Abstract
Background
Older age and medical comorbidities are identified risk factors for developing severe coronavirus disease 2019. However, there are limited data on risk stratification, clinical and laboratory course, and optimal management of coronavirus disease 2019 in pregnancy.
Objective
Our study aimed to describe the clinical course of coronavirus disease 2019, effect of comorbidities on disease severity, laboratory trends, and pregnancy outcomes of symptomatic and asymptomatic severe acute respiratory syndrome coronavirus 2–positive pregnant women.
Study Design
This is a case series of pregnant and postpartum women who received positive test results for severe acute respiratory syndrome coronavirus 2 between March 3, 2020, and May 11, 2020, within 3 hospitals of the Yale New Haven Health delivery network. Charts were reviewed for basic sociodemographic and prepregnancy characteristics, coronavirus disease 2019 course, laboratory values, and pregnancy outcomes.
Results
Of the 1567 tested pregnant and postpartum women between March 3, 2020, and May 11, 2020, 9% (n=141) had a positive severe acute respiratory syndrome coronavirus 2 result. Hispanic women were overrepresented in the severe acute respiratory syndrome coronavirus 2–positive group (n=61; 43.8%). In addition, Hispanic ethnicity was associated with a higher rate of moderate and severe diseases than non-Hispanic (18% [11/61] vs 3.8% [3/78], respectively; odds ratio, 5.5; 95% confidence interval, 1.46–20.7; P=.01). Of note, 44 women (31.2%) were asymptomatic, 37 of whom (26.2%) were diagnosed on universal screening upon admission for delivery. Moreover, 59% (n=83) were diagnosed before delivery, 36% (n=51) upon presentation for childbirth, and 5% (n=7) after delivery. Severe disease was diagnosed in 6 cases (4.3%), and there was 1 maternal death. Obese women were more likely to develop moderate and severe diseases than nonobese women (16.4% [9/55] vs 3.8% [3/79]; odds ratio, 4.96; 95% confidence interval, 1.28–19.25; P=.02). Hypertensive disorders of pregnancy were diagnosed in 22.3% of women (17/77) who delivered after 20 weeks’ gestation. Higher levels of C-reactive protein during antepartum coronavirus disease 2019–related admission were more common in women with worse clinical course; however, this association did not reach statistical significance.
Conclusion
Coronavirus disease 2019 in pregnancy may result in severe disease and death. Hispanic women were more likely to receive a positive test result for severe acute respiratory syndrome 2 than other ethnic groups. Obesity and Hispanic ethnicity represent risk factors for moderate and severe diseases.
Key words: coronavirus, coronavirus disease 2019, Hispanic ethnicity, pregnancy, severe acute respiratory syndrome coronavirus 2
AJOG MFM at a Glance.
Why was this study conducted?
There are limited data on coronavirus disease 2019 (COVID-19) outcomes when contracted during pregnancy. In this study, we describe 141 cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnancy and postpartum period in a racially and ethnically diverse population. We sought to describe the demographics of the pregnant population with COVID-19, identify risk factors for worse clinical course, review laboratory trends, and provide perinatal outcomes.
Key findings
The overall rate of moderate and severe diseases was low in pregnant women in our series (4.3%); however, there was 1 maternal death. Hispanic women were disproportionately affected by SARS-CoV-2 compared with other racial and ethnic groups. Hispanic ethnicity and obesity were risk factors for worse clinical course.
What does this add to what is known?
Our study identifies Hispanic ethnicity and obesity as risk factors for worse clinical course of COVID-19 in pregnancy.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single-stranded RNA virus, causes coronavirus disease 2019 (COVID-19) and is responsible for a global health emergency. This pandemic has led to more than 29 million people infected and more than 925,000 deaths worldwide (as of September 14, 2020).1 This health crisis has spared no demographic, causing concern about its impact on vulnerable populations, such as pregnant women.2 , 3
Since the start of the pandemic, clinicians and researchers have steadily expanded the understanding of COVID-19 in pregnancy. However, the total number of cases reported in the literature remains limited. This study aimed to describe the clinical course of pregnant women and their neonates in a large, diverse hospital system in a markedly affected region adjacent to New York City, one of the United States’ initial infectious epicenters. Medical comorbidities and sociodemographic factors were examined for association with COVID-19 severity and clinical course. Finally, we report the laboratory trends for SARS-CoV-2–positive pregnant women admitted to the hospital.
Materials and Methods
Study population
This is a case series of all pregnant and postpartum women with positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) tests between March 3, 2020, and May 11, 2020, from 3 Yale New Haven Health hospitals (Yale New Haven, Bridgeport and Greenwich hospitals). Subjects were identified using an electronic health record (EHR) search for an open pregnancy episode and a SARS-CoV-2 RT-PCR laboratory result within the time frame. Ambulatory and inpatient testing was included. Each chart was individually reviewed for current pregnant status (positive pregnancy test with or without ultrasound confirmation) or pregnancy resolution within 6 weeks of SARS-CoV-2 test for inclusion into the study cohort. Subjects with a positive test result were included for analysis. Each case was individually reviewed to collect the following: baseline sociodemographic factors; past medical, surgical, and obstetrical history; antenatal course; and COVID-19 course including symptoms, laboratory and imaging studies, management, and maternal and neonatal outcomes. The study was approved by Yale University Institutional Review Board with a waiver of consent (HIC2000027797).
Testing and diagnosis of coronavirus disease 2019
The SARS-CoV-2 testing used RT-PCR analysis of nasopharyngeal swab specimens. Testing criteria generally consisted of either (1) patients with symptoms of COVID-19 as deemed by their healthcare provider or the institutional COVID-19 call center or (2) universal testing of all pregnant women who were admitted after April 1, 2020, for delivery or antepartum management. The testing criteria of symptomatic patients evolved during the study period and were set by institutional committees guided by Centers for Disease Control and Prevention (CDC) recommendations. Neonatal testing was indicated for all newborns born to mothers who had a positive result for SARS-CoV-2 within 2 weeks of the delivery and was performed by RT-PCR of nasopharyngeal samples between 24 and 48 hours of birth.4
Disease severity was classified per the World Health Organization (WHO) into asymptomatic (no current or previous symptoms), mild (symptomatic patients without evidence of viral pneumonia or hypoxia), moderate (clinical signs of pneumonia without signs of severe pneumonia and no need for supplemental oxygen), severe (signs of severe pneumonia, ie, respiratory rate of 30/minute or more, blood oxygen saturation of <95% [the threshold for oxygen supplementation in pregnancy], severe respiratory distress), and critical (acute respiratory distress syndrome, sepsis, or septic shock).5 Outpatient triage of the pregnant population with COVID-19 was performed per institutional guidelines (Supplemental Figure). For analysis, severe and critical diseases were combined, resulting in a total of 4 groups. The final disease severity was assigned retrospectively according to the abovementioned definitions, which were set up a priori by a panel of maternal-fetal medicine subspecialists based on the entire course of the disease.
Race and ethnicity information were self-reported at the time of hospital registration and abstracted directly from the EHR. Hypertensive disorders of pregnancy (HDP), including gestational hypertension, preeclampsia without and with severe features, eclampsia, and hemolysis, elevated liver enzymes, and low platelet count (HELLP), were identified during the individual chart review. All diagnoses were confirmed to meet the American College of Obstetricians and Gynecologists (ACOG) criteria of HDP.6 Laboratory testing guidelines for admitted patients varied among the hospitals and evolved over time. D-dimer and C-reactive protein (CRP) were chosen for analysis as the most consistently tested and trended laboratory studies. Because the occurrence of birth affects the levels of these laboratory values, we divided our cohort into the following 2 groups for the purpose of our analysis: women admitted for delivery (symptomatic and asymptomatic) and women admitted in the antepartum period and discharged undelivered.
Statistical analysis
Patient characteristics including sociodemographics, pregnancy outcomes, comorbidities, and disease severity are reported descriptively and presented as percentages of the total cohort. Continuous variables were not normally distributed and thus reported as median and interquartile range (IQR). Bivariate analysis to evaluate the association among patient characteristics, comorbidities, and disease severity was performed using the Fisher exact test. Owing to the low number of subjects in the moderate and severe groups, to further examine the association between ethnicity (Hispanic and non-Hispanic) and obesity (prepregnancy body mass index [BMI] of ≥30 and <30 kg/m2) with severity of the disease, the cohort was organized into the following 2 groups: (1) asymptomatic and mild disease; and (2) moderate and severe disease. Unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for these dichotomous measures. Adjusted ORs were unable to be calculated owing to the small sample size. Tests of association between specific symptoms and disease severity were restricted to those with symptoms (n=97). In this group, we evaluated the association between COVID-19 severity as a 3-level categorical measure (mild, moderate, and severe) and dichotomous measures of symptoms using the Fisher exact test. Nonparametric Mann-Whitney U test was used to compare nonnormally distributed continuous variables (CRP values). P<.05 was considered statistically significant.
Results
During the study period, 1567 pregnant and postpartum women were evaluated for SARS-CoV-2 based on symptoms or as part of the universal testing protocol upon presentation for delivery or antepartum admission; 8.9% (141/1567) had a positive result. Notably, 59% of positive patients (84/141) received their care at Yale New Haven Hospital, 24.1% (34/141) at Bridgeport Hospital, and 16.3% (23/141) at Greenwich Hospital. The median age of the cohort was 30 years (IQR, 25–34) (Table 1 ). The median prepregnancy BMI was 28.4 kg/m2 (IQR, 24.1–35.1). Moreover, 44% of women (61/141) were Hispanic, 27.3% (38/141) were non-Hispanic white, 21.6% (30/141) were non-Hispanic black, and 7.2% (10/141) were of Asian or other race. Among all tested women, the racial and ethnic breakdown was as follows: Hispanic, 23.5% (356/1567); non-Hispanic white, 54.2% (823/1567); non-Hispanic black, 13.8% (209/1567); and Asian and other, 8.6% (130/1567); data were missing on 49 tested women (data not indicated). Comparison of race and ethnicity breakdown between SARS-CoV-2–positive and SARS-CoV-2–negative groups revealed overrepresentation of Hispanic women in the positive cohort (P<.001). For reference, the racial and ethnic distribution of all women across 3 hospitals admitted for delivery during the study frame was as follows: Hispanic, 23.1% (480/2082); non-Hispanic white, 56.3% (1172/2082); non-Hispanic black, 12.7% (265/2082); and Asian and other, 7.9% (165/2082). When evaluated as a dichotomous measure, Hispanic ethnicity was associated with increased odds of moderate and severe COVID-19 course compared with non-Hispanic ethnicity (18% [11/61] vs 3.8% [3/78]; unadjusted OR, 5.5; 95% CI, 1.46–20.71; P=.01).
Table 1.
Patient characteristics, comorbidities, and COVID-19 severity
| Characteristic | Total 141 (100%) |
COVID-19 severity |
P value | |||
|---|---|---|---|---|---|---|
| Asymptomatic 44 (31.2%) |
Mild 82 (58.2%) |
Moderate 9 (6.4%) |
Severe or critical 6 (4.3%) |
|||
| Age, y (continuous) | ||||||
| Median (IQR) | 30 (25–34) | 30 (24–33.5) | 30 (25–35) | 34 (30–35) | 30.5 (23–35) | .62 |
| Age, y (categorical) | ||||||
| <25 | 32 (22.7) | 13 (29.5) | 16 (19.5) | 1 (11.1) | 2 (33.3) | .40 |
| 25–35 | 74 (52.5) | 23 (52.3) | 45 (54.9) | 5 (55.6) | 1 (16.7) | |
| 35–40 | 27 (19.1) | 6 (13.6) | 15 (18.3) | 3 (33.3) | 3 (50.0) | |
| >40 | 8 (5.7) | 2 (4.6) | 6 (7.3) | 0 (0.0) | 0 (0.0) | |
| Race and ethnicity | ||||||
| Hispanic | 61 (43.9) | 17 (38.6) | 33 (40.7) | 7 (87.5) | 4 (66.7) | .19 |
| Non-Hispanic white | 38 (27.3) | 13 (29.6) | 25 (30.9) | 0 (0.0) | 0 (0.0) | |
| Non-Hispanic black | 30 (21.6) | 9 (20.5) | 19 (23.5) | 1 (12.5) | 1 (16.7) | |
| Asian and other | 10 (7.2) | 5 (11.4) | 4 (4.9) | 0 (0.0) | 1 (16.7) | |
| Ethnicity | ||||||
| Hispanic | 61 (43.9) | 17 (38.6) | 33 (40.7) | 7 (87.5) | 4 (66.7) | .04 |
| Non-Hispanic | 78 (56.1) | 27 (61.4) | 48 (59.3) | 1 (12.5) | 2 (33.3) | |
| Insurance | ||||||
| Commercial | 54 (40.6) | 20 (50.0) | 29 (37.2) | 4 (44.4) | 1 (16.7) | .32 |
| State | 60 (45.1) | 18 (45.0) | 33 (42.3) | 4 (44.4) | 5 (83.3) | |
| Hospital program | 15 (11.3) | 1 (2.5) | 13 (16.7) | 1 (11.1) | 0 (0.0) | |
| None, self-pay | 4 (3.0) | 1 (2.5) | 3 (3.8) | 0 (0.0) | 0 (0.0) | |
| Marital status | ||||||
| Married with partner | 67 (47.9) | 23 (53.5) | 36 (43.9) | 4 (44.4) | 4 (66.2) | .58 |
| Single, widowed, other | 73 (52.1) | 20 (46.5) | 46 (56.1) | 5 (55.6) | 2 (33.3) | |
| Known COVID-19 exposure | ||||||
| No | 56 (40.3) | 17 (38.6) | 34 (41.5) | 1 (14.3) | 4 (66.7) | .04 |
| Yes | 48 (34.5) | 11 (25.0) | 32 (39.0) | 5 (71.4) | 0 (0.0) | |
| Unknown | 35 (25.2) | 16 (36.4) | 16 (19.5) | 1 (14.3) | 2 (33.3) | |
| Timing of COVID-19 diagnosis | ||||||
| First trimester | 11 (7.8) | 0 (0.0) | 10 (12.2) | 0 (0.0) | 1 (16.7) | NAC |
| Second trimester | 37 (26.2) | 4 (9.1) | 29 (35.4) | 1 (11.1) | 3 (50.0) | |
| Third trimester | 35 (24.8) | 2 (4.5) | 25 (30.5) | 7 (77.8) | 1 (16.7) | |
| Delivery admission | 51 (36.2) | 37 (84.1) | 12 (14.6) | 1 (11.1) | 1 (16.7) | |
| Postpartum | 7 (5.0) | 1 (2.3) | 6 (7.3) | 0 (0.0) | 0 (0.0) | |
| Gestational age at delivery (n=77) | ||||||
| Median (IQR) | 39 (38–40) | 39 (38–39) | 39 (38–40) | 38.5 (38–40) | 30 (22–38) | .35 |
| Gestational age at diagnosis before delivery (n=132) | ||||||
| Median (IQR) | 35 (22–38.5) | 39 (38-39) | 27.5 (17-36) | 35 (30-36) | 26 (22-31) | <.001 |
| Any comorbidity | ||||||
| No | 84 (59.6) | 31 (70.4) | 45 (54.9) | 6 (66.7) | 2 (33.3) | .19 |
| Yes | 57 (40.4) | 13 (29.6) | 37 (45.1) | 3 (33.3) | 4 (66.7) | |
| Obesity (prepregnancy BMI of ≥30 kg/m2) | ||||||
| <30 | 79 (59.0) | 30 (71.4) | 46 (57.5) | 3 (42.9) | 0 (0.0) | .01 |
| ≥30 | 55 (41.0) | 12 (28.6) | 34 (42.5) | 4 (57.1) | 5 (100.0) | |
| Obesity (prepregnancy BMI, kg/m2) | ||||||
| <30 | 79 (59.0) | 30 (71.4) | 46 (57.5) | 3 (42.9) | 0 (0.0) | <0.01 |
| 30–35 | 22 (16.4) | 5 (11.9) | 14 (17.5) | 1 (14.3) | 2 (40.0) | |
| 35–40 | 18 (13.4) | 2 (4.8) | 10 (12.5) | 3 (42.9) | 3 (60.0) | |
| >40 | 15 (11.7) | 5 (11.9) | 10 (12.5) | 0 (0.0) | 0 (0.0) | |
| Pregestational diabetes | ||||||
| No | 132 (95.0) | 42 (97.7) | 76 (93.8) | 9 (100.0) | 5 (83.3) | .35 |
| Yes (prepregnancy or early diagnosis) | 7 (5.0) | 1 (2.3) | 5 (6.2) | 0 (0.0) | 1 (16.7) | |
| Chronic hypertension | ||||||
| No | 126 (90.0) | 40 (90.9) | 75 (91.5) | 7 (87.5) | 4 (66.7) | .21 |
| Yes | 14 (10.0) | 4 (9.1) | 7 (8.5) | 1 (12.5) | 2 (33.3) | |
| Heart disease | ||||||
| No | 134 (95.0) | 43 (97.7) | 76 (92.7) | 9 (100.0) | 6 (100.0) | .74 |
| Yes | 7 (5.0) | 1 (14.3) | 6 (7.3) | 0 (0.0) | 0 (0.0) | |
| Asthma | ||||||
| No | 122 (87.1) | 40 (93.0) | 70 (85.4) | 8 (88.9) | 4 (66.7) | .20 |
| Yes | 18 (12.9) | 3 (7.0) | 12 (14.6) | 1 (11.1) | 2 (33.3) | |
| Smoking | ||||||
| Never | 113 (81.3) | 38 (90.5) | 64 (78.1) | 6 (66.7) | 5 (83.3) | .19 |
| Current or former | 19 (13.7) | 3 (7.1) | 13 (15.8) | 3 (33.3) | 0 (0.0) | |
| Unknown | 7 (5.0) | 1 (2.4) | 5 (6.1) | 0 (0.0) | 1 (16.7) | |
Numbers may not add to 141 owing to missing values; percentages may not add to 100 owing to rounding.
BMI, body mass index; COVID-19, coronavirus disease 2019; IQR, interquartile range; NAC, not able to calculate owing to the low number of subjects.
Grechukhina et al. A series of 141 cases of COVID-19 in pregnancy. AJOG MFM 2020.
The median gestational age at diagnosis was 35 weeks for antepartum diagnoses (IQR, 22–38.5), 39 weeks (IQR, 38–39) for asymptomatic women, and 27.5 (IQR, 17–36), 35 (IQR, 30–36), and 26 weeks (IQR, 22–31) for patients with mild, moderate, and severe or critical diseases, respectively. Additional demographic information and patient characteristics are presented in Table 1. The diagnosis was made antenatally in 58.8% of cases (83/141): 7.8% (11/141) in the first trimester, 26.2% (37/141) in the second trimester, and 24.8% (35/141) in the third trimester. Of note, 36% of women (51/141) were diagnosed on admission for childbirth; 5% (7/141) were diagnosed as having COVID-19 postpartum after discharge from their childbirth admission. Moreover, 31% of women (44/141) were asymptomatic; 58% of women (82/141) had a mild disease, 6.4% (9/141) had a moderate disease, and 5 women had a severe or critical disease. In addition, 1 woman died in the emergency room. This woman, with a prepregnancy BMI of 35 kg/m2, was diagnosed as having COVID-19 in ambulatory care in the first trimester of pregnancy. She experienced respiratory distress at home 13 days after the initial symptom onset and arrived at the emergency department profoundly hypoxemic and experiencing cardiac arrest and ultimately died despite prolonged attempts at cardiopulmonary resuscitation. No autopsy was performed. Including this case, the rate of severe/critical disease in our population was 4.3% (6/141). The timing of the diagnoses and disease severity are reflected in Figure 1 .
Figure 1.
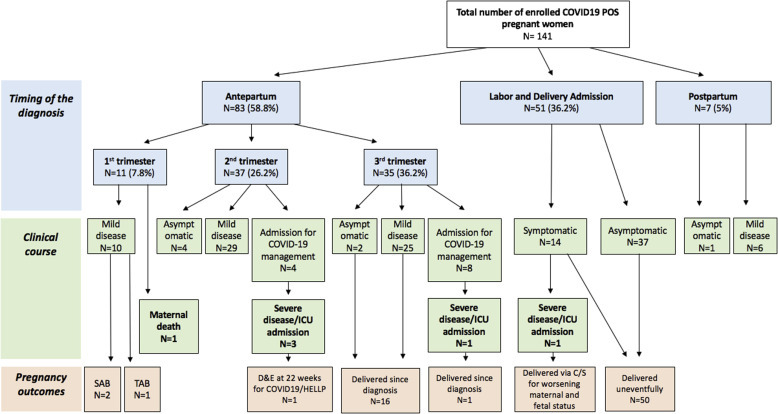
Timing of diagnosis, clinical course, and pregnancy outcomes in COVID-19–positive women
C/S, cesarean delivery; COVID-19, coronavirus disease 2019; D&E, dilation and evacuation; HELLP, hemolysis, elevated liver enzymes, and low platelet count; ICU, intensive care unit; SAB, spontaneous abortion; TAB, therapeutic abortion.
Grechukhina et al. A series of 141 cases of COVID-19 in pregnancy. AJOG MFM 2020.
Maternal medical comorbidities and their relation to COVID-19 course are presented in Table 1. The severity of disease was associated with obesity, both as a dichotomous measure and by obesity class (P=.01 and P<.01, respectively) but not with any other comorbidity. Obese women had higher rates of moderate and severe disease than nonobese women (16.4% [9/55] vs 3.8% [3 /79]; unadjusted OR, 4.96; 95% CI, 1.28–19.25). The distribution of obesity among racial and ethnic groups was as follows: Hispanic, 38.6% (22/57); non-Hispanic white, 23.7% (9/38); non-Hispanic black, 73.3% (22/30); and Asian or other, 25% (2/8) (P<.001), suggesting that Hispanic ethnicity is unlikely to be solely related to the effect of obesity on the clinical course of COVID-19. Obese Hispanic women were also more likely to develop moderate and severe COVID-19 than nonobese Hispanic women (31.8% [7/22] vs 8.6% [3/35]; OR, 4.98; 95% CI, 1.13–21.98).
Among symptomatic women, the most common symptoms in our cohort were cough (70.1%), muscle aches (51.6%), and sore throat (47.4%) (Figure 2 ). The most common symptoms in women with a severe disease were muscle aches, fever, shortness of breath, nausea, chest pain, and abdominal pain.
Figure 2.
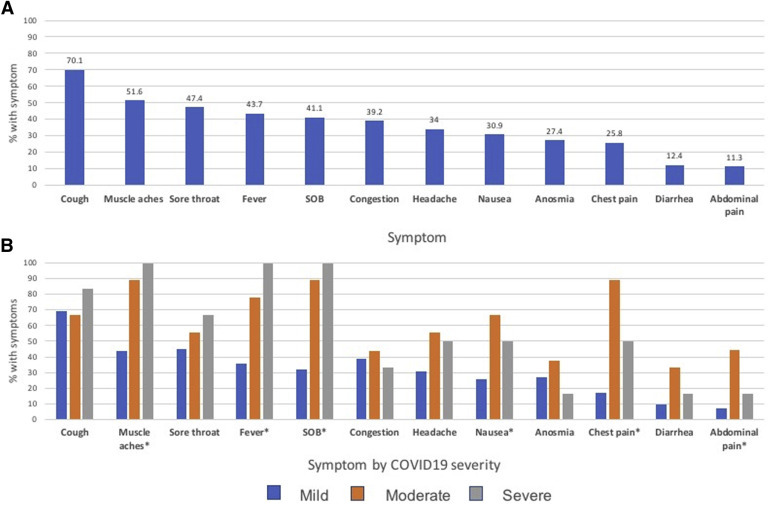
Symptom frequency among different groups of symptomatic COVID-19–positive patients
A, Overall symptom frequency in the symptomatic group. B, Symptom frequency in symptomatic group stratified by the severity of COVID-19 disease. The P values were based on Fisher exact test of association between the 3-level severity and dichotomous symptoms. Symptoms with P<.05 were marked with an asterisk.
COVID-19, coronavirus disease 2019; SOB, shortness of breath.
Grechukhina et al. A series of 141 cases of COVID-19 in pregnancy. AJOG MFM 2020.
D-dimer and CRP trends, grouped by the type of admission, are presented in Figure 3 . Notably, D-dimer values varied greatly within the group who had a positive result for SARS-CoV-2 during childbirth admission. However, most had a substantial increase in D-dimer value shortly after birth with a subsequent decline within 48 hours. D-dimer took longer to normalize in 1 patient (5 days after delivery) whose respiratory status deteriorated in labor necessitating cesarean delivery followed by intensive care unit (ICU) admission for COVID-19–related respiratory failure. There were no cases of venous thromboembolism diagnosed during the study period. CRP also peaked after delivery. Women admitted before delivery for COVID-19 management who developed a severe disease seemed to have higher initial CRP values than those with a milder disease. However, comparison between these 2 groups did not reach statistical significance (P=.057).
Figure 3.
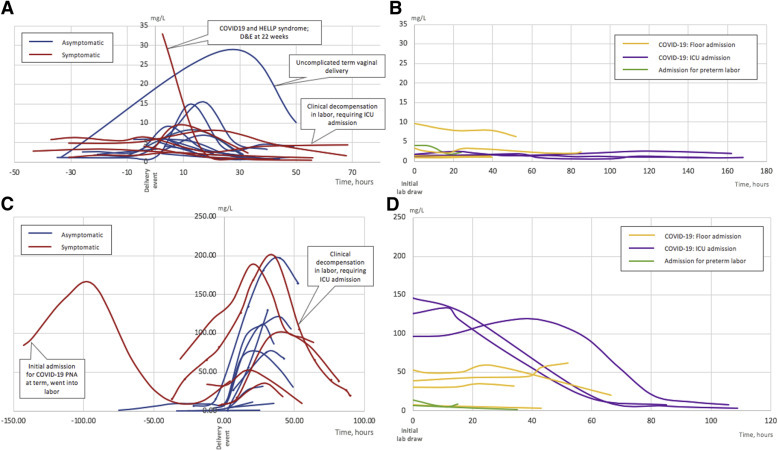
Laboratory trends in admitted symptomatic and asymptomatic COVID-19-positive women
A, D-dimer trends in asymptomatic and symptomatic patients with COVID-19 during delivery encounter. B, D-dimer trends in COVID-19–positive patients admitted before delivery (for COVID-19 and non–COVID-19 indications), who were discharged undelivered. C, CRP trends in asymptomatic and symptomatic patients with COVID-19 during delivery encounter. Marked lines indicate cesarean delivery. D, CRP trends in COVID-19–positive patients admitted before delivery (for COVID-19 and non–COVID-19 indications), who were discharged undelivered.
COVID-19, coronavirus disease 2019; CRP, C-reactive protein; D&E, dilation and evacuation; ICU, intensive care unit; PNA, pneumonia.
Grechukhina et al. A series of 141 cases of COVID-19 in pregnancy. AJOG MFM 2020.
Pregnancy outcomes were available for 56.7% of women (80/141) (Figure 1; Table 2 ). Of note, 1 woman underwent termination by dilation and evacuation at 22 weeks’ gestation owing to severe preterm preeclampsia syndrome in the setting of COVID-19 infection.7 Moreover, 95% of pregnancies (76/80) resulted in a live birth, 3 of which were preterm (2 spontaneous; 1 indicated by preeclampsia with severe features). Vaginal delivery occurred in 67.5% of cases (52/77), and cesarean delivery in 31.1% of cases (24/77). In addition, 1 cesarean delivery was indicated for maternal and fetal decompensation secondary to COVID-19.
Table 2.
Pregnancy and neonatal outcomes in COVID-19–positive women
| Pregnancy outcomes | n (%) |
|---|---|
| Pregnancy resolved since diagnosis | 80 (56.7) |
| Type of pregnancy outcome (n=80) | |
| Pregnancy termination | 4 (5.0) |
| Spontaneous | 2 (50.0) |
| Elective, not medically indicated | 1 (25.0) |
| Medically indicated (COVID-19 related) | 1 (25.0) |
| Live births | 76 (95.0) |
| Preterm birth | 3 (3.9) |
| Spontaneous preterm birth | 2 (66.7) |
| Medically indicated preterm birth | 1 (33.3) |
| COVID-19 related | 0 (0.0) |
| Term delivery | 73 (96.1) |
| Spontaneous | 40 (54.8) |
| Scheduled cesarean delivery | 6 (8.2) |
| Medically indicated | 27 (37.0) |
| COVID-19 related | 0 (0.0) |
| Mode of delivery (n=77) | |
| Vaginal | 52 (67.5) |
| Cesarean delivery by type | 24 (31.2) |
| Indicated by COVID-19 | 1 (4.2) |
| Previous cesarean delivery, no labor | 6 (25.0) |
| Fetal distress | 8 (33.3) |
| Failed induction | 1 (4.2) |
| Arrest of dilation | 1 (4.2) |
| Arrest of descent | 0 (0.0) |
| Malpresentation | 4 (16.7) |
| Other, N/A | 3 (12.5) |
| Dilation and evacuation | 1 (1.3) |
| Hypertensive disorders of pregnancy (n=77) | |
| Any | 17 (22.1) |
| Gestational hypertension | 4 (5.2) |
| Preeclampsia without severe features | 4 (5.2) |
| Preeclampsia with severe features | 8 (10.4) |
| HELLP | 1 (1.3) |
| Gestational diabetes (n=75) | |
| None | 68 (90.7) |
| A1 | 3 (4.0) |
| A2 | 4 (5.3) |
| Neonatal outcomes (n=73) | |
| Newborn SARS-CoV-2 test of nasopharyngeal swabs | |
| Negative | 60 (82.2) |
| Positive | 0 (0) |
| Not tested | 13 (17.8) |
| Neonatal intensive care unit admission | |
| No | 63 (86.3) |
| Admission COVID19 related | 0 (0.0) |
| Admission not COVID-19 related | 10 (13.7) |
COVID-19, coronavirus disease 2019; HELLP, hemolysis, elevated liver enzymes, and low platelet count; N/A, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus disease 2.
Grechukhina et al. A series of 141 cases of COVID-19 in pregnancy. AJOG MFM 2020.
HDP affected 22.1% of COVID-19–positive pregnancies (17/77). The cumulative rate of preeclampsia, eclampsia and HELLP was 16.9% (13/77); 12% of COVID-19–positive women (2/17) had preexisting hypertension. For comparison, the overall rates of HDP, cumulative preeclampsia, eclampsia and HELLP and preexisting hypertension at Yale New Haven Hospital in 2018 to 2019 among singleton pregnancies were 18.5% (1601/8691), 7.6% (668/8691), and 8.9% (770/8691), respectively.
Nasopharyngeal swab SARS-CoV-2 RT-PCR results for all tested newborns (n=60) were negative. Placental tissue from the 22-week termination for severe preterm preeclampsia syndrome had a positive result for SARS-CoV-2 RNA.7 None of the newborns required COVID-19–related ICU admission.
Comment
Principal findings
This is a series of 141 cases of COVID-19 in pregnant and postpartum women within a diverse population of Southern Connecticut. Although the rate of severe disease in our population is low (4.3%), our cohort includes 1 maternal death. In our cohort, Hispanic women were disproportionately affected by COVID-19 and seemed to have an increased risk of moderate and severe disease. This finding is unlikely to be related to a disproportionate testing in Hispanic population because all 3 hospital sites implemented universal SARS-CoV-2 testing upon admission for childbirth. Prepregnancy obesity was associated with a higher disease severity category. HDP affected approximately 1 of every 5 women with COVID-19 after 20 weeks’ gestation with the majority diagnosed as having preeclampsia with severe features or HELLP syndrome. Our study reports that delivery is associated with transient increases in D-dimer and CRP levels in all COVID-19–positive women regardless of symptomatic status. D-dimer returned to predelivery values within 24 to 48 hours in most women. D-dimer did not seem to be a useful marker to distinguish COVID-19 disease severity category. All newborns born to COVID-19–positive women had a negative result for SARS-CoV-2 RNA via nasopharyngeal swab after 24 hours of life; however, there was 1 case with a positive placental SARS-CoV-2 test result.7
Results in the context of what is known
Early reports of SARS-CoV-2 infection during pregnancy are encouraging because they failed to indicate higher susceptibility or morbidity in pregnant women than the general population.10, 11, 8, 9 More recent reports have described severe and critical diseases in pregnancy and maternal deaths from COVID-19, indicating a potential for severe maternal morbidity and mortality.12, 13, 14 The case of maternal death in our series highlights the potential for the disease course to be protracted with seemingly unpredictable and abrupt deterioration in health after 10 to 14 days.15
One of the most important goals of the healthcare community during SARS-CoV-2 pandemic is the identification of populations at risk of severe disease and death. Racial and ethnic disparities as risk factors for severe COVID-19 are an emerging focus of COVID-19 studies in the United States.16, 17, 18, 19 Our data raise a concern about the role of social determinants of health and systemic inequities specific to SARS-CoV-2 transmission and healthcare access. Our findings are further supported by a recent study by Moore et al20 that reported a disproportionate number of COVID-19 cases among underrepresented racial and ethnic groups (with Hispanic population being the largest affected group) in COVID-19 pandemic hotspots.20 We report that prepregnancy obesity is associated with more severe COVID-19, which is consistent with previous studies in nonpregnant adults and a small study of pregnant women.13 , 21
In nonpregnant adults, higher D-dimer levels are associated with an increased risk of critical COVID-19 course and death.22 , 23 Anticoagulation, guided by D-dimer levels, has been found to decrease mortality in this population.24 However, in both complicated and uncomplicated pregnancies, D-dimer levels are known to increase above baseline, although reference ranges are inconsistent.25 Our study presents novel data on D-dimer trends in SARS-CoV-2–positive symptomatic and asymptomatic women in relation to delivery. CRP has emerged as another independent predictor of adverse outcomes in nonpregnant patients with COVID-19.26 Our data suggest that D-dimer may not be helpful in determining disease severity in a pregnant and peripartum COVID-19 population. Its use for anticoagulation guidance needs to be further evaluated. Similar to D-dimer, there are no well-established reference ranges for CRP in pregnancy and there are limited data for the use of this parameter in COVID-19–positive pregnant women.27 Our data suggest that admission CRP values in antepartum women may emerge as more helpful in predicting disease severity.
Clinical and research implications
The overrepresentation of Hispanic women in our SARS-CoV-2–positive cohort and concern for increased severity of COVID-19 disease in this group indicate an urgent need to further characterize and address the causes of these disparities. Additional large-scale studies are needed to address the mounting evidence that racial and ethnic disparities are central to the myriad factors (eg, healthcare access, housing, and ability to socially distance) that lead to the unequal distribution of SARS-CoV-2 infection and COVID-19 severity and mortality seen throughout the United States.16
The CDC guidelines include only severe obesity (BMI, >40 mg/m2) as a risk factor for severe illness in the nonpregnant population whereas our study links prepregnancy BMI of ≥30 kg/m2 with worse clinical course during pregnancy.28 The current ACOG–Society for Maternal-Fetal Medicine COVID-19 guidelines do not list obesity as a comorbidity placing pregnant women at risk of more severe disease.29 Given our findings, consideration should be made to include all classes of obesity as a risk factor in pregnancy for progression to moderate and severe diseases.
Finally, larger studies are required to review the possible association between SARS-CoV-2 infection and HDP.
Strengths and limitations
Our study was performed in a diverse healthcare system consisting of academic and community hospitals with a racially and ethnically diverse population; however, the study is limited to a single geographic location and may not be generalizable to other regions of the country with different patient populations and prevalence of SARS-CoV-2. In addition, this population is heterogenous with both symptomatic and asymptomatic women being tested for SARS-CoV-2. We acknowledge that many women with symptoms were likely never tested and new commercial tests performed outside of hospital laboratories emerged during the course of this study, the results of which may have not been incorporated in the EHR and identified for review. Furthermore, testing guidelines and management strategies evolved during the study period, thus contributing to the variation in clinical decision making. The WHO COVID-19 severity assignment criteria were used for this study because this classification system was the only one explicitly applicable for pregnancy at the time. Furthermore, we adjusted the oxygen saturation criterion for severe disease from <90% on room air in nonpregnancy to <95% in pregnancy.30 The racial and ethnic composition of the tested population may not be an accurate representation of the overall pregnant population. We were unable to compare the rates of HDP in COVID-19–positive and COVID-19–negative patients because we had limited access to the data on the latter group. CRP value comparisons among COVID-19 severity groups were limited by small sample size. We were unable to perform a multivariate analysis to assess for confounding because of the small sample size. Finally, unlike other literature, we failed to report associations between preexisting hypertension and diabetes mellitus with worse COVID-19 course; this may be because of a relatively small sample size.31 Larger registry studies are needed to examine the risk factors associated with COVID-19 progression in pregnancy.
Conclusions
This study demonstrates that most pregnant women with COVID-19 remain either asymptomatic or have mild disease; however, severe illness and death can occur. Prepregnancy obesity was associated with an increased risk of severe illness. Furthermore, the Hispanic population in this cohort seemed to be at an increased risk of severe illness. Large-scale studies are required to develop better risk stratification strategies for COVID-19 in pregnancy.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
Cite this article as: Grechukhina O, Greenberg V, Lundsberg LS, et al. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am J Obstet Gynecol MFM 2020;2:100246.
Appendix
References
- 1.Johns Hopkins University Coronavirus Resource Center COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2020. https://coronavirus.jhu.edu/map Available at: Accessed September 14, 2020.
- 2.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N., Han L., Peng M., et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics FAQs: management of infants born to mothers with suspected or confirmed COVID-19. 2020. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/ Available at: Accessed September 17, 2020.
- 5.World Health Organization Clinical management of COVID-19. Interim guidance. 2020. https://www.who.int/publications-detail/clinical-management-of-covid-19 Available at: Accessed June 5, 2020.
- 6.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237–e260. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 7.Hosier H., Farhadian S.F., Morotti R.A., et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu N., Li W., Kang Q., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong S.F., Chow K.M., Leung T.N., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East Respiratory Syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52:501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dashraath P., Wong J.L.J., Lim M.X.K., et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce-Williams R.A.M., Burd J., Felder L., et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2:100134. doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blitz M.J., Rochelson B., Minkoff H., et al. Maternal mortality Among women with coronavirus disease 2019 admitted to the intensive care unit. Am J Obstet Gynecol. 2020;223:595–599.e5. doi: 10.1016/j.ajog.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009575. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadhera R.K., Wadhera P., Gaba P., et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldfarb I.T., Clapp M.A., Soffer M.D., et al. Prevalence and severity of coronavirus disease 2019 (COVID-19) illness in symptomatic pregnant and postpartum women stratified by Hispanic ethnicity. Obstet Gynecol. 2020;136:300–302. doi: 10.1097/AOG.0000000000004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore J.T., Ricaldi J.N., Rose C.E., et al. Disparities in incidence of COVID-19 Among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020 - 22 states, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baboolall U., Zha Y., Gong X., Deng D.R., Qiao F., Liu H. Variations of plasma D-dimer level at various points of normal pregnancy and its trends in complicated pregnancies: a retrospective observational cohort study. Medicine (Baltimore) 2019;98:e15903. doi: 10.1097/MD.0000000000015903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo X., Zhou W., Yan X., et al. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa641. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99:839–847. doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention People who are at higher risk for severe illness. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html Available at: Accessed July 8, 2020.
- 29.The American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine Outpatient assessment and management for pregnant women with suspected or confirmed novel coronavirus (COVID-19) 2020. https://www.smfm.org/covidclinical Available at: Accessed July 8, 2020.
- 30.Society for Maternal-Fetal Medicine Management considerations for pregnant patients with COVID-19. 2020. https://www.smfm.org/covidclinical Available at: Accessed September 14, 2020.
- 31.Andrikopoulou M., Madden N., Wen T., et al. Symptoms and critical illness among obstetric patients With coronavirus Disease 2019 (COVID-19) infection. Obstet Gynecol. 2020;136:291–299. doi: 10.1097/AOG.0000000000003996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


