Abstract
Objective: Epidemiologic studies have shown that, compared to men, women have better prognoses in chronic renal diseases. This indicates that gender hormones may have some effect on renal function. The present study aimed to investigate the expression and relationship with prognosis of estrogen receptor alpha (ERα) in renal tissue of immunoglobulin A nephropathy (IgAN) patients. Methods: Expression of ERα was detected by immunohistochemistry. Specimens were collected from forty-five IgAN patients and five normal healthy participants. In addition to expression, the relationship of ERα with clinical data, prognosis, and the degree of glomerular damage were analyzed. Results: ERα was expressed in the glomeruli and renal tubules. The expression of ERα in the glomeruli of IgAN renal tissue decreased gradually (P = 0.001, P < 0.05) with the increasing severity of disease and its expression was associated with estimated glomerular filtration rate (eGFR), serum creatinine (Scr) and pathological grade (r = 0.876, -0.818 and -0.736, P < 0.05). In addition, the expression of ERα was significantly decreased in hypertensive IgAN patients compared with the non-hypertensive group (P = 0.011). We found that ERα was of great significance for the prognosis of IgAN (P = 0.01, P < 0.05). Conclusions: The expression of ERα in the renal tissue of IgAN patients was reduced and there was a correlation with the degree of kidney damage and prognosis, suggesting that ERα may play a role in the progression of IgAN.
Keywords: Estrogen receptor alpha, immunoglobulin A nephropathy, immunohistochemistry, kidney disease, prognosis
Introduction
Epidemiological studies have shown that the incidence of chronic renal failure in male patients with kidney disease is higher than in females. However, the proportion of postmenopausal women has increased significantly [1,2]. It has been estimated that the number of postmenopausal women with end-stage kidney disease (ESKD) is increasing by greater than 4% each year [3]. During the physiologic process, the glomerular filtration rate (GFR) declined faster in males than in females between 20 and 50 years of age [4]. In a mouse model of adenine-induced renal failure, there was a significantly greater reduction of ERα in male mice than in female mice, accompanied by renal impairment. However, modulating the ERα-mediated pathway with Tamoxifen in vitro significantly ameliorated renal tubulointerstitial fibrosis [5,6]. Addressing the crucial role of gender, both in humans and animals, for chronic disease, we investigated ERα expression in renal tissue and the relationship with the prognosis in IgAN, since IgAN is a common chronic primary glomerulonephritis and a regular cause of chronic renal failure.
Materials and methods
Specimen selection
Tissue samples from 45 patients with IgAN who were hospitalized at the Second Affiliated Hospital of Nanjing Medical University between January 2004 to December 2005 were selected for inclusion in the present study. Those with kidney disease caused by allergic purpura, systemic lupus erythematosus, or nodular vasculitis were excluded through clinicopathologic diagnosis. 5 normal, healthy cases were chosen from resected tissues adjacent to renal cell carcinoma.
Clinical data
Patient medical history, status, and laboratory results were recorded.
Pathologic changes of renal tissue and evaluation measures
The samples were divided into Lee’s grades I (11 cases), II (10 cases), III (8 cases), IV (9 cases), and V (7 cases). The glomeruli and renal tubular areas were counted in each specimen. The cultured cells and positive cells were averaged from 15 non-overlapping fields, including 5 glomerular regions, 5 proximal tubule regions, and 5 distal tubule regions chosen randomly on microscopic slides. We evaluated the results by combining stain strength and percentage of positive cells. The samples were scored according to the degree of staining, referring to the literature, where 0 was no staining, 1 was weak staining, 2 was moderate staining and 3 was strong staining. Similarly, the percentage of positive cells was scored, where 0 referred to no positive cells, a score of 1 was < 1%, 2 included 1-10% positive cells, 3 from 10-30%, 4 from 30-66% and 5 above 66% [7,8]. The result was based on the sum of these two scores. Pathologic readings were carried out by two experienced pathologists. In order to resolve incongruent scoring, the pathologists reached a consensus by using a double-headed microscope together.
Immunohistochemical detection of ERα
For pathologic analysis, 4 μm-thick renal sections were cut from the paraffin blocks. DAB immunohistochemistry staining was performed using the ZSGB-BIO System (ZLI9018, Beijing, China) for renal immunohistochemical assays. The slides were incubated with a primary antibody against ERα (1:200; Abcam, Cambridge, UK).
Follow-up
Patients were monitored for 5 years until ESRD (eGFR < 15 mL/min per 1.73 m2 or renal replacement therapy (RRT) for at least 3 months) or until death.
Statistical analysis
Data were reported as mean ± standard deviation or median (quartile spacing). Statistical analysis of data was performed with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Comparisons among groups were made with analysis of variance (ANOVA) or Rank-Sum test. Spearman correlation was used for the degree and correlation of these variables. Counted data were expressed as a percentage and the chi-square (Χ2) test was used for comparisons among groups. The survival rate based on kidney disease was estimated by Kaplan-Meier method. The log-rank test was used to compare the survival curves. The Cox proportional hazards model was established to analyze the risk factors affecting the prognosis of the kidney. P < 0.05 was considered significant.
Results
Distribution of ERα in normal renal tissue
Immunohistochemical staining of human renal biopsies showed ERα protein in both the glomeruli and renal tubules (Figure 1A).
Figure 1.
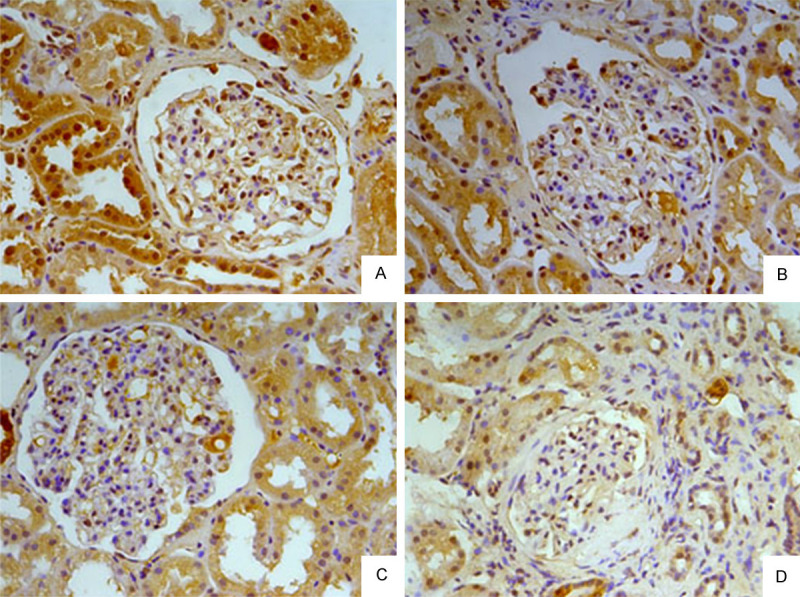
Distribution of ERα in renal tissue. A. Normal renal tissue. B. IgAN renal tissue of Lee’s grade II. C. IgAN renal tissue of Lee’s grade III. D. IgAN renal tissue of Lee’s grade V.
Distribution of ERα in IgAN renal tissue
The expression of ERα was markedly reduced in IgAN renal tissue, particularly in the glomeruli. In grade I lesions, ERα levels were similar to normal (P > 0.05). With the increasing severity of the disease, glomerular ERα gradually lessened (P = 0.001) (Figure 1B-D; Table 1).
Table 1.
ERα expression in different pathologic types of kidney (score, x̅ ± s)
| Kidney pathology type | Numbers | Glomerular ERα | Tubule ERα |
|---|---|---|---|
| Normal group | 5 | 6.40 ± 0.55 | 6.60 ± 0.55 |
| I grade | 11 | 6.18 ± 1.40 | 6.27 ± 1.19 |
| II grade | 10 | 5.60 ± 1.26 | 5.50 ± 1.08 |
| III grade | 8 | 4.88 ± 0.64 | 6.38 ± 1.30 |
| IV grade | 9 | 3.89 ± 1.36 | 6.33 ± 1.66 |
| V grade | 7 | 2.14 ± 0.38 | 4.57 ± 1.51 |
Clinical data
Of the 45 patients with IgAN, 13 were male, 32 were female and the average age was 37.93 ± 11.53 years. The cases of IgAN were divided into Lee’s grades I (n = 11), II (n = 10), III (n = 8), IV (n = 9), V (n = 7). There were differences in hemoglobin, urea, creatinine, and the glomerular filtration rates in the patients with different histologic grades at the time of diagnosis. However, there were no differences in urinary protein, urinary erythrocyte count, or serum albumin among the different histologic grades. As the lesion progressed, glomerular filtration rate decreased significantly (Table 2).
Table 2.
Analysis of clinical data in different pathologic types
| Lee I | Lee II | Lee III | Lee IV | Lee V | P | |
|---|---|---|---|---|---|---|
| (n = 11) | (n = 10) | (n = 8) | (n = 9) | (n = 7) | ||
| Age (year) | 31.82 ± 8.40 | 30.60 ± 7.40 | 40.63 ± 9.77 | 46.33 ± 12.81 | 44.14 ± 11.25 | 0.003 |
| male, n (%) | 3 (27.27) | 4 (40) | 1 (12.5) | 3 (33.33) | 2 (28.57) | 0.782 |
| Upro (g/24 h) | 0.9 (0.13-10.2) | 0.54 (0.08-4.6) | 0.3 (0.08-10) | 0.8 (0.07-11) | 2.3 (0.1-5.36) | 0.471 |
| RBC (million/ml) | 80 (3-1012) | 21.5 (0-95) | 100 (0-220) | 43 (6-560) | 14 (5-1200) | 0.450 |
| ALB (g/L) | 40 (14.8-47) | 42 (22.2-48) | 43.35 (25.3-50.4) | 38.5 (19-47) | 35.9 (18.7-44.4) | 0.134 |
| Hb (g/L) | 124.82 ± 16.63 | 137.90 ± 20.48 | 135.75 ± 13.37 | 125.78 ± 14.54 | 101.14 ± 18.69 | 0.001 |
| BUN (mmol/L) | 4.38 ± 1.11 | 5.38 ± 0.92 | 5.18 ± 1.60 | 6.88 ± 2.78 | 9.48 ± 2.09 | 0.000 |
| Scr (µmmol/L) | 70 (44.7-82) | 81.6 (62.3-105.6) | 89 (42.8-102.9) | 98.5 (66-155) | 209 (140-309) | 0.000 |
| eGFR (ml/min) | 113.58 ± 44.32 | 86.32 ± 9.69 | 76.69 ± 28.37 | 65.41 ± 23.41 | 31.93 ± 12.55 | 0.000 |
| hypertension, n (%) | 2 (18.18) | 5 (50) | 5 (62.5) | 3 (33.33) | 4 (57.14) | 0.274 |
RBC: urine sediment red cell blood count; ALB: albumin; Hb: hemoglobin; BUN: usea nitrogen.
Relationship between ERα and clinical data
Expression of ERα in the glomeruli was positively correlated with the estimated glomerular filtration rate (eGFR) (r = 0.876, P < 0.01) and negatively related to Scr and the pathologic grade of IgAN (r = -0.818, -0.736; P < 0.01). Glomerular ERα was decreased significantly in IgAN patients with hypertension (P = 0.011, P < 0.01; Table 3). ERα expression and pathologic grade of IgAN had no relevance to urine protein (r = 0.091, 0.172; P > 0.05). The pathologic grade of IgA nephropathy was positively correlated with Scr and negatively correlated with eGFR (r = 0.757, -0.781; P < 0.05).
Table 3.
Relationship between ERα expression and hypertension (score, x̅ ± s)
| Numbers | Glomerular ERα | |
|---|---|---|
| hypertensive group | 21 | 4.0 ± 1.82 |
| non-hypertensive group | 24 | 5.38 ± 1.47 |
Survival analysis
The forty-five IgAN patients were followed up for 12-60 months (median 60 months). A total of 8 (17.8%) patients were admitted to ESRD or died and 2 patients ceased participation in the follow-up examinations. Because of the small number of cases, we divided them into two groups of Lee’s grade I-III and IV-V for survival analysis, which showed a significant difference in renal survival between the two groups (P < 0.01) (Figure 2).
Figure 2.
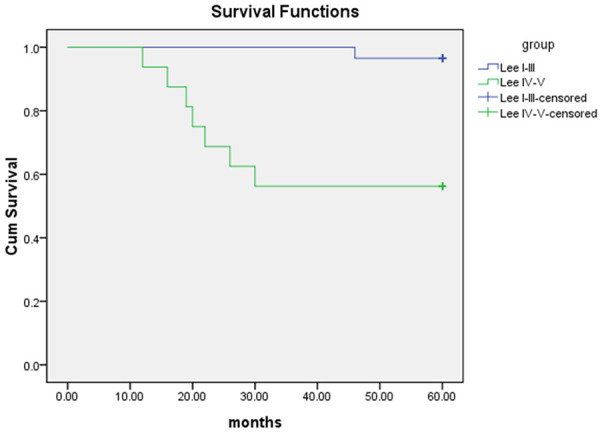
Survival curve of patients with IgAN.
The clinical and pathologic data of patients were analyzed by single-factor Cox regression, showing that low hemoglobin levels (Hb < 90 g/L), eGFR < 60 ml/min, pathologic grade (Lee’s grade I-III and IV-V groups), glomerular ERα (score 1-4 and 5-8 groups) and tubule ERα (score 1-4 and 5-8 groups) correlated with renal survival (Table 4). Multivariate Cox regression analysis showed that pathologic grade (HR 12.690, P < 0.05) and glomerular ERα expression (HR 0.059, P < 0.05) were independent factors affecting the prognosis of patients with IgAN.
Table 4.
Factors affecting the prognosis of patients with IgAN
| Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|
|
|
|
|||
| X2 | P | HR (95% CI) | P | |
| hypertension | 0.2 | 0.655 | ||
| Low Hb (< 90 g/L) | 13.949 | 0.000 | ||
| Low ALB (< 35 g/L) | 0.190 | 0.633 | ||
| eGFR < 60 ml/min | 19.40 | 0.000 | ||
| Pathologic grade | 12.751 | 0.000 | 12.690 (1.762-91.387) | 0.012 |
| Glomerular ERα | 11.206 | 0.001 | 0.059 (0.007-0.511) | 0.01 |
| Tubule ERα | 17.787 | 0.000 | ||
In the univariate Cox regression, pathologic grade was divided into two groups of Lee’s grade I-III and IV-V; Glomerular ERα and tubule ERα were divided into two groups of score 1-4 and 5-8.
Discussion
While clinical and epidemiologic studies have provided growing evidence for a substantial gender difference in the progression of chronic glomerular diseases, the current report suggests a protective effect of ER on IgAN.
ERs are members of the nuclear receptor (NR) superfamily. They have been identified as two subtypes, ERα and ERβ. Studies have shown that ERs are significantly expressed in glomerular podocytes, mesangial cells, and renal tubular epithelial cells [9-11]. Research in cultured mouse podocytes showed that ERα existed both in the cytoplasm and in the nucleus. Short-term (24 h) stimulation of estrogen induced an increase of nuclear ERα, while long-term (> 48 h) stimulation increased cytoplasmic expression [12]. Our previous study showed that Tamoxifen, an agonist of estrogen receptor, inhibited the expression of α-smooth muscle actin and ameliorated renal interstitial fibrosis [13].
In human renal biopsies, immunohistochemical staining showed the ERα protein had cytoplasmic and nuclear localization. The expression of ERα in IgAN renal tissue was significantly decreased, especially in the glomeruli. With increasing severity of the lesion, glomerular ERα gradually decreased, and our further analysis showed a certain correlation between the expression of ERα and pathologic grade of IgAN together with eGFR. Based on the above results, it could be concluded that ERα played a role in the development of IgAN, but the specific mechanism remains to be explored further.
Increasingly, recent studies have shown that estrogen regulates blood pressure by promoting vasodilation, reducing the inflammatory response, regulating oxidative stress, and regulating the activity of the renin-angiotensin-aldosterone system [14-16]. Our study also showed that, compared with IgA nephropathy patients without hypertension, those with hypertension often had significantly reduced glomerular ERα expression, suggesting that ERα may be involved in the regulation of blood pressure.
Through Cox risk analysis, we found that renal pathology and ERα expression were of great significance in the prognosis of IgAN. From a single-center study in Korean adults, the author found that when IgAN patients were categorized as Lee’s classification grade I/II/III and grade IV/V, grade IV/V was associated with a significantly increased risk of ESRD. Histopathologic classifications should be developed to predict renal outcome [17]. By utilizing a refined version of H. S. Lee’s glomerular grading system in IgAN, research indicated that glomerular grade was an independent prognostic factor for progressive renal disease [18]. Because of the small sample size, we also divided the pathologic classification into two categories, grade I/II/III and grade IV/V, for survival analysis. Compared to grade I/II/III, the prognosis of grade IV/V patients was poor. This was consistent with previous results.
Though the complexity of the physiologic and clinical interaction between female sex hormones and kidney function remains poorly understood, evidence suggests that estrogen has renoprotective effects. For example, ovariectomized glomerulosclerosis-prone ROP Os/þ mice developed more severe glomerular lesions and renal dysfunction than did age-matched female controls [19]. Several findings in humans suggested that postmenopausal hormone therapy and selective estrogen receptor modulators may play beneficial roles with respect to kidney function. The selective estrogen receptor modulator raloxifene has been associated with renoprotection in women with kidney disease [20,21]. Other animal model data also reported a renoprotective role for estrogen [22-26]. In summary, we infer that ER has renal protective effects both in people and in animals, which also supports our result that ER expression was associated with the degree and prognosis of IgAN. The specific mechanism of ER in IgAN requires further exploration, in multicenter studies with a sample size larger than ours, to verify our results.
Disclosure of conflict of interest
None.
References
- 1.Turin TC, Tonelli M, Manns BJ, Ahmed SB, Ravani P, James M, Hemmelgarn BR. Lifetime risk of ESRD. J Am Soc Nephrol. 2012;23:1569–1578. doi: 10.1681/ASN.2012020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 3.Xue JL, Ma JZ, Louis TA, Collins AJ. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12:2753–2758. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- 4.Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006;21:2577–2582. doi: 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- 5.Diwan V, Small D, Kauter K, Gobe GC, Brown L. Gender differences inadenine-induced chronic kidney disease and cardiovascular complicationsin rats. Am J Physiol Renal Physiol. 2014;307:1169–78. doi: 10.1152/ajprenal.00676.2013. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, Lee AS, Jung YJ, Yang KH, Lee S, Park SK, Kim W, Kang KP. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor α-mediated transforming growth factor-β1/Smad signaling pathway. Nephrol Dial Transplant. 2014;29:2043–53. doi: 10.1093/ndt/gfu240. [DOI] [PubMed] [Google Scholar]
- 7.Rizzardi AE, Johnson AT, Vogel RI, Pambuccian SE, Henriksen J, Skubitz AP, Metzger GJ, Schmechel SC. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn Pathol. 2012;7:42. doi: 10.1186/1746-1596-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, Iwata T, Onitsuka T, Yasumoto K. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J. Clin. Oncol. 2009;27:411–7. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 9.Yu WJ, Zhao B, Zhong HZ, et al. Expression and significance of estrogen receptor alpha in renal tissue of IgA nephropathy. Journal of Chinese Physician. 2016;18:1316–9. [Google Scholar]
- 10.Burris D, Webster R, Sheriff S, Faroqui R, Levi M, Hawse JR, Amlal H. Estrogen directly and specifically downregulates NaPi-IIa through the activation of both estrogen receptor isoforms (ERα and ERβ) in rat kidney proximal tubule. Am J Physiol Renal Physiol. 2015;308:F522–34. doi: 10.1152/ajprenal.00386.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svenson J, Cunningham M, Dasgupta S, Gilkeson GS. Estrogen receptor alpha modulates mesangial cell responses to toll-like receptor ligands. Am J Med Sci. 2014;348:492–500. doi: 10.1097/MAJ.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 12.Kummer S, Jeruschke S, Wegerich LV, Peters A, Lehmann P, Seibt A, Mueller F, Koleganova N, Halbenz E, Schmitt CP, Bettendorf M, Mayatepek E, Gross-Weissmann ML, Oh J. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PLoS One. 2011;6:e27457. doi: 10.1371/journal.pone.0027457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao G, Xu HY, Yang C, et al. Effect of Tamoxifen on interstitial fibrosis in UUO rats. Acta Univ Med Nanjing. 2007;27:480–2. [Google Scholar]
- 14.Sandberg K, Ji H. Is β the α dog in estrogen receptor-mediated protection from hypertension? Hypertension. 2013;61:1153–4. doi: 10.1161/HYPERTENSIONAHA.113.01191. [DOI] [PubMed] [Google Scholar]
- 15.Prabhushankar R, Krueger C, Manrique C. Membrane estrogen receptors: their role in blood pressure regulation and cardiovascular disease. Curr Hypertens Rep. 2014;16:408. doi: 10.1007/s11906-013-0408-6. [DOI] [PubMed] [Google Scholar]
- 16.Arias-Loza PA, Muehlfelder M, Pelzer T. Estrogen and estrogen receptors in cardiovascular oxidative stress. Pflugers Arch. 2013;465:739–46. doi: 10.1007/s00424-013-1247-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Yi SH, Seo MS, Hyun JN, Jeon JS, Noh H, Han DC, Hwang SD, Jin SY, Kwon SH. Validation of the Oxford classification of IgA nephropathy: a single-center study in Korean adults. Korean J Intern Med. 2012;27:293–300. doi: 10.3904/kjim.2012.27.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, Park SY, Han JS, Kim S, Lee JS. Histological grading of IgA nephropathy predicting renal outcome: revisiting H. S. Lee’s glomerular grading system. Nephrol Dial Transplant. 2005;20:342–8. doi: 10.1093/ndt/gfh633. [DOI] [PubMed] [Google Scholar]
- 19.Elliot SJ, Karl M, Berho M, Potier M, Zheng F, Leclercq B, Striker GE, Striker LJ. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol. 2003;162:1441–8. doi: 10.1016/S0002-9440(10)64277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumanski SM, Ramesh S, James MT, Metcalfe A, Nerenberg K, Seely EW, Robertson HL, Ahmed SB. The effect and safety of postmenopausal hormone therapy and selective estrogen receptor modulators on kidney outcomes in women: a protocol for systematic review and meta-analysis. Syst Rev. 2017;6:134. doi: 10.1186/s13643-017-0519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed SB, Ramesh S. Sex hormones in women with kidney disease. Nephrol Dial Transplant. 2016;31:1787–95. doi: 10.1093/ndt/gfw084. [DOI] [PubMed] [Google Scholar]
- 22.Stringer KD, Komers R, Osman SA, Oyama TT, Lindsley JN, Anderson S. Gender hormones and the progression of experimental polycystic kidney disease. Kidney Int. 2005;68:1729–39. doi: 10.1111/j.1523-1755.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 23.Catanuto P, Doublier S, Lupia E, Fornoni A, Berho M, Karl M, Striker GE, Xia X, Elliot S. 17beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009;75:1194–201. doi: 10.1038/ki.2009.69. [DOI] [PubMed] [Google Scholar]
- 24.Özdemir Kumral ZN, Kolgazi M, Üstünova S, Kasımay Çakır Ö, Çevik ÖD, Şener G, Yeğen BÇ. Estrogen receptor agonists alleviate cardiac and renal oxidative injury in rats with renovascular hypertension. Clin Exp Hypertens. 2016;38:500–9. doi: 10.3109/10641963.2015.1116550. [DOI] [PubMed] [Google Scholar]
- 25.Mao S, Xu H, Zou L, Xu G, Wu Z, Ding Q, Jiang H. Estrogen preserves split renal function in a chronic complete unilateral ureteral obstruction animal model. Exp Ther Med. 2014;7:1555–62. doi: 10.3892/etm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichii O, Nakamura T, Irie T, Kouguchi H, Sotozaki K, Horino T, Sunden Y, Elewa YHA, Kon Y. Close pathological correlations between chronic kidney disease and reproductive organ-associated abnormalities in female cotton rats. Exp Biol Med. 2018;243:418–27. doi: 10.1177/1535370218758250. [DOI] [PMC free article] [PubMed] [Google Scholar]


