Abstract
Background
Initial recommendations discouraged high flow nasal cannula (HFNC) in COVID-19 patients, driven by concern for healthcare worker (HCW) exposure. Noting high morbidity and mortality from early invasive mechanical ventilation, we implemented a COVID-19 respiratory protocol employing HFNC in severe COVID-19 and HCW exposed to COVID-19 patients on HFNC wore N95/KN95 masks. Utilization of HFNC increased significantly but questions remained regarding HCW infection rate.
Methods
We performed a retrospective evaluation of employee infections in our healthcare system using the Employee Health Services database and unit records of employees tested between March 15, 2020 and May 23, 2020. We assessed the incidence of infections before and after the implementation of the protocol, stratifying by clinical or non-clinical role as well as inpatient COVID-19 unit.
Results
During the study period, 13.9% (228/1635) of employees tested for COVID-19 were positive. Forty-six percent of infections were in non-clinical staff. After implementation of the respiratory protocol, the proportion of positive tests in clinical staff (41.5%) was not higher than that in non-clinical staff (43.8%). Of the clinicians working in the high-risk COVID-19 unit, there was no increase in infections after protocol implementation compared with clinicians working in COVID-19 units that did not use HFNC.
Conclusion
We found no evidence of increased COVID-19 infections in HCW after the implementation of a respiratory protocol that increased use of HFNC in patients with COVID-19; however, these results are hypothesis generating.
Keywords: COVID-19, High flow nasal cannula, Health care worker infections
1. Introduction
Early treatment recommendations for patients with COVID-19 discouraged noninvasive oxygenation methods for respiratory support, including non-invasive ventilation (NIV) and high flow nasal cannula (HFNC), instead favoring early endotracheal intubation [1,2]. This was largely driven by concerns regarding health care workers' (HCW) exposure to aerosolized SARS-CoV-2. In response, many hospitals restrict HFNC and NIV use despite emerging evidence that non-invasive modalities effectively prevented intubation in many patients and that aerosolization of SARS-CoV-2 from HFNC may be minimal [[3], [4], [5], [6]].
Between April 3, 2020, and April 7, 2020 we implemented a multidisciplinary respiratory protocol for patients with suspected or confirmed COVID-19) across the healthcare system. The protocol (Supplementary Materials) increased use of HFNC for patients admitted to the hospital with COVID-19, utilizing non-negative pressure rooms in a dedicated COVID-19 intermediate care (COVID Intercare) unit [7]. Staff safety and infection rates are critical metrics in the evaluation of COVID-19 interventions [8]. The objective of this study was to explore whether the increased the use of HFNC and NIV also resulted in an increased rate of COVID-19 infections in HCWs.
2. Methods
2.1. Study design
We performed a retrospective evaluation of an existing Employee Health Services (EHS) database at XXX, an 800-bed tertiary care center with over 12,000 clinical and non-clinical employees. We obtained data for all employees tested for COVID-19 between March 15, 2020 and May 23, 2020. Beginning March 14, 2020, all symptomatic employees were referred to EHS for reverse transcriptase-polymerase chain reaction (RT-PCR) nasopharyngeal swab for SARS-CoV-2 (Roche COBAS 6800). The database also included employees tested during any hospital visit. Available data included the date of the test result, department of employment, and job title but did not include demographic information. To triangulate our data, we obtained numbers of positive tests and dates for clinical staff who worked in the COVID-19 Intercare unit and were exposed to COVID-19 patients using HFNC/NIV and numbers of positive tests for staff working in a similar sized COVID-19 unit where HFNC/NIV were not used. The medical director of Hospital Medicine and unit leadership independently kept records of hospitalists and staff on the COVID-19 units who were diagnosed with COVID-19. The XXX Institutional Review Board determined our evaluation was not human subjects research. We adhered to the SQUIRE reporting standards [9].
2.2. Measurements
Cases were listed on the day the result was reported. In mid-March the time to result for outpatient testing was 3–5 days, which shortened to 1–2 days in early April. To account for batching of tests, we used 3-day averages of positive tests. We stratified employees as “clinical,” involving direct patient interactions, or “non-clinical.” Nursing, physicians, advanced practitioners, respiratory therapists, and “clinical support staff” were categorized as clinical, while administrative support, information technology, “trades/engineering,” behavioral health, finance, and contractors were categorized as non-clinical. Not all staff classified as “clinical” treated COVID-19 patients; however, some were redeployed to COVID-19 units, thus it's likely some clinical staff did not have in-hospital exposures. We classified cases as pre-implementation or post-implementation of the respiratory protocol. Tests that resulted after April 7 were considered post-implementation.
2.3. Outcomes
We report descriptive data of the incidence of positive tests before and after the implementation of the respiratory protocol, when HFNC use increased, stratified by clinical or non-clinical role. We report cases over time in relation to the COVID-19 daily census. We also compare the number of employees who tested positive from the dedicated COVID-19 Intercare unit with the number of employees who tested positive from the acute care COVID-19 unit which did not use HFNC and NIV.
2.4. Analysis
To estimate community prevalence and account for the risk of an increasing daily census of COVID-19 positive patients, we compared the daily COVID-19 inpatient census during the study period (median 100, IQR, 82–145) to the number of COVID-19 cases in employees and stratified by clinical or non-clinical positions. We compared the proportion of clinical staff testing positive post-intervention to the proportion of non-clinical staff testing positive. We estimate that with a median of 15 patients/day on HFNC/ NIV (range 12–23), any given day would have resulted in the exposure of 35 staff members across all shifts. With 45 days of exposure, we would expect to see an increase in cases if these exposures were causing infection.
3. Results
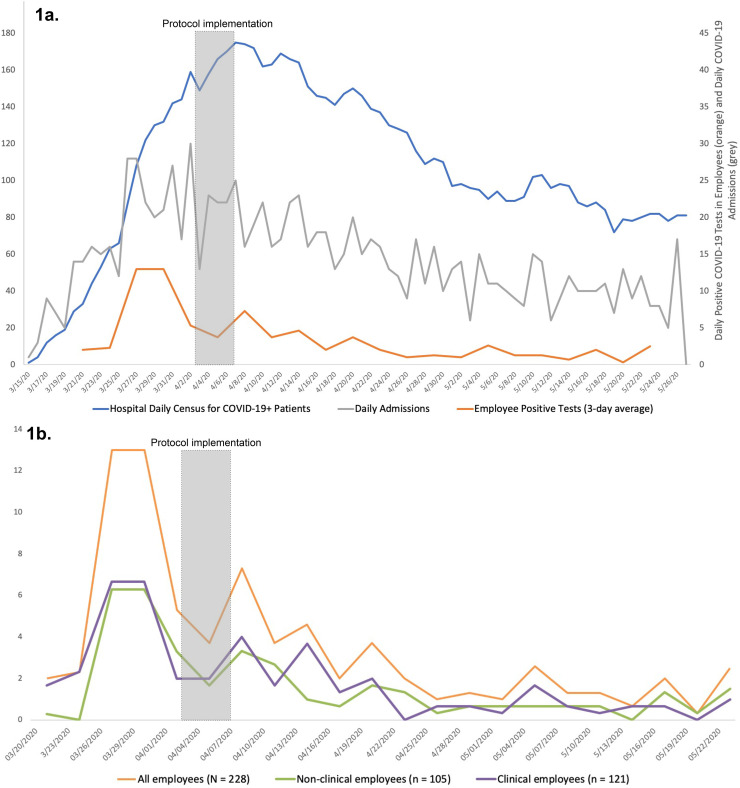
From March 15, 2020 through May 26, 2020, 13.9% (228/1635) of employees tested were positive for COVID-19. Employee cases peaked in March, during the peak of COVID-19 at the hospital level, and were decreasing during protocol implementation (4/3/20-4/7/20). After implementation, employee cases continued to decrease, mirroring the daily hospital COVID-19 census trend (Fig. 1a).
Fig. 1.
Trends in COVID-19 infections in hospital employees.
a. Comparison of trend in daily hospital census for patients with COVID-19 (bluet), daily admissions (grey*), and employee positive rate (orange*).
tScale on left.
*Scale on right.
b. Graph of 3-day average positive tests for employees, separated by clinical versus non- clinical department.
Of the 228 confirmed infections, 105 (46%) were in non-clinical staff and 123 (54%) were in clinical staff (Fig. 1b). For non-clinical staff 43.8% (46/105) of positive tests resulted after protocol implementation whereas for clinical staff 41.5% (51/123) of positive tests resulted during that same period.
Of the 79 staff members (nurses and patient care technicians (PCTs)) exposed to HFNC/NIV in the COVID-19 Intercare unit, 2 tested positive, one during the pre-intervention period (3/24/20) and one during the post-intervention period (4/18/20). Of the 67 staff members in the general COVID-19 unit (no HFNC/NIV use) 4 tested positive, three during the pre-intervention period (3/26/20, 4/3/20, 4/5/20) and one during the post intervention period (5/18/20). The hospitalist service had 2 COVID-19 cases, one in the designated group of 20 hospitalists that exclusively cared for patients with COVID-19 (4/26/20), and one in the “non-COVID-ist” group of 50 hospitalists (3/25/20).
4. Discussion
Guided by early research highlighting the dangers of early intubation and ability of non-invasive ventilation methods to prevent intubation in many patients, we implemented a protocol that increased the use of HFNC in patients with severe COVID-19. Despite initial concerns for increased transmission of SARS-CoV-2 in patients using HFNC/NIV, we did not find evidence of an increase in employee infection rates in clinical staff compared to non-clinical staff, in dedicated COVID-19 hospitalists compared to non-COVID-19 hospitalists, or in nursing staff working in the HFNC/NIV COVID-19 unit compared to a similar unit with no HFNC/NIV. Rather, clinical and non-clinical employee infection rates appeared to parallel the community transmission of COVID-19.
Our study reports employee COVID-19 testing results in a health system with over 45 days experience using HFNC/NIV in patients admitted with COVID-19. Further, our study benefits from an integrated employee health record, as well as independent triangulation and verification of infection rates in clinicians with confirmed contact with patients with COVID-19.
It is important to note that our airway protocol was designed with safety measures to prevent potential transmission of SARS-CoV-2. First, clinical staff caring for COVID-19 patients were universally instructed to wear full personal protective equipment and N95/KN95 masks during aerosolizing procedures throughout the study period. N95/KN95s became mandatory for all care of patients with COVID-19 on April 7, 2020. Additionally, admitted patients requiring HFNC/NIV were cohorted into a single unit to limit staff exposure, although HFNC/NIV were also used in the emergency department and critical care units. Finally, the protocol required patients receiving HFNC to wear standard surgical masks to limit droplet spread; however, in practice this often did not happen. It remains unclear which safety measures, if any, may have helped to limit SARS-CoV-2 transmission. However, these safety measures do not require specialized equipment or training to implement.
5. Limitations
Our findings should be interpreted in the context of several limitations. As in other regions, our hospital struggled with access to PPE; however, staff caring for COVID-19 patients wore N95s/KN95s during aerosolizing procedures throughout the study period. HFNC may be less safe for HCWs in a facility without appropriate PPE [10]. Second, hospital infection control policies changed during the course of the study. Universal masking was “encouraged” on March 28 but was not mandatory until April 7. Additionally, employee temperature screening began April 8, which could have increased the number of employees tested. Although we found no increase in SARS-CoV-2 transmission, the number of providers with direct contact with patients with COVID-19 on HFNC was small – 20 hospitalists and less than 70 nursing staff. Though larger studies are needed to confirm the safety of HFNC/NIV for hospital employees, we hope our results, combined with appropriate safety measures, may encourage other institutions to consider HFNC/NIV for patients admitted with COVID-19.
6. Conclusion
We are reassured that, at our institution, a respiratory protocol that included HFNC and NIV, combined with appropriate PPE and cohorting precautions, did not lead to a measurable increase in symptomatic COVID-19 infections in our HCWs; however, our results should be viewed as hypothesis generating and additional research on this topic is needed.
Funding sources
Dr. Soares is supported by a K08 from NIDA (1K08DA045933-01).
Dr. Schoenfeld is supported by a K08 from AHRQ (5K08HS025701-02).
Dr. Westafer is supported by a K12 from the NHLBI (1K12HL138049-01).
Author contributions
ES, LW, VM, WS conceived the study. DS collected the data. ES, LW, WS designed the study. ES analyzed the data. ES, LW drafted the manuscript and all authors contributed substantially to its revision.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajem.2020.09.086.
Appendix A. Supplementary data
Supplementary material
References
- 1.Brown C.A., Mosier J.M., Carlson J.N., Gibbs M.A. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020:80–84. doi: 10.1002/emp2.12063. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH . 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is Suspected; pp. 1–21. [Google Scholar]
- 3.Colla J., Rodos A., Seyller H., Weingart S. Fighting COVID-19 hypoxia with one hand tied behind our back: blanket prohibition of high flow oxygen and non-invasive positive end-expiratory pressure in United States hospitals. Ann Emerg Med [Internet] 2020 doi: 10.1016/j.annemergmed.2020.04.015. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson S., Gottlieb D. Breaking news: what’s working for COVID-19 patients in the epicenter. Emerg Med News. 2020;42(5):37–49. [Google Scholar]
- 5.Ding L., Wang L., Ma W., He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(28) doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonard S., Volakis L., DeBellis R., Kahlon A., Mayar S. 2020. TRANSMISSION ASSESSMENT REPORT: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19 [Internet]. Vapotherm Blog; pp. 1–25.https://content.vapotherm.com/hubfs/COVID-19 Transmission Assessment Report.pdf [cited 2020 Mar 25]. Available from: [Google Scholar]
- 7.Westafer L.M., Elia T., Medarametla V., Lagu T. A Transdisciplinary COVID-19 early respiratory intervention protocol: an implementation story. 2020;15(6):1–3. doi: 10.12788/jhm.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean S.A. A critical COVID metric: your ED staff infection rate. Acad Emerg Med. 2020;27(4):341–342. doi: 10.1111/acem.13974. [DOI] [PubMed] [Google Scholar]
- 9.Ogrinc G., Davies L., Goodman D., Batalden P., Davidoff F., Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf [Internet] 2016;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. http://www.ncbi.nlm.nih.gov/pubmed/26369893 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artenstein A.W. In pursuit of PPE. N Engl J med [internet] 2020;382(18):e46. doi: 10.1056/NEJMc2010025. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material