Abstract
Atypical teratoid rhabdoid tumor (AT/RT) is a highly aggressive central nervous system embryonic tumor predominantly occurring in infants and young children. The AT/RT can occur in the cerebellopontine angle and cause facial nerve palsy as a presenting sign. We present a case of a 2-year-old girl with a cerebellopontine angle tumor who initially presented with acute facial palsy. Later, asubsequent diagnosis of AT/RT was made.
Keywords: Atypical teratoid rhabdoid tumor, cerebellopontine angle, acoustic nerve, facial nerve palsy
Introduction
Atypical teratoid rhabdoid tumors (AT/RTs) are highly malignant central nervous system (CNS) neoplasm predominantly occurring in infants or young children younger than 3 years. AT/RTs are a type of embryonic CNS tumor demonstrating histologic and immunohistochemical evidence of polyphenotypic differentiation with neuroectodermal, epithelial, and mesenchymal lines [1]. AT/RT corresponds histologically to WHO grade IV neoplasms that carry a poor prognosis with a 3-year overall survival rate of 22% [1,2]. AT/RTs can occur in both supra- and infra-tentorial regions including cerebral hemispheres, ventricular system, suprasellar region, pineal gland, cerebellar hemispheres, brain stem, and cerebellopontine angle (CP angle) [3,4]. With AT/RT in the CP angle it is relatively difficult for cytoreductive surgery to decrease tumor burden, because the CP angle contains many critical structures [5]. Moreover, a more common and frequent lesion in CP angle, schwannoma, might be assumed and would delay treatment. Thus, preoperative differential diagnosis is important for surgical planning and prognostication. Here we report a case of a 2-year-old girl who presented with facial nerve palsy. Subsequent diagnosis was a CP angle AR/RT.
Case report
A 2-year-old girl presented with acute left facial palsy and hearing loss 4 months before visiting our hospital with an initial diagnosis of viral infection. Administration of anti-viral agents and steroids did not relieve the symptoms. In our hospital, brain computed tomography (CT) and magnetic resonance imaging (MRI) examinations were performed and the results revealed a 1.6 × 1.5 cm solid mass in the left CP angle with widened internal auditory meatus (Figure 1A, 1B). The initial radiological impression was benign schwannoma arising from acoustic nerve. She underwent a craniectomy to obtain a biopsy specimen of the lesion.
Figure 1.
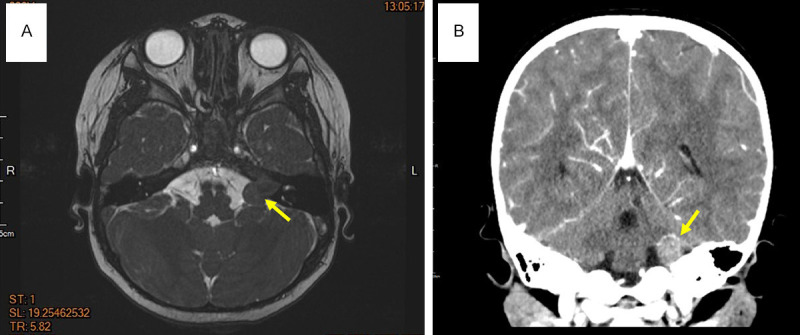
Brain MRI and CT images showed a 1.6 × 1.5 cm heterogeneously enhancing tumor in the left CP angle cistern with extension into the left internal acoustic canal. (A) Transverse view (MRI) (yellow arrow), and (B) coronal view (CT) (yellow arrow).
Histopathologically, the tumor showed high cellularity and was composed of primitive small blue round cells with abundant eosinophilic cytoplasm, eccentrically located nuclei containing vesicular chromatin, and conspicuous eosinophilic nucleoli (Figure 2A). Immu-nohistochemically, tumor cells were strongly diffuse positive for vimentin, and focally positive for smooth muscle actin (SMA) (Figure 2B, 2C), while negative for lymphoma marker CD45, rhabdomyosarcoma marker MyoD1, and Ewing sarcoma/PNET (primitive neuroectodermal tumor) marker NKX2.2 (not shown). The Ki-67 proliferation index was high at about 45% (not shown). Pathognomonic immunostain was the complete loss of INI1/SMARCB1 in tumor cells (Figure 2D), supporting the diagnosis of AT/RT.
Figure 2.
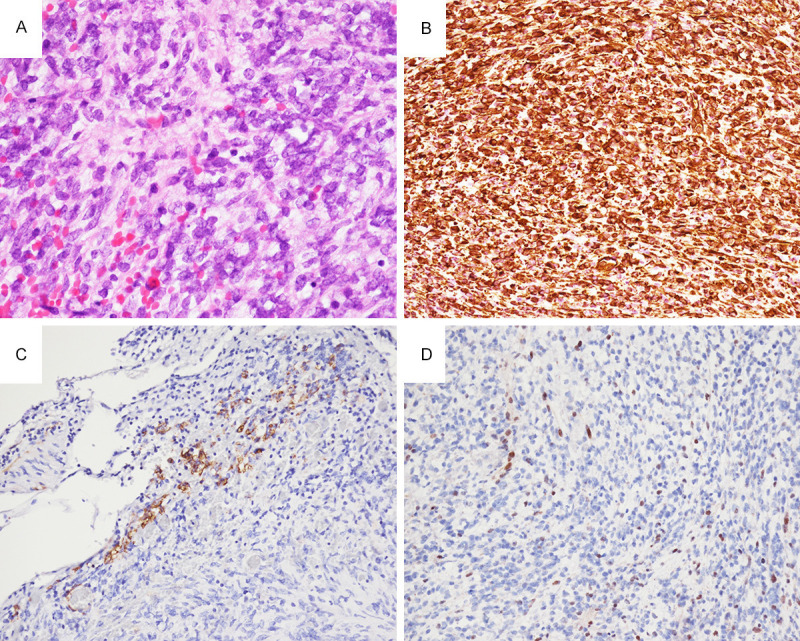
(A) Hematoxylin and eosin stain of the tumor showed primitive small round to rhabdoid cells with abundant eosinophilic cytoplasm, vesicular chromatin, and conspicuous eosinophilic nucleoli. Tumor cells strongly express vimentin (B), and focally express smooth muscle actin (C). (D) Loss of expression of INI1/SMARCB1 in nuclei of tumor cells, but with retained expression in intratumoral lymphocytes and endothelial cells of blood vessel (200 ×).
The patient recovered well from the surgery and was transferred to pediatric oncology department for the subsequent chemotherapy plan. Further physical survey revealed the dissemination of AT/RT tumor cells in cerebrospinal fluid. She subsequently received systemic chemotherapy including cisplatin and vincristine regime and was in good condition one year after the surgery.
Discussion
AT/RTs account for approximately 1-2% of pediatric brain tumors but are estimated to account for > 10% of CNS tumors in infants due to the preponderance of cases in children < 3 years [6,7]. On the other hand, cerebellopontine angle tumors in young children are infrequent and accounting for less than 3% of pediatric brain tumors [8]. The clinical presentation of AT/RT is variable and depending on the age of the patient and the size and location of the tumor. Infants are more often present with non-specific signs of vomiting, lethargy or failure to thrive. More specific signs include head tilt and cranial nerve palsy, most commonly sixth and seventh paresis [1]. A series study showed up to 46% of patients with AT/RT present with cranial nerve palsies as the initial symptom [9]. The nature of AT/RT as it relates to specific cranial nerves such as facial nerve is unknown.
The exact histogenesis of AT/RT is not well understood. Although the cell of origin in AT/RTs is unclear, it has been suggested that they derive from pluripotent fetal cells since neural, epithelial, and mesenchymal markers can all be expressed [10,11]. AT/RTs can occur sporadically or as part of a rhabdoid tumor predisposition syndrome [6]. Mutation or loss of INI1/SMARCB1 locus at 22q11.2 is the genetic hallmark of AT/RT [12]. The INI1/SMARCB1 protein is one component of the mammalian SWI/SNF complex, which functions as an ATP-dependent chromatin remodeler and regulates a plethora of gene expressions [13]. Loss of INI1/SMAR-CB1 results in widespread but specific deregulation of genes and pathways associated with cell cycle, cell survival, differentiation and the epigenome [14,15].
The differential diagnosis in young children with CP angle tumors, according to several large case series, commonly includes medulloblastoma, schwannoma, ependymoma, and astrocytoma [8,16,17]. However, no specific symptoms are correlated to pathologic findings on the above studies. Siu et al. reported that in three cases, acute facial nerve palsy is highly associated with CP angle AT/RT, indicating acute facial nerve neuropathy may serve as an adjunct that should raise the suspicion for AT/RT in CP angle lesions in children [5]. Albeit rare, clinicians and pathologists should be aware of the acuity of facial nerve deficits as this may raise the concern for AT/RT in the preoperative differential diagnosis and could guide the treatment course and prompt implementation of a multimodal therapy plan as early as possible.
Disclosure of conflict of interest
None.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. 4th edition. Lyon: IARC; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Hoff K, Hinkes B, Dannenmann-Stern E, von Bueren AO, Warmuth-Metz M, Soerensen N, Emser A, Zwiener I, Schlegel PG, Kuehl J, Frühwald MC, Kortmann RD, Pietsch T, Rutkowski S. Frequency, risk-factors and survival of children with atypical teratoid rhabdoid tumors (AT/RT) of the CNS diagnosed between 1988 and 2004, and registered to the German HIT database. Pediatr Blood Cancer. 2011;57:978–985. doi: 10.1002/pbc.23236. [DOI] [PubMed] [Google Scholar]
- 3.Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, Walter AW, Rorke LB, Biegel JA. Central Nervous system atypical teratoid rhabdoid tumor: results of therapy in children enrolled in a registry. J. Clin. Oncol. 2004;22:2877–2884. doi: 10.1200/JCO.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 4.Tekautz TM, Fuller CE, Blaney S, Fouladi M, Broniscer A, Merchant TE, Krasin M, Dalton J, Hale G, Kun LE, Wallace D, Gilbertson RJ, Gajjar A. Atypical teratoid/rhabdoid tumors (AT/RT) improve survival in children 3 years of age and older with radiation therapy and high-does alkylator-based chemotherapy. J. Clin. Oncol. 2005;23:1491–1499. doi: 10.1200/JCO.2005.05.187. [DOI] [PubMed] [Google Scholar]
- 5.Siu A, Lee M, Rice R, Myseros JS. Association of cerebellopontine angle atypical teratoid/rhabdoid tumors with acute facial nerve palsy in infants. J Neurosurg Pediatr. 2014;13:29–32. doi: 10.3171/2013.10.PEDS13292. [DOI] [PubMed] [Google Scholar]
- 6.Biegel JA. Molecular genetics of atypical teratoid/rhabdoid tumor. Neurosurg Focus. 2006;20:E11. doi: 10.3171/foc.2006.20.1.12. [DOI] [PubMed] [Google Scholar]
- 7.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 8.Zuccaro G, Sosa F. Cerebellopontine angle lesions in children. Childs Nerv Syst. 2007;23:177–183. doi: 10.1007/s00381-006-0208-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen ML, McComb JG, Krieger MD. Atypical teratoid/rhabdoid tumors of the central nervous system: management and outcomes. Neurosurg Focus. 2005;18:E8. [PubMed] [Google Scholar]
- 10.Bouffard JP, Sandberg GD, Golden JA, Rorke LB. Double immunolabeling of central nervous system atypical teratoid/rhabdoid tumors. Mod Pathol. 2004;17:679–683. doi: 10.1038/modpathol.3800099. [DOI] [PubMed] [Google Scholar]
- 11.Ota S, Crabbe DC, Tran TN, Triche TJ, Shimada H. Malignant rhabdoid tumor. A study with two established cell lines. Cancer. 1993;71:2862–2872. doi: 10.1002/1097-0142(19930501)71:9<2862::aid-cncr2820710930>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 13.Roberts CW, Orkin SH. The SWI/SNF complex-chromatin and cancer. Nat Rev Cancer. 2004;4:133–142. doi: 10.1038/nrc1273. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Cimica V, Ramachandra N, Zagzag D, Kalpana GV. Aurora A is a repressed effector target of chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011;71:3225–3235. doi: 10.1158/0008-5472.CAN-10-2167. [DOI] [PubMed] [Google Scholar]
- 15.Tsikitis M, Zhang Z, Edelman W, Zagzag D, Kalpana GV. Genetic ablation of Cyclin D1 abrogates genesis of rhabdoid tumors resulting from Ini1 loss. Proc Natl Acad Sci U S A. 2005;102:12129–12134. doi: 10.1073/pnas.0505300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phi JH, Wang KC, Kim IO, Cheon JE, Choi JW, Cho BK, Kim SK. Tumors in the cerebellopontine angle in children: warning of a high probability of malignancy. J Neurooncol. 2013;112:383–391. doi: 10.1007/s11060-013-1067-9. [DOI] [PubMed] [Google Scholar]
- 17.Tsai MH, Wong AM, Jaing TH, Wang HS, Hsueh C, Wu CT. Treatment of cerebellopontine angle tumors in children: a single institution’s experience. J Pediatr Hematol Oncol. 2009;21:832–834. doi: 10.1097/MPH.0b013e3181acd842. [DOI] [PubMed] [Google Scholar]


