Abstract
Phyllodes tumor of the breast is a rare fibroepithelial lesion characterized by a propensity for local recurrence and distant metastasis. Its histologic classification, based on morphology, mitotic index and tumor margin, includes malignant, borderline, and benign. These tumors show similar cellular morphology, which may contribute to difficulty in classifying them histologically and in prediction of their clinical behavior. Thus, the identification of new biomarkers detectable also by in situ methods has become indispensable. Paralogous HOX13 genes (HOX A13, HOX B13, HOX C13 and HOX D13) play a relevant role in tumor development and progression in particular in breast cancer. In this study we analyzed the immunohistochemical expression of paralogous HOX13 homeoproteins on a phyllodes tumor case series to validate their usefulness in histologic classification.
Keywords: Paralogous 13 HOX genes, breast phyllodes tumors, diagnostic marker
Introduction
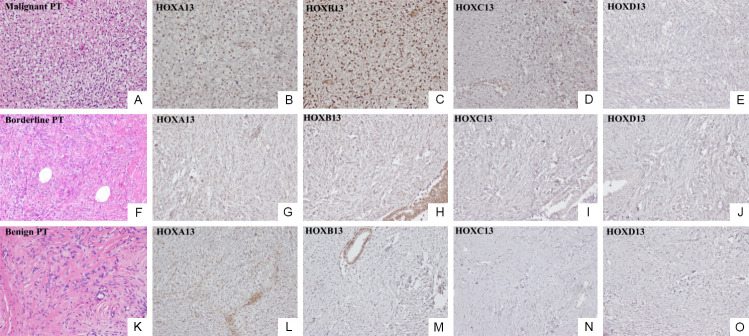
Phyllodes tumor (PT) of the breast is a rare fibroepithelial neoplasm, accounting for 0.3% to 1% of all breast tumors and characterized by a propensity for local recurrence and distant metastasis [1]. Morphologically, PT is characterized by prominent leaf-like fronds, but it may exhibit histological features similar to the more common benign fibroadenoma. Both fibroadenoma and PT are biphasic breast tumors harboring both a mesenchymal and an epithelial component. During PT tumor evolution the loss of stromal-epithelial interdependency, leads to an increase of stromal proliferation/transformation that becomes prevalent. The histologic classification of PT proposed by the World Health Organization (WHO) [2] graded them into malignant (Figure 1A), borderline (Figure 1F), and benign (Figure 1K) based on morphology (stromal cellularity, nuclear atypia, stromal overgrowth), mitotic index and tumor margin. Clinically, benign phyllodes tumors do not show a propensity to metastasize, but they may recur locally. Borderline tumors show a higher local recurrence ability and may rarely metastasize, while malignant tumors show higher local recurrence and distant metastasis propensity [1].
Figure 1.
Phyllodes tumors of the breast: (A) Malignant tumor: H&E staining (20×); (B) HOXA13, (C) HOXB13, (D) HOXC13, and (E) HOXD13 IHC expression (20×); (F) Borderline tumor: H&E staining (20×); (G) HOXA13, (H) HOXB13, (I) HOXC13, and (J) HOXD13 IHC expression (20×); (K) Benign tumor: H&E staining (20×); (L) HOXA13, (M) HOXB13, (N) HOXC13, and (O) HOXD13 IHC expression (20×).
Whereas it is fairly easy to distinguish fibroadenoma from PT, benign, borderline and malignant PTs showed similar cellular morphology and a documented genetic heterogeneity, which may contribute to difficulty in classifying them histologically and in prediction of their clinical behavior. In addition, since the diagnosis of these lesions is performed in the first instance on micro-histologic samples (core biopsy) considering only the morphological characteristics, the absence of specific biomarkers makes the differential diagnosis very difficult. This led to the production of a series of molecular studies for the identification of differentially expressed genes. Comparing expression signatures of PT and fibroadenomas revealed several genes that may be involved in clonal progression of fibroadenoma to PT, in particular overexpression of the transcriptional factor HOXC13 [3]. Moreover, the aberrant expression of another member of the paralogous 13 HOX genes family, HOXB13, in stromal cells of PT has been significantly correlated with tumor grade and histologic features and can distinguish borderline and malignant phyllodes tumors [4].
HOX genes represent key determinants of embryonic development and their deregulation was associated with structural abnormalities and different human diseases, especially cancer [5]. Paralogous HOX13 genes (HOXA13, HOXB13, HOXC13 and HOXD13) play a relevant role in tumor development and progression, in particular in breast cancer [6].
Although the genomic landscapes of PT provided insights into molecular pathogenesis of PT and help to improve diagnostic accuracy, also identifying potential drug targets in malignant PT, in situ methods are the most accessible in the pathology laboratory, and an immunophenotypic characterization of PTs would represent a helpful diagnostic tool.
In this study, we analyzed the immunohistochemical (IHC) expression of HOXA13, HOXB13, HOXC13, and HOXD13 homeoproteins on a PT case series to validate their usefulness in classifying phyllodes tumors.
From 2005 to 2018, 28 Formalin Fixed Paraffin Embedded (FFPE) samples from breast cancer patients were retrieved from the athology unit, at the National Cancer Institute “Giovanni Pascale Foundation” of Naples, Italy. All selected cases were phyllodes breast tumors histologically classified as benign (10 samples), borderline (10 samples), and malignant (8 samples) PT tumors. This classification focused on the degree of stromal hypercellularity, stromal cell atypia, stromal overgrowth, mitotic count, and nature of the microscopic borders.
Immunohistochemical staining was done on slides from formalin-fixed, paraffin embedded tissues to evaluate the expression of paralogous 13 HOX genes as previously described [7-9] with anti-HOXA13 antibody (Rabbit polyclonal, Abcam, dilution 1:200), anti-HOXB13 antibody (Mouse monoclonal, Abcam, dilution 1:1200), anti-HOXC13 antibody (Mouse polyclonal, Abcam, 1:50), anti-HOXD13 antibody (Rabbit polyclonal, Abcam, dilution 1:100). The sections were counterstained with hematoxylin and mounted and results were interpreted by two pathologists (GB and MDB) using a light microscope. For each sample, at least five fields (inside the tumor and in the peripheral areas) and >500 cells were analysed. Using a semi-quantitative scoring system microscopically and referring to each antigen scoring method in other studies, each observer evaluated the intensity, extent, and subcellular distribution. For all paralogous 13 HOX genes we only considered nuclear staining in the epithelial and stromal cells of the tumor. Staining intensity was graded as follow: absent, weak, moderate, and intense (0, +/-, + and ++).
In all analyzed cases, HOXA13 IHC expression had a similar trend in malignant, borderline, (Figure 1G) and benign (Figure 1L) PTs; only an upregulation (++) was observed in tumor stomal cells of malignant PTs (Figure 1B). HOXB13 showed an overexpression (++) in tumor stromal cells of malignant PTs (Figure 1C), a low expression (-/+) in borderline PTs (Figure 1H), and absence of expression in tumor stromal cells of benign PTs (Figure 1M), as previously demonstrated [4]. A high HOXC13 protein expression was predominantly present only in malignant PT (Figure 1D). For HOXA13, HOXB13, and HOXC13 markers, weak expression was present in epithelial cells of all three PT types. HOXD13 was silent in all PTs analyzed (Figure 1E, 1J, 1O).
These results highlighted an important role of the paralogous 13 HOX genes, in particular HOXA13, HOXB13, and HOXC13, in PT pathogenesis, suggesting the possibility of using an immunohistochemical panel (Table 1) to discriminate mainly malignant from borderline PTs. The use of these markers could be useful not only to classify phyllodes tumors but also to predict the biologic evolution of these tumors since their prognostic value is amply demonstrated in different solid tumors [10].
Table 1.
Schematic representation of paralogous 13 HOX proteins’ IHC expression in epithelial and tumor stromal cells of phyllodes tumors
| HOXA13 | HOXB13 | HOXC13 | HOXD13 | |
|---|---|---|---|---|
| Malignant PT | Epithelial cells + | Epithelial cells + | Epithelial cells + | Epithelial cells - |
| Tumor stromal cells ++ | Tumor stromal cells ++ | Tumor stromal cells ++ | Tumor stromal cells - | |
| Borderline PT | Epithelial cells + | Epithelial cells + | Epithelial cells - | Epithelial cells - |
| Tumor stromal cells + | Tumor stromal cells -/+ | Tumor stromal cells - | Tumor stromal cells - | |
| Benign PT | Epithelial cells + | Epithelial cells + | Epithelial cells - | Epithelial cells - |
| Tumor stromal cells + | Tumor stromal cells - | Tumor stromal cells - | Tumor stromal cells - |
Disclosure of conflict of interest
None.
References
- 1.Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, Brogi E, Calvo JP, Dabbs DJ, Ellis IO, Eusebi V, Farshid G, Fox SB, Ichihara S, Lakhani SR, Rakha EA, Reis-Filho JS, Richardson AL, Sahin A, Schmitt FC, Schnitt SJ, Siziopikou KP, Soares FA, Tse GM, Vincent-Salomon A, Tan PH. Phyllodes tumours of the breast: a consensus review. Histopathology. 2016;68:5–21. doi: 10.1111/his.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press; 2012. pp. 143–7. [Google Scholar]
- 3.Kuijper A. Pathogenesis and progression of fibroepithelial breast tumors. The Netherlands: Utrecht University; 2006. [Google Scholar]
- 4.Chong LY, Cheok PY, Tan WJ, Thike AA, Allen G, Ang MK, Ooi AS, Tan P, Teh BT, Tan PH. Keratin 15, transcobalamin I and homeobox gene Hox-B13 expression in breast phyllodes tumors: novel markers in biological classification. Breast Cancer Res Treat. 2012;132:143–51. doi: 10.1007/s10549-011-1555-6. [DOI] [PubMed] [Google Scholar]
- 5.Cillo C, Cantile M, Faiella A, Boncinelli E. Homeobox genes in normal and malignant cells. J Cell Physiol. 2001;188:161–9. doi: 10.1002/jcp.1115. [DOI] [PubMed] [Google Scholar]
- 6.Botti G, Cillo C, De Cecio R, Malzone MG, Cantile M. Paralogous HOX13 genes in human cancers. Cancers (Basel) 2019;11 doi: 10.3390/cancers11050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantile M, Scognamiglio G, La Sala L, La Mantia E, Scaramuzza V, Valentino E, Tatangelo F, Losito S, Pezzullo L, Chiofalo MG, Fulciniti F, Franco R, Botti G. Aberrant expression of posterior HOX genes in well differentiated histotypes of thyroid cancers. Int J Mol Sci. 2013;14:21727–40. doi: 10.3390/ijms141121727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatangelo F, Di Mauro A, Scognamiglio G, Aquino G, Lettiero A, Delrio P, Avallone A, Cantile M, Botti G. Posterior HOX genes and HOTAIR expression in the proximal and distal colon cancer pathogenesis. J Transl Med. 2018;16:350. doi: 10.1186/s12967-018-1725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aquino G, Franco R, Sabatino R, Mantia EL, Scognamiglio G, Collina F, Longo F, Ionna F, Losito NS, Liguori G, Botti G, Cantile M. Deregulation of paralogous 13 HOX genes in oral squamous cell carcinoma. Am J Cancer Res. 2015;5:3042–55. [PMC free article] [PubMed] [Google Scholar]
- 10.Li B, Huang Q, Wei GH. The role of HOX transcription factors in cancer predisposition and progression. Cancers (Basel) 2019;11:528. doi: 10.3390/cancers11040528. [DOI] [PMC free article] [PubMed] [Google Scholar]