Abstract
Leiomyosarcoma is the most frequent subtype of the deadly uterine sarcoma and shares many common clinical grounds with leiomyoma, which is in turn the most common solid benign uterine neoplasm. With the recent progress in minimally invasive techniques for managing leiomyomas, accurate preoperative diagnosis of uterine masses has become the most important selection criterion for the safest therapeutic option. Therefore, different imaging modalities would be playing a key role in management of uterine masses. Testing for a sarcoma-specific promoter that expresses its downstream reporter gene only in leiomyosarcoma and not in leiomyoma or healthy uterine tissue. Adenoviral vectors were utilized both in vitro and in vivo to test the specificity of the promoters. Quantitative studies of downstream gene expression of these promoters was carried out both in vitro and in vivo. Our data indicated that human leiomyosarcoma cells highly expressed the reporter gene downstream to survivin promoter (Ad-SUR-LUC) when compared with benign leiomyoma or normal cells (p value of 0.05). Our study suggested that survivin is the unique promoter capable of distinguishing between the deadly sarcoma and the benign counterparts.
Keywords: Molecular imaging probe, Leiomyosarcoma, Sarcoma-specific promoter
Introduction
Uterine sarcomas are a highly aggressive, heterogeneous group of gynecologic malignancies. Uterine leiomyosarcoma (LMS) is the most common type of uterine sarcoma, accounting for approximately 42–60% of all uterine sarcomas. Although fewer than 3000 women are diagnosed with this disease in the USA each year, LMS is the most common sarcoma arising in the genital tract of reproductive-aged women.
Sarcomas have been challenging to diagnose before surgery because of limitations in clinical and radiographic predictors. Millions of American women undergo hysterectomies for different indications [1]. According to the Centers for Disease Control and Prevention (CDC), from 2006 to 2010, 11.7 percent of women between the ages of 40 and 44 had a hysterectomy for medical indications [2]. In November 2014, the US Food and Drug Administration (FDA) issued a safety communication severely restricting the use of power morcellators during minimally invasive surgery for women with uterine leiomyomas (LMA) [3]. This guidance was prompted by concern that if a patient had an undiagnosed leiomyosarcoma, the power morcellator might inadvertently spread tumor cells within the peritoneal cavity, and in turn worse the patient prognosis [4]. Accurate assessment of the incidence of occult malignancies encountered during surgery for suspected benign gynecologic conditions is critical for various reasons, including counseling regarding the risks and benefits of surgical approach, appropriate planning of a specific surgical approach (for example, open versus minimally invasive), and avoidance of iatrogenic complications [5].
Though the vast majority of women with uterine masses do indeed have benign leiomyomas, the current controversy over power morcellation in gynecologic surgery has highlighted how little we know about differentiating between benign myomas and sarcomas before surgery or in our gynecologic practice(s) in general [6].
Importantly, diagnostic biomarkers that can distinguish between human uterine LMS and LMA are not yet established [7]. There have been several reports that serum CA125 and/or LDH were elevated in uterine leiomyosarcoma; however, the sensitivity and specificity were low [8].
There is a concern that radiographic evaluation with combined positron emission tomography/computed tomography (PET/CT), which is commonly used to aid assessment of patient prognosis, might not necessarily be effective for diagnosis and surveillance of human uterine LMS [9]. Unfortunately, until now radiographic imaging cannot provide any medical information to help distinguish between fibroid and malignant uterine LMS [9]. Ultrasound imaging has only limited information on uterine smooth muscle tumors. There are no specific ultrasound features described for LMS tumors compared with fibroids [10]. Although certain MRI features may indicate differences in tissue intensity, there are no definitive imaging findings that reliably determine ordinary leiomyomas from leiomyoma variants [10]. The utility of PET/CT to differentiate ordinary leiomyomas, leiomyoma variants, and leiomyosarcoma remains limited because ordinary leiomyomas can take up fluorodeoxyglucose (FDG) on PET (as a marker for cellular proliferation) [11].
Given the above limitations, sarcoma until now, is a histologic diagnosis based upon pathology evaluation after resection of uterine tissue (myomectomy or hysterectomy), and there is no reliable test or imaging modality yet that can definitively determine whether a mass thought to be a fibroid is a cancer or not.
Because there are currently no imaging modalities or biomarkers to distinguish between a benign and a sarcomatous uterine mass [12], the power morcellation controversy highlights the urgent need to develop a simple safe and non-invasive reliable diagnostic tool that can triage suspicious uterine masses into benign versus malignant assignment [13].
Targeted imaging of cancer remains a major scientific goal. Accuracy, sensitivity, and specificity together in a cancer-imaging tool would provide early diagnostic and/or a screening tool, which represents a paradigm shift in treatment planning and substantially benefit therapeutic intervention.
A focused look at the expanding list of the molecular profiles of different malignant tumors and their microenvironment, molecular imaging has the potential to be well designed and could be generated utilizing tumor-specific targets [14]. The challenge of nonspecific localization of the putative targeted agents, eliciting unacceptably high background signals from naive tissues or cells, is real and does indeed impede further development in this field. Molecular-genetic imaging is an evolving exciting field that image molecular genetic processes rather than anatomy and provides visualization in space and time of normal as well as abnormal cellular processes at a molecular or genetic level [15].
A well-characterized human gene encoding a structurally unique inhibitor of apoptosis (IAP), designated survivin was first described in 1997 [16]. Present during fetal development, survivin is undetectable in terminally differentiated adult tissues. However, survivin becomes prominently expressed in transformed cell lines and in most common human cancers, e.g., lung [16], colon [17], pancreas [18], prostate [19], and breast [20]. Survivin is also found in approximately 50% of high-grade non-Hodgkin’s lymphomas (centroblastic, immunoblastic), but not in low-grade lymphomas (lymphocytic) [21]. Survivin functions as a key regulator of mitosis and programmed cell death. Initially, survivin was described as an inhibitor of caspase-9 [22]. More recently, research studies have shown that the role of survivin in cancer pathogenesis is not limited to apoptosis inhibition but also involves the regulation of the mitotic spindle checkpoint and the promotion of angiogenesis and chemoresistance [22]. Not more than 10 years of its discovery, survivin was established as a cancer-specific promoter in many studies [23].
Our diagnostic strategy make use of this cancer-specific enhanced survivin expression in malignant versus normal/benign cells to test its promoter driving potential of a downstream reporter gene which, once activated, will enable the detection of cancer cells [24].
The “Cancer specific promoters Hypothesis” is serving here for early detection of cancer cells and translating this into an imaging strategy. This new approach would be an effective one to clearly triage suspicious lesions in situ into either benign or malignant preoperatively in an outpatient (office) setting.
Serum survivin protein levels were evaluated as possible biomarkers for cancer diagnostics. Unfortunately, due to wide variations and large overlaps with normal controls, its clinical utility is currently restricted mainly for prognostic purposes [25].
The failures of these trials tracking the serum levels of the protein product of this cancer-specific promoter drove our attention to apply this direct in situ survivin promoter imaging probe strategy to assess the malignant status of a suspicious uterine mass. Our group had previously tested many different promoters to compare its specificity and ability to drive gene expression in human uterine leiomyoma in a different context of developing novel-localized gene therapy strategies against that common disease [26].
Here, we show that the enhanced survivin promoter in leiomyosarcoma versus leiomyoma cells can be exploited, using bioluminescence-based molecular imaging techniques, to drive imaging reporters selectively to enable detection of human leiomyosarcoma lesions in vivo in a preclinical animal model. Because of its strong promoter activity, tumor specificity, and capacity for clinical translation, survivin promoter–driven gene expression may represent a practical, new system to facilitate early leiomyosarcoma diagnosis.
Materials and Methods
Recombinant Adenovirus
Large scale production of adenovirus (Ad) vectors was performed as we have described previously (Al-Hendy et al. [27]) with a typical batch yield of 210 plaque-forming units (PFU)/ml.
Promoter Characteristics
Human survivin promoter
Complete promoter size, 268 bp
Adenovirus constructs, Ad-Survivin Ad-Heparanase (Ad-HEP-LUC), and Ad-Secretory leukoprotease inhibitor (Ad-SLPI-LUC) (Titer 5.0 × 1012 VP/ml 1.6 × 1011 IFU/ml, volume 10 × 200 μl construct)
The viral backbone adenoviral—type 5 (dE1/E3) promoter human survivin transgene human SLC5A5 RefSeq Accession: BC105047.1 Marker/Tag 2 × CsCl, purification Lot # 20151113, viral solution component: PBS with 5% glycerol
Cells
Fibroid tumor cells (F) derived from intramural fibroids of African American patients with size range of 4–8 cm. According to Augusta University Institutional Review Board, protocol No. 644354-6, which approves all human studies (40). Both F and Myo cells were cultured and maintained in smooth muscle cell basal medium (Lonza) containing 10% fetal bovine serum (Lonza) and 1% P/S. Human leiomyosarcoma cell line (SKUT-1) ATCC® HTB-114™ is a grade III mesodermal tumor (mixed) consistent with leiomyosarcoma cell derived from a 75-year-old adult Caucasian female. It was purchased from American Type Culture Collection (Manassas, VA, USA). The base medium for this cell line is ATCC-formulated Eagle’s Minimum Essential Medium. To make the complete growth medium, we add the following components to the base medium, i.e., fetal bovine serum to a final concentration of 10%. All cells were maintained at 37 °C and 5% CO2/air.
X-gal Staining of Fixed Leiomyosarcoma Cells
The susceptibility of SKUT-1 cells to transfection by wild type adenovirus serotype 5 was evaluated using β-Galactosidase as a reporter gene. Three different multiplicity of infections (MOIs) of 1, 3, and 5 were used. The viral particles were mixed with cell culture media followed by 1 hr of mild shaking, regular cell culture conditions were applied. Twenty four hrs post-transfection, X-gal staining was performed on the cells.
Transfection with Adenovirus Vectors, Imaging, and Luciferase Reporter Assays
The transfection with different Ad vectors was conducted as we have described previously (Al-Hendy et al., [28]; Salama et al., [29]). Briefly, various cell lines (F, Myo, and SKUT-1) were cultured in 60 mm plates. For the screening, the three cell types were transfected with either 1 or 5 PFU/cell of various Ad vectors using transfection medium containing 1% antibiotic and 2% FBS for 4 hrs with continuous gentle shaking. The media was then replaced with fresh media, and incubation was continued for 48 hrs. Luciferase transactivation was determined using luciferase enzyme assay systems according to the manufacturer’s protocol (Promega, Madison, WI, USA). Where, the growth medium was removed from cultured cells, and the cells were rinsed in 1 × PBS. Then, without dislodging cells much of the final wash was removed as possible. A total of 400 μl volume of 1 × cell lysis buffer was dispensed (CCLR) into each culture vessel, then attached cells were scraped from the dish, and the cells and solution were transferred to a microcentrifuge tube. Debris was separated by brief centrifugation, and the supernatant was transferred to a new tube. A total of 20 μl of cell lysate was used with 100 μl of luciferase assay reagent and measured the light produced by Synergy HT microplate reader utilizing Gen-5 software for bioluminescence detection.
For whole plate imaging, the three cell types were transfected with 1, 2, 3, 5, or 10 PFU/cell of Ad-survivin-luc then spectral imaging system AMIX was done to detect the total photon emission (TPE) for each plate per manufacturer’s instruction (see below).
Ex Vivo and In Vivo Studies
Female BALB/c 4–6 weeks old nude mice (Charles River, San Diego, CA) were housed in accordance with the Augusta University Institutional Animal Care and Use Committee (IACUC) guidelines. All procedures performed on animals were approved by the Augusta University’s IACUC and were within the guidelines of humane care of laboratory animals. In the ex vivo case, transfected cells were implanted in the animals either subcutaneously or intrauterine. While in the in vivo case, untransfected cells were implanted in the nude mice, and then when the tumor developed, the virus was injected intravenously. D-luciferin 15 mg/ml in PBS was intraperitoneally injected at 260 μl per mouse followed by isoflurane inhalation anesthesia 10 min later. The anesthetized animals were then placed in the AMIX chamber. The software mode was set to luminescence, photography, and X-ray. Exposure was 600 with low binning. [30]. Adult female BALB/c nude mice, 4–6 weeks of age, weighing between 20 and 25 g either subcutaneously or intrauterine, D-luciferin 15 mg/ml in PBS was intraperitoneally injected followed by isoflurane inhalation anesthesia 10 min later as described earlier (40). Anesthetized animals were then placed in the AMIX chamber and luminescence emission (TPE) was evaluated.
Spectral Imaging AMIX
Spectral Amix Imaging Systems (Spectral Instruments Imaging, LLC, Tucson, AZ) with an excitation and emission wavelength of 745 nm and 790 nm, respectively, were utilized in this work. AMIX machine located in the Core Imaging Facility for Small Animals, Georgia Cancer Center, Augusta University, was utilized for these experiments. The system includes animal handling features such as a heated sample shelf and a full gas anesthesia system. It is highly automated with all hardware motor movement, imaging parameters, and image analysis controlled via Living Image® software. Animals were injected with 260 μl intraperitoneal (IP) of D-luciferin dissolved in PBS (15 mg/ml). All measurements using AMIX were done using standard protocol per manufacturer’s instructions with software mode luminescence, photography at exposure 600, binning low, X-ray low in chamber AMIX flow.
MTT Assay
The MTT assay was done using the protocol described by Sigma. The assay was optimized for the cell lines used in the experiments. Briefly, cells were cultured in 96 well plate at density of 10,000 cells/well; the next day, we made the transfection using Ad-SUR-LUC (100, 500, and 1000 MOIs). At 24, 48, and 72 hrs post-transfection, we add 100 μl of MTT reagent (0.5 mg/ml in phenol red free culture media) to each well and incubate for 2 hrs then remove the reagent followed by the addition of DMSO (100 μl), gentle shaking for 10 min so that complete dissolution of formazan crystals was achieved. Absorbance was recorded at 560 nm using the microplate spectrophotometer system (Gen-5 Molecular Devices). Results were analyzed with the Gen-5 software.
Statistical Analysis of the Data
Data were fed to the computer and analyzed using the IBM SPSS software package version 20.0. Quantitative data were described using mean, standard error, and lower and upper 95% confidence limits. Significance of the obtained results was judged at the 5% level. The used tests were Student’s t test for normally quantitative variables, to compare between the two studied groups [31]. F-test (ANOVA) for normally quantitative variables was used to compare between more than two studied groups and post hoc test (LSD) for pairwise comparisons.
Results
Adenovirus Is Highly Effective Vector in Transfecting Human Leiomyosarcoma Cells
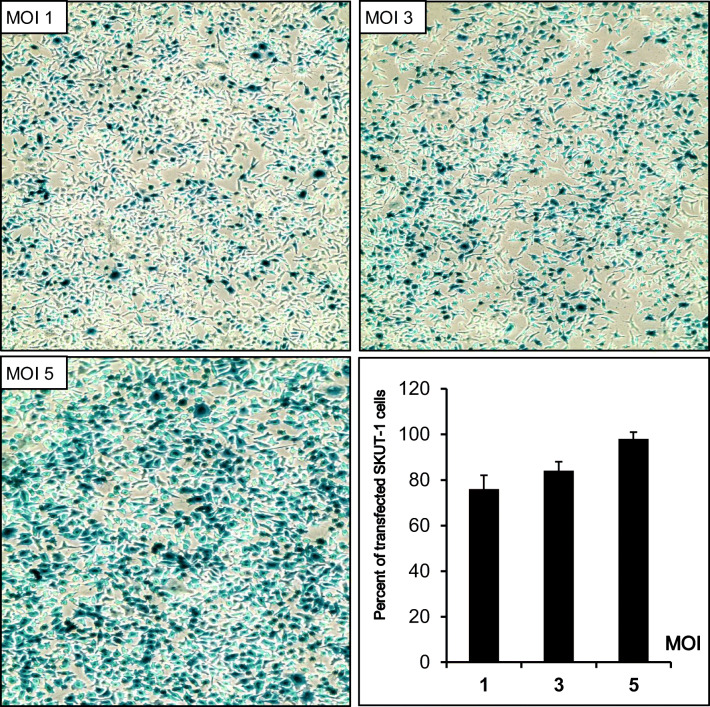
Myometrial and fibroid cells are susceptible to adenovirus transfection (Nair et al. [32]), however adenoviral vectors have not been tested prior against human sarcoma cells. This experiment was done to evaluate the susceptibility of SKUT-1 [33] human leiomyosarcoma cells to transfection by replication incompetent adenovirus serotype 5 expressing β-galactosidase as a reporter gene. We used 3 different MOIs1, 3, or 5 PFU/cell. Viral particles were mixed with cell culture media followed by 1 h of mild shaking, then regular cell culture conditions were applied as described in the “Materials and Methods” section. The expression of the transgene of the bacterial enzyme β-galactosidase was easily visualized using the artificial substrate X-gal, which turns blue when cleaved by β-galactosidase. Light microscopy of LMS cells transfected with 3 MOIs (1, 3, or 5) shows 76%, 84%, 98% transfection of the LMS cells respectively. Negative control (no adenovirus added) showed no background staining (Fig. 1).
Fig. 1.
Susceptibility of SKUT-1 cells to transfection by adenovirus LacZ and magnitude of transfection at 3 different multiplicity of infections (MOIs)
Adenovirus Survivin Promoter Construct Supports Highest Gene Expression in Human Leiomyosarcoma Cells
To identify a sarcoma-ON/leiomyoma-OFF–specific promoter, we screened several candidate promoters and short-listed 3 finalist promoters of interest. We transfected 60 mm2 cell culture dishes of SKUT-1 human LMS, F, and Myo cells at 80–90% confluence with 3 different adenoviral constructs harboring different promoters, all driving a luciferase marker gene; Ad-Survivin (Ad-SUR-LUC), Ad-Heparanase (Ad-HEP-LUC), and Ad-Secretory leukoprotease Inhibitor (Ad-SLPI-LUC) have all at the same MOI using the same technique as described above.
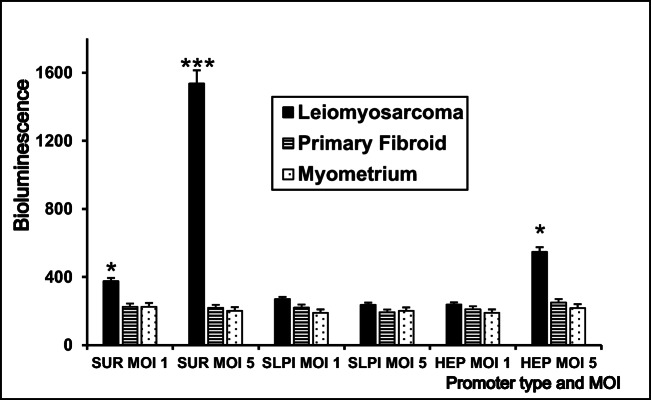
Luciferase assay was then used to differentiate between gene expression levels in the three cell types under the control of the 3 finalist promoters. We used the standard luciferase assay to compare the degree of reporter gene expression in leiomyosarcoma cells under different promoters. As shown in Fig. 2, luciferase assay showed (survivin) promoter is expressed at least 10–12 times higher than other constructs, where at MOI 1 or 5 of Adeno-sur-luc shows a statistically significant p value of 0.03, 0.01, respectively.
Fig. 2.
Comparison of transfection potential of adenovirus luciferase constructs under 3 different promoters Ad-Survivin (Ad-SUR-LUC), Ad-Heparanase (Ad-HEP-LUC), and Ad-Secretory leukoprotease inhibitor (Ad-SLPI-LUC) at 3 different MOIs. The SKUT-1 cells transfected with Ad-SUR-LUC showed significantly higher bioluminescence than other constructs
Safety Studies of Adenovirus-Survivin-Luciferase on the Selected Three-Cell Lines
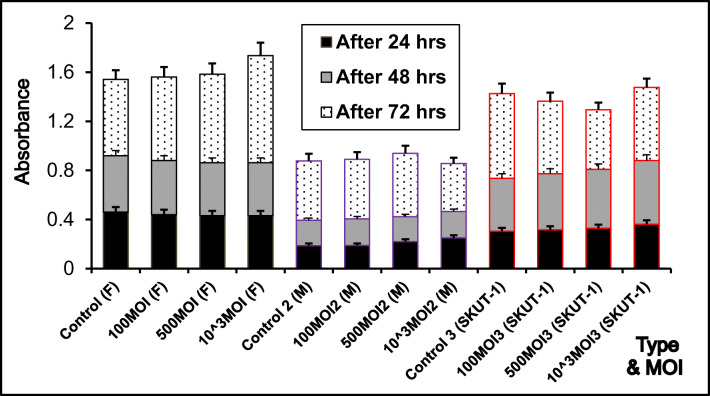
We transfected our cells (F, Myo, and SKUT-1 cells) with Ad-SUR-LUC at different MOIs 100, 500, and 1000. Then, we performed the MTT assay at three different time points after 24, 48, and 72 h for the three cell lines. As it appears from Fig. 3 there is no statistically significant effect of the Adenovirus construct on the cell survival and growth plateau (p value of 0.670681, 0.9965, 0. 955626).
Fig. 3.
MTT assay for comparison of cell viability after Ad-SUR-LUC transfection in normal myometrium (Myo), primary fibroid cells (F), and leiomyosarcoma cells (SKUT-1)
Ad-SUR-LUC Elicits High Luminescence Emission in Human Leiomyosarcoma Cells
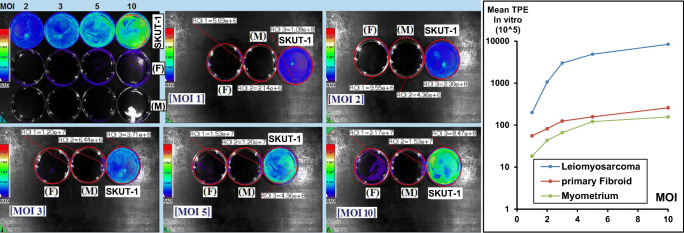
In preparation for in vivo work, we also wanted to confirm the superior Ad-SUR-LUC controlled reporter gene expression by AMIX live cell imaging. Cells were cultured in 60 mm dishes, and at 70% confluence, we did transfection using Ad-SUR-LUC. Firefly D-luciferin dissolved in PBS 15 mg/ml was added to cell culture media. The luminescence imaging was performed as reported previously [30]. After processing the data as shown in Fig. 4, it showed statistically significant higher luminescence only in SKUT-1 cells (p value < 0.0081).
Fig. 4.
Comparison of luminescence signal in the normal myometrial cells, primary fibroid cells, and leiomyosarcoma cells at 5 MOIs using AMIX spectral imaging system to detect the in vitro bioluminescence signal. There was significantly high MOI-dependent bioluminescence signal in leiomyosarcoma cells when compared with normal and benign cells (p < 0.05) (n = 4)
Ex Vivo Delivered Ad-SUR-LUC Successfully Differentiate Between Leiomyosarcoma and Leiomyoma
Evaluating the efficacy of Ad-SUR-LUC in vivo in an appropriate animal model of human leiomyosarcoma was the next step. Ad-SUR-LUC pre-transfected cells were implanted in adult female BALB/c nude mice, 4–6 weeks of age, weighing between 20 and 25 g either subcutaneously (Fig. 5) or intrauterine (Fig. 6). D-luciferin 15 mg/ml in PBS was intraperitoneally injected followed by isoflurane inhalation anesthesia 10 min later as described earlier (29). Anesthetized animals were then placed in the AMIX chamber, and luminescence emission (TPE) was evaluated. Multiple comparison shows that in case of subcutaneously injected pre-transfected SKUT-1 versus either fibroid or untransfected SKUT-1, the TPE is statistically significant with a p value range (0.01–< 0.001). While in the case of intrauterine injected cells, the TPE signal was recorded immediately, and 48 h post-operation until it dwindled after 6 days and pre-transfected SKUT-1(IU) show a statistically significant TPE versus fibroid or non-transfected LMS with p value of < 0.001.
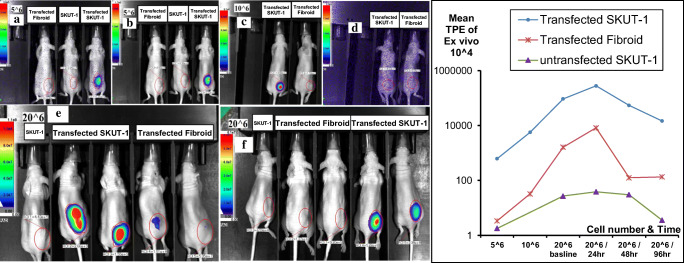
Fig. 5.
Ex vivo subcutaneous animal model for comparison of gene expression in different cell types transplanted subcutaneously in nude mice. 5^6 cells of transfected Fibroid, SKUT-1 “control”, & transfected SKUT-1; (a) immediate imaging after injecting luciferin (IP), (b) after 30 minutes. (c) Imaging after injecting 10^6 cells/animal of transfected SKUT-1 and transfected Fibroid, the signal disappeared 9 days post transfection as shown in (d). (e) 20^6 cells were injected in each mouse subcutaneously, Bioluminescence imaging showing higher signal in the transfected SKUT-1 lesions compared to the non-transfected as well as the begin leiomyoma case after 24 hours (e) and after 48 hours as in (f). There was a significant high expression and hence bioluminescence in animals bearing SKUT-1 cells
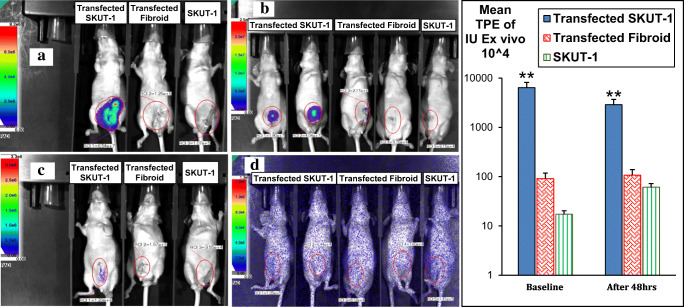
Fig. 6.
Ex vivo comparison of gene expression in the 3 different cell types transplanted intrauterine in nude mice. There was a significant higher expression and hence bioluminescence in animals bearing transfected SKUT-1 cells as shown in both (a & b), the signal faded (disappeared) after 6 days as in (c & d)
Intravenous Delivered Ad-SUR-LUC Successfully Differentiate Between Pre-Existing Human Leiomyosarcoma Versus Human Leiomyoma in Mouse Model
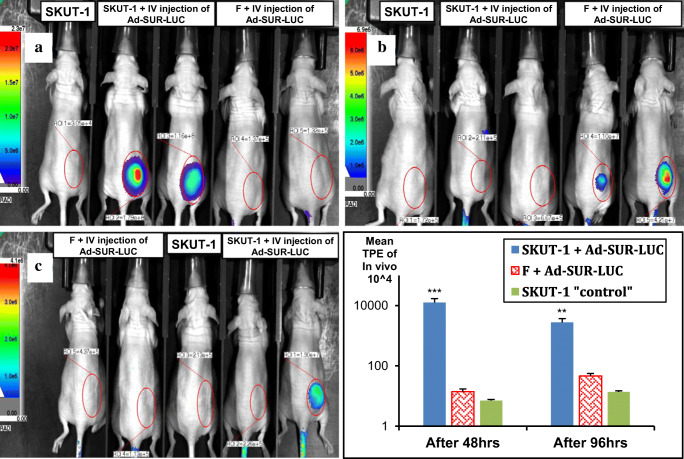
The ultimate test of our strategy was to validate the ability of Ad-SUR-LUC to specifically and safely discriminate between pre-existing human leiomyosarcoma lesions versus human leiomyoma lesions after systemically delivering this vector via intravenous injection. We implanted non-transfected SKUT-1 cells (20 × 106 cells/mouse) or human leiomyoma cells (20 × 106 cells/mouse) subcutaneously into female nude mice, and corresponding tumors were allowed to develop for 14 days. We, then proceeded to injected Ad-SUR-LUC intravenously (1 × 109 PFU/mouse) via the tail vein. As shown in Fig. 7, bioluminescence imaging shows signals only in mice bearing SKUT-1 lesions that were injected intravenously with the Ad-SUR-LUC and started to appear significantly after 48 and 96 hrs (Fig. 7a and b). While, no signal in mice with SKUT-1 lesions, and didn't get the IV Ad-SUR-LUC as well as the benign leiomyoma bearing mice injected intravenously with the Ad-SUR-LUC with a p value of < 0.001). The signal faded 8 days post Intravenous injection of Ad-survivin-luciferase.
Fig. 7.
Intravenously injected virus (Ad-SUR-LUC) animal model to differentiate gene expression in the different cell types previously transplanted subcutaneously in nude mice. There was a significant high expression and hence bioluminescence in animals bearing SKUT-1 cells 24 (a), 48 (b), and 96 (c) hrs after viral I.V. injection in comparison to mice bearing F cells
Discussion
Molecular-genetic imaging is advancing from a valuable preclinical tool to becoming a clinical reality that drives appropriate patient management. Our goal is to develop a systemically deliverable construct that would enable preoperative molecular-genetic imaging of leiomyosarcoma.
The necessary elements for such a construct include a sufficiently strong promoter with high sarcoma specificity and very low activity in benign leiomyoma and normal myometrium (Sarcoma/ON-Leiomyoma/OFF) design. Our strategy involved pairing an imaging reporter gene with a complementary imaging agent in a system that can be used to measure gene expression or protein interaction, or track gene tagged cells in vivo. We were able to detect the extent of promoter gene expression in each cell type and subsequently were able to distinguish normal or benign (leiomyoma) from malignant (leiomyosarcoma) cells by the extent of appreciation of significant difference in survivin gene expression. Promoters derived from the human telomerase reverse transcriptase [34], survivin [35] and carcinoembryonic antigen [36] promoters, and enhancer elements have been previously used in molecular-genetic imaging to provide tumor-specific reporter expression. Often, promoter activity must be amplified to drive the downstream gene for purposes of imaging or therapy. One such strategy involves the two-step transcriptional amplification system [37] using the GAL4-VP16 fusion protein and GAL4 response elements [38, 39]. However in our study, survivin promoter did not require such amplification to achieve the extremely high sensitivity imaging that we observed in this work.
There is a growing evidence indicating that survivin is expressed in some normal adult cells, particularly primitive hematopoietic cells, T lymphocytes, polymorphonuclear neutrophils, and vascular endothelial cells, and may regulate their proliferation or survival. In our situation, this fact may be responsible of the presence of background during imaging as we are going to inject the adenovirus systemically, which means that wherever there is survivin expression, there will be some luciferase expression and a luminescent signal. Even though, in a normal woman, there should be minimal survivin expression except if she has leiomyosarcoma, so there should be minimal background, but it will be way much less in intensity compared with a leiomyosarcoma lesion. Understanding the distribution of survivin expressing cells in normal versus malignant tissues and cells is very important in identifying strategies that maximally discriminate survivin expressing cancer cells. Relevance of survivin high expression in cancers justifies the pursuit of survivin-based diagnostics depending mainly on appreciating differences in survivin expression between normal and cancer cells.
Adenovirus have shown tremendous promise as delivery vehicles for recombinant genes and gene therapy. Some of the attributes which make Ad vectors suitable candidates for gene delivery applications include (1) the safety and relative ease of vector development; (2) the ability to infect a wide range of actively dividing and non-dividing mammalian cells and to induce a high level of transgene expression; (3) the minimum risk of integration into the host genome; (4) the capacity to be grown to very high titers in tissue culture; (5) numerous strategies that have been developed to construct Ad vectors carrying a foreign gene insert. While by now, virus-based vectors have proven to show efficient and safe transduction of various cell types in many ex vivo or in local treatments, the use of such vectors for systemic delivery is still unsatisfactory. One of the most important limitations to adenovirus use is the activation of both innate and adaptive immune responses [40]. Fortunately, chemical shielding and use of different Ad vectors based on different adenovirus serotypes could be considered successful strategies to circumvent these immunologic barriers [41].
A limitation in translating this diagnostic system to the clinical arena is the lack of well-optimized chemiluminescence–based robust human imaging devices [42]. In our future work, we will upgrade this imaging probe to humanize it and adapt it to currently available human imaging techniques such as positron emission tomography and magnetic resonance imaging.
Conclusion
Survivin promoter, which is associated with cancer genetic makeup and cancer progression, was exploited for its possible utility in directing expression-targeted genetic imaging. It was found that survivin promoter provides high specificity to distinguish between human leiomyosarcoma cancer cells on one hand versus both normal human myometrial cells as well as human benign leiomyoma cells on the other hand, in vitro. In a murine model of leiomyosarcoma, this promoter-based imaging technique was capable of distinguishing sarcomatous lesions from normal uterine tissue or benign leiomyoma lesions with promising accuracy.
This system can be considered promising and is candidate of future development and optimization as it has the potential of having an impact to improve management of suspicious uterine masses, a current major challenge in clinical gynecology.
Authors Contribution
Dr. Al-Hendy conceived the idea, developed the theory, designed the study, edited and revised the manuscript; Dr. Shahinaz Shalaby and Dr. Mostafa Khater collected, analyzed the data, wrote the manuscript. Ms. Archana Laknaur prepared for the experiments. Dr. Arbab facilitated the spectral imaging studies, revised the protocols, and edited the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Funding Information
This study was funded by the National Institute of Health (R01 ES 028615–01, R01HD 087417, R01 HD 094378, R01 HD 094380).
Compliance with Ethical Standards
Conflict of Interest
Dr. Ayman Al-Hendy is consulting Abbvie, Bayer, Allergan, MD Stem Cells for research support. Dr. Ali Arbab declares that he has no conflict of interest. Ms. Archana Laknaur declares that she has no conflict of interest. Dr.Mostafa Khater declares that he has no conflict of interest. Dr. Shahinaz Shalaby declares that she has no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and IACUC approval of Augusta University was taken. This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perri T, Korach J, Sadetzki S, Oberman B, Fridman E, Ben-Baruch G. Uterine leiomyosarcoma: does the surgical procedure matter? Int J Gynecol Cancer. 2009;19(2):257–260. doi: 10.1111/IGC.0b013e31819a1f8f. [DOI] [PubMed] [Google Scholar]
- 2.Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33. doi: 10.1016/j.jmig.2014.08.781. [DOI] [PubMed] [Google Scholar]
- 3.Ottarsdottir H, Cohen SL, Cox M, Vitonis A, Einarsson JI. Trends in mode of hysterectomy after the US Food and Drug Administration power morcellation advisory. Obstet Gynecol. 2017;129(6):1014–1021. doi: 10.1097/AOG.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 4.Schatz F, et al. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum Reprod Update. 2016;22(4):497–515. [DOI] [PMC free article] [PubMed]
- 5.Wright JD, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107(11):djv251. doi: 10.1093/jnci/djv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao J, et al. Population-based estimates of the prevalence of uterine sarcoma among patients with leiomyomata undergoing surgical treatment. JAMA Surgery. 2015;150(4):368–370. doi: 10.1001/jamasurg.2014.3518. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, et al. A novel diagnostic biomarker for human uterine leiomyosarcoma: PSMB9/β1i. Chin Clin Oncol. 2017;6(2). [DOI] [PubMed]
- 8.Jitsumori M, et al. Hyperphosphatasemia in leiomyosarcoma of the uterus: two case reports and a literature review. J Obstet Gynaecol Res. 2017;43(9):1498–503. [DOI] [PubMed]
- 9.Wu TI, et al. Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol Oncol. 2006;100(1):166–172. doi: 10.1016/j.ygyno.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.White M. Uterine smooth muscle tumors of uncertain malignant potential (stump): review of pathophysiology, classification, diagnosis, treatment, and surveillance. J Health Commun. 2017;2:4. 10.4172/2472-1654.100080.
- 11.Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002;12(4):354–361. doi: 10.1046/j.1525-1438.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- 12.Cui RR, Wright JD. Risk of occult uterine sarcoma in presumed uterine fibroids. Clin Obstet Gynecol. 2016;59(1):103–118. doi: 10.1097/GRF.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 13.Peters A, Sadecky AM, Winger DG, Guido RS, Lee TTM, Mansuria SM, Donnellan NM. Characterization and preoperative risk analysis of leiomyosarcomas at a high-volume tertiary care center. Int J Gynecol Cancer. 2017;27(6):1183–1190. doi: 10.1097/IGC.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyoeun CB, et al. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nat Med. 2011;17(1):123–129. doi: 10.1038/nm.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohan K, et al. Sub-nanomolar detection of prostate-specific membrane antigen in synthetic urine by synergistic, dual-ligand phage. J Am Chem Soc. 2013;135(20):7761–7767. doi: 10.1021/ja4028082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 17.Gianani R, Jarboe E, Orlicky D, Frost M, Bobak J, Lehner R, Shroyer KR. Expression of survivin in normal, hyperplastic, and neoplastic colonic mucosa. Hum Pathol. 2001;32(1):119–125. doi: 10.1053/hupa.2001.21897. [DOI] [PubMed] [Google Scholar]
- 18.Kami K, Doi R, Koizumi M, Toyoda E, Mori T, Ito D, Fujimoto K, Wada M, Miyatake S, Imamura M. Survivin expression is a prognostic marker in pancreatic cancer patients. Surgery. 2004;136(2):443–448. doi: 10.1016/j.surg.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Shariat SF, Lotan Y, Saboorian H, Khoddami SM, Roehrborn CG, Slawin KM, Ashfaq R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer. 2004;100(4):751–757. doi: 10.1002/cncr.20039. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6(1):127–134. [PubMed] [Google Scholar]
- 21.Ansell S, Arendt BK, Grote DM, Jelinek DF, Novak AJ, Wellik LE, Remstein ED, Bennett CF, Fielding A. Inhibition of survivin expression suppresses the growth of aggressive non-Hodgkin's lymphoma. Leukemia. 2004;18(3):616–623. doi: 10.1038/sj.leu.2403281. [DOI] [PubMed] [Google Scholar]
- 22.Mita AC, et al. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14(16):5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 23.Zhu ZB, et al. Transcriptional targeting of tumors with a novel tumor-specific survivin promoter. Cancer Gene Ther. 2004;11(4):256–262. doi: 10.1038/sj.cgt.7700679. [DOI] [PubMed] [Google Scholar]
- 24.Bao R, Connolly DC, Murphy M, Green J, Weinstein JK, Pisarcik DA, Hamilton TC. Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst. 2002;94(7):522–528. doi: 10.1093/jnci/94.7.522. [DOI] [PubMed] [Google Scholar]
- 25.Monzó M, et al. A novel anti-apoptosis gene: re-expression of survivin messenger RNA as a prognosis marker in non–small-cell lung cancers. J Clin Oncol. 1999;17(7):2100. doi: 10.1200/JCO.1999.17.7.2100. [DOI] [PubMed] [Google Scholar]
- 26.Hassan MH, Khatoon N, Curiel DT, Hamada FM, Arafa HM, al-Hendy A. Toward gene therapy of uterine fibroids: targeting modified adenovirus to human leiomyoma cells. Hum Reprod. 2008;23(3):514–524. doi: 10.1093/humrep/dem410. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hendy, Ayman, et al. "Ovarian cancer gene therapy: repeated treatment with thymidine kinase in an adenovirus vector and ganciclovir improves survival in a novel immunocompetent murine model." American journal of obstetrics and gynecology 182.3 (2000);553-559. [DOI] [PubMed]
- 28.Al-Hendy, A., Lee, E. J., Wang, H. Q., & Copland, J. A. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. American journal of obstetrics and gynecology, (2004);191(5):1621-1631. [DOI] [PubMed]
- 29.Salama, S. A., Kamel, M., Christman, G., Wang, H. Q., Fouad, H. M., & Al-Hendy, A. Gene therapy of uterine leiomyoma: adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir treatment inhibits growth of human and rat leiomyoma cells in vitro and in a nude mouse model. Gynecologic and obstetric investigation, (2007);63(2):61-70. [DOI] [PubMed]
- 30.Henriques C, et al. In vivo imaging of mice infected with bioluminescent Trypanosoma cruzi unveils novel sites of infection. Parasit Vectors. 2014;7:89. doi: 10.1186/1756-3305-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotz S, Balakrishnan N, Read CB, Vidakovic B. Encyclopedia of statistical sciences. 2. Hoboken: Wiley-Interscience; 2006. [Google Scholar]
- 32.Nair, S., Curiel, D. T., Rajaratnam, V., Thota, C., & Al-Hendy, A. Targeting adenoviral vectors for enhanced gene therapy of uterine leiomyomas. Human Reproduction, (2013);28(9):2398-2406. [DOI] [PMC free article] [PubMed]
- 33.Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, Teraishi F, Kyo S, Mizuguchi H, Hashimoto Y, Urata Y, Tanaka N, Fujiwara T. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med. 2006;12(10):1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 35.Ray S, Paulmurugan R, Patel MR, Ahn BC, Wu L, Carey M, Gambhir SS. Noninvasive imaging of therapeutic gene expression using a bidirectional transcriptional amplification strategy. Mol Ther. 2008;16(11):1848–1856. doi: 10.1038/mt.2008.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao J, Doubrovin M, Sauter BV, Huang Y, Guo ZS, Balatoni J, Akhurst T, Blasberg RG, Tjuvajev JG, Chen SH, Woo SL. Tumor-specific transcriptional targeting of suicide gene therapy. Gene Ther. 2002;9(3):168–175. doi: 10.1038/sj.gt.3301618. [DOI] [PubMed] [Google Scholar]
- 37.Iyer M, et al. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci. 2001;98(25):14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iyer M, Salazar FB, Lewis X, Zhang L, Carey M, Wu L, Gambhir SS. Noninvasive imaging of enhanced prostate-specific gene expression using a two-step transcriptional amplification-based lentivirus vector. Mol Ther. 2004;10(3):545–552. doi: 10.1016/j.ymthe.2004.06.118. [DOI] [PubMed] [Google Scholar]
- 39.Huyn ST, et al. A potent, imaging adenoviral vector driven by the cancer-selective mucin-1 promoter that targets breast cancer metastasis. Clin Cancer Res. 2009;15(9):3126–3134. doi: 10.1158/1078-0432.CCR-08-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JW, et al. A comparative study of replication-incompetent and-competent adenoviral therapy-mediated immune response in a murine glioma model. Mol Ther Oncol. 2017;5:97–104. doi: 10.1016/j.omto.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jönsson F, Kreppel F. Barriers to systemic application of virus-based vectors in gene therapy: lessons from adenovirus type 5. Virus Genes. 2017;53(5):692–9. [DOI] [PubMed]
- 42.Park JY, Kricka LJ. Prospects for the commercialization of chemiluminescence-based point-of-care and on-site testing devices. Anal Bioanal Chem. 2014;406(23):5631–5637. doi: 10.1007/s00216-014-7697-8. [DOI] [PubMed] [Google Scholar]