Abstract
Paracrine interactions between ovarian theca-interstitial cells (TICs) and granulosa cells (GCs) play an important role in the regulation of follicular steroidogenesis. Androgens serve as substrates for aromatization as well as affect GC function. This study evaluated the effects of co-culture of GC with TICs and the role of testosterone (T) and 5-alpha-dihydrotestosterone (DHT), and estradiol (E2) in modulation of GC expression of genes involved in the production of progesterone: 3β-hydroxysteroid dehydrogenase/Δ5–4 isomerase (Hsd3b) and cholesterol side-chain cleavage (Cyp11). GCs obtained from immature Sprague-Dawley rats and were cultured in chemically defined media without or with TICs, DHT, or T. Hsd3b and Cyp11 transcripts were analyzed by qt-PCR. Co-culture of GCs with TICs stimulated Hsd3b and CYP11 expression in GCs. DHT and T induced a concentration-dependent upregulation of Hsd3b and CYP11 expression, as well as increased progesterone concentrations in spent media. E2 also increased expression of Hsd3b, and Cyp11. Effects of androgens were abrogated in the presence of an anti-androgen bicalutamide and the antiestrogen ICI 182780 (ICI). In conclusion, present findings demonstrate that androgens upregulate production of progesterone in GCs; these effects are likely due to a combination of direct action on androgen receptors and effects mediated by estrogen receptors.
Keywords: Granulosa cells, Androgens, Rat, Gene expression
Introduction
Ovarian steroidogenesis involves coordinated interactions between the theca cells (TCs) and granulosa cells (GCs) of the follicle. While it is well established that communication between the oocyte and GCs plays an important role in folliculogenesis, communication between GCs and TCs is still not well understood. The two-cell–two-gonadotropin theory acknowledges the relevance of TCs-derived testosterone as a substrate for aromatization by GCs [1]. However, androgens also exert other important effects on the developing follicle. Several studies have shown that androgens stimulate early follicle growth [2, 3]. Consistent with these observations, follicular development is impaired in GC-specific androgen receptor knockout animals [4]. In addition, mouse follicles grown in the presence of androgen receptor (AR) antagonists grow more slowly than control follicles [5]. These data suggest that in addition to being a substrate for steroid production, androgens have important effects on follicle development and function.
GCs serve two important functions in the follicle. First, GCs produce factors such as Kit ligand that regulate oocyte maturation and follicle development [6]. Second, GCs are the primary source of the sex steroids estrogen (E2) and progesterone. While there is a sizable body of literature evaluating the effect of androgens on follicle development, little is known about the effects of androgens on the steroidogenic function of GCs, in particular, progesterone production. While early studies of GCs indicated that androgen treatment may increase progesterone production [7, 8], the mechanisms driving this increase were not known.
We hypothesize that androgens may serve as a TC-derived paracrine signal that can regulate GC steroidogenesis. To address this issue, a study was conducted to determine the effects of TC/GC co-culture on steroidogenic genes with emphasis on the impact of androgens on GC progesterone production.
Materials and Methods
Animals
Female Sprague-Dawley rats were obtained at age 24 days from Envigo (Placentia, CA). At the age of 27, 28, and 29 days, the rats were injected with 17β-estradiol (1 mg/0.3 ml sesame oil, s.c.; Milipore Sigma, St. Louis, MO) to stimulate ovarian development and growth of antral follicles as described previously [9, 10]. For 24 h after the last injection, the animals were anesthetized using ketamine and xylazine (i.p.) and euthanized by intracardiac perfusion using 0.9% saline. The ovaries were removed from the animals and dissected free of oviducts and fat under a dissecting microscope. GCs were released by puncturing antral follicles with a 26.5-gauge needle. After puncture, remaining ovarian tissue was minced and treated with a 60-min collagenase digestion. The resulting theca-interstitial cells (TICs) were purified using discontinuous Percoll gradient centrifugation. Both cell types were then resuspended in McCoy’s 5A culture medium supplemented with 1% antibiotic/antimycotic mix, 0.1% BSA, and 2-mm l-glutamine (Gibco, Life Technologies, Carlsbad, CA).
Co-culture Experiments
To study effects of TICs on mRNA expression of relevant genes, 24-well transwell plates (Corning Life Science, Tewksbury, MA) were used. Wells were coated with human fibronectin (Corning Life Science) as follows: inserts were removed from the plate and placed into a separate 24-well plate, fibronectin was diluted with DPBS to a concentration of 26 μg/mL, and this solution was added to wells and incubated at room temperature for 1 h. Fibronectin solution was then aspirated and wells were rinsed. Plates were then placed at 4 °C overnight. Matrigel (Corning Life Science) was diluted to 0.3 mg/ml with serum-free culture medium, and 40uL of diluted matrigel was placed into each insert of a 24-transwell plate, and inserts were incubated at room temperature until gelled. GCs (200,000 per well) were then added to insert. Inserts were then transferred to fibronectin-coated transwell plates with wells already plated with TICs (100,000 and 200,000 cells/well). These cultures were maintained for 48 h. GCs and TICs were then lysed in lysis/binding solution for RNA isolation.
Granulosa Cell Treatments
Isolated GCs were plated into fibronectin-coated 24-well plates at a density of 300,000 cells/well. DHT and T were then added at the listed concentrations and cells cultured for 48 h. For time-course experiments, cells were cultured for 2, 24, and 48 h. Cells and supernatant were then collected for RNA extraction and progesterone quantification.
RNA Isolation
RNA was isolated using the MagMAX-96 Total RNA Isolation Kit (Applied Biosystems, Foster City, CA) and the KingFisher robot (Thermo Scientific, Vantaa, Finland). Reverse transcription of total RNA to cDNA was performed using High Capacity cDNA Reverse Transcription Kit for rt-PCR (Applied Biosystems, Foster City, CA). PCR reactions were set up in 20-μl volumes, consisting of 5 μl cDNA (5 ng), 2.5 μl forward and 2.5 μl reverse 200 nM primers, and 10 μl of 2X SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The primers are listed in Table 1. QPCR was run on an ABI 7300 (Applied Biosystems, Foster City, CA). Transcript levels of Hsd3b, Cyp11, and StAR were quantified using the delta-delta Ct method using Hprt as housekeeping reference gene.
Table 1.
Sequences of primers
| Gene | RefSeq | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|---|
| Hprt1 | NM_012583 | TTGTTGGATATGCCCTTGACT | CCGCTGTCTTTTAGGCTTTG | 105 |
| Hsd3b1 | NM_001007719 | ATATTGGAGGCCTGCGTCG | CGGCCATCCTTTTGCTGTA | 166 |
| Cyp11a1 | NM_017286.3 | GCTGGAAGGTGTAGCTCAGG | CAC TGG TGT GGA ACA TCT GG | 224 |
| Star | NM_031558 | GCCTGAGCAAAGCGGTGTC | CTG GCG AAC TCT ATC TGG GTC TGT | 180 |
Rat primers for qPCR analysis. All primers listed in 5′ to 3′ direction
Progesterone Quantification
Spent cell culture media was collected after 48 h of treatment and kept frozen at −80 °C prior to testing. Progesterone concentration was then quantified using the Abcam Human saliva progesterone ELISA kit (Abcam, Cambridge, MA). Samples were assayed in duplicates.
Statistical Analysis
Statistical analysis was performed using JMP 13.0 (SAS, Cary, NC). P-values < 0.05 were considered significant. Values were expressed as mean ± SEM. Comparisons were performed by one-way analysis of variance followed by post hoc pairwise comparisons of individual means (Dunnett’s test). Normality of distribution was assessed by the Shapiro-Wilk W test. In the absence of normality, data were transformed (Box-Cox), and/or nonparametric testing (Kruskal-Wallis, Dunn) was performed when appropriate.
Results
Co-culture Experiments
Figure 1 summarizes effects of TICs on GC expression of key genes relevant to progesterone production. Co-culture with TICs at a concentration of 100,000 and 200,000 cells per well, respectively, led to a 1.5-fold (P = 0.02) and a 2-fold (P = 0.0001) increase of Hsd3b transcription in comparison to cultures of GCs alone. Significant increase of Cyp11 transcription (1.8-fold) by GCs was observed in the presence of 200,000 TICs per well (P = 0.003).
Fig. 1.
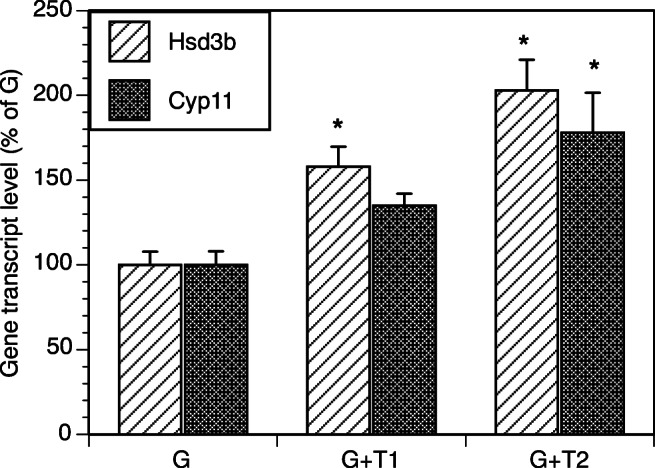
Effect of co-culture of TICs with GCs on mRNA expression of Hsd3b and Cyp11 in GCs. GCs (200,000 cells/well) were cultured in the absence (G) or in the presence of TICs at a concentration of 100,000 cells per well (G + T1) or 200,000 cells/well (G + T2). TICs were placed in the fibronectin-coated 24-well plates, while Gs were placed in the matrigel-coated inserts. All cultures were carried out for 48 h in chemically defined media. Each bar represents mean ± SEM; * denotes means significantly different (P < 0.05) from control cultures (G)
Effects of Androgens and Anti-androgen on GCs
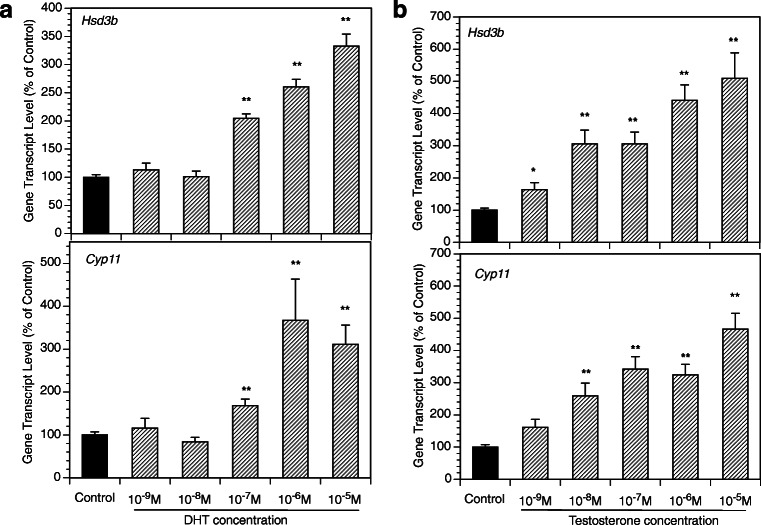
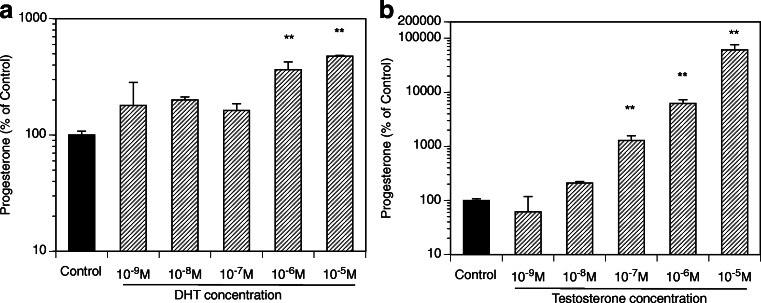
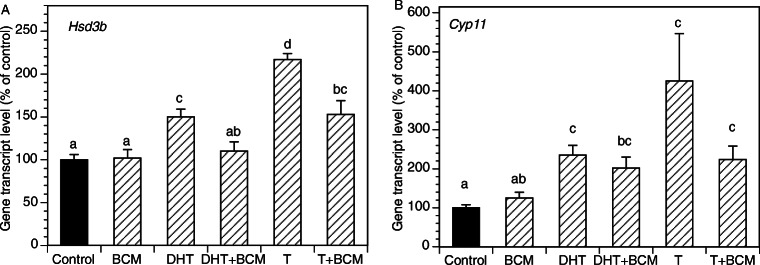
Figure 2 presents the time course of the effects of the exposure of GCs to DHT (10 μM, 2–48 h) on the expression of key genes relevant to progesterone production. The greatest effects were observed at 48 h with a significant increase of mRNA expression of Hsd3b and CYP11. Subsequent experiments evaluated androgen effects across a wide range of DHT and T concentrations (Fig. 3). DHT significantly increased Hsd3b at 100 nM to 10 μM range (by up to 3.3-fold (P < 0.0001); DHT also increased Cyp11 at 100 nM to 10 μM range by up to 3.7-fold (P < 0.0001). T significantly increased Hsd3b expression at all concentrations tested by up to 5.1-fold (P < 0.0001); T also significantly increased Cyp11 expression at concentrations ranging from 10 nM to 10 μM by up to 11.1-fold (P < 0.0001). Figure 4 presents effects of androgens on progesterone accumulation in the conditioned culture media. Both androgens induced concentration-dependent effects with up to a 4.7-fold increase of progesterone in response to DHT at 10 μM (P < 0.0001) and a 606-fold increase in response to T at 10 μM (P < 0.0001). To test whether the effects of androgens on Cyp11 and Hsd3b were mediated by a direct action on androgen receptors, GCs were exposed to DHT, T, and/or the nonsteroidal anti-androgen bicalutamide (BCM). BCM partly abrogated DHT and T-mediated Hsd3b upregulation but had no significant effect on Cyp11 (Figs. 5a and b).
Fig. 2.
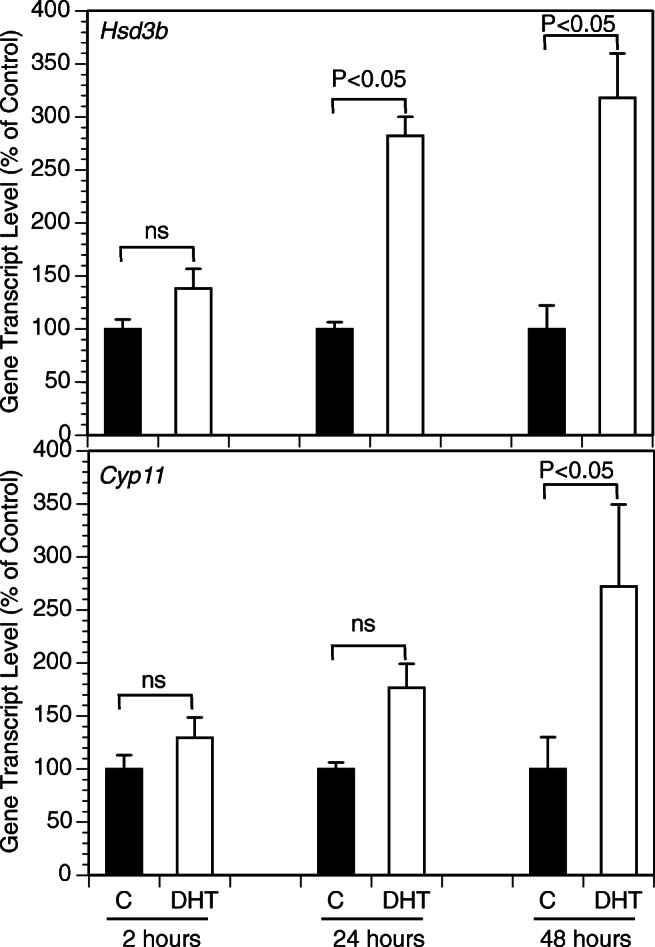
Time course of effects of DHT on mRNA expression of select genes by GCs cultured for 2, 24, and 48 h in the absence (C) or in the presence of DHT (10 μM). GCs were plated in fibronectin-coated 24-well plates and incubated in chemically defined media. Each bar represents mean ± SEM; significant differences are denoted by P-values above brackets
Fig. 3.
Effects of various concentrations of DHT (A, left panel) and T (B, right panel) on mRNA expression of Hsd3b and Cyp11 by GCs. All cultures were carried out for 48 h in chemically defined media. Each bar represents mean ± SEM; means significantly different from cultures in the absence of androgens (Control) are denoted by * (P < 0.05) or ** (P < 0.01)
Fig. 4.
Effects of various concentrations of DHT (A, left panel) and T (B, right panel) on progesterone level in culture media. All cultures were carried out for 48 h in chemically defined media. Each bar represents mean ± SEM; means significantly different from cultures in the absence of androgens (Control) are denoted by ** (P < 0.01)
Fig. 5.
Effects of an anti-androgen BCM on basal and androgen-induced mRNA expression of Hsd3b and Cyp11 by GCs. All cultures were carried out for 48 h in chemically defined media. Each bar represents mean ± SEM; means with no superscripts in common are significantly different (P < 0.05)
Effects of Estradiol and Antiestrogen on GCs
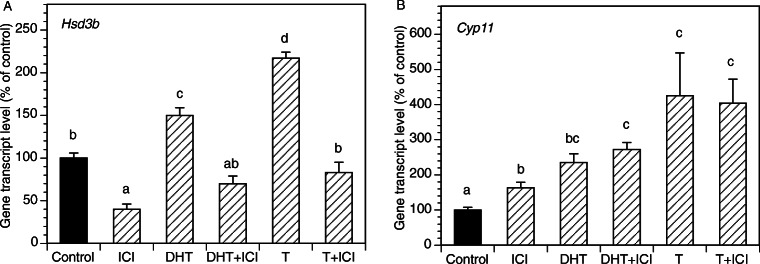
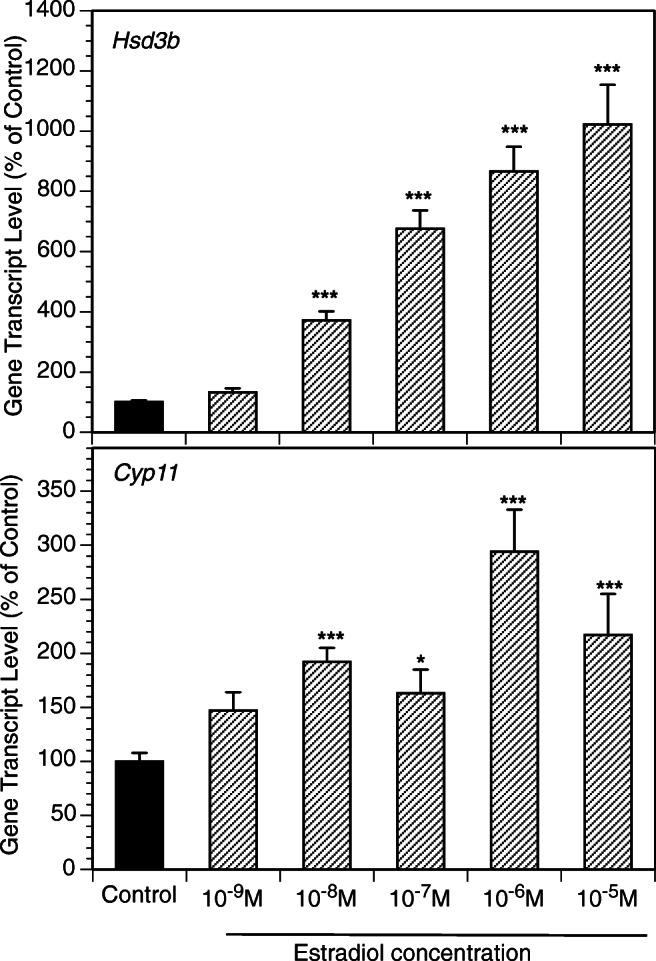
E2 at 10 nM-10 μM significantly increased Cyp11 and Hsd3b expression (Fig. 6). To determine whether actions of androgens on expression of Cyp11 and Hsd3b were mediated by estrogen receptors, GCs were cultured with DHT, T, and/or the antiestrogen ICI (Fig. 7). ICI decreased basal Hsd3b expression and entirely reversed androgen mediated Hsd3b upregulation. In contrast, ICI had a slight stimulatory effect on Cyp11 expression and had no effect on androgen-induced Cyp11 expression.
Fig. 6.
Effects of various concentrations of E2 on mRNA expression of Hsd3b and Cyp11 by GCs. All cultures were carried out for 48 h in chemically defined media. Each bar represents mean ± SEM; means significantly different from cultures in the absence of androgens (control) are denoted by * (P < 0.05), ** (P < 0.01), or *** (P < 0.001)
Fig. 7.
Effects of an anti-androgen ICI on basal and androgen-induced mRNA expression of Hsd3b and Cyp11 by GCs. All cultures were carried out for 48 h in chemically defined media. Each bar represents mean ± SEM; means with no superscripts in common are significantly different (P < 0.05)
Discussion
The results of this study demonstrate that TCs exert paracrine actions on GCs by increasing mRNA expression of several key genes involved in the regulation of progesterone production. Furthermore, both aromatizable (T) and non-aromatizable (DHT) androgens greatly stimulate progesterone production by GCs while significantly upregulating expression of Hsd3b and Cyp11 transcripts. This effect appears to be mediated by two mechanisms: directly via androgen receptors and indirectly via E2 receptors. These findings extend previous studies by Ross et al. [7, 8] and provide a mechanistic explanation for androgen-induced progesterone production by isolated rat granulosa cells.
Recently, Stocco and colleagues observed that in the rat granulosa cells, androgens induced expression of Cyp11 and Cyp19 [11]. Our data confirm and extend this report and demonstrate that androgens not only increase Cyp11 transcripts, but also markedly upregulate mRNA expression of Hsd3b, a key enzyme involved in the production of progesterone. Like Stocco, we also found that T led to a greater increase in gene transcription than DHT. Interestingly, Orisaka et al. evaluated early antral follicles in cattle and found that TCs had no significant effect on GC progesterone production or transcription of Cyp11 and Hsd3b [12].
Our observations provide new insight into the actions of androgens on GCs and the regulation of progesterone production. Progesterone is an important steroid intermediate and is produced in greatest amounts by the corpus luteum after ovulation. This increase in luteal progesterone production is primarily driven by an LH-mediated increase in StAR expression [13]. The findings of this study reveal that progesterone production can also be driven independently of LH through a novel, androgen-mediated increase in Cyp11 and Hsd3b transcription. Our results are consistent with previous studies that have demonstrated co-culture of GCs with TCs is associated with increased androgen production by TCs [14, 15]. This putative additional mechanism of progesterone production may be relevant to anovulatory conditions, such as in women with polycystic ovary syndrome, who exhibit hyperandrogenemia [16]. Small antral follicles from these individuals have been shown to hypersecrete progesterone [16]. This may account for higher serum progesterone levels in PCOS women compared to women with normal androgen levels [17]. On a cautionary note, the present study was performed on murine cells, and it is possible that responses of granulosa cells to androgens may vary across different species.
In summary, the results of this study demonstrate an interaction between GCs and TCs in the regulation of steroidogenesis. Our findings provide further evidence for the role of paracrine factors in the regulation of follicular function independent of gonadotropins. In particular, our work suggests that androgen plays a direct stimulatory role in the production of progesterone. Further work is needed to investigate the mechanisms underlying these actions of androgens and to evaluate this phenomenon in GCs of different maturity and in the presence of gonadotropins.
Funding Information
This work was supported by NCTRI Center Grant P50 HD012303 and by T32 Training Grant HD007203.
Footnotes
Summary Sentence
In the rat granulosa cells, androgens increase expression of Hsd3b and Cyp11 and stimulate progesterone production.
References
- 1.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the 'two-cell, two-gonadotrophin' model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-X. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Andoh K, Hagiwara H, Xiaowei L, Kikuchi N, Abe Y, Yamada K, Fatima R, Mizunuma H. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology. 2001;142:4930–4936. doi: 10.1210/endo.142.11.8482. [DOI] [PubMed] [Google Scholar]
- 3.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403. doi: 10.1210/me.2010-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113:27–33. doi: 10.1530/jrf.0.1130027. [DOI] [PubMed] [Google Scholar]
- 6.Monniaux D. Driving folliculogenesis by the oocyte-somatic cell dialog: lessons from genetic models. Theriogenology. 2016;86:41–53. doi: 10.1016/j.theriogenology.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Lucky AW, Schreiber JR, Hillier SG, Schulman JD, Ross GT. Progesterone production by cultured preantral rat granulosa cells? Stimulation by androgens. Endocrinology. 1977;100:128–133. doi: 10.1210/endo-100-1-128. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SG, Knazek RA, Ross GT. Androgenic stimulation of progesterone production by granulosa cells from preantral ovarian follicles: further in vitro studies using replicate cell cultures. Endocrinology. 1977;100:1539–1549. doi: 10.1210/endo-100-6-1539. [DOI] [PubMed] [Google Scholar]
- 9.Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. doi: 10.1242/dev.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology. 1988;122:2345–2347. doi: 10.1210/endo-122-5-2345. [DOI] [PubMed] [Google Scholar]
- 11.Wu YG, Bennett J, Talla D, Stocco C. Testosterone, not 5alpha-dihydrotestosterone, stimulates LRH-1 leading to FSH-independent expression of Cyp19 and P450scc in granulosa cells. Mol Endocrinol. 2011;25:656–668. doi: 10.1210/me.2010-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orisaka M, Mizutani T, Tajima K, Orisaka S, Shukunami K, Miyamoto K, Kotsuji F. Effects of ovarian theca cells on granulosa cell differentiation during gonadotropin-independent follicular growth in cattle. Mol Reprod Dev. 2006;73:737–744. doi: 10.1002/mrd.20246. [DOI] [PubMed] [Google Scholar]
- 13.Devoto L, Kohen P, Vega M, Castro O, González RR, Retamales I, Carvallo P, Christenson LK, Strauss JF. Control of human luteal steroidogenesis. Mol Cell Endocrinol. 2002;186:137–141. doi: 10.1016/S0303-7207(01)00654-2. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Qiao P, Jiang A, Jiang J, Han H, Wang L, Ren C. Paracrine regulation of steroidogenesis in theca cells by granulosa cells derived from mouse preantral follicles. Biomed Res Int. 2015;2015:925691. doi: 10.1155/2015/925691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang YD, McTavish KJ, Chang RJ, Shimasaki S. Paracrine regulation of theca androgen production by granulosa cells in the ovary. Fertil Steril. 2013;100:561–567. doi: 10.1016/j.fertnstert.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumesic DA, Abbott DH. Implications of polycystic ovary syndrome on oocyte development. Semin Reprod Med. 2008;26:53–61. doi: 10.1055/s-2007-992925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosencrantz MA, Wachs DS, Coffler MS, Malcom PJ, Donohue M, Chang RJ. Comparison of inhibin B and estradiol responses to intravenous FSH in women with polycystic ovary syndrome and normal women. Hum Reprod. 2010;25:198–203. doi: 10.1093/humrep/dep373. [DOI] [PMC free article] [PubMed] [Google Scholar]