Abstract
Objective
To describe the characteristics of COVID-19 patients seen in March-April and June-August 2020 in Marseille, France with the aim to investigate possible changes in the disease between these two time periods.
Methods
Demographics, hospitalization rate, transfer to intensive care unit (ICU), lethality, clinical and biological parameters were investigated.
Results
Compared to those seen in March-April, COVID-19 patients seen in June-August were significantly younger (39.2 vs. 45.3 years), more likely to be male (52.9% vs. 45.6%), and less likely to be hospitalized (10.7 vs. 18.0%), to be transferred to ICU (0.9% vs. 1.8%) and to die (0.1% vs. 1.1%). Their mean fibrinogen and D-dimer blood levels were lower (1.0 vs. 1.5 g/L and 0.6 vs. 1.1 μg/mL, respectively). By contrast, their viral load was higher (cycle threshold ≤16 = 5.1% vs. 3.7%).
Conclusions
Patients in the two periods did not present marked age and sex differences, but markers of severity were undoubtedly less prevalent in the summer period, associating with a 10 times decrease in the lethality rate.
Keywords: COVID-19, Seasonality, Severity markers, Lethality
Since its emergence in China at the end of 2019, SARS-CoV-2 spread worldwide with more than 29 million cases of COVID-19 reported, as of 15 September 2020. Currently, most cases have been reported from the US, India, Brazil and Russia (https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6). In Europe, since the beginning of the pandemic, the EU/EEA and the UK have reported 1,733,550 COVID-19 cases and 182,639 deaths, as of 2 August 2020 (10% of all cases reported worldwide). In early April, the EU/EEA and the UK reached a peak in reported cases. Then the trend declined until June to reach a plateau. however in recent weeks there has been a resurgence, although it is currently lower than the first peak which occurred in April (European Center for Disease Prevention and Control, 2020). In France, the incidence of PCR-confirmed cases peaked on early April with 50.1/100,000 and declined to less than 10/100,000 in early May, however, on late July the incidence rose again over this threshold and reached 75/1,000,000 in early September (Santé Publique France).
In Marseille, the largest city in south of France and the second largest in France, our institute adopted a strategy consisting in early and massive screening of SARS-CoV-2 infections and COVID-19 treatment using hydroxychloroquine and azithromycin, since the first case was documented. We recently described the characteristics of 3737 adult COVID-19 patients seen at our institute between 3 March and 27 April (Lagier et al., 2020). In this paper, we describe these characteristics in 743 new adult patients seen between 15 June and 15 August with the aim to investigate possible changes in the disease between these two time periods.
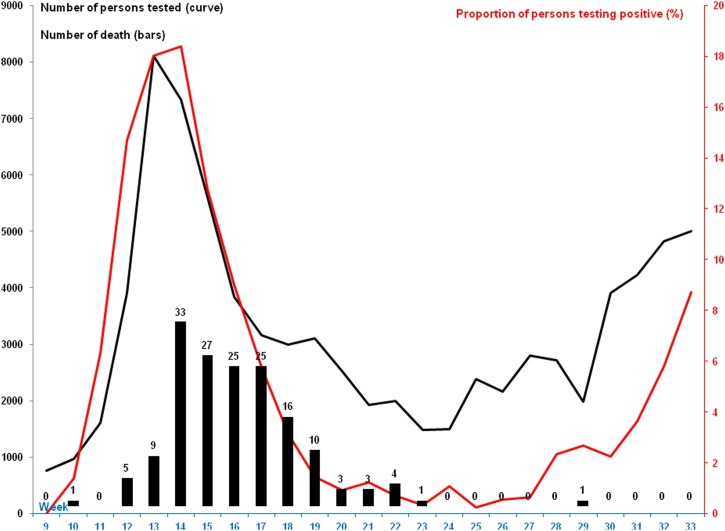
Starting from early March, our institute proposed massive testing of any person presenting at our facility regardless of whether or not they had COVID-19 symptoms. On 16 March, the WHO Director-General called for testing every suspected COVID-19 case (World Health Organization, 2020). In France, the generalization of SARS-CoV-2 PCR testing was officially authorized by the Ministry of Health on 4 May (Ministère des Solidarités et de la Santé, 2020). The number of individuals newly-tested at our institute for SARS-CoV-2 infection by PCR sharply increased from 765 per week in late February (week 9) up to 8105 per week at the peak at the end of March (week 13), and then progressively decreased to 1481 patients per week in early June (week 24) (Figure 1 ). It then increased again to reach 5007 per week in mid August (week 33). Interestingly, the proportion of individuals testing positive paralleled this curve with a slight shift of one week later. By contrast, numbers of deaths peaked to 33 per week during week 14 and progressively decreased to reach 0 week 24.
Figure 1.
Number of persons tested for SARS-CoV-2 (black curve), proportion testing positive (red curve) and number of deaths (black bars) in COVID-19 patients per week, at Méditerranée Infection Institute.
Compared to patients seen during late winter and spring, those seen in summer were significantly younger with a lower proportion of patients aged ≥65 years, and were more likely to be male, although differences were not marked (Table 1 ). No significant differences were seen with regards to anosmia and agueusia prevalence, according to period of study. Hospitalization rate, proportion of patients transferred to ICU, and lethality rates were significantly lower in patients seen in summer than in those seen earlier. The mean Ct value of positive PCR results was significantly lower in patients seen in summer than in those seen earlier, with a proportion of patients with high viral load (Ct≤ 16) tending to be higher in summer. Lymphocyte and platelet counts and fibrinogen and D-dimer levels were significantly lower in patients seen in summer as compared to those seen earlier.
Table 1.
Characteristics of COVID-19 patients seen at Méditerannée Infection Institute according to seasons.
| Late winter and spring 3 March-27 April | Summer 15 June-15 August | P valuea | |
|---|---|---|---|
| N patients | 3737 | 768 | |
| Mean age | 45.3 years Sd=16.8 |
39.2 years Sd = 17.3 |
<2.2 10−16 |
| <65 years ≥65 years | 3284 (87.9%) 453 (12.1%) |
696 (90.6%) 72 (9.4%) |
0.0358 |
| Male sex | 1704 (45.6%) | 406 (52.9%) | 0.00028 |
| Hospitalization | 673 (18.0%) | 82 (10.7%) | 9.499 10−7 |
| Transfert to ICU | 67 (1.8%) | 7 (0.9%) | 0.048 (unilat.) |
| Death (all patients) Death (under 60 years) | 41 (1.1%) 0 (0%) |
1 (0.1%) 0 (0%) |
0.0098 (unilat.) |
| Anosmia | 1442/3676 (39.2%) | 249/644 (38.7%) | 0.7931 |
| Agueusia | 1389/3676 (37.8%) | 255/644 (39.6%) | 0.4032 |
| CTb value ≤16 at admission Mean Sd |
113/3056 (3.7%) 25.0 5.3 |
36/700 (5.1%) 23.6 5.1 |
0.108 4.051 10−10 |
| Mean lymphocyte counts (G/L) | 1.8 Sd=0.9 |
1.6 Sd=0.8 |
1.15 10−6 |
| Mean platelet count (G/L) | 233.7 Sd=74.8 |
225.9 Sd=62.4 |
0.005 |
| Mean fibrinogen blood level (g/L) | 4.7 Sd=1.5 |
3.1 Sd = 1.0 |
< 2.2 10−16 |
| Mean D-dimer blood level (μg/mL) | 1.1 | 0.6 | 2.8 10−16 (Wilcoxon) |
Fisher exact test was used to compare differences between proportions (unilateral test used when indicated). Quantitative data means were compared using Student’s t-test or Wilcoxon’s rank test.
CT = cycle threshold.
The current resurgence of COVID-19 in Marseille that started in June shows a marked dissociation between numbers of cases and numbers of death. The number of deaths among COVID-19 patients seen at our institute did not show a significant rebound, so far, while the number of cases clearly re-increased. Since the kinetic of the proportion of individuals testing positive paralleled the total number of tested individuals, the current increase in the number of new patients is not an artifact due to an increase in the number of tested individuals.
In this report we also show that the presentation of the disease in patients seen in summer is different than that of patients seen earlier. Patients in the two periods do not present marked age and sex differences, but markers of severity are undoubtedly less prevalent in the summer period, associating with a 10 times decrease in the lethality rate from 1.1 to 0.1%. At the time of writing, it seems, as described in other diseases (Nicolle, 1939), that the start of the epidemic was characterized by higher lethality rates, in comparison with the current phase of the epidemic. This could be possibly due to mutations of the virus. The current situation of the COVID-19 epidemic seems to be similar to that of other endemic coronavirus infections (Su et al., 2016, Yin and Wunderink, 2018). Continuous analysis of epidemiological data is needed in the following months to confirm that this trend is continuous.
Author’s note
Since this analysis was completed, and as of the 30 September 2020, 3557 additional patients were diagnosed at the Institut Méditerranée Infection with 33 deaths, resulting in an overall lethality rate of 0.8% in the 4325 patients seen between 15 June and 30 September 2020.
Competing interest
The authors declare that they have no competing interests.
Funding source
None
Ethical approval
Approval was not required
References
- European Center for Disease Prevention and Control . 2020. Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK – eleventh update: resurgence of cases. [Google Scholar]
- Lagier J.C., Million M., Gautret P. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministère des Solidarités et de la Santé . 2020. Arrêté du 3 mai 2020 complétant l’arrêté du 23 mars 2020 prescrivant les mesures d’organisation et de fonctionnement du système de santé nécessaires pour faire face à l’épidémie de covid-19 dans le cadre de l’état d’urgence sanitaire : NOR : SSAZ2011117A-https://www.legifrance.gouv.fr/jo_pdf.do?id=JORFTEXT000041842843 [Google Scholar]
- Nicolle C. Presse Universitaire de France; Paris: 1939. Destin des maladies infectieuses. [Google Scholar]
- https://www.gouvernement.fr/info-coronavirus/carte-et-donnees?xtor=SEC-3-GOO-[425080454101]-S-[covid]Santé Publique France. 2020.
- Su S., Wong G., Shi W. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 16 March 2020. [Google Scholar]
- Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]