Abstract
SARS-CoV2 might conduce to rapid respiratory complications challenging healthcare systems worldwide. Immunological mechanisms associated to SARS-CoV2 infection are complex and not yet clearly elucidated. Arguments are in favour of a well host-adapted virus. Here I draw a systemic immunological representation linking actual SARS-CoV2 infection literature that hopefully might guide healthcare decisions to treat COVID-19. I suggest HLA-G and HLA-E, non classical HLA class I molecules, in the core of COVID-19 complications. These molecules are powerful in immune tolerance and might inhibit/suppress immune cells functions during SARS-CoV2 infection promoting virus subversion. Dosing soluble forms of these molecules in COVID-19 patients’ plasma might help the identification of critical cases. I recommend also developing new SARS-CoV2 therapies based on the use of HLA-G and HLA-E or their specific receptors antibodies in combination with FDA approved therapeutics to combat efficiently COVID-19.
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, Coronavirus disease-2019; FDA, Food and Drug Administration; HLA, Human Leukocyte Antigen; IDSA, Infectious Diseases Society of America; MMP, matrix metalloproteinases; NK, natural killer; ROS, reactive oxygen species; SARS-CoV2, Severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
Keywords: SARS-CoV2, COVID-19, HLA-G, HLA-E, NKG2A
1. Introduction
SARS-CoV2 (Severe acute respiratory syndrome coronavirus 2) is the novel enveloped RNA betacoronavirus [1], [2] causative of the Coronavirus disease-2019 (COVID-19) [3]. The World Health Organization (WHO) has declared, in 11 March 2020, the COVID-19 as a pandemic public health disease [4]. This new disease could be either mastered or could evolve to more severe disease with worsening respiratory symptoms leading to pneumonitis and to acute respiratory distress syndrome (ARDS) with high morbidity and mortality [5], [6], [7]. Roughly SARS-CoV2 infection is divided into three stages: Stage I, an early asymptomatic stage with or without detectable virus; stage II, a non-severe symptomatic stage with mild COVID-19 symptoms; and stage III, a severe symptomatic stage with advanced and significant respiratory symptoms with high viral load [6], [7], [8].
Since specific COVID-19 drugs will take several years to be developed, and based on the international concern over SARS-CoV2 fast spread and huge number of dead people, many strategies to treat COVID-19 were proposed [9]. Different clinical trials were launched using current marketed drugs and some new ones (3237 trials recorded in American ClinicalTrials.gov; in 06 September 2020). At emergency, these numerous clinical trials were mandatory to manage severe complications and avoid death. However, after nine months from the emergence of the SARS-CoV2 in December 2019, new clinical trials should be benefit from recent results. Indeed, the pace of clinical trials should be reduced and more focused. In fact, most efficient therapies will be those integrating tangible proofs associating both rational knowledge and recent results from fundamental studies and clinical trials on SARS-CoV2.
In this article, I ruled out the possible implications of HLA-G and HLA-E as well as their specific receptors in SARS-CoV2 spread based on published literature. HLA-G and HLA-E are both non classical molecules with tolerogenic properties [10], [11]. Indeed, they could inhibit both innate and adaptative immune responses. Seven Isoforms are known for HLA-G including membrane-bound Isoforms (HLA-G1, -G2, -G3, -G4) and soluble Isoforms (sHLA-G: HLA-G5, -G6, -G7). One additional soluble form of HLA-G is the shedding HLA-G generated by matrix metalloproteinases (MMP) cleavage of the membrane-bound HLA-G [12]. Recently, novel spliced forms were reported with extended 5′-region and no transmembrane or alpha 1 domains [13]. Currently, six receptors are known to interact with HLA-G (ILT-2, ILT-4, KIR2DL4, CD8, CD160 and NKG2A/CD94) [14], [15].
Discovered at the maternal-fetal interface, HLA-G molecule has been implicated in the fetus maintain in physiological context [16], [17]. After that, several studies demonstrated the expression of HLA-G in pathological context including cancers [18], [19], [20], auto-immune [21], and inflammatory diseases [22]. This molecule was also expressed in infection diseases including parasites and viruses [23], [24], [25]. In these contexts, HLA-G molecule functions to suppress the immune system [26]. Indeed, HLA-G inhibits functions of many immune cells including NK cells, CD8+ T cells, CD4+ T cells as well as dendritic cells [27], [28]. Particularly, HLA-G has been shown to attenuate cytotoxicity of NK and CD8+ T cells, to decrease alloproliferation of CD4+ T cells and to decrease dendritic cells maturation and functions [27], [28]. In addition, HLA-G has the potential to shift cytokines production from Th1 to Th2 cytokines [29] that strongly establish an immune tolerance microenvironment. This has led to the suggestion of HLA-G as a checkpoint immune molecule [28].
Many viruses spread implicates HLA-G molecule. Indeed, influenza A virus, herpes, rabies, hepatite C, hepatite B viruses developed a subversive strategy to avoid immune system detection and destruction [23], [30], [31], [32], [33]. Human viruses up-regulate the expression of HLA-G on the surface of infected cells. By this way, viruses can replicate and spread in the host without great host-immune responses.
HLA-E molecule expression is closely related to HLA-I molecules including HLA-G. Indeed, peptides derived from the leader sequence of HLA-I molecules are linked to HLA-E, allowing its externalization [34]. HLA-E can interact with its receptors (inhibitory receptors: NKG2A/CD94, NKG2B/CD94 and activating receptor: NKG2C/CD94) [35] and regulate immune cells functions [11]. Interestingly, HLA-E affinity to NKG2A/CD94 receptor is six fold higher than its affinity to NKG2C/CD94 receptor [36].
2. Insights into immunological mechanism underlying SARS-CoV2 spread: potential roles of HLA-G and HLA-E molecules
The novel strain of coronavirus, SARS-CoV2 is associated with a huge upheaval immunological change. Three immunological phases could be described. The first phase is associated to the virus infection, the second is associated to the massive replication of the virus, and the third is the convalescence phase.
To infect host cells and massively replicate, SARS-CoV2 needs to counter and subvert immune responses. The first type of immunity facing the SARS-CoV2 is the innate immunity. This first line defence could be stronger enough to inhibit the virus spread. This defence encloses potent immune cells and soluble molecules. The first line cells include phagocytes, natural killer (NK) cells as well as polymorphonuclear leukocytes. The second line includes soluble molecules with the cytokines and chemokines. The entry of SARS-CoV2 may induce a “cytokines storm” [37] majorly produced by macrophages and monocytes. Indeed, enhanced serum/plasma levels of IL-1β, IL-2, IL-6, IL-7, IL-8, IL-10, IL-17, IFN-γ, TNF-α, G-CSF, GM-CSF, IP10, MCP1, MIP1A and MIP1B were reported [38], [39], [40], [41] (Fig. 1 ). In a recent case report, TNF-α and IL-2 were not increased in the early inflammation stage [39].
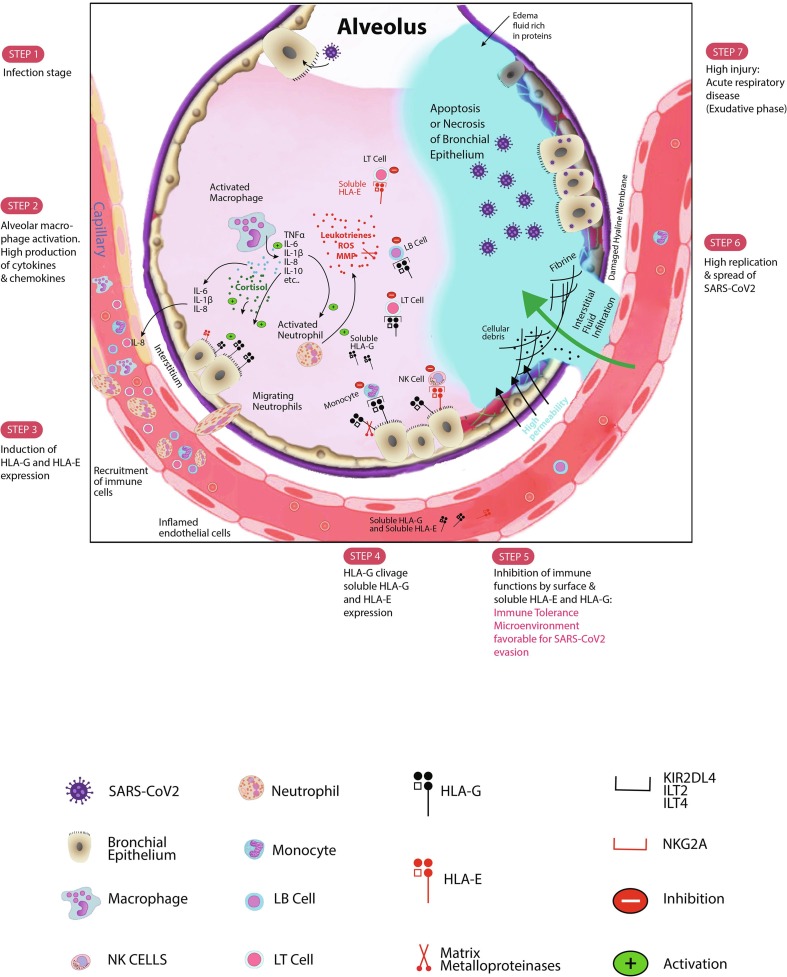
Fig. 1.
Schematic SARS-CoV2 infection evolution steps. After infecting bronchial epithelial cells, SARS-CoV2 decreases the IFN-γ production. Activated macrophages and monocytes particularly produce high levels of cytokines, chemokines, and also growth factors. Some cytokines and chemokines including IL-6, IL-1β, and IL-8 recruit immune cells to the site of SARS-CoV2 infection. The released IL-10, an anti-inflammatory cytokine, is produced to counteract the exacerbated inflammation. IL-10 might then stimulate the expression of HLA-G molecule at the surface of infected cells as well as microenvironment immune cells. Membrane-bound HLA-G strongly binds its inhibitory receptors on immune cells (NK, CD8+ T cells, LB cells, monocytes/dendritic cells) to disrupt their functions inducing immune system inhibition. This peripheral tolerance might be in favour of SARS-CoV2 subversion allowing high replication. Moreover, the probable presence of cortisol might stimulate both HLA-G production and lymphopenia. The activation of Neutrophil cells will increase production of leukotrienes, reactive oxygen species (ROS) and matrix metalloproteinases (MMP) causing pneumocytes and endothelial cells injury. The high injury will promote the constitution of the edema fluid causing the acute respiratory distress syndrome (ARDS). The production of MMP triggers the clivage of HLA-G and its release in bronchial cells microenvironment and in plasma that empower the inhibition of immune cells functions. Furthermore, HLA-E molecule either in its membrane-bound form or in its soluble form might downregulate synergically the immune cells functions. Soluble HLA-G/HLA-E together with membrane-bound HLA-G/HLA-E production may be detrimental for the SARS-CoV2 infected host. Along with current results and our proposed mechanism, we think that targeting these checkpoint molecules and/or their specific receptors will impair considerably the SARS-CoV2 progression.
In early inflammation, IL-1β acts together with the TNF-α by promoting Th17 responses and enhances vascular permeability [38]. There is a substantial evidence suggesting the involvement of IL-6 and IL-1β in the recruitment of neutrophils and cytotoxic CD8+ cells [37] (Fig. 1). Moreover, chemokines like IL-8 promote the recruitment of more immune infiltrates. Additionally, IL-1β enhances neutrophil cytotoxicity [42]. Activated neutrophils releasing leukotrienes, reactive oxygen species (ROS), and MMP, promote pneumocytes and endothelial cells injury [37] (Fig. 1). Furthermore, activated macrophages normally promoting viral control, can also emphasize tissue damage [37]. As explained this inflammasome constitutes an important arm for innate immunity [42], however, high levels of cytokines and chemokines, as well as immune cells activation may induce extreme damages to host tissues. To avoid high injury, the host might produce, in the early inflammation stage, IL-10, an anti-inflammatory cytokine [43].
In a recent study, Zhang and collaborators demonstrate, in the early inflammation stage, a high membrane expression of HLA-G, in a Chinese patient infected by SARS-CoV2 [39]. By flow cytometry, HLA-G molecules were highly expressed at the surface of immune cells including T cells, B cells and monocytes [39]. Because HLA-G, a non classical HLA class I molecule, is considered as a potent immune tolerance molecule, I think that HLA-G may initially modulate the hyperinflammation induced by the SARS-CoV2. Moreover, the high production of IL-10 might enhance HLA-G expression (i) in infected cells as previously reported in cancers and other inflammatory contexts [44]; as well as (ii) in immune cells [45] as reported by Zhang et al. [39] (Fig. 1).
To maintain homeostasis during infection, IL-6 not only induces IL-10 production but could also enhance cortisol levels in plasma [46], [47], [48]. The produced cortisol might reduce the lymphocytes number observed in patients with SARS-CoV2 infection. Until now, no study has investigated the cortisol level in SARS-CoV2 patients [49]. However, based on the fact that SARS-CoV2 patients have commonly observed lymphopenia [39], [40], [41], Pal and collaborators think that cortisol is increased in SARS-CoV2 patients during the replication stage [49]. I postulate that cortisol might enhance HLA-G expression as do IL-10 in infected cells and in immune cells (Fig. 1). Glucorticoids including dexamethasone and hydrocortisone are known to be potent inducers of HLA-G [50], [51], [52].
Interestingly, HLA-G expression at the surface of infected cells has been associated with the disease progression and its complications. Actually, no studies shed light about HLA-G surface expression in lung infected cells.
Zhang et al. reported a decrease in HLA-G expression in immune cells in SARS-CoV2 positive stage (T cells, B cells and Monocytes; checked by flow cytometry). I suggest that the observed membrane-bound HLA-G decrease is the consequence of its delocalization into plasma after cleavage by MMP, massively produced by connective tissue and pro-inflammatory cells including activated neutrophils [53] (Fig. 1). The produced Shedding HLA-G molecules might be highly loaded in plasma of SARS-CoV2 patients. This hypothesis is supported by findings showing: (i) A high level of MMP in SARS-CoV2 patients, necessary for tissue damage and remodelling [37], [38], [54], and (ii) A high expression of MMP-2, in human coronavirus infection [55], [56], responsible of HLA-G cleavage [12].
The probable soluble shedding HLA-G production might stimulate the dissemination of SARS-CoV2 viruses. This is of considerable relevance to make sHLA-G dosage for SARS-CoV2 patients. As the increased level of sHLA-G is frequently associated with a worse prognostic for infection diseases and cancers [57], we think that sHLA-G dosage in blood might provide the rational basis to strategically guide physicians to prioritize the reanimation beds. A systematic dosage might be considered to identify patients with high sHLA-G probably associated with a worse prognostic COVID-19.
In the replication stage of SARS-CoV2, the total number of NK and CD8+ T cells is markedly decreased [39], [58] thus favouring the spread of the virus. The study of these cells markers revealed the increased expression of the inhibitory receptor NKG2A/CD94 and reduced percentages of CD107a and granzyme B expression in CD8+ T cells in COVID-19 patients compared to healthy controls [58]. This observed function deregulation of CD8+ T cells might be attributed to NKG2A/CD94 signalling [11], [26].
Because HLA-E surface expression preferentially require peptides derived from HLA-G [59], we suggest a high expression of HLA-E in infected cells of SARS-CoV2 patients. Interestingly, Bortolotti et al. demonstrated a high expression of HLA-E and an increase of NKG2A/CD94 receptor expression in NK cells co-cultured with Spike SP1-transfected lung epithelial cell lines (Beas-2B) both at transcriptional and at protein levels [60]. The interaction between NKG2A/CD94 with HLA-E molecule conduced to a significant decrease of NK degranulation (decrease of CD107a) [60].
As recently demonstrated by Hò et al., HLA-G links NKG2A/CD94 [14], I suggest that this interaction may occur also in SARS-CoV2 infection decreasing probably NK degranulation as previously demonstrated for HLA-E [60]. Further experiments might elucidate this potential function.
The expression of HLA-G, HLA-E, their soluble forms and their specific receptors could explain the exhaustion of cytotoxic CD8+ T lymphocytes and NK cells probably promoting SARS-CoV2 progression (Fig. 1).
Finally, during convalescence, Zhang et al. demonstrated that HLA-G expression is increased at surface of immune cells in the SARS-CoV2 patient [39]. This expression is simply restored through a limitation of soluble HLA-G production after medication and/or a reinforced immunity.
3. Potential future therapeutic strategies targeting HLA-G and HLA-E molecules and their receptors
Although strides have been made in the treatment of other human coronaviruses, SARS-CoV2 infection remains difficult to treat. As currently, neither approved vaccine nor specific therapies targeting SARS-CoV2 were found, the unique and reasonable strategy remains to use repurposed drugs previously approved by the Food and Drug Administration (FDA) for other diseases. Therein lays the challenge to identify effective drugs and associated appropriate therapies for COVID-19.
In the beginning of COVID-19 spread, many approaches have been proposed to treat the COVID-19. SARS-CoV2 pharmacotherapeutics included antiviral therapy, antibiotics, systemic corticosteroids, anti-inflammatory drugs, neuraminidase inhibitors, RNA synthesis inhibitors, convalescent plasma and traditional herbal medicines [9]. Recent the FDA revised recommendations to combat COVID-19 with therapeutics included convalescent plasma and Remdesivir antiviral drug (https://www.fda.gov/media/136832/download). The Infectious Diseases Society of America (IDSA; https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-3) recommended: (i) convalescent plasma, Lopinavir-Ritonavir antiviral drugs and Tocilizumab (Anti-IL-6) only in the context of a clinical trial, and (ii) Glucocorticoids and Remdesivir antiviral drug only for hospitalized patients with severe COVID-19.
Based on the puzzling out reflections here proposed to link global literature results concerning SARS-CoV2, I think that the missing “core” drugs that should be associated with FDA and IDSA recommended drugs are immune tolerance drug blockers. Indeed, blocking HLA-G and/or HLA-E interaction with their specific receptors either by blocking HLA-G/HLA-E molecules, or blocking their specific receptors (Anti-NKG2A, anti-ILT2, anti-ILT4, and anti-KIR2DL4) may enhance immune system efficiency.
Actually, there is no known FDA approved monoclonal antibodies against HLA-G, or HLA-E. However, a novel HLA-G antagonist has been produced by Tizona Therapeutics (TTX-080), that is currently being investigated in monotherapy dose-escalation in advanced or metastatic solid cancers expressing HLA-G (NCT04485013, Phase 1 multicenter study, started in 14 July 2020). This HLA-G antagonist could potentially be a new medicine targeting HLA-G bolstering immune responses in SARS-CoV2 infection by the restoration of immune cells functions.
Additionally, the Monalizumab, a humanized anti-NKG2A antibody has been approved for poor prognosis tumors therapy [61]. Blocking the inhibitory NKG2A/CD94 receptor has improved tumor immunity by promoting NK and CD8+ T cells functions both in mice and humans; and by advancing their ADCC activity [62], [63]. In the context of the SARS-CoV2, I think that targeting NKG2A/CD94 receptor may empower the immune system. NKG2A/CD94 receptor is known to interact with HLA-E molecules [36], and has been recently proposed as a new receptor for HLA-G [14]. Active immunotherapy using anti-NKG2A antibodies may block both HLA-G and HLA-E signalling pathways enhancing the NK and LT CD8+ cytotoxicity.
Passive conditioned immunotherapy could be also proposed. A pre-selection of convalescent COVID-19 plasma with high levels of plasma antibodies to HLA-G and HLA-E could be envisaged. This hypothesis could be supported by the study of Jucaud et al. having previously reported the relevance of serum non classical HLA-I antibodies in patients with systemic lupus erythematosus during disease flares compared to controls [64]. Passive immunotherapy by the injection of anti-HLA-G and/or anti-HLA-E antibodies might be of great interest. Further studies will be necessary to propose an accurate clinical protocol.
4. Conclusions
The probable implication of HLA-G and HLA-E molecules, either in their membrane-bound or soluble forms, in the progression of SARS-CoV2 infection was supported by several previous results. The complications associated to SARS-CoV2 might be in part associated with these checkpoint molecules productions. It is our hope that our systemic immunological proposition could be useful for: (i) Future monitoring and management of COVID-19 patients through HLA-G/HLA-E expression exploration, and soluble HLA-G/soluble HLA-E dosage in plasma, and through (ii) Future prospection and design of COVID-19 treatments with more specific inhibitors. To achieve the highest possible benefit, I propose associating specific blockers of HLA-G and HLA-E molecules or their receptors with FDA recommended therapeutics. Further evaluations targeting the axes HLA-G/HLA-E and their specific receptors still warranted to guide future therapeutics for monitoring and control of the COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The figure was technically illustrated by Mr Oussema KESSENTINI, an Engineer, Technical designer and Technology entrepreneur. Co-founder & CEO Junior Network Tunisia.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London, England) 2020;395:565. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y., Ho W., Huang Y., Jin D.-Y., Li S., Liu S.-L. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet (London, England) 2020;395:949. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang S., Shi Z., Shu Y., Song J., Gao G.F., Tan W. A distinct name is needed for the new coronavirus. Lancet (London, England) 2020;395:949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO: WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020, 2020.
- 5.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 6.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical Characteristics of Coronavirus Disease 2019 in China. New England J. Med. 2020;382:1708. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R. Wu, L. Wang, H.-C.D. Kuo, A. Shannar, R. Peter, P.J. Chou, et al., An Update on Current Therapeutic Drugs Treating COVID-19. Current pharmacology reports, 2020, p. 1. [DOI] [PMC free article] [PubMed]
- 10.Rouas-Freiss N., Khalil-Daher I., Riteau B., Menier C., Paul P., Dausset J. The immunotolerance role of HLA-G. Semin. Cancer Biol. 1999;9:3. doi: 10.1006/scbi.1998.0103. [DOI] [PubMed] [Google Scholar]
- 11.Morandi F., Pistoia V. Interactions between HLA-G and HLA-E in physiological and pathological conditions. Front. Immunol. 2014;5:394. doi: 10.3389/fimmu.2014.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo R., Trentini A., Bortolotti D., Manfrinato M.C., Rotola A., Castellazzi M. Matrix metalloproteinase-2 (MMP-2) generates soluble HLA-G1 by cell surface proteolytic shedding. Mol. Cell. Biochem. 2013;381:243. doi: 10.1007/s11010-013-1708-5. [DOI] [PubMed] [Google Scholar]
- 13.Tronik-Le Roux D., Renard J., Vérine J., Renault V., Tubacher E., LeMaoult J. Novel landscape of HLA-G isoforms expressed in clear cell renal cell carcinoma patients. Mol. Oncol. 2017;11:1561. doi: 10.1002/1878-0261.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hò G.T., Celik A.A., Huyton T., Hiemisch W., Blasczyk R., Simper G.S. NKG2A/CD94 Is a New Immune Receptor for HLA-G and Distinguishes Amino Acid Differences in the HLA-G Heavy Chain. Int. J. Mol. Sci. 2020;21:4362. doi: 10.3390/ijms21124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carosella E.D., Gregori S., Rouas-Freiss N., LeMaoult J., Menier C., Favier B. The role of HLA-G in immunity and hematopoiesis. Cell Mol. Life Sci. 2011;68:353. doi: 10.1007/s00018-010-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovats S., Main E., Librach C., Stubblebine M., Fisher S., DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt C.M., Orr H.T. Maternal/fetal interactions: the role of the MHC class I molecule HLA-G. Crit. Rev. Immunol. 1993;13:207. [PubMed] [Google Scholar]
- 18.Shih I.-M. Application of human leukocyte antigen-G expression in the diagnosis of human cancer. Hum. Immunol. 2007;68:272. doi: 10.1016/j.humimm.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheu J., Shih I.-M. HLA-G and Immune Evasion in Cancer Cells. J. Formos. Med. Assoc. 2010;109:248. doi: 10.1016/S0929-6646(10)60050-2. [DOI] [PubMed] [Google Scholar]
- 20.Amiot L., Ferrone S., Grosse-Wilde H., Seliger B. Biology of HLA-G in cancer: a candidate molecule for therapeutic intervention? Cellular Molecular Life Sci.: CMLS. 2011;68:417. doi: 10.1007/s00018-010-0583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzo R., Bortolotti D., Bolzani S., Fainardi E. HLA-G molecules in autoimmune diseases and infections. Front. Immunol. 2014;5:592. doi: 10.3389/fimmu.2014.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olavio R.B., Marina S., Loredana M., Roberta R. HLA-G and inflammatory diseases. Inflammation & Allergy - Drug Targets (Discontinued) 2008;7:67. doi: 10.2174/187152808785107615. [DOI] [PubMed] [Google Scholar]
- 23.Catamo E., Zupin L., Crovella S., Celsi F., Segat L. Non-classical MHC-I human leukocyte antigen (HLA-G) in hepatotropic viral infections and in hepatocellular carcinoma. Hum. Immunol. 2014;75:1225. doi: 10.1016/j.humimm.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Dias F.C., Castelli E.C., Collares C.V.A., Moreau P., Donadi E.A. The Role of HLA-G Molecule and HLA-G Gene Polymorphisms in Tumors, Viral Hepatitis, and Parasitic Diseases. Front. Immunol. 2015;6:9. doi: 10.3389/fimmu.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabbagh A., Sonon P., Sadissou I., Mendes-Junior C.T., Garcia A., Donadi E.A. The role of HLA-G in parasitic diseases. HLA. 2018;91:255. doi: 10.1111/tan.13196. [DOI] [PubMed] [Google Scholar]
- 26.Seliger B. The non-classical antigens of HLA-G and HLA-E as diagnostic and prognostic biomarkers and as therapeutic targets in transplantation and tumors. Clin. Transpl. 2013;465 [PubMed] [Google Scholar]
- 27.Amodio G., Sales de Albuquerque R., Gregori S. New insights into HLA-G mediated tolerance. Tissue Antigens. 2014;84:255. doi: 10.1111/tan.12427. [DOI] [PubMed] [Google Scholar]
- 28.E.D. Carosella, N. Rouas-Freiss, D.T.-L. Roux, P. Moreau, J. LeMaoult, Chapter Two - HLA-G: An Immune Checkpoint Molecule, in: Alt FW (Ed.), Advances in Immunology, vol. 127, Academic Press, 2015, p. 33. [DOI] [PubMed]
- 29.Kanai T., Fujii T., Unno N., Yamashita T., Hyodo H., Miki A. Human Leukocyte Antigen-G-Expressing Cells Differently Modulate the Release of Cytokines from Mononuclear Cells Present in the Decidua Versus Peripheral Blood. Am. J. Reprod. Immunol. 2001;45:94. doi: 10.1111/j.8755-8920.2001.450205.x. [DOI] [PubMed] [Google Scholar]
- 30.Schust D.J., Tortorella D., Ploegh H.L. HLA-G and HLA-C at the feto–maternal interface: lessons learned from pathogenic viruses. Semin. Cancer Biol. 1999;9:37. doi: 10.1006/scbi.1998.0106. [DOI] [PubMed] [Google Scholar]
- 31.Lafon M. Immune evasion, a critical strategy for rabies virus. Dev. Biol. (Basel) 2008;131:413. [PubMed] [Google Scholar]
- 32.LeBouder F., Khoufache K., Menier C., Mandouri Y., Keffous M., Lejal N. Immunosuppressive HLA-G molecule is upregulated in alveolar epithelial cells after influenza A virus infection. Hum. Immunol. 2009;70:1016. doi: 10.1016/j.humimm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Foucault M.L., Moules V., Rosa-Calatrava M., Riteau B. Role for proteases and HLA-G in the pathogenicity of influenza A viruses. J. Clin. Virol. 2011;51:155. doi: 10.1016/j.jcv.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Braud V.M., Allan D.S., Wilson D., McMichael A.J. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr. Biol. 1998;8:1. doi: 10.1016/s0960-9822(98)70014-4. [DOI] [PubMed] [Google Scholar]
- 35.Braud V.M., Allan D.S., O'Callaghan C.A., Söderström K., D'Andrea A., Ogg G.S. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser B.K., Pizarro J.C., Kerns J., Strong R.K. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc. Natl. Acad. Sci. U S A. 2008;105:6696. doi: 10.1073/pnas.0802736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S., Gan J., Chen B.G., Zheng D., Zhang J.G., Lin R.H. Dynamics of peripheral immune cells and their HLA-G and receptor expressions in a patient suffering from critical COVID-19 pneumonia to convalescence. Clin. Transl. Immunol. 2020;9 doi: 10.1002/cti2.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130:2620. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.F. Martinon, K. Burns, J. Tschopp, The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Molecular Cell 10 (2002) 417. [DOI] [PubMed]
- 43.Hutchinson K.L., Rollin P.E. Cytokine and Chemokine Expression in Humans Infected with Sudan Ebola Virus. J. Infect. Dis. 2007;196:S357. doi: 10.1086/520611. [DOI] [PubMed] [Google Scholar]
- 44.Urosevic M., Kurrer M.O., Kamarashev J., Mueller B., Weder W., Burg G. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am. J. Pathol. 2001;159:817. doi: 10.1016/S0002-9440(10)61756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreau P., Adrian-Cabestre F., Menier C., Guiard V., Gourand L., Dausset J. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int. Immunol. 1999;11:803. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 46.Bethin K.E., Vogt S.K., Muglia L.J. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. PNAS. 2000;97:9317. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steensberg A., Fischer C.P., Keller C., Møller K., Pedersen B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol.-Endocrinol. Metabolism. 2003;285:E433. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 48.Mahmud-Al-Rafat A., Majumder A., Taufiqur Rahman K.M., Mahedi Hasan A.M., Didarul Islam K.M., Taylor-Robinson A.W. Decoding the enigma of antiviral crisis: Does one target molecule regulate all? Cytokine. 2019;115:13. doi: 10.1016/j.cyto.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine. 2020;1 doi: 10.1007/s12020-020-02325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreau P., Faure O., Lefebvre S., Ibrahim E.C., O’Brien M., Gourand L. Glucocorticoid hormones upregulate levels of HLA-G transcripts in trophoblasts. Transpl. Proc. 2001;33:2277. doi: 10.1016/s0041-1345(01)01990-x. [DOI] [PubMed] [Google Scholar]
- 51.Akhter A., Das V., Naik S., Faridi R.M., Pandey A., Agrawal S. Upregulation of HLA-G in JEG-3 cells by dexamethasone and hydrocortisone. Arch. Gynecol. Obstet. 2012;285:7. doi: 10.1007/s00404-011-1880-3. [DOI] [PubMed] [Google Scholar]
- 52.Akhter A., Faridi R.M., Das V., Pandey A., Naik S., Agrawal S. In vitro up-regulation of HLA-G using dexamethasone and hydrocortisone in first-trimester trophoblast cells of women experiencing recurrent miscarriage. Tissue Antigens. 2012;80:126. doi: 10.1111/j.1399-0039.2012.01884.x. [DOI] [PubMed] [Google Scholar]
- 53.Verma R.P., Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007;15:2223. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Quiros Roldan E., Biasiotto G., Magro P., Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): A role for iron homeostasis? Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards J.A., Denis F., Talbot P.J. Activation of glial cells by human coronavirus OC43 infection. J. Neuroimmunol. 2000;108:73. doi: 10.1016/S0165-5728(00)00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desforges M., Miletti T.C., Gagnon M., Talbot P.J. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130:228. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pistoia V., Morandi F., Wang X., Ferrone S. Soluble HLA-G: Are they clinically relevant? Semin. Cancer Biol. 2007;17:469. doi: 10.1016/j.semcancer.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishitani A., Sageshima N., Lee N., Dorofeeva N., Hatake K., Marquardt H. Protein Expression and Peptide Binding Suggest Unique and Interacting Functional Roles for HLA-E, F, and G in Maternal-Placental Immune Recognition. J. Immunol. 2003;171:1376. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 60.Bortolotti D., Gentili V., Rizzo S., Rotola A., Rizzo R. SARS-CoV-2 Spike 1 Protein Controls Natural Killer Cell Activation via the HLA-E/NKG2A Pathway. Cells. 2020;9:E1975. doi: 10.3390/cells9091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mingari M.C., Pietra G., Moretta L. Immune Checkpoint Inhibitors: Anti-NKG2A Antibodies on Board. Trends Immunol. 2019;40:83. doi: 10.1016/j.it.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 62.André P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175:1731. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haanen J.B., Cerundolo V. NKG2A, a New Kid on the Immune Checkpoint Block. Cell. 2018;175:1720. doi: 10.1016/j.cell.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 64.Jucaud V., Ravindranath M.H., Terasaki P.I., Morales-Buenrostro L.E., Hiepe F., Rose T. Serum antibodies to human leucocyte antigen (HLA)-E, HLA-F and HLA-G in patients with systemic lupus erythematosus (SLE) during disease flares: Clinical relevance of HLA-F autoantibodies. Clin. Exp. Immunol. 2016;183:326. doi: 10.1111/cei.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]