Abstract
Despite mounting evidence that large intramural leiomyomas decrease fecundity during in vitro fertilization cycles, few studies have demonstrated a mechanism for this impact. We hypothesize that large intramural leiomyomas (IM) decrease the expression of endometrial implantation factors during the window of implantation. We prospectively recruited sub-fertile patients with IM 3 cm or greater in size planning myomectomy and performed endometrial biopsies the day of planned myomectomy (n = 9). Preoperative screening demonstrated no intercavitary lesions. Control endometrial samples were obtained from young, normally menstruating women free of uterine leiomyomas (n = 8). Endometrial samples were obtained in the mid-secretory phase (average cycle day for control patients and intramural leiomyoma patients were 24.5 and 21.3, respectively). Expression of implantation markers HOXA10, leukemia inhibitory factor (LIF), ER-α, and PR was compared using quantitative immunohistochemistry. Standard descriptive statistics were used to compare H-scores between the cohorts. Patients with intramural leiomyomas were found to have decreased LIF compared to controls (p value < 0.001). Expressions of HOXA10 and PR were no different between cohorts; however, ER-α showed a trend toward increased expression in the fibroid cohort (p value 0.07). LIF is downregulated in the endometrium of patients with large IM. This study is among the first to show decreased LIF expression in patients with uterine leiomyomas. We hypothesize that this difference from previously published work is due to sampling the endometrium at the height of LIF expression. Further work is needed to show if LIF downregulation is corrected with leiomyoma resection.
Keywords: Leukemia inhibitory factor, Intramural fibroid, Leiomyoma
Introduction
Human embryo implantation requires complex interactions between the blastocyst and the endometrium, the latter of which undergoes necessary molecular changes in the luteal phase to ready for this event. The presence of gynecologic diseases may disrupt the mid-luteal phase endometrium, leading to decreased success of implantation of the human blastocyst.1 In sub-fertile women embarking upon assisted reproductive technology, it is therefore advisable to assess the uterine cavity to rule out gynecologic pathologic conditions such as leiomyoma, endometrial polyps, or synechiae.2
Benign leiomyomas, also known as uterine fibroids, are present in 20–50% of reproductive age women and may represent the sole cause of infertility in up to 2.4% of presenting couples. 3, 4 Submucosal fibroids have been shown to reduce endometrial factors associated with embryo implantation, and the removal of submucosal fibroids is associated with increased fertility.5 The association between intramural fibroids and infertility is unclear. While past studies have been inconsistent regarding a relationship between non-cavity distorting intramural fibroids and reduced live birth rates, a recent meta-analysis demonstrated a significant reduction in pregnancy rates of as high as 18% 6–8. Studies have also demonstrated a size relationship between intramural fibroids and IVF success, with fibroids 2.85 cm or greater being associated with IVF implantation failure.9
The pathophysiologic cause of implantation failure in patient with non-cavity distorting intramural fibroids is not currently clear. Several endometrial implantation factors have been studied to provide an understanding of subfertility in patients with non-cavity distorting fibroids. HOXA10 is a widely studied homeobox gene which is responsible for cellular differentiation in the human uterus.10 HOXA10 expression increases in the mid-secretory phase under the influence of a rise in progesterone, and HOXA10 is known to regulate pro-embryo adhesion molecules such as pinopodes, beta3-integrin, and insulin-like growth factor binding protein-1.11, 12 Embryo implantation failure occurs in mice with HOXA10 mutations, demonstrating its vital importance.13 HOXA10 has been shown to be reduced in the setting of submucosal fibroids, but the findings in intramural fibroids are less clear.5 While one previous study showed no difference in HOXA10 between the endometrium of control patients and patients with intramural fibroids, another demonstrated significant difference in HOXA10 between these cohorts. Key differences in the studies were differing menstrual cycle phases when the sample was obtained.14 The cytokine leukemia inhibitory factor (LIF) has also been shown to play a critical role in the implantation process, promoting apposition and attachment to the endometrium along with promoting growth, differentiation, and function of the trophoblast.15 The importance of LIF in implantation is suggested strongly by its marked increase in expression during the mid- and late-secretory phases of the menstrual cycle, as well as expression of its receptor within the blastocyst. Furthermore, studies in LIF-knockout mice have demonstrated failure of embryonal implantation.16 No previous studies to our knowledge have demonstrated a decrease in LIF expression in non-cavity distorting intramural fibroids.
Several factors needed for implantation are expressed as a result of the hormones progesterone and estrogen binding to their prospective receptor.17 Given the limited published research on the effects of non-cavity distorting intramural fibroids on implantation factors and the lack of studies completed assessing implantation factors in patients with large intramural fibroids, we designed a study to assess the expression of estrogen receptor alpha (ER-α) and progesterone receptor (PR), and downstream implantation factors LIF and HOXA10, in patients with large intramural fibroids compared to control patients with normal uteri. We hypothesized that patients with non-cavity distorting intramural fibroids 3 cm and greater would have decreased expression of ER-α, PR, LIF, and HOXA10 in luteal phase endometrial biopsy samples compared to normal controls.
Materials and Methods
Study Population
This prospective cohort study was performed on nine prospectively collected endometrial specimens from women planning myomectomy with intramural leiomyomas 3 cm or greater. All tissues were obtained in accordance with the Committee for the Protection of Human Subjects at the University of Alabama at Birmingham. Each participant signed an informed consent for an Institutional Review Board approved protocol (UAB #00000726) to obtain an endometrial biopsy at time of scheduled myomectomy.
All samples were obtained in the secretory phase of the menstrual cycle. Subjects were nulliparous premenopausal women, or patients with a history of recurrent pregnancy loss (age range, 25–40 years) with regular menstrual cycles, scheduled for a laparoscopic, robot-assisted, or open myomectomy. Subjects had a hysterosalpingogram, saline-infusion sonogram, or magnetic resonance imaging (MRI) suggesting no compression of the uterine cavity by the intramural leiomyoma. Subjects using any form of hormonal treatment within 3 months of surgery were excluded. Additional exclusion criteria included current pregnancy or postpartum within 6 months of delivery, current breastfeeding, or pelvic malignancy. As controls, eight paid volunteer female subjects were recruited who demonstrated regular cyclic menses using a separate Institutional Review Board protocol at UNC (05-1757). None of these subjects had signs or symptoms of endometriosis or a history of infertility, and all were in good health. There was no attempt to match controls to the cases in terms of age, body mass index (BMI), or gravidity. All subjects underwent an endometrial biopsy, timed to the mid-secretory phase using urinary LH testing.
Endometrial biopsies were performed using a pipelle suction curettage during the luteal phase for patients undergoing myomectomy (cycle day 16–29) and during luteal phase for control endometrial samples on LH + 7–10 (cycle day 21–24). The menstrual cycle stage was determined by a single pathologist (L.N.) using the dating criteria of Noyes et al.18 Portions of endometrial biopsies were placed in 10% buffered formalin for paraffin embedding and sectioning.
Tissue Specimens
Endometrium specimens were collected from patients with an endometrial pipelle before the myomectomy surgery began. The segment of endometrium was fixed immediately in 10% buffered formalin for 24 h at room temperature before paraffin embedding.
Immunohistochemistry of Endometrium for HOXA10, LIF, ER-α, and PR
Formalin-fixed paraffin embedded samples (FFPE) were cut into 5-μm sections and placed on positively charged slides. Hematoxylin and eosin-stained tissue were analyzed by a gynecologic pathologist to confirm histology. Slides were deparaffinized and rehydrated to prepare for immunohistochemistry (IHC) of estrogen receptor (α), progesterone receptor, LIF (N-18), and HOXA10. Antigen retrieval was with 10-mM sodium citrate at pH 6.0 under pressure for 5 min. Slides were washed in PBS. Endogenous peroxidases were blocked with 3% H2O2 in methanol for 15 min. Slides were then blocked with 10% normal goat serum (Vector Labs) plus 1% normal horse serum (Vector Labs) in PBS, followed by primary antibody incubation in same blocking buffer. After primary antibody, slides were washed in PBS. Primary antibody detection was achieved with Imm-Press anti-rabbit IgG (MP-6401) for estrogen receptor- α and progesterone receptor IHC’s anti-goat IgG (MP-7405) kit for LIF and HOXA10 IHC, followed by 3,3′-diaminobenzidine incubation. Slides were counterstained with Gill’s hematoxylin and then washed in water and PBS. Slides were sealed with Permount (SP15-100). Antibody concentrations were as follows: estrogen receptor(α), 1:100 (Abcam); progesterone receptor, 1:100 (Abcam); LIF (N-18), 1:100 (Santa Cruz); and HOXA10 (Santa Cruz).
Histopathologic Evaluation of the Immunostaining
Immunostained endometrial tissue was examined in a blinded fashion by two gynecologic pathologists. Independent grading of the intensity of each type of staining and percentage of the area stained have been reported and results further analyzed.
Statistical Analysis
Mean demographic parameters between groups were compared using an independent t test for continuous data and c2 analysis for proportions. An independent-samples t-test and Mann–Whitney rank sum testing were used to analyze the results of quantitative immunohistochemistry results between groups. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 18.0.3 (SPSS, Chicago, IL).
Results
The baseline demographics of the study cohorts are demonstrated in Table 1. As expected, the control patient group was younger and more lean when compared to the study cohort (25.9 years compared to 37.7, 23.3 m2 compared to 31.2 m2). There were more African-American women in the study cohort as compared to the control cohort. All study patients had fibroids 4 cm or larger, except for two patients who had multiple leiomyomas with the largest measuring 3 cm. All samples were taken in the luteal phase for all patients (Table 2).
Table 1.
Study participant characteristics
| Control (n = 8) | Fibroid cohort (n = 9) | |
|---|---|---|
| Age at time of endometrial biopsy, years | 25.9 ± 4.3 | 37.7 ± 1.9a |
| Body mass index, kg/m2 | 23.3 ± 3.0 | 31.2 ± 5.4a |
| Race | ||
| African American | 0/8 | 5/9a |
| Caucasian | 5/8 | 3/9 |
| Asian | 3/8 | 1/9 |
| Prior parity | 0/8 | 1/9 |
| Cycle day | 24.5 ± 4.5 | 21.3 ± 4.2 |
ap < 0.05 versus control
Table 2.
Fibroid cohort specific characteristics
| Patient ID | Cycle day | Maximum leiomyoma size (CM) |
|
|---|---|---|---|
| 1 | 16 | 7 | |
| 2 | 21 | 11 | |
| 3 | 29 | 5 | |
| 4 | 27 | 3 | |
| 5 | 20 | 13 | |
| 6 | 20 | 10 | |
| 7 | 21 | 8 | |
| 8 | 21 | 10 | |
| 9 | 17 | 3 |
Expression of HOXA10 did not differ between patients with intramural leiomyomas and control patients. Patients with intramural leiomyomas were found, however, to have decreased leukemia inhibitory factor (LIF) when compared to controls (p value < 0.001). Expression of progesterone receptor (PR) did not different between cohorts; however, estrogen receptor alpha (ER-α) showed a trend toward increased expression in the fibroid cohort (p value 0.07) (Fig. 1).
Fig. 1.
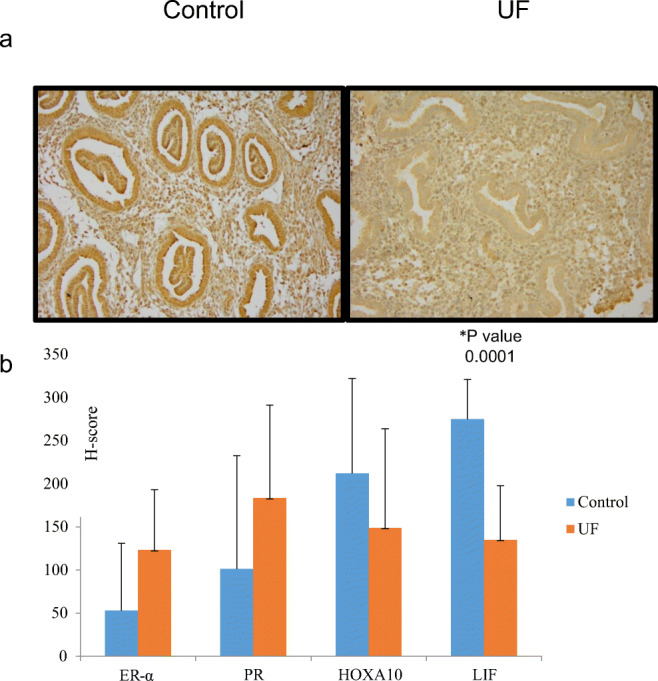
(a) Representative tissue sections of control group endometrium [control] and endometrium from uterine fibroid patients [UF]; immunohistochemical staining was performed with antibodies to leukemia inhibitory factor (LIF). (b) H-scores (mean ± SD) for expression of estrogen receptor α (ER- α), progesterone receptor (PR), homeobox gene A10 (HOXA10), and leukemia inhibitory factor
Conclusion
Implantation of the blastocyst into the human endometrium requires a series of molecular events during the window of implantation, primarily coordinated by the effects of estrogen and progesterone. Many necessary implantation factors, such as leukemia inhibitory factor, are transcribed as downstream effects by the activation of estrogen receptor alpha (ER-α) and progesterone receptor (PR).19 Previous studies suggest increased ER-α and PR expression in leiomyoma tissue and decreased expression of endometrial ER-α and PR in patients with leiomyoma compared to those without.20, 21 Our study was set to determine if implantation factors HOXA10 and LIF decreased in the endometrium of patients with large, non-cavity distorting intramural leiomyomas compared to patients without myoma, as well as to assess for alterations in endometrium ER-α and PR.
To our knowledge, this study marks one of the first to demonstrate that LIF is downregulated in the endometrium of patients with large, non-cavity distorting intramural leiomyomas. We hypothesize that this difference from previously published work is due to sampling the endometrium at the height of LIF expression. Leukemia inhibitory factor (LIF) is a member of the gp130 cytokine family and is critical for embryo implantation. LIF expression in the endometrium changes during the menstrual cycle, with an increase in expression during the window of implantation.22 LIF acts as a stimulus for endometrial cell proliferation, angiogenesis, cell differentiation and survival, and change in epithelial polarity. It appears that calreticulin (mediated by cyclic AMP regulator EPCA2) is critical for LIF expression and LIF expression leads to the expression of several signaling pathways (JAK/STAT, MAPK, P13-kinase) (PIPK).23, 24 Through these signaling pathways, LIF increases factors needed for invasion in trophoblastic cells.25 In mice, LIF binds both to its receptor at the level of the endometrium, along with an LIF receptor in the surface of the blastocyst.26 Previous studies have demonstrated decreasing levels of LIF in women with recurrent implantation failure, adenomyosis, and unexplained infertility.27–30 Our work demonstrates that despite no compression seen on imaging, large fibroids 3 cm or greater exert local changes that downregulate LIF expression. LIF expression has been shown to be decreased previously in cavity-distorting leiomyoma, but to our knowledge, no previous study demonstrated lower LIF expression in non-cavity distorting intramural fibroids.31 Our findings are likely a result of sampling in the luteal phase specifically, as well as recruiting only patients with large leiomyomas. One recent study looked at microarray data from patients with and without uterine fibroids and found no difference in LIF expression. This study had seven patients with non-cavity distorting fibroids, only four of which were mid-luteal, and most patients in their leiomyoma cohort were over the age of 40. It could be these differences that led them to not detect changes in LIF expression.32 Further studies are needed to know if medical or surgical therapies restore LIF expression in patient with large, non-cavity distorting leiomyomas.
In our study, HOXA10 expression in the endometrium of patients with non-cavity distorting leiomyomas was lower when compared to control patients, but this difference did not reach significance. HOXA10 expression in the mid-luteal phase regulates expression of needed implantation factors, such as β3-integrin, and is known to be downregulated in patients with adenomyosis, recurrent pregnancy loss, and recurrent implantation factor.33–35 Previous studies have demonstrated mixed results when detecting HOXA10 in the presence of intramural fibroids, with the most recently study demonstrating decreased expression via a microarray study when compared to control patients.5, 14, 32 Recent studies demonstrate altered expression of HOXA10 mRNA in patients with non-cavity distorting intramural fibroids, with one study noting a significant rise in HOXA10 mRNA expression after myomectomy.36, 37 Proposed mechanisms for non-cavity distorting fibroids causing abnormal HOXA10 expression include leiomyoma TGF-β effects on BMP-2 expression (leading to decreased HOXA10 downstream) and change in methylation in HOXA10.38, 39 Studies that demonstrated lower HOXA10 expression sampled the endometrium in the luteal phase, as our study has. Perhaps increased study power would lead to a significant difference presence in our patient cohort.
Progesterone receptor (PR) isoforms induce genes necessary for embryo attachment to the endometrium.40 Specifically working with other transcription factors such as STAT3 to regulate needed target genes, PR is responsible for the needed implantation mechanisms such as uterine epithelial proliferation and local immune responses.41 PR expression decreases near the window of implantation, leading to the expression of Forkhead Box 01 (FOXO1), an important mediator of endometrial epithelial cell polarity and apoptosis.42, 43 PR expression has been shown to be significantly reduced in infertile women in the mid-luteal phase compared to control, and PR deficiency is also found in patients with endometriosis.44, 45 Our study demonstrated a nonsignificant elevation in PR in patient with uterine fibroids, perhaps suggesting a dysfunctional expression during the window of implantation.
The strength of our study lies in the study design and patient recruitment. Few previous studies addressing implantation factors in patients with intramural fibroids have addressed size and location with respect to the endometrial cavity. We screened each patient in our study with imaging of the uterine cavity to ensure the leiomyoma did not distort the endometrium. The timing of endometrial biopsy was also important, as we sampled most patients during the window of implantation. There are some weaknesses of our study. Our control group was not age matched and was significantly thinner, than our study cohort. While this does represent possible confounders, the profile of our fibroid patient cohort is consistent with the common clinical presentation of patients with fibroids-sub-fertile, over the age of 35, and more commonly African-American. Also, our study did not follow patients after myomectomy to determine if they conceived, which may have been used as a marker of the restoration of normal implantation factors.
Our study demonstrated decreased LIF in patients with non-cavity distorting leiomyomas greater than 3 cm compared to patients with a normal uterus and regular menstrual cycles. Further work is needed to show if LIF downregulation is corrected with medical leiomyoma volume reduction or with leiomyoma resection, in patients with non-cavity distorting leiomyomas.
Acknowledgments
This research was completed at the University of Alabama at Birmingham.
Funding Information
This study was supported by a Research Grant from the Scientific Advisory Board of Vivere Health (2014–2015).
Footnotes
The views expressed in this abstract/manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Contributor Information
Bruce Pier, Email: bruce.d.pier3.mil@mail.mil.
Christopher Crellin, Email: cristopher.j.crellin.mil@mail.mil.
Ashwini Katre, Email: akatre@uabmc.edu.
Michael G. Conner, Email: mgconner@uabmc.edu
Lea Novak, Email: lnovak@uabmc.edu.
Steven L Young, Email: youngs@med.unc.edu.
Rebecca Arend, Email: rarend@uab.edu.
References
- 1.Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–253. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6) e44-.e50. [DOI] [PubMed]
- 3.Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002;17(6):1424–1430. doi: 10.1093/humrep/17.6.1424. [DOI] [PubMed] [Google Scholar]
- 4.Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–1223. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93:2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klatsky PC, Lane DE, Ryan IP, Fujimoto VY. The effect of fibroids without cavity involvement on ART outcomes independent of ovarian age. Hum Reprod. 2007;22:521–526. doi: 10.1093/humrep/del370. [DOI] [PubMed] [Google Scholar]
- 7.Somigliana E, Vercellini P, Daguati R, Pasin R, Giorgi O, Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update. 2007;13:465–476. doi: 10.1093/humupd/dmm013. [DOI] [PubMed] [Google Scholar]
- 8.Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod. 2010;25:418–429. doi: 10.1093/humrep/dep396. [DOI] [PubMed] [Google Scholar]
- 9.Yan L, Ding L, Li C, Wang Y, Tang R, Chen ZJ. Effect of fibroids not distorting the endometrial cavity on the outcome of in vitro fertilization treatment: a retrospective cohort study. Fertil Steril. 2014;101:716–721. doi: 10.1016/j.fertnstert.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6(1):75–79. doi: 10.1093/humupd/6.1.75. [DOI] [PubMed] [Google Scholar]
- 11.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med. 2010;28(1):69–74. doi: 10.1055/s-0029-1242996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daftary GS, Taylor HS. Molecular markers of implantation: clinical implications. Curr Opin Obstet Gynecol. 2001;13(3):269–274. doi: 10.1097/00001703-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki S, Canis M, Darcha C, Pouly JL, Mage G. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24:3180–3318. doi: 10.1093/humrep/dep306. [DOI] [PubMed] [Google Scholar]
- 15.Aghajanova L. Leukemia inhibitory factor and human embryo implantation. Ann N Y Acad Sci. 2004;1034:176–183. doi: 10.1196/annals.1335.020. [DOI] [PubMed] [Google Scholar]
- 16.Salleh N, Giribabu N. Leukemia inhibitory factor: roles in embryo implantation and in nonhormonal contraception. Sci World J. 2014;1:1–10. doi: 10.1155/2014/201514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young SL. Oestrogen and progesterone action on endometrium: a translational approach to understanding endometrial receptivity. Reprod BioMed Online. 2013;27:497–505. doi: 10.1016/j.rbmo.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 19.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gellersen B, Brosens J. Cyclic Decidualization of the human endometrium. Endocr Rev. 2014;35(6):851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 21.Jakimiuk A, Michal Bogusiewicz M, Tarkowski R, et al. Estrogen receptor and expression in uterine leiomyomas from premenopausal women. Fertil Steril. 2004;82(1):1244–1249. doi: 10.1016/j.fertnstert.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 22.Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med. 2009;27(1):62–79. doi: 10.1055/s-0028-1108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosario GX, Stewart CL. The multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation. Am J Reprod Immunol. 2016;75(3):246–255. doi: 10.1111/aji.12474. [DOI] [PubMed] [Google Scholar]
- 24.Kusama K, Yoshie M, Tamura K, Imakawa K, Tachikawa E. EPAC2-mediated calreticulin regulates LIF and COX2 expression in human endometrial glandular cells. J Mol Endocrinol. 2015;54(1):17–24. doi: 10.1530/JME-14-0162. [DOI] [PubMed] [Google Scholar]
- 25.Suman P, Shembekar N, Gupta SK. Leukemia inhibitory factor increases the invasiveness of trophoblastic cells through integrated increase in the expression of adhesion molecules and pappalysin 1 with a concomitant decrease in the expression of tissue inhibitor of matrix metalloproteinases. Fertil Steril. 2013;99(2):533–542. doi: 10.1016/j.fertnstert.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. Leukemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil. 1994;101:421–426. doi: 10.1530/jrf.0.1010421. [DOI] [PubMed] [Google Scholar]
- 27.Choi Y, Kim HR, Lim EJ, Park M, Yoon JA, Kim YS, Kim EK, Shin JE, Kim JH, Kwon H, Song H, Choi DH. Integrative analyses of uterine transcriptome and microRNAome reveal compromised LIF-STAT3 signaling and progesterone response in the endometrium of patients with recurrent/repeated implantation failure (RIF) PLoS One. 2016;11(6):e0157696. doi: 10.1371/journal.pone.0157696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen CF, Liao SK, Huang SJ, Tabak S, Arcuri F, Lee CL, Arici A, Petraglia F, Wang HS, Kayisli UA. Decreased endometrial expression of leukemia inhibitory factor receptor disrupts the STAT3 signaling in adenomyosis during the implantation window. Reprod Sci. 2017;24(8):1176–1186. doi: 10.1177/1933719116681515. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Y, Sun X, Yang X, Zhang J, Xue Q, Cai B, Zhou Y. Leukemia inhibitory factor is dysregulated in the endometrium and uterine flushing fluid of patients with adenomyosis during implantation window. Fertil Steril. 2010;94(1):85–89. doi: 10.1016/j.fertnstert.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Franasiak J, Holoch K, Yuan L, Schamme D, Young S, Lessey B. Prospective assessment of midsecretory endometrial leukemia inhibitor factor expression versus anb3 testing in women with unexplained infertility. Fertil Steril. 2014;101(6):1724–1731. doi: 10.1016/j.fertnstert.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasegawa E, Ito H, Hasegawa F, Hatano K, Kazuka M, Usuda S, Isaka K. Expression of leukemia inhibitory factor in the endometrium in abnormal uterine cavities during the implantation window. Fertil Steril. 2012;97:953–958. doi: 10.1016/j.fertnstert.2012.01.113. [DOI] [PubMed] [Google Scholar]
- 32.Aghajanova L, Houshdaran S, Irwin J, Giudice L. Effects of noncavity-distorting fibroids on endometrial gene expression and function. Biol Reprod. 2017;97(4):564–576. doi: 10.1093/biolre/iox107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu LH, Sun LH, Hu YL, Jiang Y, Liu HY, Shen XY, Jin XY, Zhen X, Sun HX, Yan GJ. PCAF impairs endometrial receptivity and embryo implantation by down-regulating β3-integrin expression via HOXA10 acetylation. J Clin Endocrinol Metab. 2013;98(11):4417–4428. doi: 10.1210/jc.2013-1429. [DOI] [PubMed] [Google Scholar]
- 34.Fischer CP, Kayisili U, Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil Steril. 2011;95(3):1133–1136. doi: 10.1016/j.fertnstert.2010.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Chen X, Saravelos SH, Liu Y, Huang J, Zhang J, Li TC. HOXA-10 and E-cadherin expression in the endometrium of women with recurrent implantation failure and recurrent miscarriage. Fertil Steril. 2017;107(1):136–143. doi: 10.1016/j.fertnstert.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Makker A, Goel MM, Nigam D, Bhatia V, Mahdi AA, Das V, Pandey A. Endometrial expression of homeobox genes and cell adhesion molecules in infertile women with intramural fibroids during window of implantation. Reprod Sci. 2017;24(3):435–444. doi: 10.1177/1933719116657196. [DOI] [PubMed] [Google Scholar]
- 37.Unlu C, Celik O, Celik N, Otlu B. Expression of endometrial receptivity genes increase after myomectomy of intramural leiomyomas not distorting the endometrial cavity. Reprod Sci. 2016;23(1):31–41. doi: 10.1177/1933719115612929. [DOI] [PubMed] [Google Scholar]
- 38.Doherty LF, Taylor HS. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil Steril. 2015;103(3):845–852. doi: 10.1016/j.fertnstert.2014.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulp JL, Mamillapalli R, Taylor HS. Aberrant HOXA10 methylation in patients with common gynecologic disorders: implications for reproductive outcomes. Reprod Sci. 2016;23(4):455–463. doi: 10.1177/1933719116630427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halasz M, Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol. 2013;97(1):43–50. doi: 10.1016/j.jri.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Bhurke AS, Bagchi IC, Bagchi MK. Progesterone-regulated endometrial factors controlling implantation. Am J Reprod Immunol. 2016;75(3):237–245. doi: 10.1111/aji.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lessey BA, Yeh I, Castelbaum AJ, Fritz MA, Ilesanmi AO, Korzeniowski P, Sun J, Chwalisz K. Endometrial progesterone receptors and markers of uterine receptivity in the window of implantation. Fertil Steril. 1996;65(3):477–483. [PubMed] [Google Scholar]
- 43.Vasquez YM, Wang X, Wetendorf M, Franco HL, Mo Q, Wang T, Lanz RB, Young SL, Lessey BA, Spencer TE, Lydon JP, DeMayo F. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018;14(11):e1007787. doi: 10.1371/journal.pgen.1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petousis S, Prapas Y, Margioula-Siarkou C, Milias S, Ravanos K, Kalogiannidis I, Haitoglou C, Prapas N, Rousso D. Expression of progesterone receptors is significantly impaired in the endometrium of infertile women during the implantation window: a prospective observational study. J Matern Fetal Neonatal Med. 2016;29(23):3912–3919. doi: 10.3109/14767058.2016.1152244. [DOI] [PubMed] [Google Scholar]
- 45.Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, Lin Z, Imir G, Thung S, Su EJ, Kim JJ. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]


