, including inflammatory bowel disease.
Keywords: enteroendocrine cells, gut microbiota, inflammatory bowel disease, immune system, gut dysfunction
Abstract
Host sensing in the gut microbiota has been crucial in the regulation of intestinal homeostasis. Although inflammatory bowel diseases (IBDs), multifactorial chronic inflammatory conditions of the gastrointestinal tract, have been associated with intestinal dysbiosis, the detailed interactions between host and gut microbiota are still not completely understood. Enteroendocrine cells (EECs) represent 1% of the intestinal epithelium. Accumulating evidence indicates that EECs are key sensors of gut microbiota and/or microbial metabolites. They can secrete cytokines and peptide hormones in response to microbiota, either in traditional endocrine regulation or by paracrine impact on proximal tissues and/or cells or via afferent nerve fibers. Enteroendocrine cells also play crucial roles in mucosal immunity, gut barrier function, visceral hyperalgesia, and gastrointestinal (GI) motility, thereby regulating several GI diseases, including IBD. In this review, we will focus on EECs in sensing microbiota, correlating enteroendocrine perturbations with IBD, and the underlying mechanisms.
INTRODUCTION
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC) and Crohn’s disease (CD), is a group of chronic, recurrent, and relapsing diseases in the human gastrointestinal tract characterized by chronic intestinal inflammation and extra-intestinal manifestations.1 The incidence of IBD has been increasing worldwide in recent years, consequently leading to an economic burden to both patients and society.2, 3 Etiological studies of IBD have proposed several factors contributing to the occurrence of IBD, including the altered gut microbiota, abnormal host immune responses, genetic predispositions, and environmental factors.4, 5 Enteroendocrine cells (EECs) represent around 1% of the total intestinal epithelium. Accumulating studies indicate that interactions between EECs and the gut microbiota may play an essential role in the pathogenesis of IBD. In this review, we will present an overview of the latest advances for roles of EECs in maintaining intestinal homeostasis. A comprehensive understanding of the functions of EECs would broaden our understanding of the mechanisms underlying the pathophysiology of IBD, and could thus identify potential therapeutic options for treatment of these diseases. For readers who are interested in the roles of enteroendocrine cells in inflammation beyond IBD, please read the elegant review article by Worthington et al.6
ENTEROENDOCRINE CELLS
Enteroendocrine cells are dispersed as single cells along the gastrointestinal mucosa in the crypts and villi and represent the largest endocrine system of the human body (Fig. 1).7, 8 Traditionally, EECs can be divided into more than 10 different cell types based on their major secretory hormones (Table 1), including members of the chromogranin/secretogranin family, serotonin (5-HT), somatostatin, neuropeptide Y (NPY), vasoactive intestinal peptide (VIP), substance P (SP), cholecystokinin (CKK), glucagon-like peptide (GLP)-1/2, and Ghrelin.9, 10 Of note, enterochromaffin cells, the largest population of EECs, can synthesize and secret 5-HT, accounting for >95% of the whole body’s 5-HT.11 Emerging evidence also demonstrates that several peptide hormones are likely to be co-expressed in the same EECs.12, 13 For instance, enteroendocrine L-cells have been shown to express glucose-dependent insulinotropic peptide (GIP), cholecystokinin (CCK) neurotensin, and somatostatin in addition to GLP and peptide YY (PYY).14, 15 The hormones produced by EECs may play essential roles in the regulation of nutrient absorption, intestinal immune response, epithelial barrier defense, visceral hyperalgesia, and colonic motility.16 Chromogranin-A (CgA) is a heat-stable and soluble protein, which is stored and released from storage granules in the EECs.17 Within the gut, several CgA-derived peptides (CgDPs) are generated by proteolytic cleavage of CgA (Table 2), such as vasostatin (VS), catestatin (CST), chromofungin (CHR), pancreastatin (PST), and serpinin.18–20
FIGURE 1.
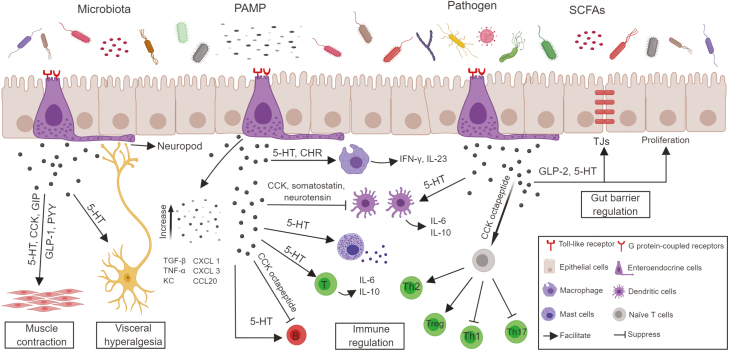
Enteroendocrine cell influence on gut function in inflammatory bowel disease. Enteroendocrine cells possess multiple chemosensory receptors that can detect intestinal microbiota and microbial metabolites. In response, EECs can secrete peptide hormones and classical cytokines to the surrounding immune cells and modulate both the innate and adaptive immune systems. Besides that, EECs possess cytoplasmic processes in close proximity to enteric nerve terminals. The released hormones may regulate the visceral hyperalgesia and intestinal motility in a paracrine fashion, along with synaptic transmission. Furthermore, enteroendocrine hormones can modulate the intestinal epithelial barrier function through both transcellular and paracellular pathways. All this evidence predicts the crucial role of EECs in the pathophysiology of IBD.
TABLE 1.
EEC Cell Subtypes Based on Major Secretary Hormones
| Cell Types | Major Secretory Hormones | Digestive Function |
|---|---|---|
| A (X-like) cell | Ghrelin, nesfatin-1 | Appetite control, growth hormone release |
| G cell | Gastrin | Acidity, GI motility |
| D cell | Somatostatin | GI hormone release, GI motility, mucosal immunity |
| L cell | GLP-1, GLP-2, PYY | Appetite control, GI motility, energy homeostasis |
| K cell | GIP | Insulin secretion |
| I cell | CCK | Appetite control, GI motility, bile acid and digestive enzyme release, mucosal immunity |
| Enterochromaffin cell | 5-HT | Appetite control, GI motor and secretory function, mucosal immunity |
| N cell | Neurotensin | GI motility, mucosal immunity |
| M cell | Motilin | GI motility |
| S cell | Secretin | Acidity, body fluid homeostasis |
| Enterochromaffin-like cell | Histamine | Acidity, mucosal immunity |
| P cell | Leptin | Appetite control, nutrients absorption, mucosal immunity |
TABLE 2.
Physiological Functions of Chromogranin-A and its Derived Peptides in Intestinal Homeostasis
| Molecule | Physiological Functions |
|---|---|
| CgA | Hormone and/or neuropeptide storage and release, gut microbiota composition, mucosal immunity, Ca2+ homeostasis |
| VS | Regulate intestinal permeability, repair intestinal mucosa, intestinal inflammation, GI motility |
| CST | Modulate leptin signaling, intestinal inflammation |
| CHR | Regulate GI motility, intestinal inflammation, epithelial tight junctions |
| PST | Regulate insulin secretion, gastric acid secretion, intestinal inflammation |
| Prochromacin | Antimicrobial activities |
| WE-14 | Regulate intestinal inflammation |
| Parastatin | Regulate inflammation through vitamin D metabolic pathway |
| Serpinin | Regulate plasmin-induced inflammation and decrease cell death |
Abbreviation: WE-14, a neuropeptide derived from the post-translational processing of CgA.
Enteroendocrine cells have specialized sensory microvilli reaching to the lumen and respond to intestinal luminal nutrients and/or microbiota by releasing hormones in a classical endocrine fashion. Further, morphological studies have revealed that several long pseudopod-like basal processes named “neuropods” extend from EECs, and the terminal end of these processes often resembles a synapse, which seems to interface with intestinal neurons or epithelial cells.21, 22 These studies suggest that the EECs may affect neighboring cells or neurons by exocytosis of biological mediators through a paracrine effect, or by directly activating the afferent synaptic transmission.23, 24
GUT MICROBIOTA–ENTEROENDOCRINE CELL AXIS
The gastrointestinal microbiota consists of a group of microorganisms (bacteria, viruses, and some eukaryotes), with concentrations of up to 1011–1012 cells/g luminal contents.25 A healthy gut is colonized by >500 different microbial species.26 The microbiota has established a harmonious ecosystem within the human organism, which contributes to the maintenance of normal immunological functions, facilitates nutrient digestion and absorption, prevents growth of pathogenic bacteria, and produces a variety of biologically important compounds, such as short-chain fatty acids (SCFAs), including principally acetate, propionate, and butyrate.27, 28 Within the gut, acetate and propionate are mainly produced by bacteria of the Bacteroidetes phylum, whereas butyrate is generated by those of the Firmicutes phylum.29 Notably, Akkermansia muciniphila, which is a mucin-degrading bacterium located in the colonic mucus layer, has been shown to produce SCFAs such as acetate and propionate.30
For the last 2 decades, IBD has been one of the most extensively investigated inflammatory disorders that are closely related with the altered gut microbiota. Increasing evidence has shown that reduced intestinal microbiota biodiversity and microbial dysbiosis appear to be important factors in the pathogenesis of IBD.31–34 Large-scale gut microbiome sequencing associated with IBD reveals an increased prevalence of proteobacteria, including Escherichia coli and Shigella, and a significant decline of known beneficial bacteria, such as Firmicutes, Enterobacteriaceae, Bacteroidetes, Roseburia intestinalis, and the Clostridium XIVa and IV groups.31–35 Further, an increased number of the genus Fusobacterium has been reported in the colonic mucosa of patients with UC.36 Additionally, decreased butyrate-producing bacteria such as Faecalibacterium prausnitzii have been shown in IBD patients, which may account for the decreased amount of SCFAs in fecal samples from these patients.37
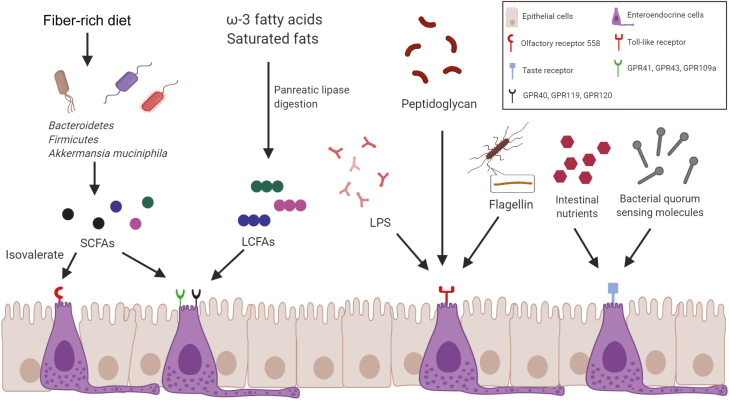
One potential mechanism underlying the interaction between the gut microbiota and IBD is microbial endocrinology, in which the bacteria regulate neuroendocrine hormone production in the host, which has been a focus of intense interest in recent years. Enteroendocrine cells express a vast array of receptors that play key roles in gut sensing (Fig. 2). Enteroendocrine cells express G protein-coupled receptors (GPCRs), including GPR40, GPR41, GPR43, GPR119, and GPR120, which have been identified as sensing receptors for gut microbiota-derived SCFAs,38 or long-chain fatty acids (LCFAs) from triglyceride metabolites by pancreatic lipase digestion.39 Both in vitro and in vivo studies demonstrate that EECs also express functional Toll-like receptors (TLRs; eg, TLR1, TLR2, TLR4, etc.) and respond to intestinal bacterial TLR ligands,40 indicating that EECs might play an essential role in immune surveillance of luminal contents. Taste receptors (T1Rs and T2Rs, etc.) can respond to intestinal nutrients and beneficial compounds and play critical roles in nutrient assimilation and regulation of glucose homeostasis.41, 42 EECs respond to bacterial quorum sensing molecules called acyl homoserine lactones from Gram-negative bacteria through activation of taste receptor type 2 member 38 (T2R38).43Clostridium sporogenes expresses tryptophan decarboxylases to generate tryptamine, which is able to stimulate the production of 5-HT from enterochromaffin cells and modify the whole-body homeostasis.44 In the gut mucosa of CD patients, enterochromaffin cells exhibit elevated transcripts for tryptophan hydroxylase 1 (TPH1) and TLR4, and E. coli lipopolysaccharides (LPS) stimulates more 5-HT release from the enterochromaffin cells of CD patients than those of healthy volunteers.45 A concentration-dependent inhibitory effect of 5-HT on the A. muciniphila has been clarified using Tph1 gene knockout mice, indicating the role of 5-HT in regulating the gut microbiota and altering susceptibility to DSS-induced colitis.46 CgA levels in EECs have been associated with gut microbial composition and diversity, in which the strongest association to CgA is observed from the Archaea species Methanobrevibacter smithii, whereas a negative association was reported from the phylum Bacteroidetes.47 Furthermore, CgA levels in EECs have been considered a biomarker for colitis activity and response to therapy in patients with IBD.48 Isovalerate, a microbial metabolite, can activate olfactory receptor 558 (Olfr558), which is a microbial metabolite detector on enterochromaffin cells, and lead to voltage-gated Ca2+ channel-dependent 5-HT release from these cells.49 Flagellin and bacterial LPS may act on the Toll-like receptor of EECs to enhance the inflammatory status associated with human IBD.50
FIGURE 2.
Interactions between the various receptors of enteroendocrine cells and the intestinal microbiota and/or microbial metabolites. G protein-coupled receptors, including GPR40, GPR41, GPR43, GPR119, and GPR120, have been recognized as sensing receptors for gut microbiota–derived SCFAs or LCFAs. Olfactory receptor 558, a microbial metabolite detector on EECs, can sense isovalerate, which is an SCFA. Toll-like receptors, such as TLR1, TLR2, and TLR4, can detect multiple intestinal bacterial TLR ligands (eg, bacterial LPS, flagellin, peptidoglycan). Taste receptors can respond to intestinal nutrients and bacterial quorum sensing molecules from Gram-negative bacteria.
ENTEROENDOCRINE CELLS IN IBD
Inflammatory bowel disease is characterized by a dysregulated immune response and inflammation-mediated mucosal damage, and its clinical manifestations include abnormal mucosal cytokine secretion, visceral hyperalgesia, and motility disorder. Paired-like homeobox 2b (Phox2B) and ubiquitination factor E4A (UBE4A),51, 52 2 enteroendocrine markers identified by genome-wide association studies, are increased in the terminal ileal tissue of CD patients, indicating a critical role for EECs in the pathogenesis of intestinal inflammatory disorders.53 Alterations in enteroendocrine cell numbers and hormone secretion in the intestine have been demonstrated in both patients with IBD and animal models of colitis. By immunohistochemistry, the number of 5-HT-immunoreactive cells in the colon was significantly increased in both UC and CD patients.54 Meanwhile, the densities of colonic PYY, pancreatic polypeptide (PP), and oxyntomodulin-producing endocrine cells were decreased in CD patients.54 Consistent with IBD patients, the percentages of 5-HT-positive enterochromaffin cells were also increased in the inflamed ileum of guinea pigs suffering from trinitrobenzene sulfonic acid (TNBS)–induced colitis.55 Additionally, the densities of 5-HT and oxyntomodulin-producing endocrine cells were increased, whereas PP production was decreased in the colon of rats after treatment with TNBS or dextran sulfate sodium (DSS).55, 56 However, intestinal levels of CgA were increased in rats after administration with DSS but were decreased in the model of TNBS-induced colitis,55, 56 indicating distinct EEC hormone responses to colitis under different triggers and pathogenic factors. Furthermore, altered circulating neuroendocrine synthesis and release have also been described in IBD. Patients with IBD exhibited elevated serum and plasma CgA,57 whereas elevated fecal CgA levels were only found in patients with UC, but were not related to disease activity.58 CgA and its derived peptides (eg, VS, CST, CHR, and chromacin) have been shown to participate in regulating antimicrobial activity, suggesting that altered CgA production in EECs may contribute to alterations in intestinal microbial composition, diversity, and functional richness.47, 59 Significant alterations have also been demonstrated in other circulating EEC secretory products, such as PYY, CCK, GLP-1, 5-HT, somatostatin, gastrin, and motilin during the course of IBD.60–66 Furthermore, neutrophil gelatinase-associated lipocalin (NGAL), a potential antimicrobial glycoprotein in human neutrophils, has been found to be expressed in EECs in the healthy gut and in CD.67
MICROBIOTA–EECS AXIS IN THE REGULATION OF MUCOSAL IMMUNITY IN IBD
It has been well established that IBD is a result of an inappropriate mucosal immune response to gut microbiota, leading to chronic immune activation.68 Among multiple levels of immune regulation that have been implicated in the pathogenesis of IBD, EECs, acting as the first line of pathogen detection, can secrete classical inflammatory cytokines and peptide hormones and have been widely studied in multiple inflammation- and infection-driven diseases of the gut.6, 69, 70 In recent years, EECs have increasingly become of particular interest in understanding the pathogenesis of IBD.
Intestinal immune cells can express various receptors for secreted hormone peptides from EECs, including 5-HT, CCK, GLP-1, GLP-2, and neurotensin.71 For instance, the expression of 7 isoforms of 5-HT receptor has been confirmed in mast cells, monocytes, dendritic cells (DCs), lymphocytes, and neutrophils.72 The role of 5-HT as an immunomodulatory factor has been well established, including activating macrophages,73 inducing proliferation of lymphocytes,74 protecting natural killer (NK) cells from oxidative damage,75 and promoting recruitment of T cells.76 The amelioration of TNBS-induced colitis in monocyte chemoattractant protein-1-deficient (MCP-1-/-) mice is associated with decreased infiltration of CD3+ T cells and macrophages, along with decreased 5-HT-expressing enterochromaffin cells in the colonic mucosa.77 Itgb7tm1Cgn(β7-/-) mice, which lack natural gut intraepithelial T lymphocytes (natural IELs), were resistant to cardiovascular disease through GLP-1 production.78 Several CgA-derived peptides have been described in the pathophysiology of intestinal inflammation of IBD. Chromofungin activates neutrophils and regulates the functions of macrophages in the colon through an NF-κB dependent pathway.79, 80 Pancreastatin can upregulate the expressions of pro-inflammatory mediators, including interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)–α, IL-12p70, and interferon (IFN)-γ,81 and downregulate anti-inflammatory cytokines such as IL-10.82 Catestatin has been demonstrated to have a beneficial effect against intestinal inflammation via modulation of the innate immune cells and gut microbial composition.83 Accumulating evidence indicates a significant correlation between the abnormalities of intestinal endocrine cells and the mucosal recruitment of activated cells from both the innate and adaptive immune systems in IBD.55, 84–88
Innate immune cells, such as macrophages and DCs, can sense the intestinal microbiota and initiate the innate immune response, which represents the first line of defense against the microorganisms of the intestinal flora. Higher frequencies of macrophages and DCs are present in the intestinal inflammatory lesions of human IBD.89–93 In the inflamed mucosa of CD patients, macrophages are increased and produce large amounts of pro-inflammatory cytokines (IFN-γ, IL-23) in response to commensal bacteria, such as Escherichia faecalis.90 Carboxypeptidase E (CPE), an enzyme critical for the synthesis of most hormones, is specifically expressed in EECs. Deficiency of CPE in mice leads to increased migration of myeloid macrophages into the mucosa and subsequently an aggravated severity of DSS-induced colitis.94 Higher levels of activated DCs have been found during a flare-up in IBD patients.95, 96 Several EEC peptide hormones, including CCK, somatostatin, and neurotensin, are reported to be inhibitory to DC activation,97–99 which might be beneficial for managing these patients. By binding to 5-HTR3, 5-HTR4, and 5-HTR7 receptors, 5-HT regulates DCs to release pro-inflammatory cytokines, such as IL-6, and promote CCL22/MDC chemokine production.100 In tryptophan hydroxylase-1-deficient (TPH1-/-) mice, the decreased levels of 5-HT are accompanied by the reduced production of IL-12p40 by DCs, which accounts for the attenuation of colitis after exposure to DSS.101 A positive correlation between mast cell number and the density of enterochromaffin cells has been found in the ileum of IBD patients,102 and application of 5-HT receptor antagonists (methysergide or ketanserin) could reduce endotoxin-induced mesenteric mast cell activation in vivo, probably via the 5-HT(2A) receptor subtype.103
Enteroendocrine cells possess functional TLRs, which are recognized as crucial components of the innate immune response. In response to LPS stimulation, STC-1 cells, a murine EEC line, neutralize intestinal bacteria by releasing keratinocyte-derived chemokine and β-defensin 2104 and promoting the expression of the proinflammatory cytokine TNF-α and the anti-inflammatory cytokine TGF-β through the activation of intracellular NF-κB and MAPK pathways.105 Consistently, LCC-18 cells, a human enteroendocrine cell line, produce chemoattractant molecules (eg, CXCL 1 and 3 and CCL20) to recruit innate immune cells from the colonic lamina propria in response to stimulation of LPS and flagellin.50
The potential interactions between EECs and the surrounding adaptive immune cells in the pathogenesis of IBD remain largely unexplored. In the colonic mucosa of rhesus monkeys, 5-HT-positive EECs are in contact with, or in close proximity to, both CD3+ T cells and CD20+ B cells, which suggests a possible role of 5-HT in the regulation of intestinal adaptive immune responses.106 Further investigations have demonstrated that 5-HT could directly act on T and B cells to regulate their activation and proliferation through its receptors expressed on lymphocytes.107, 108 Cholecystokinin octapeptide can directly affect T cells and B cells. It has been shown that CCK octapeptide suppresses Th1 and Th17 differentiation but promotes Th2 and Treg development.109, 110 Cholecystokinin octapeptide also inhibits the production of co-stimulatory molecules (CD80, CD86) in LPS-activated B cells.109, 110 The presence of IL-13 receptor α1 on both 5-HT and CgA-expressing enteroendocrine cells further verifies the importance of the immunoendocrine axis in the gut.111 A better understanding of the interaction of EECs and immune cells in the regulation of the pathogenesis of IBD is required in future investigations.
MICROBIOTA–EECS INTERACTION IN THE REGULATION OF GUT BARRIER FUNCTION IN IBD
The intestinal epithelium, composed of intestinal epithelial cells (IECs), goblet cells, Paneth cells, tuft cells, and EECs, serves as the interface for digestion and nutrient absorption and is a critical defensive barrier against toxins and microorganisms. The intestinal barrier is regulated by a number of intercellular junctional complexes, including tight junctions (TJs). Accumulating evidence shows that epithelial morphological changes, including increased TJ breaks in IBD, contribute to an increase in intestinal epithelium permeability, which promotes abnormal translocation of the gut microbiota or gut microbiota components from the gut lumen to the host blood and tissues.112, 113
GLP-2, secreted from EECs in the intestine after food intake, is upregulated in patients with IBD.114 It can act directly on human IECs to promote their proliferation115 and wound healing through induction of epithelial cell migration mediated by TGF-β.116 GLP-2 has also been demonstrated to decrease colonic crypt cell apoptosis, increase crypt depth and colon length, and protect colonic mucosal architecture in DSS-induced colitis.117–119 Subcutaneous administration of GLP-2 in CD-1 mice decreases the intestinal conductance and unidirectional fluxes, indicating that GLP-2 treatment might improve intestinal barrier function by affecting both paracellular and transcellular pathways.120 Furthermore, specific tight junction protein expression, such as ZO-1 and occludin, is upregulated in GLP-2-treated Caco-2 cells via a TNFα-mediated process.121 Additionally, Akkermansia muciniphila, which resides in the mucus layer and plays a role in gut barrier function, has been shown to be decreased in patients with IBD.122 Its effects on barrier function could be mediated by 2-oleoylglycerol (2-OG), which stimulates the secretion of GLP through enteroendocrine L cells.123, 124
Piezo2, a mechanosensitive ion channel, has been demonstrated to be expressed in a subset of human and mouse EECs.125 Mechanical stimulation of these EECs leads to an intracellular Ca2+ increase, 5-HT release, and pressure-induced epithelial fluid secretion.126 Furthermore, human enteric adenovirus 41 could infect enterochromaffin cells and induce the release of 5-HT,127 which might activate enteric glia.128 Furthermore, EECs in the Drosophila midgut could respond to the pathogenic bacterium Pseudomonas entomophila by expressing the prosecretory transcription factor dimm, which is necessary for the induction of antimicrobial peptides at the barrier epithelium.129
MICROBIOTA–EECS INTERACTION IN THE REGULATION OF VISCERAL HYPERALGESIA IN IBD
Visceral hyperalgesia or an increased sensation of physical stimuli in the gut is relatively common in patients with IBD. A correlation between visceral pain disorders and alteration of the intestinal microbiota or microbial products has been demonstrated in these patients. Inflammatory bowel disease patients show decreased numbers of butyrate-producing bacteria (eg, Roseburia inulinivorans, Ruminococcus torques, C. lavalense, B. uniformis, and F. prausnitzii.) and a subsequent reduction of butyrate levels in the gut, resulting in changes in visceral hyperalgesia.130, 131 In germ-free mice, inflammatory hypernociception, which is induced by various stimuli, including LPS and IL-1β, is reduced.132 Accumulating evidence indicates that EECs regulate visceral hyperalgesia in IBD.
Enteroendocrine cells communicate with the enteric nervous system (ENS) through hormone secretion.133 Serotonin is a key neurotransmitter in control of nociceptive responses, with receptors located in the peripheral and central nervous systems. Increased secretion of 5-HT has been shown in the enterochromaffin cells of patients with IBD and experimental colitis models,54–56, 134–136 which could stimulate the 5-HT3 and 5-HT4 receptors expressed on the primary afferent neurons of both the splanchnic and vagal fibers and correlate with the severity of abdominal pain.137 Specific receptors on enterochromaffin cells, including transient receptor potential ankyrin 1 channel (TRPA1) sensing irritation, Olfr558 sensing microbial metabolites, and TRPC4, an α2A adrenoreceptor sensing stress-related catecholamines, are proposed to be involved in sensory transduction pathways by controlling 5-HT release.49
Using 3D reconstruction of confocal microscopic images, recent evidence demonstrates that EECs possess cytoplasmic processes termed neuropods, which are surrounded by glia and in close contact with enteric nerve terminals, including sensory nerve endings.21, 22 These findings reveal the possibility of a novel neurotransmission mediating the responsiveness of EECs to the bacterial by-products in the lumen of the intestine. Isovalerate, a microbial metabolite, could bind to Olfr558 on enterochromaffin cells and consequently modulate 5HT3R-expressing primary afferent nerve fibers via synaptic connections.49 Furthermore, using serial block face scanning electron microscopy, the neuropods of enteroendocrine cells are escorted by enteric glial cells, which have a similar morphology and function as astrocytes in the brain and have been shown to be involved in the inducing process of visceral hyperalgesia.138 A recent study demonstrated that neuropod cells transduced glucose stimuli to vagal neurons by releasing the neurotransmitter glutamate, helping the brain make sense of the gut luminal signals rapidly.139
MICROBIOTA–EECS INTERACTION IN THE REGULATION OF GUT MOTILITY IN IBD
A change in gut motility has been found in symptomatic IBD, such as decreased segmenting colonic contractions and increased colonic propagating contractions, which may induce efficient anterograde movement of luminal contents,140–142 resulting in symptoms of diarrhea, at least in UC patients.141 Several enteroendocrine hormones, such as CCK, GIP, GLP-1, and PYY, are essential mediators of postprandial gastrointestinal motility.143 SCFAs, acting through GRP41 and GPR43 on EECs,144 could stimulate the release of 5-HT and subsequently provoke the secretion of acetylcholine from the colonic myenteric plexus, resulting in muscle contractions.145 Indigenous spore-forming bacteria from the gut microbiota might regulate 5-HT biosynthesis in EECs through promoting TPH1 expression.146 Non-neural pathways, such as the cyclo-oxygenase products prostaglandins, have been reported to be involved in the propionate-induced colonic tonic contraction through their direct actions on circular muscle.147 TRPA1, activated by thermal nociception, natural plant-derived products, and inflammatory hyperalgesia, along with olfactory receptor,148–150 has been shown to be expressed in enterochromaffin cells and contributes to the production of 5-HT.151 The release of 5-HT could evoke intestinal contractions of isolated guinea pig ileum, which is significantly inhibited via the 5-HT3 receptor antagonist.152Drosophila provides the ideal intestinal model system to investigate the functional implication of EECs in the context of the host–microbiota interaction. Peptide hormone Diuretic Hormone 31, expressed by a group of EECs, is responsible for peristalsis in the junction region of the Drosophila larval midgut.153 The hypochlorous acid–sensitive receptor expressed in a subset of midgut EECs might facilitate enteric expulsion of the opportunistic pathogen Erwinia carotovora through activation of the Duox pathway, thus maintaining the microbiota’s homeostasis.154 These results indicate that the gut microbiota and enteroendocrine hormones might play pivotal roles in GI motility; however, the role of EECs in microbiota-induced gut motility disorders remains to be further clarified in the pathophysiology of IBD.
CONCLUSIONS
Inflammatory bowel disease is a chronic intestinal inflammatory disease affecting patients’ quality of life. Accumulating evidence has demonstrated that the interactions of enteroendocrine cells and the gut microbiota contribute to the maintenance of intestinal homeostasis. Enteroendocrine cells, as firstline sensors for the microbiota, can secrete classical cytokines and hormonal peptides to directly and indirectly regulate several functions in different cell types in the GI tract. Their involvement in microbial sensing and their roles in the regulation of innate and adaptive immune responses, the intestinal epithelial barrier, visceral hyperalgesia, and gut motility emphasize the importance of EECs in the regulation of the pathogenesis of IBD. Investigation of the gut microbiota–EEC interaction in IBD will provide great insights into the pathogenesis of IBD and the development of tools for the prediction, diagnosis, and treatment of IBD.
Supported by: This work was supported by the National Natural Science Foundation of China (NSFC 81670486) and the Fundamental Research Funds of Shandong University (2017JC036 to Y.Y.).
Conflicts of interest: The authors declare no competing interests.
REFERENCES
- 1. Targan SR, Karp LC. Inflammatory bowel disease diagnosis, evaluation and classification: state-of-the art approach. Curr Opin Gastroenterol. 2007;23:390–394. [DOI] [PubMed] [Google Scholar]
- 2. Hammer T, Nielsen KR, Munkholm P, et al. . The Faroese IBD Study: incidence of inflammatory bowel diseases across 54 years of population-based data. J Crohns Colitis. 2016;10:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. [DOI] [PubMed] [Google Scholar]
- 4. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogler G, Biedermann L, Scharl M. New insights into the pathophysiology of inflammatory bowel disease: microbiota, epigenetics and common signalling pathways. Swiss Med Wkly. 2018;148:w14599. [DOI] [PubMed] [Google Scholar]
- 6. Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018;11:3–20. [DOI] [PubMed] [Google Scholar]
- 7. Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res. 2004;36:735–741. [DOI] [PubMed] [Google Scholar]
- 9. Fothergill LJ, Furness JB. Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification scheme. Histochem Cell Biol. 2018;150:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gehart H, van Es JH, Hamer K, et al. . Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. 2019;176:1158–1173.e16. [DOI] [PubMed] [Google Scholar]
- 13. Gribble FM, Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–299. [DOI] [PubMed] [Google Scholar]
- 14. Habib AM, Richards P, Cairns LS, et al. . Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Habib AM, Richards P, Rogers GJ, et al. . Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia. 2013;56:1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eissa N, Hussein H, Hendy GN, et al. . Chromogranin-A and its derived peptides and their pharmacological effects during intestinal inflammation. Biochem Pharmacol. 2018;152:315–326. [DOI] [PubMed] [Google Scholar]
- 17. Engelstoft MS, Lund ML, Grunddal KV, et al. . Research resource: a chromogranin a reporter for serotonin and histamine secreting enteroendocrine cells. Mol Endocrinol. 2015;29:1658–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helle KB, Corti A, Metz-Boutigue MH, et al. . The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loh YP, Cheng Y, Mahata SK, et al. . Chromogranin A and derived peptides in health and disease. J Mol Neurosci. 2012;48:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koshimizu H, Cawley NX, Kim T, et al. . Serpinin: a novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol Endocrinol. 2011;25:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohórquez DV, Shahid RA, Erdmann A, et al. . Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bohórquez DV, Chandra R, Samsa LA, et al. . Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. 2011;42:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47–57. [DOI] [PubMed] [Google Scholar]
- 24. Bertrand PP. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. [DOI] [PubMed] [Google Scholar]
- 26. Rajilić-Stojanović M, Smidt H, de Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9:2125–2136. [DOI] [PubMed] [Google Scholar]
- 27. Chow J, Lee SM, Shen Y, et al. . Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hooper LV, Wong MH, Thelin A, et al. . Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. [DOI] [PubMed] [Google Scholar]
- 29. Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. [DOI] [PubMed] [Google Scholar]
- 30. Derrien M, Vaughan EE, Plugge CM, et al. . Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–1476. [DOI] [PubMed] [Google Scholar]
- 31. Baumgart M, Dogan B, Rishniw M, et al. . Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. Isme J. 2007;1:403–418. [DOI] [PubMed] [Google Scholar]
- 32. Frank DN, St Amand AL, Feldman RA, et al. . Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ott SJ, Musfeldt M, Wenderoth DF, et al. . Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gevers D, Kugathasan S, Denson LA, et al. . The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gophna U, Sommerfeld K, Gophna S, et al. . Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. [DOI] [PubMed] [Google Scholar]
- 37. Sokol H, Pigneur B, Watterlot L, et al. . Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cummings JH, Pomare EW, Branch WJ, et al. . Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katsuma S, Hatae N, Yano T, et al. . Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem. 2005;280:19507–19515. [DOI] [PubMed] [Google Scholar]
- 40. Furness JB, Rivera LR, Cho HJ, et al. . The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–740. [DOI] [PubMed] [Google Scholar]
- 41. Janssen S, Laermans J, Verhulst PJ, et al. . Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A. 2011;108:2094–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dotson CD, Zhang L, Xu H, et al. . Bitter taste receptors influence glucose homeostasis. PLoS One. 2008;3:e3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viswanathan VK. Sensing bacteria, without bitterness? Gut Microbes. 2013;4:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takaki M, Mawe GM, Barasch JM, et al. . Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience. 1985;16:223–240. [DOI] [PubMed] [Google Scholar]
- 45. Kidd M, Gustafsson BI, Drozdov I, et al. . IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil. 2009;21:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kwon YH, Wang H, Denou E, et al. . Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell Mol Gastroenterol Hepatol. 2019;7:709–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhernakova A, Kurilshikov A, Bonder MJ, et al. ; LifeLines Cohort Study Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zissimopoulos A, Vradelis S, Konialis M, et al. . Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: a prospective observational study. Scand J Gastroenterol. 2014;49:942–949. [DOI] [PubMed] [Google Scholar]
- 49. Bellono NW, Bayrer JR, Leitch DB, et al. . Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:185–198.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Selleri S, Palazzo M, Deola S, et al. . Induction of pro-inflammatory programs in enteroendocrine cells by the Toll-like receptor agonists flagellin and bacterial LPS. Int Immunol. 2008;20:961–970. [DOI] [PubMed] [Google Scholar]
- 51. Rioux JD, Xavier RJ, Taylor KD, et al. . Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sakiyama T, Fujita H, Tsubouchi H. Autoantibodies against ubiquitination factor E4A (UBE4A) are associated with severity of Crohn’s disease. Inflamm Bowel Dis. 2008;14:310–317. [DOI] [PubMed] [Google Scholar]
- 53. Moran GW, Pennock J, McLaughlin JT. Enteroendocrine cells in terminal ileal Crohn’s disease. J Crohns Colitis. 2012;6:871–880. [DOI] [PubMed] [Google Scholar]
- 54. El-Salhy M, Danielsson A, Stenling R, et al. . Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. [DOI] [PubMed] [Google Scholar]
- 55. El-Salhy M, Hatlebakk JG. Changes in enteroendocrine and immune cells following colitis induction by TNBS in rats. Mol Med Rep. 2016;14:4967–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El-Salhy M, Hatlebakk JG, Gilja OH. Abnormalities in endocrine and immune cells are correlated in dextran‑sulfate‑sodium‑induced colitis in rats. Mol Med Rep. 2017;15:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rindi G, Leiter AB, Kopin AS, et al. . The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. [DOI] [PubMed] [Google Scholar]
- 58. Strid H, Simrén M, Lasson A, et al. . Fecal chromogranins and secretogranins are increased in patients with ulcerative colitis but are not associated with disease activity. J Crohns Colitis. 2013;7:e615–e622. [DOI] [PubMed] [Google Scholar]
- 59. Briolat J, Wu SD, Mahata SK, et al. . New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell Mol Life Sci. 2005;62:377–385. [DOI] [PubMed] [Google Scholar]
- 60. Koch TR, Roddy DR, Go VL. Abnormalities of fasting serum concentrations of peptide YY in the idiopathic inflammatory bowel diseases. Am J Gastroenterol. 1987;82:321–326. [PubMed] [Google Scholar]
- 61. Moran GW, Leslie FC, McLaughlin JT. Crohn’s disease affecting the small bowel is associated with reduced appetite and elevated levels of circulating gut peptides. Clin Nutr. 2013;32:404–411. [DOI] [PubMed] [Google Scholar]
- 62. Karmiris K, Koutroubakis IE, Xidakis C, et al. . Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. [DOI] [PubMed] [Google Scholar]
- 63. Bendet N, Scapa E, Cohen O, et al. . Enhanced glucose-dependent glucagon-like peptide-1 and insulin secretion in Crohn patients with terminal ileum disease is unrelated to disease activity or ileal resection. Scand J Gastroenterol. 2004;39:650–656. [DOI] [PubMed] [Google Scholar]
- 64. Keller J, Beglinger C, Holst JJ, et al. . Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;297:G861–G868. [DOI] [PubMed] [Google Scholar]
- 65. Nishi Y, Isomoto H, Ueno H, et al. . Plasma leptin and ghrelin concentrations in patients with Crohn’s disease. World J Gastroenterol. 2005;11:7314–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Binimelis J, Webb SM, Monés J, et al. . Circulating immunoreactive somatostatin in gastrointestinal diseases. Decrease after vagotomy and enhancement in active ulcerative colitis, irritable bowel syndrome, and duodenal ulcer. Scand J Gastroenterol. 1987;22:931–937. [DOI] [PubMed] [Google Scholar]
- 67. Thorsvik S, Bakke I, van Beelen Granlund A, et al. . Expression of neutrophil gelatinase-associated lipocalin (NGAL) in the gut in Crohn’s disease. Cell Tissue Res. 2018;374:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- 69. Di Sabatino A, Giuffrida P, Vanoli A, et al. . Increase in neuroendocrine cells in the duodenal mucosa of patients with refractory celiac disease. Am J Gastroenterol. 2014;109:258–269. [DOI] [PubMed] [Google Scholar]
- 70. Dlugosz A, Törnblom H, Mohammadian G, et al. . Chlamydia trachomatis antigens in enteroendocrine cells and macrophages of the small bowel in patients with severe irritable bowel syndrome. BMC Gastroenterol. 2010;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg. 2003;186:253–258. [DOI] [PubMed] [Google Scholar]
- 72. Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf). 2015;213:561–574. [DOI] [PubMed] [Google Scholar]
- 73. Ghia JE, Li N, Wang H, et al. . Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. [DOI] [PubMed] [Google Scholar]
- 74. Stefulj J, Cicin-Sain L, Schauenstein K, et al. . Serotonin and immune response: effect of the amine on in vitro proliferation of rat lymphocytes. Neuroimmunomodulation. 2001;9:103–108. [DOI] [PubMed] [Google Scholar]
- 75. Betten A, Dahlgren C, Hermodsson S, et al. . Serotonin protects NK cells against oxidatively induced functional inhibition and apoptosis. J Leukoc Biol. 2001;70:65–72. [PubMed] [Google Scholar]
- 76. Laberge S, Cruikshank WW, Beer DJ, et al. . Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J Immunol. 1996;156:310–315. [PubMed] [Google Scholar]
- 77. Khan WI, Motomura Y, Wang H, et al. . Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G803–G811. [DOI] [PubMed] [Google Scholar]
- 78. He S, Kahles F, Rattik S, et al. . Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. 2019;566:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eissa N, Hussein H, Kermarrec L, et al. . Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-κB signaling. Biochem Pharmacol. 2017;145:102–113. [DOI] [PubMed] [Google Scholar]
- 80. Eissa N, Hussein H, Kermarrec L, et al. . Chromofungin ameliorates the progression of colitis by regulating alternatively activated macrophages. Front Immunol. 2017;8:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bandyopadhyay GK, Lu M, Avolio E, et al. . Pancreastatin-dependent inflammatory signaling mediates obesity-induced insulin resistance. Diabetes. 2015;64:104–116. [DOI] [PubMed] [Google Scholar]
- 82. Eissa N, Hussein H, Kermarrec L, et al. . Chromogranin-A regulates macrophage function and the apoptotic pathway in murine DSS colitis. J Mol Med (Berl). 2018;96:183–198. [DOI] [PubMed] [Google Scholar]
- 83. Radek KA, Elias PM, Taupenot L, et al. . Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe. 2010;7:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:503–511. [DOI] [PubMed] [Google Scholar]
- 86. Ameri P, Ferone D. Diffuse endocrine system, neuroendocrine tumors and immunity: what’s new? Neuroendocrinology. 2012;95:267–276. [DOI] [PubMed] [Google Scholar]
- 87. El-Salhy M, Hausken T. The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD). Neuropeptides. 2016;55:137–144. [DOI] [PubMed] [Google Scholar]
- 88. Tari A, Teshima H, Sumii K, et al. . Peptide YY abnormalities in patients with ulcerative colitis. Jpn J Med. 1988;27:49–55. [DOI] [PubMed] [Google Scholar]
- 89. Rugtveit J, Bakka A, Brandtzaeg P. Differential distribution of B7.1 (CD80) and B7.2 (CD86) costimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD). Clin Exp Immunol. 1997;110:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kamada N, Hisamatsu T, Okamoto S, et al. . Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hart AL, Al-Hassi HO, Rigby RJ, et al. . Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. [DOI] [PubMed] [Google Scholar]
- 92. Baumgart DC, Thomas S, Przesdzing I, et al. . Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin Exp Immunol. 2009;157:423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Verstege MI, ten Kate FJ, Reinartz SM, et al. . Dendritic cell populations in colon and mesenteric lymph nodes of patients with Crohn’s disease. J Histochem Cytochem. 2008;56:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bär F, Föh B, Pagel R, et al. . Carboxypeptidase E modulates intestinal immune homeostasis and protects against experimental colitis in mice. PLoS One. 2014;9:e102347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baumgart DC, Metzke D, Schmitz J, et al. . Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vuckovic S, Florin TH, Khalil D, et al. . CD40 and CD86 upregulation with divergent CMRF44 expression on blood dendritic cells in inflammatory bowel diseases. Am J Gastroenterol. 2001;96:2946–2956. [DOI] [PubMed] [Google Scholar]
- 97. Jia X, Cong B, Zhang J, et al. . CCK8 negatively regulates the TLR9-induced activation of human peripheral blood pDCs by targeting TRAF6 signaling. Eur J Immunol. 2014;44:489–499. [DOI] [PubMed] [Google Scholar]
- 98. Kao JY, Pierzchala A, Rathinavelu S, et al. . Somatostatin inhibits dendritic cell responsiveness to Helicobacter pylori. Regul Pept. 2006;134:23–29. [DOI] [PubMed] [Google Scholar]
- 99. da Silva L, Neves BM, Moura L, et al. . Neurotensin downregulates the pro-inflammatory properties of skin dendritic cells and increases epidermal growth factor expression. Biochim Biophys Acta. 2011;1813:1863–1871. [DOI] [PubMed] [Google Scholar]
- 100. Müller T, Dürk T, Blumenthal B, et al. . 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One. 2009;4:e6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li N, Ghia JE, Wang H, et al. . Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yu Y, Daly DM, Adam IJ, et al. . Interplay between mast cells, enterochromaffin cells, and sensory signaling in the aging human bowel. Neurogastroenterol Motil. 2016;28:1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Walther A, Peter C, Yilmaz N, et al. . Influence of serotonin-receptor antagonism on mast cell activation during endotoxemia. Pathophysiology. 2002;8:161–165. [DOI] [PubMed] [Google Scholar]
- 104. Palazzo M, Balsari A, Rossini A, et al. . Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol. 2007;178:4296–4303. [DOI] [PubMed] [Google Scholar]
- 105. Bogunovic M, Davé SH, Tilstra JS, et al. . Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–G1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang GB, Lackner AA. Proximity between 5-HT secreting enteroendocrine cells and lymphocytes in the gut mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role for enterochromaffin cell 5-HT in mucosal immunity. J Neuroimmunol. 2004;146:46–49. [DOI] [PubMed] [Google Scholar]
- 107. Matsumura Y, Byrne SN, Nghiem DX, et al. . A role for inflammatory mediators in the induction of immunoregulatory B cells. J Immunol. 2006;177:4810–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ahern GP. 5-HT and the immune system. Curr Opin Pharmacol. 2011;11:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang JG, Cong B, Li QX, et al. . Cholecystokinin octapeptide regulates lipopolysaccharide-activated B cells co-stimulatory molecule expression and cytokines production in vitro. Immunopharmacol Immunotoxicol. 2011;33:157–163. [DOI] [PubMed] [Google Scholar]
- 110. Zhang JG, Liu JX, Jia XX, et al. . Cholecystokinin octapeptide regulates the differentiation and effector cytokine production of CD4(+) T cells in vitro. Int Immunopharmacol. 2014;20:307–315. [DOI] [PubMed] [Google Scholar]
- 111. Wang H, Steeds J, Motomura Y, et al. . CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schmitz H, Barmeyer C, Fromm M, et al. . Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. [DOI] [PubMed] [Google Scholar]
- 113. Zeissig S, Bürgel N, Günzel D, et al. . Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xiao Q, Boushey RP, Cino M, et al. . Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1057–R1063. [DOI] [PubMed] [Google Scholar]
- 115. Jasleen J, Ashley SW, Shimoda N, et al. . Glucagon-like peptide 2 stimulates intestinal epithelial proliferation in vitro. Dig Dis Sci. 2002;47:1135–1140. [DOI] [PubMed] [Google Scholar]
- 116. Bulut K, Meier JJ, Ansorge N, et al. . Glucagon-like peptide 2 improves intestinal wound healing through induction of epithelial cell migration in vitro-evidence for a TGF–beta-mediated effect. Regul Pept. 2004;121:137–143. [DOI] [PubMed] [Google Scholar]
- 117. L’Heureux MC, Brubaker PL. Glucagon-like peptide-2 and common therapeutics in a murine model of ulcerative colitis. J Pharmacol Exp Ther. 2003;306:347–354. [DOI] [PubMed] [Google Scholar]
- 118. Drucker DJ, Yusta B, Boushey RP, et al. . Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol. 1999;276:G79–G91. [DOI] [PubMed] [Google Scholar]
- 119. Sigalet DL, Wallace LE, Holst JJ, et al. . Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol. 2007;293:G211–G221. [DOI] [PubMed] [Google Scholar]
- 120. Benjamin MA, McKay DM, Yang PC, et al. . Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Moran GW, O’Neill C, McLaughlin JT. GLP-2 enhances barrier formation and attenuates TNFα-induced changes in a Caco-2 cell model of the intestinal barrier. Regul Pept. 2012;178:95–101. [DOI] [PubMed] [Google Scholar]
- 122. Png CW, Lindén SK, Gilshenan KS, et al. . Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–2428. [DOI] [PubMed] [Google Scholar]
- 123. Hansen KB, Rosenkilde MM, Knop FK, et al. . 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–E1417. [DOI] [PubMed] [Google Scholar]
- 124. Everard A, Belzer C, Geurts L, et al. . Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang F, Knutson K, Alcaino C, et al. . Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol. 2017;595:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Alcaino C, Knutson KR, Treichel AJ, et al. . A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A. 2018;115:E7632–E7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Westerberg S, Hagbom M, Rajan A, et al. . Interaction of human enterochromaffin cells with human enteric adenovirus 41 leads to serotonin release and subsequent activation of enteric glia cells. J Virol. 2018;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Savidge TC, Newman P, Pothoulakis C, et al. . Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. [DOI] [PubMed] [Google Scholar]
- 129. Beebe K, Park D, Taghert PH, et al. . The drosophila prosecretory transcription factor dimmed is dynamically regulated in adult enteroendocrine cells and protects against Gram-negative infection. G3 (Bethesda). 2015;5:1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Buttó LF, Schaubeck M, Haller D. Mechanisms of microbe-host interaction in Crohn’s disease: dysbiosis vs. pathobiont selection. Front Immunol. 2015;6:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Takahashi K, Nishida A, Fujimoto T, et al. . Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion. 2016;93:59–65. [DOI] [PubMed] [Google Scholar]
- 132. Gottesfeld JM, Murphy RF, Bonner J. Structure of transcriptionally active chromatin. Proc Natl Acad Sci U S A. 1975;72:4404–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Okano-Matsumoto S, McRoberts JA, Taché Y, et al. . Electrophysiological evidence for distinct vagal pathways mediating CCK-evoked motor effects in the proximal versus distal stomach. J Physiol. 2011;589:371–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Coates MD, Mahoney CR, Linden DR, et al. . Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. [DOI] [PubMed] [Google Scholar]
- 135. Linden DR, Chen JX, Gershon MD, et al. . Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. [DOI] [PubMed] [Google Scholar]
- 136. O’Morain C, Bishop AE, McGregor GP, et al. . Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut. 1984;25:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cremon C, Carini G, Wang B, et al. . Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. [DOI] [PubMed] [Google Scholar]
- 138. Wang P, Du C, Chen FX, et al. . BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2016;6:20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kaelberer MM, Buchanan KL, Klein ME, et al. . A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rao SS, Read NW. Gastrointestinal motility in patients with ulcerative colitis. Scand J Gastroenterol Suppl. 1990;172:22–28. [DOI] [PubMed] [Google Scholar]
- 141. Reddy SN, Bazzocchi G, Chan S, et al. . Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289–1297. [DOI] [PubMed] [Google Scholar]
- 142. Menys A, Puylaert C, Tutein Nolthenius CE, et al. . Quantified terminal ileal motility during MR enterography as a biomarker of Crohn disease activity: prospective multi-institution study. Radiology. 2018;289:428–435. [DOI] [PubMed] [Google Scholar]
- 143. Yabe D, Seino Y. Incretin actions beyond the pancreas: lessons from knockout mice. Curr Opin Pharmacol. 2013;13:946–953. [DOI] [PubMed] [Google Scholar]
- 144. Tazoe H, Otomo Y, Kaji I, et al. . Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–262. [PubMed] [Google Scholar]
- 145. Fukumoto S, Tatewaki M, Yamada T, et al. . Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. [DOI] [PubMed] [Google Scholar]
- 146. Yano JM, Yu K, Donaldson GP, et al. . Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mitsui R, Ono S, Karaki S, et al. . Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–594. [DOI] [PubMed] [Google Scholar]
- 148. Bautista DM, Jordt SE, Nikai T, et al. . TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. [DOI] [PubMed] [Google Scholar]
- 149. Jordt SE, Bautista DM, Chuang HH, et al. . Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. [DOI] [PubMed] [Google Scholar]
- 150. Bandell M, Story GM, Hwang SW, et al. . Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. [DOI] [PubMed] [Google Scholar]
- 151. Braun T, Voland P, Kunz L, et al. . Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. [DOI] [PubMed] [Google Scholar]
- 152. Nozawa K, Kawabata-Shoda E, Doihara H, et al. . TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A. 2009;106:3408–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. LaJeunesse DR, Johnson B, Presnell JS, et al. . Peristalsis in the junction region of the Drosophila larval midgut is modulated by DH31 expressing enteroendocrine cells. BMC Physiol. 2010;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Du EJ, Ahn TJ, Kwon I, et al. . TrpA1 regulates defecation of food-borne pathogens under the control of the duox pathway. PLoS Genet. 2016;12:e1005773. [DOI] [PMC free article] [PubMed] [Google Scholar]