Abstract
Cell-free fetal DNA in the maternal circulation has been associated with the onset of labor at term. Moreover, clinical studies have suggested that cell-free fetal DNA has value to predict pregnancy complications such as spontaneous preterm labor leading to preterm birth. However, a mechanistic link between cell-free fetal DNA and preterm labor and birth has not been established. Herein, using an allogeneic mouse model in which a paternal green fluorescent protein (GFP) can be tracked in the fetuses, we established that cell-free fetal DNA (Egfp) concentrations were higher in late gestation compared to mid-pregnancy and were maintained at increased levels during the onset of labor at term, followed by a rapid decrease after birth. A positive correlation between cell-free fetal DNA concentrations and the number of GFP-positive pups was also observed. The increase in cell-free fetal DNA concentrations prior to labor at term was not linked to a surge in any specific cytokine/chemokine; yet, specific chemokines (i.e., CCL2, CCL7, and CXCL2) increased as gestation progressed and maintained elevated levels in the postpartum period. In addition, cell-free fetal DNA concentrations increased prior to systemic inflammation-induced preterm birth, which was associated with a strong cytokine response in the maternal circulation. However, cell-free fetal DNA concentrations were not increased prior to intra-amniotic inflammation-induced preterm birth, but in this model, a mild inflammatory response was observed in the maternal circulation. Collectively, these findings suggest that an elevation in cell-free fetal DNA concentrations in the maternal circulation precedes the physiological process of labor at term and the pathological process of preterm labor linked with systemic inflammation, but not that associated with intra-amniotic inflammation.
Electronic supplementary material
The online version of this article (10.1007/s43032-019-00023-6) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Chorioamnionitis, Prematurity, Fetal inflammatory response, Funisitis
Introduction
Labor is a state of physiological inflammation [1–6] that is characterized by an increase in cellular and soluble inflammatory mediators in the cervix [1, 7–19], myometrium [8, 9, 11, 12, 20–24], chorioamniotic membranes [9, 11, 25–32], and decidua (e.g., maternal-fetal interface) [5, 9, 11, 25, 26, 29, 33–38]. This concept is supported by transcriptomic studies showing that gestational tissues from women who underwent spontaneous labor at term display a gene expression signature consistent with acute inflammation [28, 39–50]. In addition to this local inflammation, labor is accompanied by a maternal systemic inflammatory response [12, 51–57]. Such an immune process is thought to be initiated by cell-free fetal DNA (cffDNA) [58–62], since its concentrations increase from mid to late pregnancy in humans [60, 63–73] and animals [74–77] and are even greater during the onset of term parturition [60, 66].
Human studies have shown that there is a positive association between maternal circulating cffDNA and adverse pregnancy outcomes such as early- and late-onset preeclampsia [78–81], intrauterine growth restriction [81, 82], and spontaneous preterm labor [83–88]. However, the predictive value of cffDNA in the maternal circulation is controversial [89–93]. Animal studies have also mechanistically shown that the administration of CpG (oligodeoxynucleotide) to pregnant Il10−/−or NOD mice induces an inflammatory response leading to fetal resorption and preterm birth in a TLR9-dependent manner [94, 95]. It was further established that such a TLR9-mediated inflammatory process was triggered by fetal, but not adult, cell-free DNA [86], suggesting that fetal cell-free nucleic acids are capable of inducing pregnancy complications. However, such mechanistic studies have relied on the administration of exogenous DNA. Herein, using an animal model of allogenic pregnancy, we investigated whether the presence of the gene for the green fluorescent protein can be detected in the cell-free DNA fraction of the maternal circulation from mid to late gestation, before and during term labor, and prior to preterm labor and birth. In addition, maternal inflammatory responses were evaluated by measuring plasma cytokine concentrations.
Methods
Mice
C57BL/6 Actb-Egfp (GFP-expressing) males and DBA/2 virgin females were purchased from The Jackson Laboratory in Bar Harbor, ME, USA, and bred in the animal care facility at C.S. Mott Center for Human Growth and Development at Wayne State University, Detroit, MI. All mice were kept under a circadian cycle (light:dark = 12:12 h). Females, 8–12 weeks old, were bred with males of proven fertility. Female mice were checked daily between 8:00 and 9:00 a.m. for the appearance of a vaginal plug, which indicated 0.5 days post coitum (dpc). Females were then housed separately from the males, their weights were monitored, and a gain of 2 or more grams by 12.5 dpc confirmed pregnancy. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Wayne State University (Protocol No. A 07-03-15).
Plasma Collection During Mid and Late Pregnancy and Postpartum
Ten groups of pregnant DBA/2 mice were used for experiments at a designated time of gestation: 12.5 dpc, 13.5 dpc, 14.5 dpc, 15.5 dpc, 16.5 dpc, 17.5 dpc, 18.5 dpc, 19.5 dpc, in labor, and postpartum (n = 5–11 per group). The in labor group was defined as dams on 20.5 dpc that were actively delivering as indicated by the presence of the first pup in the cage bedding or a pup in the birth canal. The postpartum group was defined as dams that delivered at 20.5 dpc, from which samples were collected 12–36 h after delivery. On the designated day of gestation or postpartum, dams were anesthetized by the intraperitoneal injection of 250 mg/kg of Avertin [tribromoethanol (Sigma-Aldrich, St. Louis, MO, USA) dissolved in amylene hydrate (Sigma-Aldrich) and distilled water]. Whole blood (0.5–1.2 mL) was collected by cardiac puncture using a 22-gauge needle (BD PrecisionGlide Needle, Becton Dickinson, Franklin Lakes, NJ, USA) and immediately dispensed into a K2 EDTA MiniCollect® tube (Greiner Bio-one GmbH, Kremsmünster, Austria). Anticoagulated blood was centrifuged at 800 x g for 10 min at 4 °C. The plasma was collected and stored in a DNA LoBind Eppendorf tube (Eppendorf, Hamburg, Germany) at −20 °C until analysis.
Epifluorescence Imaging of the Green Fluorescent Protein-Positive Pups
Once the blood was collected, the whole uterus with the cervix and ovaries still intact was removed and placed in a Petri dish with PBS. The number of fetuses expressing green fluorescent protein (GFP) from each dam was determined by ex vivo imaging with the IVIS Spectrum in vivo imaging system (Perkin Elmer, Waltham, MA, USA) and recorded. An excitation filter of 465 nm and an emission filter of 520 nm were used to determine GFP expression.
Intra-amniotic Administration of Lipopolysaccharide
Pregnant DBA/2 mice were anesthetized on 16.5 dpc by inhalation of 2–3% isoflurane (Aerrane, Baxter Healthcare Corporation, Deerfield, IL, USA) and 1–2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.75–2% isoflurane and 2.0 L/min of oxygen. Dams were positioned on a heating pad and stabilized with adhesive tape. Fur removal from the abdomen was accomplished by applying Nair depilatory cream (Church & Dwight Co., Inc., Ewing, NJ, USA). Body temperature was maintained at 37 ± 2 °C and detected using a rectal probe (VisualSonics, Toronto, Ontario, Canada). Respiratory and heart rates were monitored by electrodes embedded in the heating pad. An ultrasound probe (VisualSonics) was fixed and mobilized with a mechanical holder, and the transducer was slowly moved toward the abdomen. Ultrasound-guided intra-amniotic injection of lipopolysaccharide (LPS; Escherichia coli O111:B4; Sigma-Aldrich) at a concentration of 5 μg/100 μL of sterile 1X phosphate-buffered saline (PBS) (Fisher Scientific Bioreagents, Fair Lawn, NJ, USA) was performed in each amniotic sac (3-10 pups per dam) using a 30-gauge needle (BD PrecisionGlide Needle, Becton Dickinson) (n = 15). Controls were injected with 100 μL of PBS into each amniotic sac (n = 13). The syringe was stabilized with a mechanical holder (VisualSonics Inc.). Following the ultrasound, dams were placed under a heat lamp until they regained full motor function, which typically occurred 5–10 min after heating.
Intraperitoneal Administration of Lipopolysaccharide
Pregnant DBA/2 mice were intraperitoneally injected on 16.5 dpc with 20 μg of LPS (Escherichia coli O55:B5; Sigma-Aldrich) in 200 μL PBS using a 26-gauge needle (BD PrecisionGlide Needle, Becton Dickinson) (n = 10). Controls were injected with 200 μL of PBS (n = 9).
Plasma Collection Prior to Inflammation-Induced Preterm Birth
Dams were anesthetized 21–22 h after intra-amniotic injection of LPS or 10–11 h after intraperitoneal injection of LPS. Whole blood (0.5–1.2 mL) was collected by cardiac puncture using a 22-gauge needle (BD PrecisionGlide Needle, Becton Dickinson) and immediately dispensed into a K2 EDTA MiniCollect® tube (Greiner Bio-one GmbH). Anticoagulated blood was centrifuged at 800 x g for 10 min at 4 °C. The plasma was collected and stored in a DNA LoBind Eppendorf tube (Eppendorf) at −20 °C until analysis.
Cell-Free DNA Extraction and Real-Time PCR
Cell-free DNA was extracted from 200 μL of the previously collected maternal plasma using the Plasma/Serum Cell-Free Circulating DNA Purification Micro Kit (Norgen Biotek Corp, Thorold, ON, Canada), following the manufacturer’s protocol (steps 1 through 9). Three positive controls for Egfp, obtained using DNA extracted from the semen of transgenic GFP-expressing male mice, were serially diluted tenfold through six concentrations (10 ng/μL–0.1 pg/mL) and used to generate standard curves. Samples and controls were preamplified with the Taqman™ PreAmp Master Mix (Life Technologies, Carlsbad, CA, USA), following the manufacturer’s instructions for reaction volumes, to increase the amount of DNA. The final concentration of each primer was 0.18 μM, and the final concentration of each probe was 0.05 μM. Fourteen cycles of the preamplification PCR were run on the Applied Biosystems QuantStudio 12K Flex Real-Time PCR instrument (Life Technologies).
A total of 1 μL of preamplified DNA was used for quantification of the amount of Egfp DNA present in the extracted cell-free DNA fraction. Kapa Probe Fast ABI Prism 2x qPCR Master Mix was used as recommended in the manufacturer’s protocol. A 75 base pair region of Egfp was detected with primers and probed as previously described [75, 96] (forward primer, 5’-ACTACAACAGCCACAACGTCTATATCA-3’; reverse primer, 5’-GGCGGATCTTGAAGTTCACC-3’; and Taqman probe, 5’-FAM-CCGACAAGCAGAAGAACGGCATCA-TAMRA-3’, where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). Apolipoprotein B (Apob) was used as a housekeeping gene (forward primer, 5’-CGTGGGCTCCAGCATTCTA-3’; reverse primer, 5’-TCACCAGTCATTTCTGCCTTTG-3’; and Taqman probe, 5’-FAM-CCTTGAGCAGTGCCCGACCATTC-TAMRA-3’). Egfp primers had a final concentration of 900 nM each and a final probe concentration of 200 nM. Apob primers had a final concentration of 300 nM each and a final probe concentration of 200 nM. The samples were amplified using the Applied Biosystems QuantStudio 12K Flex Real-Time PCR instrument (Life Technologies). All samples were run in triplicate. Results were presented as relative concentrations of cell-free fetal DNA (Egfp, ng/μL) which were calculated by extrapolation from the standard curve established above.
Cytokine/Chemokine Plasma Concentrations
The ProcartaPlex Mouse Cytokine & Chemokine Panel 1A 36-plex (Invitrogen by Thermo Fisher Scientific, Vienna, Austria) was used to measure the concentrations of IFNγ, IL-12p70, IL-1β, TNFα, GM-CSF, IL-18, IL-17A, IL-22, IL-23, IL-27, IL-9, IL-15/IL-15R, IL-13, IL-4, IL-5, IL-6, IL-10, Eotaxin (CCL11), IFNα, IL-28, IL-2, IL-3, LIF, IL-1α, IL-31, GRO-α (CXCL1), MIP-1α (CCL3), IP-10 (CXCL10), MCP-1 (CCL2), MCP-3 (CCL7), MIP-1β (CCL4), MIP-2 (CXCL2), RANTES (CCL5), G-CSF, M-CSF, and ENA-78 (CXCL5) in the plasma samples, according to the manufacturer’s instructions. Plates were read using the Luminex 100 SystemFill (Luminex, Austin, TX, USA), and analyte concentrations were calculated with ProcartaPlex Analyst 1.0 software from Affymetrix, San Diego, CA, USA. The sensitivities of the assays were as noted in Supplementary Table 1. Inter-assay and intra-assay coefficients of variation were less than 10%.
Statistical Analysis
The following data analysis was completed using SPSS version 19.0 software (IBM Corp, Armonk, NY, USA). Maternal plasma concentrations of cell-free fetal DNA (Egfp) throughout normal pregnancy were analyzed by the Kruskal-Wallis test followed by correction for multiple comparisons. The p values shown in Fig. 1 represent the comparisons between the 12.5 dpc group and the following groups: 17.5 dpc, 18.5 dpc, 19.5 dpc, and in labor. The Spearman rank correlation was used to analyze the relationship between the maternal plasma concentrations of cell-free fetal DNA (Egfp) and the number of GFP-positive pups per litter (Fig. 2). Cytokine and chemokine concentrations throughout normal pregnancy were analyzed using the Kruskal-Wallis test; the p values shown in Fig. 3B, 3C, 3D, and 3E represent the overall difference among groups. The Spearman rank correlation was used to analyze the relationship between the cytokine and chemokine concentrations and gestational age (Fig. 3F, 3G, 3H, and 3I). The Spearman rank correlation was also used to analyze the relationship between the cytokine and chemokine concentrations and number of GFP-positive pups or cell-free fetal DNA concentrations (Supplementary Figs. 2 and 3 ). Maternal plasma cell-free fetal DNA (Egfp) and cytokine/chemokine concentrations prior to preterm birth were compared using the Mann-Whitney U test. A p value ≤ 0.05 was regarded as statistically significant.
Fig. 1.
Cell-free fetal DNA concentrations from mid to late pregnancy, in labor, and postpartum. A Animal model of a paternal antigen (Egfp) expressed by some of the concepti, and timeline for the collection of maternal plasma for the quantification of cell-free fetal DNA (Egfp). B Concentrations of cell-free fetal DNA (Egfp) in the maternal plasma at 12.5–19.5 days post coitum (dpc), in labor, and 12–36 h postpartum. Each dot represents one maternal plasma sample. Midlines = medians, boxes = interquartile range, whiskers = minimum and maximum range. *p = 0.04, **p = 0.02. N = 5–11 dams per group
Fig. 2.
Correlation between maternal concentrations of cell-free fetal DNA (Egfp) and the number of GFP-positive pups. A Timeline for the collection of the whole uterus with pups for epifluorescence imaging. B Representative epifluorescence images of the whole uterus including GFP-positive pups at (from top to bottom) 12.5–19.5 days post coitum (dpc) and in labor. Implantation sites and GFP-positive pups are also shown 12–36 h postpartum. Upper left panel shows the epifluorescence color scale (red = lowest expression, yellow = highest expression). Noncolored pups are GFP-negative. C Correlation between maternal plasma concentrations of cell-free fetal DNA (Egfp) and the number of GFP-positive pups per litter. Each dot represents one maternal plasma sample. Spearman correlation coefficient ρ = 0.399, correlation significance p < 0.001. N = 88 dams
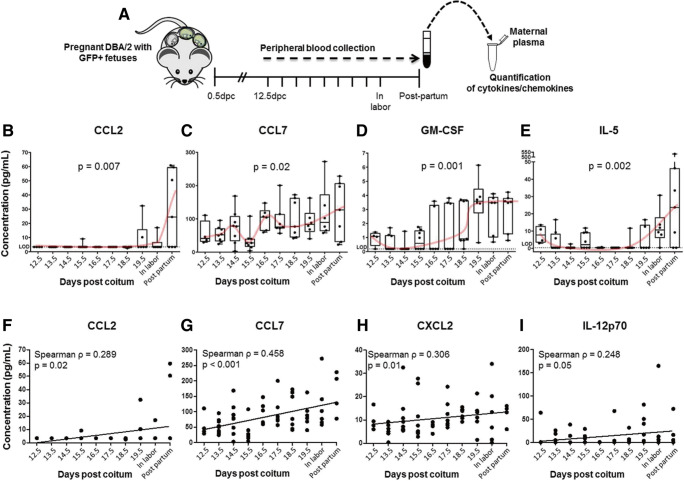
Fig. 3.
Maternal cytokine/chemokine plasma concentrations from mid to late pregnancy, in labor, and postpartum. A Timeline for the collection of maternal plasma for the quantification of cytokines/chemokines. Plots showing the maternal plasma concentrations of B CCL2, C CCL7, D GM-CSF, and E IL-5 at 12.5–19.5 days post coitum (dpc), in labor, and 12–36 h postpartum. Plots showing the positive correlation between gestational age and plasma concentrations of F CCL2, G CCL7, H CXCL2, and I IL-12p70 at 12.5–19.5 days post coitum (dpc), in labor, and 12–36 h postpartum. Each dot represents one maternal plasma sample. Midlines = medians, boxes = interquartile range, whiskers = minimum and maximum range. Red curves indicate trends over time. P values reflect overall difference among the groups (B–E) or correlation significance (F–I). Spearman correlation coefficients are also displayed. N = 5–11 dams per group
Results
Cell-Free Fetal DNA Concentrations During Mid and Late Pregnancy, in Labor, and Postpartum
A previous study indicated that cell-free fetal DNA levels are higher in allogeneic pregnancies compared to those which are congenic [75]. Therefore, we used an allogeneic murine model of pregnancy to evaluate whether cell-free fetal DNA concentrations increase prior to normal parturition. The mating strategy and sample collection time points are described in Fig. 1A.
The concentrations of cell-free fetal DNA (Egfp) in the maternal plasma were significantly elevated on 17.5 dpc compared to 12.5 dpc and were maintained until the onset of labor (Fig. 1B). Yet, cell-free fetal DNA (Egfp) concentrations dropped rapidly during the postpartum period (Fig. 1B).
Next, we evaluated whether the number of GFP-positive pups per litter would correlate with the cell-free fetal DNA (Egfp) concentration in the maternal circulation. To investigate this research question, we utilized an epifluorescence imaging system that allowed us to track the number of GFP-positive pups during mid and late pregnancy, in labor, and during the postpartum period (Fig. 2A). In Fig. 2B, representative images of the uterine horns including GFP-positive pups, placentas, and implantation sites (red-yellow color) are shown. The epifluorescence scale used to detect the green fluorescent protein is also shown in Fig. 2B. There was a positive and significant correlation between the number of GFP-positive pups per litter and the concentrations of cell-free fetal DNA (Egfp) (Spearman correlation coefficient ρ = 0.3989, p < 0.001; Fig. 2C).
Cytokine/Chemokine Plasma Concentrations During Mid and Late Pregnancy, in Labor, and Postpartum
Given that the cell-free fetal DNA concentrations are associated with a maternal systemic inflammatory response [60, 61, 86, 94, 95], we investigated the plasma cytokine/chemokine concentrations in the maternal circulation during mid and late pregnancy, in labor, and postpartum (Fig. 3A). Out of the 36 cytokines/chemokines determined in the maternal plasma, only four of them changed significantly during pregnancy, in labor, and the postpartum period after adjustment for multiple comparisons (data not shown). Specifically, CCL2 plasma concentrations were maintained at low levels throughout mid and late pregnancy and in labor but peaked during the postpartum period (p = 0.007; Fig. 3B). CCL7 plasma concentrations oscillated during mid and late pregnancy and were maintained at high levels during the postpartum period (p = 0.02; Fig. 3C). In addition, GM-CSF plasma concentrations increased from mid to late pregnancy, peaking on 19.5 dpc, and were maintained until the postpartum period (p = 0.001; Fig. 3D). On the other hand, IL-5 plasma concentrations were decreased in late gestation (14.5–18.5 dpc) compared to mid-pregnancy (12.5 dpc), resurged during labor, and were maintained in the postpartum period (p = 0.002; Fig. 3E). Interestingly, IL-10 plasma concentrations increased prior to labor and dropped soon after; yet, these changes were not statistically significant (Supplementary Fig. 1).
We also determined whether maternal cytokine/chemokine plasma concentrations were correlated with increasing gestational age. Maternal systemic concentrations of CCL2 (Spearman correlation coefficient ρ = 0.289, p = 0.02; Fig. 3F), CCL7 (Spearman correlation coefficient ρ = 0.458, p < 0.001; Fig. 3G), CXCL2 (Spearman correlation coefficient ρ = 0.306, p = 0.01; Fig. 3H), and IL-12p70 (Spearman correlation coefficient ρ = 0.248, p = 0.05; Fig. 3I) were positively correlated with increasing gestational age. All of these mediators were maintained elevated in the postpartum period. Furthermore, no significant correlations between plasma concentrations of cytokines/chemokines and the number of GFP-positive pups or cell-free fetal DNA concentrations were observed throughout gestation (data not shown).
Cell-Free Fetal DNA Concentrations Prior to Inflammation-Induced Preterm Birth
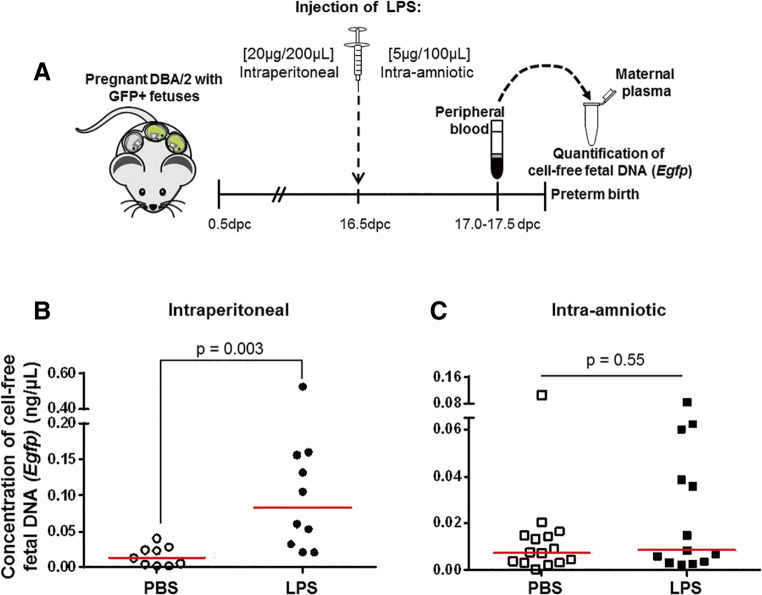
Given that cell-free fetal DNA concentrations were increased prior to term parturition (Fig. 1B) and cell-free fetal DNA levels are associated with preterm labor and birth [83–88], we investigated whether the levels of cell-free Egfp were increased prior to inflammation-induced preterm birth. To address this research question, we used two previously established models of endotoxin-induced preterm birth: a systemic model of inflammation (i.e., intraperitoneal administration of LPS) [97–106] and an intra-amniotic model of inflammation (i.e., intra-amniotic administration of LPS) [107, 108]. The animal models of inflammation-induced preterm birth are displayed in Fig. 4A. Maternal plasma samples were collected prior to preterm parturition (Fig. 4A). The rate of preterm birth induced by systemic inflammation was 72.7% (8/11 mice), similar to what has been reported [107, 108]. Concentrations of cell-free fetal DNA (Egfp) were increased prior to systemic inflammation-induced preterm birth (Fig. 4B). The rate of preterm birth induced by intra-amniotic inflammation was 87.5% (7/8 mice), as previously reported [107, 108]. Yet, concentrations of cell-free fetal DNA (Egfp) were not increased prior to intra-amniotic inflammation-induced preterm birth (Fig. 4C). It is worth mentioning that five dams intra-amniotically injected with LPS presented cell-free fetal DNA concentrations similar to those dams intraperitoneally injected with LPS (Fig. 4C vs. 4B). However, the median cell-free fetal DNA (Egfp) concentration of dams intraperitoneally injected with LPS was approximately eightfold higher than those that were intra-amniotically injected with LPS (Fig. 4B vs. 4C).
Fig. 4.
Cell-free fetal DNA (Egfp) concentrations prior to inflammation-induced preterm birth. A Timeline for the systemic (intraperitoneal) or intra-amniotic administration of lipopolysaccharide (LPS) to pregnant dams with GFP-positive pups on 16.5 days post coitum (dpc). Maternal plasma was collected at 17.0–17.5 dpc for the quantification of cell-free fetal DNA (Egfp). Concentrations of cell-free fetal DNA (Egfp) in the maternal plasma prior to B systemic (intraperitoneal) or C intra-amniotic inflammation-induced preterm birth. Each dot represents one maternal plasma sample. Red lines indicate medians. N = 9–15 dams per group
Cytokine/Chemokine Plasma Concentrations Prior to Inflammation-Induced Preterm Birth
To conclude our study, we determined the cytokine/chemokine concentrations in the maternal circulation prior to inflammation-induced preterm birth. As previously shown [108–110], the systemic concentrations of multiple cytokines/chemokines were increased prior to systemic inflammation-induced preterm birth (Fig. 5). The systemic concentrations of some cytokines/chemokines were also increased prior to intra-amniotic inflammation-induced preterm birth (Fig. 5). Yet, the systemic inflammatory response prior to intra-amniotic inflammation-induced preterm birth was milder than that of the systemic inflammation model (Fig. 5). Specifically, the plasma concentrations of GM-CSF, IL-1β, IL-6, IL-4, IL-12p70, IL-17A, CCL2, CCL3, CCL4, CCL5, CCL7, CXCL1, CXCL2, CXCL10, and G-CSF were higher prior to systemic inflammation-induced preterm birth than prior to intra-amniotic inflammation-induced preterm birth (Fig. 5).
Fig. 5.
Maternal cytokine/chemokine plasma concentrations prior to inflammation-induced preterm birth. Plots showing the maternal plasma concentrations of (left to right, top to bottom) GM-CSF, IL-1β, IL-6, IL-4, IL-12p70, IL-17A, CCL2, CCL3, CCL4, CCL5, CCL7, CXCL1, CXCL2, CXCL10, and G-CSF prior to systemic (intraperitoneal) or intra-amniotic inflammation-induced preterm birth. Each dot represents one maternal plasma sample. Red lines indicate medians. N = 9–15 dams per group
Lastly, we determined the correlations between the plasma concentrations of cytokines/chemokines and the number of GFP-positive pups or cell-free fetal DNA (Egfp) levels in our systemic and intra-amniotic inflammation-induced preterm birth models. Plasma concentrations of IL-6 and IL-4 were negatively correlated with the number of GFP-positive pups prior to systemic inflammation-induced preterm birth (Supplementary Fig. 2). Moreover, plasma concentrations of IL-4 were negatively correlated with cell-free fetal DNA (Egfp) levels prior to systemic inflammation-induced preterm birth (Supplementary Fig. 3). No significant correlations were observed between the plasma concentrations of cytokines/chemokines and the number of GFP-positive pups or cell-free fetal DNA (Egfp) levels prior to intra-amniotic inflammation-induced preterm birth (data not shown).
Discussion
Principal Findings
The principal findings of our study are as follows: 1) cell-free fetal DNA (Egfp) concentrations were higher in late gestation compared to mid-pregnancy and were maintained increased during the onset of labor at term, followed by a rapid decrease after birth; 2) there was a positive and significant correlation between cell-free fetal DNA (Egfp) concentrations and the number of GFP-positive pups; 3) cytokine/chemokine plasma concentrations followed different patterns from mid to late pregnancy, and most of them were increased in the postpartum period; 4) plasma concentrations of specific chemokines (i.e., CCL2, CCL7, and CXCL2) and IL-12p70 increased as gestation progressed and maintained elevated levels in the postpartum period; 5) cell-free fetal DNA (Egfp) concentrations increased prior to systemic inflammation-induced preterm birth, but not before intra-amniotic inflammation-induced preterm birth; and 6) cytokine/chemokine plasma concentrations increased prior to systemic inflammation- or intra-amniotic inflammation-induced preterm birth; however, the cytokine storm was greater in dams injected intraperitoneally.
Cell-Free Fetal DNA Concentrations Are Increased in Late Gestation and During Labor at Term
Consistent with Khosrotehrani et al. [75], whose animal model was used herein, cell-free fetal DNA concentrations in allogeneic pregnancies were higher in late gestation than in mid-pregnancy. The difference in cell-free fetal DNA concentrations between allogeneic and syngeneic pregnancies has been attributed to the apoptotic deletion of maternal fetal-reactive cells [75]. The activation of such cell death pathways may explain why there is a need for more immunoregulation (i.e., regulatory T cells) in allogenic than in syngeneic pregnancies [111]. While the abovementioned study included the first, second, and third weeks of murine pregnancy [75], in the current study, we determined the cell-free fetal DNA concentrations from mid to late pregnancy, since detection of circulating cffDNA during early/mid gestation is used to diagnose common fetal genetic abnormalities [112–124]. We further examined cell-free fetal DNA concentrations during the active process of labor and in the postpartum period. Consistent with human [60, 66] and animal [74–77] studies, cell-free fetal DNA concentrations were increased before and during term labor and decreased rapidly after birth. These data are consistent with the hypothesis suggesting that cell-free fetal DNA is a trigger for term parturition [58, 59, 61].
An important observation made in the current study is that the number of pups containing the paternally derived gene for green fluorescent protein (GFP) was positively correlated with the maternal concentrations of cell-free fetal DNA. One possible explanation is that greater numbers of pups containing the gene will result in a larger amount of gestational tissue that releases microparticles (e.g., syncytiotrophoblast microvilli) into the maternal circulation that can serve as a source of cell-free DNA [125–128]. Another possibility is that circulating cell-free fetal DNA is partially derived from fetal nucleated erythroid cells that enter into the maternal circulation as early as 12 weeks of gestation [129–134]. The frequency of such fetal cells increases as a function of gestation, peaking during late gestation and diminishing rapidly after birth [135], a pattern that is mirrored by cell-free fetal DNA concentrations. Yet, it is worth mentioning that the absolute quantity of fetal DNA in maternal circulation is greater in the cell-free fraction than in the cellular component [66]. Notably, maternal obesity has been shown to reduce the concentrations of circulating cell-free fetal DNA both in the clinical setting [136, 137] and in an animal model [138], possibly as a result of the differential gene methylation [139] or altered cell death pathways [140] observed in the placentas of obese women.
Telomere shortening [62, 141–143], cellular senescence [6, 141, 142, 144–148], and apoptosis [149] are thought to lead to cell-free DNA release, which in turn induces the onset of labor at term through the involvement of the TLR9 signaling pathway [58, 62]. Recent studies in humans have found a positive association between systemic cell-free fetal DNA and IL-6 concentrations [60]. In addition, DNA derived from mouse placental tissues and fetuses induced the release of IL-6 by macrophages in an in vitro model [62, 149, 150]. This pro-inflammatory effect was observed upon incubation with telomere-depleted mouse DNA and was inhibited by chloroquine, a TLR9 inhibitor [62, 150]. In the current study, the rise in cell-free fetal DNA in the maternal circulation was not linked to a surge in the systemic levels of any specific cytokine or chemokine; however, the maternal plasma levels might not accurately reflect the localized proinflammatory events occurring at the maternal-fetal interface and/or the placenta and fetal membranes. These studies did, however, demonstrate an increase in plasma concentrations of CCL7, GM-CSF, and IL-5 preceded or occurred during labor at term. In addition, we found that plasma concentrations of CCL2, CCL7, CXCL2, and IL-12p70 increased as gestation progressed. Together, these data suggest a role for these cytokines/chemokines in the physiological process of labor at term. This concept is supported by the following evidence: 1) CCL2 expressed by myometrial tissues regulates mechanical and endocrine signals implicated in the process of labor [21]; 2) GM-CSF amniotic fluid concentrations are higher in women with intra-amniotic infection [151, 152], and the blockade of this cytokine prevents preterm birth in mice [153]; 3) plasma concentrations of IL-12 are greater in mid gestation than in early pregnancy [154], and elevated levels of this cytokine in serum are associated with spontaneous preterm birth [155]; and 4) maternal plasma concentrations of IL-5 are increased in women who delivered at term with clinical chorioamnionitis [156]. Nonetheless, we found that the systemic concentrations of IL-5 and CCL7 (both anti-inflammatory mediators [157, 158]) peaked during postpartum, indicating that these cytokines, and possibly IL-10, may be important in preventing a systemic cytokine storm during normal parturition and in the healing processes taking place during the postpartum period.
Cell-Free Fetal DNA Concentrations Increased Prior to Systemic Inflammation-Induced, but Not Intra-amniotic Inflammation-Induced, Preterm Birth
We found that maternal plasma cell-free fetal DNA (Egfp) concentrations were increased prior to systemic (intraperitoneal) inflammation-induced preterm labor, but not prior to preterm labor resulting from intra-amniotic inflammation. These data indicate that not all cases of preterm labor are characterized by an increased cell-free fetal DNA concentration in the maternal circulation, which could explain why there is still controversy regarding the value of cell-free fetal DNA for predicting preterm labor and birth [83–93, 159].
Previous studies have consistently shown that Il10−/−and NOD mice injected with CpG ODN delivered preterm [94, 95, 160]. However, wild-type mice injected with CpG ODN delivered at term [94, 95, 160], indicating that the mechanisms whereby cell-free DNA induces preterm parturition involve the IL-10 pathway. It is therefore not surprising that the adoptive transfer of regulatory T cells prevents CpG-induced preterm birth [160]. Contrary to the previous reports [94, 95, 160], another study showed that injection of CpG and fetal DNA, but not adult DNA, in wild-type mice induces fetal loss [86]. Such a process is mediated via the TLR9 pathway, since Tlr9−/−mice injected with fetal DNA did not result in fetal loss [86]. This controversy has been recently reviewed [159]. Herein, we provide evidence that, in some cases of preterm labor driven by a systemic inflammatory response, a rise in cell-free fetal DNA in the maternal circulation precedes preterm birth.
Mechanistic studies have shown that intrauterine inoculation of TLR ligands (e.g., peptidoglycan and Poly:IC) caused preterm birth by inducing apoptosis [161]. This is consistent with research demonstrating that inflammatory stimuli induce apoptosis in the placental tissues [162–167]. The engagement of TLRs in the intrauterine space can also upregulate the expression of inflammasome components [161, 168–170]. In humans, the inflammasome is implicated in the mechanisms leading to spontaneous labor at term [169, 171–175] or preterm labor [171, 176–179]. Herein, we provide evidence that the systemic administration of endotoxin, the TLR4 ligand [180, 181], can lead to elevated concentrations of cell-free fetal DNA in the maternal circulation, a process that is most likely due to the activation of several apoptotic and non-apoptotic pathways.
In the current study, we also used a recently described animal model of intra-amniotic inflammation-induced preterm birth, which resembles the human syndrome of preterm labor since it occurs in the absence of a body temperature change (a readout of fever in humans) and a strong cytokine storm, both of which are observed in the intraperitoneal model [107]. Yet, the intra-amniotic administration of endotoxin induces a mild inflammatory response in the mother and the fetus [108]. Herein, we showed that intra-amniotic inflammation induced by an endotoxin leads to preterm birth in the absence of elevated cell-free fetal DNA concentrations in the maternal circulation. However, in some cases, intra-amniotic administration of endotoxin modestly increased the concentration of cell-free fetal DNA in the circulation, but these levels were not comparable to dams injected intraperitoneally with an endotoxin. These data suggest that intra-amniotic inflammation triggers inflammatory pathways (apoptotic and non-apoptotic mechanisms) leading to a minimal release of cell-free fetal DNA into the maternal circulation, which is insufficient to induce preterm labor and birth. In line with the concept that cell-free fetal DNA can activate non-apoptotic pathways, we have recently shown that intra-amniotic inflammation, either induced by microbial products or alarmins, induces pyroptosis by activating the NLRP3 inflammasome prior to preterm birth [182, 183]. Yet, other potential inflammatory pathways implicated in the mechanisms whereby cell-free fetal DNA leads to preterm birth should also be investigated.
Conclusion
In the current study, we provide evidence that cell-free fetal DNA concentrations are increased in the maternal circulation during late pregnancy and the onset of the labor and reduced rapidly in the postpartum period. In addition, cell-free fetal DNA concentrations were positively correlated with the number of pups carrying the paternally derived green fluorescent protein gene. The increase in cell-free fetal DNA concentrations was not associated with a surge in a specific cytokine/chemokine in the maternal circulation; however, these studies did not rule out the possibility of localized proinflammatory events within the maternal-fetal interface and/or placental tissues. We also found that cell-free fetal DNA concentrations were increased prior to systemic inflammation-induced preterm birth, which was associated with a strong cytokine storm in the maternal circulation. Yet, cell-free fetal DNA concentrations were not increased prior to intra-amniotic inflammation-induced preterm birth; however, in this model, a mild inflammatory response was observed in the maternal circulation. Collectively, these findings suggest that an elevation in cell-free fetal DNA concentrations in the maternal circulation precedes the process of labor at term. Furthermore, an elevation in cell-free fetal DNA concentrations in the maternal circulation is associated with a subset of preterm births that are induced by systemic inflammation.
Electronic supplementary material
Plot showing the maternal plasma concentrations of IL-10 at 12.5 – 19.5 days post coitum (dpc), in labor, and 12 – 36 h postpartum. Each dot represents one maternal plasma sample. Midlines = medians, boxes = interquartile range, whiskers = minimum and maximum range. Red curves indicate trends over time. N = 5 – 11 dams per group. (PNG 62 kb)
Plots showing the negative correlations between plasma concentrations of IL-6 or IL-4 and the number of GFP-positive pups prior to systemic inflammation-induced preterm birth. Spearman correlation coefficients and p-values are displayed. Asterisk (*) indicates p-value obtained using a one-tailed Mann-Whitney U test. N = 8 dams. (PNG 83 kb)
Plot showing a negative correlation between plasma concentrations of IL-4 and cell-free fetal DNA (Egfp) levels prior to systemic inflammation-induced preterm birth. Spearman correlation coefficients and p-values are displayed. N = 8 dams. (PNG 80 kb)
(DOC 50 kb)
Acknowledgments
We thank the research assistants from the PRB Perinatal Translational Science Laboratory for their help with carrying out the Luminex assays, and Nicole Ducharme for her help with the DNA extraction and PCR assays. Finally, we thank Derek Miller, MSc, for his critical readings of the manuscript.
Funding Information
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Roberto Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal, and Child Health.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict(s) of interest.
References
- 1.Liggins CG. Cervical ripening as an inflammatory reaction. In: Elwood DA, Andersson ABM, editors. Cervix in Pregnancy and Labour. Edinburgh: Churchill Livingstone; 1981. pp. 1–9. [Google Scholar]
- 2.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160(5 Pt 1):1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 Pt 2):S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 4.Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88(4):625–633. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 6.Norwitz Errol R., Bonney Elizabeth A., Snegovskikh Victoria V., Williams Michelle A., Phillippe Mark, Park Joong Shin, Abrahams Vikki M. Molecular Regulation of Parturition: The Role of the Decidual Clock. Cold Spring Harbor Perspectives in Medicine. 2015;5(11):a023143. doi: 10.1101/cshperspect.a023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod. 1997;12(3):586–590. doi: 10.1093/humrep/12.3.586. [DOI] [PubMed] [Google Scholar]
- 8.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61(4):879–883. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- 9.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 10.Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol. 2002;57(1-2):217–224. doi: 10.1016/s0165-0378(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 11.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 12.Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003;10(6):323–338. doi: 10.1016/s1071-5576(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66(2):161–173. doi: 10.1016/j.jri.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod. 2008;78(3):438–444. doi: 10.1095/biolreprod.107.063404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182(5):2700–2707. doi: 10.4049/jimmunol.0803138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yellon SM, Oshiro BT, Chhaya TY, et al. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod. 2011;85(3):498–502. doi: 10.1095/biolreprod.111.091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod. 2011;84(3):587–594. doi: 10.1095/biolreprod.110.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012;87(5):106. doi: 10.1095/biolreprod.112.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers DA. The recruitment and activation of leukocytes into the immune cervix: further support that cervical remodeling involves an immune and inflammatory mechanism. Biol Reprod. 2012;87(5):107. doi: 10.1095/biolreprod.112.105049. [DOI] [PubMed] [Google Scholar]
- 20.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. [PubMed] [Google Scholar]
- 21.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181(2):1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 22.Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S2–10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi A, Makieva S, Rinaldi SF, Arcuri F, Petraglia F, Norman JE. Expression of matrix metalloproteinases in the mouse uterus and human myometrium during pregnancy, labor, and preterm labor. Reprod Sci. 2018;25(6):938–949. doi: 10.1177/1933719117732158. [DOI] [PubMed] [Google Scholar]
- 25.Fidel PL, Jr, Romero R, Ramirez M, et al. Interleukin-1 receptor antagonist (IL-1ra) production by human amnion, chorion, and decidua. Am J Reprod Immunol. 1994;32(1):1–7. doi: 10.1111/j.1600-0897.1994.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 26.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181(6):1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 27.Lonergan M, Aponso D, Marvin KW, Helliwell RJ, Sato TA, Mitchell MD, Chaiwaropongsa T, Romero R, Keelan JA. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J Clin Endocrinol Metab. 2003;88(8):3835–3844. doi: 10.1210/jc.2002-021905. [DOI] [PubMed] [Google Scholar]
- 28.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, Branch DW, Silver RM, Adashi EY. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26(8-9):661–671. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80(1-2):122–131. doi: 10.1016/j.jri.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205(3):235 e215–235 e224. doi: 10.1016/j.ajog.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204(4):364 e369–364 e316. doi: 10.1016/j.ajog.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Hadley EE, Sheller-Miller S, Saade G, et al. Amnion epithelial cell-derived exosomes induce inflammatory changes in uterine cells. Am J Obstet Gynecol. 2018;219(5):478 e471–478 e421. doi: 10.1016/j.ajog.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990;132(2):181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 34.Keski-Nisula L, Aalto ML, Katila ML, Kirkinen P. Intrauterine inflammation at term: a histopathologic study. Hum Pathol. 2000;31(7):841–846. doi: 10.1053/hupa.2000.8449. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, Jones RL. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86(2):39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69(3):212–230. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One. 2013;8(2):e56946. doi: 10.1371/journal.pone.0056946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, et al. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol. 2014;71(1):86–93. doi: 10.1111/aji.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudley DJ, Collmer D, Mitchell MD, Trautman MS. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig. 1996;3(6):328–335. [PubMed] [Google Scholar]
- 40.Ammala M, Nyman T, Salmi A, Rutanen EM. The interleukin-1 system in gestational tissues at term: effect of labour. Placenta. 1997;18(8):717–723. doi: 10.1016/s0143-4004(97)90014-x. [DOI] [PubMed] [Google Scholar]
- 41.Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195(2):394 e391–394 e324. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J Jr The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195(3):778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Hassan SS, Romero R, Tarca AL, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2007;197(3):250.e251–250.e257. doi: 10.1016/j.ajog.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nhan-Chang CL, Romero R, Tarca AL, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202(5):462.e461–462.e441. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L, Mazaki-Tovi S, Hassan SS, Mesiano S, Kim CJ. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38(6):617–643. doi: 10.1515/JPM.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaemsaithong P, Madan I, Romero R, Than NG, Tarca AL, Draghici S, Bhatti G, Yeo L, Mazor M, Kim CJ, Hassan SS, Chaiworapongsa T. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med. 2013;41(6):665–681. doi: 10.1515/jpm-2013-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Tarca AL, Chaemsaithong P, et al. Transcriptome interrogation of human myometrium identifies differentially expressed sense-antisense pairs of protein-coding and long non-coding RNA genes in spontaneous labor at term. J Matern Fetal Neonatal Med. 2014;27(14):1397–1408. doi: 10.3109/14767058.2013.860963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephen GL, Lui S, Hamilton SA, et al. Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. Am J Reprod Immunol. 2015;73(1):36–55. doi: 10.1111/aji.12328. [DOI] [PubMed] [Google Scholar]
- 49.Bukowski R, Sadovsky Y, Goodarzi H, et al. Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ. 2017;5:e3685. doi: 10.7717/peerj.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arenas-Hernandez M, Gomez-Lopez N, Garcia-Flores V, et al. Choriodecidual leukocytes display a unique gene expression signature in spontaneous labor at term. Genes Immun. 2019;20(1):56–68. doi: 10.1038/s41435-017-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 52.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185(5):1118–1123. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 53.Unal ER, Cierny JT, Roedner C, Newman R, Goetzl L. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol. 2011;204(3):223 e221–223 e225. doi: 10.1016/j.ajog.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Cierny JT, Unal ER, Flood P, et al. Maternal inflammatory markers and term labor performance. Am J Obstet Gynecol. 2014;210(5):447 e441–447 e446. doi: 10.1016/j.ajog.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 55.Neal JL, Lamp JM, Lowe NK, Gillespie SL, Sinnott LT, McCarthy DO. Differences in inflammatory markers between nulliparous women admitted to hospitals in preactive vs active labor. Am J Obstet Gynecol. 2015;212(1):68 e61–68 e68. doi: 10.1016/j.ajog.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aghaeepour N, Lehallier B, Baca Q, et al. A proteomic clock of human pregnancy. Am J Obstet Gynecol. 2018;218(3):347 e341–347 e314. doi: 10.1016/j.ajog.2017.12.208. [DOI] [PubMed] [Google Scholar]
- 57.Tarca AL, Romero R, Xu Z, et al. Targeted expression profiling by RNA-Seq improves detection of cellular dynamics during pregnancy and identifies a role for T cells in term parturition. Sci Rep. 2019;9(1):848. doi: 10.1038/s41598-018-36649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillippe M. Cell-free fetal DNA--a trigger for parturition. N Engl J Med. 2014;370(26):2534–2536. doi: 10.1056/NEJMcibr1404324. [DOI] [PubMed] [Google Scholar]
- 59.Phillippe M. Cell-free fetal DNA, telomeres, and the spontaneous onset of parturition. Reprod Sci. 2015;22(10):1186–1201. doi: 10.1177/1933719115592714. [DOI] [PubMed] [Google Scholar]
- 60.Herrera CA, Stoerker J, Carlquist J, et al. Cell-free DNA, inflammation, and the initiation of spontaneous term labor. Am J Obstet Gynecol. 2017;217(5):583 e581–583 e588. doi: 10.1016/j.ajog.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 61.Phillippe M. The link between cell-free DNA, inflammation and the initiation of spontaneous labor at term. Am J Obstet Gynecol. 2017;217(5):501–502. doi: 10.1016/j.ajog.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Goldfarb IT, Adeli S, Berk T, Phillippe M. Fetal and placental DNA stimulation of TLR9: a mechanism possibly contributing to the pro-inflammatory events during parturition. Reprod Sci. 2018;25(5):788–796. doi: 10.1177/1933719117728798. [DOI] [PubMed] [Google Scholar]
- 63.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 64.Lo YM, Tein MS, Lau TK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62(4):768–775. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet. 1999;64(1):218–224. doi: 10.1086/302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, Lee TH. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41(12):1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 67.Chan LY, Leung TN, Chan KC, Tai HL, Lau TK, Wong EM, Lo YM. Serial analysis of fetal DNA concentrations in maternal plasma in late pregnancy. Clin Chem. 2003;49(4):678–680. doi: 10.1373/49.4.678. [DOI] [PubMed] [Google Scholar]
- 68.Chan KC, Zhang J, Hui AB, et al. Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem. 2004;50(1):88–92. doi: 10.1373/clinchem.2003.024893. [DOI] [PubMed] [Google Scholar]
- 69.Bischoff FZ, Lewis DE, Simpson JL. Cell-free fetal DNA in maternal blood: kinetics, source and structure. Hum Reprod Update. 2005;11(1):59–67. doi: 10.1093/humupd/dmh053. [DOI] [PubMed] [Google Scholar]
- 70.Birch L, English CA, O'Donoghue K, Barigye O, Fisk NM, Keer JT. Accurate and robust quantification of circulating fetal and total DNA in maternal plasma from 5 to 41 weeks of gestation. Clin Chem. 2005;51(2):312–320. doi: 10.1373/clinchem.2004.042713. [DOI] [PubMed] [Google Scholar]
- 71.Majer S, Bauer M, Magnet E, Strele A, Giegerl E, Eder M, Lang U, Pertl B. Maternal urine for prenatal diagnosis--an analysis of cell-free fetal DNA in maternal urine and plasma in the third trimester. Prenat Diagn. 2007;27(13):1219–1223. doi: 10.1002/pd.1875. [DOI] [PubMed] [Google Scholar]
- 72.Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33(7):662–666. doi: 10.1002/pd.4119. [DOI] [PubMed] [Google Scholar]
- 73.Taglauer ES, Wilkins-Haug L, Bianchi DW. Review: cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta. 2014;35(Suppl):S64–S68. doi: 10.1016/j.placenta.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jimenez DF, Tarantal AF. Quantitative analysis of male fetal DNA in maternal serum of gravid rhesus monkeys (Macaca mulatta) Pediatr Res. 2003;53(1):18–23. doi: 10.1203/00006450-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Khosrotehrani K, Wataganara T, Bianchi DW, Johnson KL. Fetal cell-free DNA circulates in the plasma of pregnant mice: relevance for animal models of fetomaternal trafficking. Hum Reprod. 2004;19(11):2460–2464. doi: 10.1093/humrep/deh445. [DOI] [PubMed] [Google Scholar]
- 76.Mitsunaga F, Ueiwa M, Kamanaka Y, Morimoto M, Nakamura S. Fetal sex determination of macaque monkeys by a nested PCR using maternal plasma. Exp Anim. 2010;59(2):255–260. doi: 10.1538/expanim.59.255. [DOI] [PubMed] [Google Scholar]
- 77.Kadivar A, Hassanpour H, Mirshokraei P, Azari M, Gholamhosseini K, Karami A. Detection and quantification of cell-free fetal DNA in ovine maternal plasma; use it to predict fetal sex. Theriogenology. 2013;79(6):995–1000. doi: 10.1016/j.theriogenology.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 78.Munoz-Hernandez R, Medrano-Campillo P, Miranda ML, et al. Total and fetal circulating cell-free DNA, angiogenic, and antiangiogenic factors in preeclampsia and HELLP syndrome. Am J Hypertens. 2017;30(7):673–682. doi: 10.1093/ajh/hpx024. [DOI] [PubMed] [Google Scholar]
- 79.Contro E, Bernabini D, Farina A. Cell-free fetal DNA for the prediction of pre-eclampsia at the first and second trimesters: a systematic review and meta-analysis. Mol Diagn Ther. 2017;21(2):125–135. doi: 10.1007/s40291-016-0245-9. [DOI] [PubMed] [Google Scholar]
- 80.Rafaeli-Yehudai T, Imterat M, Douvdevani A, Tirosh D, Benshalom-Tirosh N, Mastrolia SA, Beer-Weisel R, Klaitman V, Riff R, Greenbaum S, Alioshin A, Rodavsky Hanegbi G, Loverro G, Catalano MR, Erez O. Maternal total cell-free DNA in preeclampsia and fetal growth restriction: evidence of differences in maternal response to abnormal implantation. PLoS One. 2018;13(7):e0200360. doi: 10.1371/journal.pone.0200360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rolnik D. L., da Silva Costa F., Lee T. J., Schmid M., McLennan A. C. Association between fetal fraction on cell‐free DNA testing and first‐trimester markers for pre‐eclampsia. Ultrasound in Obstetrics & Gynecology. 2018;52(6):722–727. doi: 10.1002/uog.18993. [DOI] [PubMed] [Google Scholar]
- 82.Morano D, Rossi S, Lapucci C, Pittalis MC, Farina A. Cell-free DNA (cfDNA) fetal fraction in early- and late-onset fetal growth restriction. Mol Diagn Ther. 2018;22(5):613–619. doi: 10.1007/s40291-018-0353-9. [DOI] [PubMed] [Google Scholar]
- 83.Leung TN, Zhang J, Lau TK, Hjelm NM, Lo YM. Maternal plasma fetal DNA as a marker for preterm labour. Lancet. 1998;352(9144):1904–1905. doi: 10.1016/S0140-6736(05)60395-9. [DOI] [PubMed] [Google Scholar]
- 84.Farina A, LeShane ES, Romero R, Gomez R, Chaiworapongsa T, Rizzo N, Bianchi DW. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2005;193(2):421–425. doi: 10.1016/j.ajog.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 85.Jakobsen TR, Clausen FB, Rode L, Dziegiel MH, Tabor A. High levels of fetal DNA are associated with increased risk of spontaneous preterm delivery. Prenat Diagn. 2012;32(9):840–845. doi: 10.1002/pd.3917. [DOI] [PubMed] [Google Scholar]
- 86.Scharfe-Nugent A, Corr SC, Carpenter SB, et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188(11):5706–5712. doi: 10.4049/jimmunol.1103454. [DOI] [PubMed] [Google Scholar]
- 87.Quezada MS, Francisco C, Dumitrascu-Biris D, Nicolaides KH, Poon LC. Fetal fraction of cell-free DNA in maternal plasma in the prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2015;45(1):101–105. doi: 10.1002/uog.14666. [DOI] [PubMed] [Google Scholar]
- 88.Dugoff L, Barberio A, Whittaker PG, Schwartz N, Sehdev H, Bastek JA. Cell-free DNA fetal fraction and preterm birth. Am J Obstet Gynecol. 2016;215(2):231 e231–231 e237. doi: 10.1016/j.ajog.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 89.Bauer M, Hutterer G, Eder M, et al. A prospective analysis of cell-free fetal DNA concentration in maternal plasma as an indicator for adverse pregnancy outcome. Prenat Diagn. 2006;26(9):831–836. doi: 10.1002/pd.1513. [DOI] [PubMed] [Google Scholar]
- 90.Illanes S, Gomez R, Fornes R, Figueroa-Diesel H, Schepeler M, Searovic P, Serra R, Perez A, Nien JK. Free fetal DNA levels in patients at risk of preterm labour. Prenat Diagn. 2011;31(11):1082–1085. doi: 10.1002/pd.2838. [DOI] [PubMed] [Google Scholar]
- 91.Stein W, Muller S, Gutensohn K, Emons G, Legler T. Cell-free fetal DNA and adverse outcome in low risk pregnancies. Eur J Obstet Gynecol Reprod Biol. 2013;166(1):10–13. doi: 10.1016/j.ejogrb.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Poon LC, Musci T, Song K, Syngelaki A, Nicolaides KH. Maternal plasma cell-free fetal and maternal DNA at 11-13 weeks' gestation: relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn Ther. 2013;33(4):215–223. doi: 10.1159/000346806. [DOI] [PubMed] [Google Scholar]
- 93.Thurik FF, Lamain-de Ruiter M, Javadi A, Kwee A, Woortmeijer H, Page-Christiaens GC, Franx A, van der Schoot C, Koster MP. Absolute first trimester cell-free DNA levels and their associations with adverse pregnancy outcomes. Prenat Diagn. 2016;36(12):1104–1111. doi: 10.1002/pd.4940. [DOI] [PubMed] [Google Scholar]
- 94.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183(2):1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun Y, Qin X, Shan B, Wang W, Zhu Q, Sharma S, Wu J, Lin Y. Differential effects of the CpG-Toll-like receptor 9 axis on pregnancy outcome in nonobese diabetic mice and wild-type controls. Fertil Steril. 2013;99(6):1759–1767. doi: 10.1016/j.fertnstert.2013.01.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pan D, Gunther R, Duan W, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6(1):19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- 97.Fidel PL, Jr, Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170(5 Pt 1):1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 98.Bizargity P, Del Rio R, Phillippe M, Teuscher C, Bonney EA. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol Reprod. 2009;80(5):874–881. doi: 10.1095/biolreprod.108.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65(2):110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roy-Lacroix ME, Guerard M, Berthiaume M, Rola-Pleszczynski M, Crous-Tsanaclis AM, Pasquier JC. Time-dependent effect of in utero inflammation: a longitudinal study in rats. J Matern Fetal Neonatal Med. 2013;26(8):789–794. doi: 10.3109/14767058.2012.755164. [DOI] [PubMed] [Google Scholar]
- 101.Evangelinakis NE, Polyzou EN, Salamalekis GE, Kotsaki AJ, Chrelias CG, Giamarellos-Bourboulis EJ, Kassanos DP. Alterations in the cellular component of the maternal immune system in a murine preterm delivery model. J Matern Fetal Neonatal Med. 2013;26(10):1024–1029. doi: 10.3109/14767058.2013.765848. [DOI] [PubMed] [Google Scholar]
- 102.Gharedaghi MH, Javadi-Paydar M, Yousefzadeh-Fard Y, Salehi-Sadaghiani M, Javadian P, Fakhraei N, Tavangar SM, Dehpour AR. Muscimol delays lipopolysaccharide-induced preterm delivery in mice: role of GABA(A) receptors and nitric oxide. J Matern Fetal Neonatal Med. 2013;26(1):36–43. doi: 10.3109/14767058.2012.722715. [DOI] [PubMed] [Google Scholar]
- 103.Shynlova O, Dorogin A, Li Y, Lye S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J Cell Mol Med. 2014;18(9):1816–1829. doi: 10.1111/jcmm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toyama RP, Xikota JC, Schwarzbold ML, et al. Dose-dependent sickness behavior, abortion and inflammation induced by systemic LPS injection in pregnant mice. J Matern Fetal Neonatal Med. 2015;28(4):426–430. doi: 10.3109/14767058.2014.918600. [DOI] [PubMed] [Google Scholar]
- 105.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol. 2016;13(4):462–473. doi: 10.1038/cmi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Y, Romero R, Miller D, et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol. 2016;196(6):2476–2491. doi: 10.4049/jimmunol.1502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomez-Lopez N, Romero R, Arenas-Hernandez M, et al. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med. 2018;31(4):439–446. doi: 10.1080/14767058.2017.1287894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garcia-Flores V, Romero R, Miller D, et al. Inflammation-induced adverse pregnancy and neonatal outcomes can be improved by the immunomodulatory peptide exendin-4. Front Immunol. 2018;9:1291. doi: 10.3389/fimmu.2018.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gotsch F, Romero R, Espinoza J, Kusanovic JP, Mazaki-Tovi S, Erez O, Than NG, Edwin S, Mazor M, Yoon BH, Hassan SS. Maternal serum concentrations of the chemokine CXCL10/IP-10 are elevated in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2007;20(10):735–744. doi: 10.1080/14767050701511650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Madan I, Than NG, Romero R, et al. The peripheral whole-blood transcriptome of acute pyelonephritis in human pregnancya. J Perinat Med. 2014;42(1):31–53. doi: 10.1515/jpm-2013-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 112.Lo YM, Lun FM, Chan KC, et al. Digital PCR for the molecular detection of fetal chromosomal aneuploidy. Proc Natl Acad Sci U S A. 2007;104(32):13116–13121. doi: 10.1073/pnas.0705765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A. 2008;105(42):16266–16271. doi: 10.1073/pnas.0808319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nicolaides KH, Syngelaki A, Ashoor G, Birdir C, Touzet G. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am J Obstet Gynecol. 2012;207(5):374 e371–374 e376. doi: 10.1016/j.ajog.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 115.Song Y, Liu C, Qi H, Zhang Y, Bian X, Liu J. Noninvasive prenatal testing of fetal aneuploidies by massively parallel sequencing in a prospective Chinese population. Prenat Diagn. 2013;33(7):700–706. doi: 10.1002/pd.4160. [DOI] [PubMed] [Google Scholar]
- 116.Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, Craig JA, Chudova DI, Devers PL, Jones KW, Oliver K, Rava RP, Sehnert AJ, CARE Study Group DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808. doi: 10.1056/NEJMoa1311037. [DOI] [PubMed] [Google Scholar]
- 117.Comas C, Echevarria M, Rodriguez MA, Prats P, Rodriguez I, Serra B. Initial experience with non-invasive prenatal testing of cell-free DNA for major chromosomal anomalies in a clinical setting. J Matern Fetal Neonatal Med. 2015;28(10):1196–1201. doi: 10.3109/14767058.2014.947579. [DOI] [PubMed] [Google Scholar]
- 118.Quezada MS, Gil MM, Francisco C, Orosz G, Nicolaides KH. Screening for trisomies 21, 18 and 13 by cell-free DNA analysis of maternal blood at 10-11 weeks' gestation and the combined test at 11-13 weeks. Ultrasound Obstet Gynecol. 2015;45(1):36–41. doi: 10.1002/uog.14664. [DOI] [PubMed] [Google Scholar]
- 119.Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, Tomlinson MW, Pereira L, Spitz JL, Hollemon D, Cuckle H, Musci TJ, Wapner RJ. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597. doi: 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 120.Petersen AK, Cheung SW, Smith JL, et al. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am J Obstet Gynecol. 2017;217(6):691 e691–691 e696. doi: 10.1016/j.ajog.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 121.Iwarsson E, Jacobsson B, Dagerhamn J, Davidson T, Bernabe E, Heibert AM. Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population - a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(1):7–18. doi: 10.1111/aogs.13047. [DOI] [PubMed] [Google Scholar]
- 122.Society for Maternal-Fetal Medicine. Electronic address pso. Norton ME, Biggio JR, Kuller JA, Blackwell SC. The role of ultrasound in women who undergo cell-free DNA screening. Am J Obstet Gynecol. 2017;216(3):B2–B7. doi: 10.1016/j.ajog.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 123.Liang B, Li H, He Q, Li H, Kong L, Xuan L, Xia Y, Shen J, Mao Y, Li Y, Wang T, Zhao YL. Enrichment of the fetal fraction in non-invasive prenatal screening reduces maternal background interference. Sci Rep. 2018;8(1):17675. doi: 10.1038/s41598-018-35738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bianchi DW, Chiu RWK. Sequencing of circulating cell-free DNA during pregnancy. N Engl J Med. 2018;379(5):464–473. doi: 10.1056/NEJMra1705345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105(6):632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 126.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21(7):597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 127.Hahn S, Huppertz B, Holzgreve W. Fetal cells and cell free fetal nucleic acids in maternal blood: new tools to study abnormal placentation? Placenta. 2005;26(7):515–526. doi: 10.1016/j.placenta.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 128.Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48(1):28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 129.Bianchi DW, Flint AF, Pizzimenti MF, Knoll JH, Latt SA. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc Natl Acad Sci U S A. 1990;87(9):3279–3283. doi: 10.1073/pnas.87.9.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bianchi DW, Zickwolf GK, Yih MC, Flint AF, Geifman OH, Erikson MS, Williams JM. Erythroid-specific antibodies enhance detection of fetal nucleated erythrocytes in maternal blood. Prenat Diagn. 1993;13(4):293–300. doi: 10.1002/pd.1970130408. [DOI] [PubMed] [Google Scholar]
- 131.Lo ES, Lo YM, Hjelm NM, Thilaganathan B. Transfer of nucleated maternal cells into fetal circulation during the second trimester of pregnancy. Br J Haematol. 1998;100(3):605–606. doi: 10.1046/j.1365-2141.1998.0636a.x. [DOI] [PubMed] [Google Scholar]
- 132.Bianchi DW. Fetal cells in the mother: from genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):103–108. doi: 10.1016/s0301-2115(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 133.Lo YM, Lau TK, Chan LY, Leung TN, Chang AM. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem. 2000;46(9):1301–1309. [PubMed] [Google Scholar]
- 134.Sekizawa A, Samura O, Zhen DK, Falco V, Farina A, Bianchi DW. Apoptosis in fetal nucleated erythrocytes circulating in maternal blood. Prenat Diagn. 2000;20(11):886–889. doi: 10.1002/1097-0223(200011)20:11<886::aid-pd942>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 135.Dunsmore Garett, Koleva Petya, Ghobakhloo Nafiseh, Sutton Reed, Ambrosio Lindsy, Meng Xuanyi, Hotte Naomi, Nguyen Vivian, Madsen Karen L, Dieleman Levinus A, Huang Vivian, Elahi Shokrollah. Lower Abundance and Impaired Function of CD71+ Erythroid Cells in Inflammatory Bowel Disease Patients During Pregnancy. Journal of Crohn's and Colitis. 2018;13(2):230–244. doi: 10.1093/ecco-jcc/jjy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Livergood MC, LeChien KA, Trudell AS. Obesity and cell-free DNA "no calls": is there an optimal gestational age at time of sampling? Am J Obstet Gynecol. 2017;216(4):413 e411–413 e419. doi: 10.1016/j.ajog.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 137.Rolnik DL, Yong Y, Lee TJ, Tse C, McLennan AC, da Silva Costa F. Influence of body mass index on fetal fraction increase with gestation and cell-free DNA test failure. Obstet Gynecol. 2018;132(2):436–443. doi: 10.1097/AOG.0000000000002752. [DOI] [PubMed] [Google Scholar]
- 138.Mhatre Mohak, Adeli Sharareh, Norwitz Errol, Craigo Sabrina, Phillippe Mark, Edlow Andrea. The Effect of Maternal Obesity on Placental Cell-Free DNA Release in a Mouse Model. Reproductive Sciences. 2018;26(9):1218–1224. doi: 10.1177/1933719118811647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mitsuya K, Parker AN, Liu L, Ruan J, Vissers MCM, Myatt L. Alterations in the placental methylome with maternal obesity and evidence for metabolic regulation. PLoS One. 2017;12(10):e0186115. doi: 10.1371/journal.pone.0186115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Higgins L, Mills TA, Greenwood SL, Cowley EJ, Sibley CP, Jones RL. Maternal obesity and its effect on placental cell turnover. J Matern Fetal Neonatal Med. 2013;26(8):783–788. doi: 10.3109/14767058.2012.760539. [DOI] [PubMed] [Google Scholar]
- 141.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One. 2015;10(9):e0137188. doi: 10.1371/journal.pone.0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-associated molecular pattern markers HMGB1 and cell-free fetal telomere fragments in oxidative-stressed amnion epithelial cell-derived exosomes. J Reprod Immunol. 2017;123:3–11. doi: 10.1016/j.jri.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Phillippe Mark, Phillippe Shiela M. Birth and death: Evidence for the same biologic clock. American Journal of Reproductive Immunology. 2017;77(5):e12638. doi: 10.1111/aji.12638. [DOI] [PubMed] [Google Scholar]
- 144.Behnia F, Taylor BD, Woodson M, et al. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015;213(3):359 e351–359 e316. doi: 10.1016/j.ajog.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 145.Bonney EA, Krebs K, Saade G, et al. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta. 2016;43:26–34. doi: 10.1016/j.placenta.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Menon R. Human fetal membranes at term: dead tissue or signalers of parturition? Placenta. 2016;44:1–5. doi: 10.1016/j.placenta.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Menon R, Mesiano S, Taylor RN. Programmed fetal membrane senescence and exosome-mediated signaling: a mechanism associated with timing of human parturition. 2017;8:196–Front Endocrinol (Lausanne). [DOI] [PMC free article] [PubMed]
- 148.Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2018;218(2S):S762–S773. doi: 10.1016/j.ajog.2017.11.567. [DOI] [PubMed] [Google Scholar]
- 149.Phillippe M, Adeli S. Cell-free DNA release by mouse placental explants. PLoS One. 2017;12(6):e0178845. doi: 10.1371/journal.pone.0178845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sawyer MR, Adeli S, Phillippe M. Cell-free DNA release by mouse fetal membranes. Reprod Sci. 2018;1933719118817659. [DOI] [PubMed]
- 151.Kacerovsky M, Celec P, Vlkova B, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One. 2013;8(3):e60399. doi: 10.1371/journal.pone.0060399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213(6):836 e831–836 e818. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nold Christopher, Stone Julie, O’Hara Kathleen, Davis Patricia, Kiveliyk Vladislav, Blanchard Vanessa, Yellon Steven M., Vella Anthony T. Block of Granulocyte-Macrophage Colony-Stimulating Factor Prevents Inflammation-Induced Preterm Birth in a Mouse Model for Parturition. Reproductive Sciences. 2018;26(4):551–559. doi: 10.1177/1933719118804420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, Hougaard DM, Thorsen P. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 2008;77(2):152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 155.Ekelund CK, Vogel I, Skogstrand K, et al. Interleukin-18 and interleukin-12 in maternal serum and spontaneous preterm delivery. J Reprod Immunol. 2008;77(2):179–185. doi: 10.1016/j.jri.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 156.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Chaiyasit N, Ahmed AI, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016;44(1):77–98. doi: 10.1515/jpm-2015-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117(4):902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 159.van Boeckel SR, Davidson DJ, Norman JE, Stock SJ. Cell-free fetal DNA and spontaneous preterm birth. Reproduction. 2018;155(3):R137–R145. doi: 10.1530/REP-17-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lin Y, Liu X, Shan B, Wu J, Sharma S, Sun Y. Prevention of CpG-induced pregnancy disruption by adoptive transfer of in vitro-induced regulatory T cells. PLoS One. 2014;9(4):e94702. doi: 10.1371/journal.pone.0094702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol. 2013;191(11):5702–5713. doi: 10.4049/jimmunol.1301604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fortunato SJ, Menon R, Lombardi SJ. Support for an infection-induced apoptotic pathway in human fetal membranes. Am J Obstet Gynecol. 2001;184(7):1392–1397. doi: 10.1067/mob.2001.115434. [DOI] [PubMed] [Google Scholar]
- 163.Menon R, Lombardi SJ, Fortunato SJ. TNF-alpha promotes caspase activation and apoptosis in human fetal membranes. J Assist Reprod Genet. 2002;19(4):201–204. doi: 10.1023/A:1014898130008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23(3):709–715. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- 165.Abrahams VM, Aldo PB, Murphy SP, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180(9):6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mulla MJ, Myrtolli K, Tadesse S, et al. Cutting-edge report: TLR10 plays a role in mediating bacterial peptidoglycan-induced trophoblast apoptosis. Am J Reprod Immunol. 2013;69(5):449–453. doi: 10.1111/aji.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lappas M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are increased after labour in foetal membranes and myometrium and are essential for TNF-alpha-induced expression of pro-labour mediators. Am J Reprod Immunol. 2015;73(4):313–329. doi: 10.1111/aji.12295. [DOI] [PubMed] [Google Scholar]
- 168.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, Dong Z, Hassan SS, Gomez-Lopez N. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol Reprod. 2016;95(6):130. doi: 10.1095/biolreprod.116.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gomez-Lopez N, Romero R, Xu Y, et al. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci. 2017;24(6):934–953. doi: 10.1177/1933719116675058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Willcockson AR, Nandu T, Liu CL, Nallasamy S, Kraus WL, Mahendroo M. Transcriptome signature identifies distinct cervical pathways induced in lipopolysaccharide-mediated preterm birth. Biol Reprod. 2018;98(3):408–421. doi: 10.1093/biolre/iox180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21(9):605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gomez-Lopez Nardhy, Romero Roberto, Xu Yi, Garcia-Flores Valeria, Leng Yaozhu, Panaitescu Bogdan, Miller Derek, Abrahams Vikki M., Hassan Sonia S. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. American Journal of Reproductive Immunology. 2017;77(5):e12648. doi: 10.1111/aji.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM, Hassan SS, Yeo L, Gomez-Lopez N. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. 2018;79(6):e12440. doi: 10.1111/aji.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gomez-Lopez Nardhy, Romero Roberto, Maymon Eli, Kusanovic Juan Pedro, Panaitescu Bogdan, Miller Derek, Pacora Percy, Tarca Adi L., Motomura Kenichiro, Erez Offer, Jung Eunjung, Hassan Sonia S., Hsu Chaur-Dong. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. Journal of Perinatal Medicine. 2019;47(3):276–287. doi: 10.1515/jpm-2018-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Panaitescu Bogdan, Romero Roberto, Gomez-Lopez Nardhy, Xu Yi, Leng Yaozhu, Maymon Eli, Pacora Percy, Erez Offer, Yeo Lami, Hassan Sonia S., Hsu Chaur-Dong. In vivo evidence of inflammasome activation during spontaneous labor at term. The Journal of Maternal-Fetal & Neonatal Medicine. 2018;32(12):1978–1991. doi: 10.1080/14767058.2017.1422714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM, Yeo L, Hassan SS. A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci. 2017;24(10):1382–1401. doi: 10.1177/1933719116687656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Modi BP, Teves ME, Pearson LN, Parikh HI, Haymond-Thornburg H, Tucker JL, Chaemsaithong P, Gomez-Lopez N, York TP, Romero R, Strauss JF., 3rd Mutations in fetal genes involved in innate immunity and host defense against microbes increase risk of preterm premature rupture of membranes (PPROM) Mol Genet Genomic Med. 2017;5(6):720–729. doi: 10.1002/mgg3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, Hsu CD. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80(5):e13049. doi: 10.1111/aji.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gomez-Lopez Nardhy, Motomura Kenichiro, Miller Derek, Garcia-Flores Valeria, Galaz Jose, Romero Roberto. Inflammasomes: Their Role in Normal and Complicated Pregnancies. The Journal of Immunology. 2019;203(11):2757–2769. doi: 10.4049/jimmunol.1900901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Poltorak A, He X, Smirnova I, Liu MY, van Huffel C, du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 181.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162(7):3749–3752. [PubMed] [Google Scholar]
- 182.Gomez-Lopez Nardhy, Romero Roberto, Garcia-Flores Valeria, Leng Yaozhu, Miller Derek, Hassan Sonia S, Hsu Chaur-Dong, Panaitescu Bogdan. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes†. Biology of Reproduction. 2018;100(5):1306–1318. doi: 10.1093/biolre/ioy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Faro Jonathan, Romero Roberto, Schwenkel George, Garcia-Flores Valeria, Arenas-Hernandez Marcia, Leng Yaozhu, Xu Yi, Miller Derek, Hassan Sonia S, Gomez-Lopez Nardhy. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome†. Biology of Reproduction. 2018;100(5):1290–1305. doi: 10.1093/biolre/ioy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plot showing the maternal plasma concentrations of IL-10 at 12.5 – 19.5 days post coitum (dpc), in labor, and 12 – 36 h postpartum. Each dot represents one maternal plasma sample. Midlines = medians, boxes = interquartile range, whiskers = minimum and maximum range. Red curves indicate trends over time. N = 5 – 11 dams per group. (PNG 62 kb)
Plots showing the negative correlations between plasma concentrations of IL-6 or IL-4 and the number of GFP-positive pups prior to systemic inflammation-induced preterm birth. Spearman correlation coefficients and p-values are displayed. Asterisk (*) indicates p-value obtained using a one-tailed Mann-Whitney U test. N = 8 dams. (PNG 83 kb)
Plot showing a negative correlation between plasma concentrations of IL-4 and cell-free fetal DNA (Egfp) levels prior to systemic inflammation-induced preterm birth. Spearman correlation coefficients and p-values are displayed. N = 8 dams. (PNG 80 kb)
(DOC 50 kb)