Abstract
Objectives
As per National AIDS Control Organization (NACO) estimates, there are 2.1 million people living with HIV (PWH) in India, of whom 1.2 million are on first‐line antiretroviral therapy (ART). This study explored the use of a single‐tablet regimen containing tenofovir disoproxil fumarate 300 mg + lamivudine 300 mg + efavirenz 400 mg (TLE400 STR) as a first‐line switch strategy in PWH in Pune, India.
Methods
This retrospective cohort study was conducted in private sector ART clinics in three tertiary‐level hospitals in Pune, India. PWH > 12 years of age (n = 502) who initiated first‐line ART (predominantly TLE600 STR), completed ≥ 6 months of follow‐up and achieved virological suppression [plasma viral load (VL) < 1000 HIV‐1 RNA copies/mL] were identified and switched to TLE400 STR. The virological and immunological efficacy of TLE400 STR at 6 and 12 months of follow‐up were noted. Grade 3/4 adverse events (especially efavirenz‐related neuropsychiatric adverse events) leading to regimen discontinuation were also noted.
Results
Of 502 PWH who switched to TLE400 STR, complete virological suppression (VL < 20 copies/mL) was maintained in more than 97% of patients at follow‐up. TLE400 STR was successful in maintaining CD4 counts within the range observed at the start of the regimen. Grade 3/4 adverse events leading to TLE400 STR discontinuation were seen in 11 (2.2%) patients. Virological failure (VL > 1000 copies/mL) and treatment regimen failure were seen in six (1.2%) and 49 (9.8%) subjects, respectively.
Conclusions
TLE400 STR exhibits excellent efficacy and safety as a switch strategy and should be introduced in the Indian National ART Program, especially for PWH who are virologically suppressed on TLE600 STR.
Keywords: antiretroviral therapy optimization, cost‐effectiveness, efavirenz 400 mg, India, single‐tablet regimen
Introduction
According to the World Health Organization (WHO), there are more than 37.9 million people living with HIV (PWH) worldwide [1]. In 2018, almost 770 000 people died of HIV‐related illnesses, underscoring the importance of prioritizing HIV infection as one of the most pressing global health challenges [1, 2, 3, 4]. India has 2.1 million PWH, with 88 000 people becoming newly infected annually [5, 6]. Pune is located in the state of Maharashtra, which has the highest estimated number of PWH [0.33 million; interquartile range (IQR) 0.25–0.43 million] in India [7, 8, 9].
Antiretroviral therapy (ART) is used to suppress HIV infection. It comprises pharmacological drug classes including nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), integrase strand transfer inhibitors (INSTIs), fusion inhibitors, and chemokine receptor antagonists [10, 11]. The WHO 2013 ART guidelines recommended a fixed‐dose, single‐tablet regimen containing two NRTIs [tenofovir disoproxil fumarate (TDF) + lamivudine (3TC)] plus one NNRTI [efavirenz (EFV)] (TLE600 STR) as the preferred first‐line regimen for ART‐naïve adults and adolescents in low‐ and middle‐income countries (LMICs) such as India [12]. In such regimens, EFV is used at the standard dose of 600 mg. As of 2019, 1.2 million PWH in India were on first‐line ART, the majority being on TLE600 STR [13].
The superiority of the TLE600 reference regimen was challenged in the SINGLE trial, which compared this regimen with a dolutegravir (DTG; an INSTI)‐based regimen [14]. The DTG‐based regimen had superior virological efficacy to an EFV 600‐based regimen. The superior responses were driven primarily by a higher rate of discontinuation because of adverse events (AEs) in the TLE600 group [14]. Subsequently, a regimen consisting of TDF + 3TC + DTG (TLD) was devised, which had a high genetic barrier to resistance, was available as a fixed‐dose STR, and could be manufactured at a low cost [15]. Thus, it became an ideal candidate for universal first‐line ART and was introduced as WHO preferred first‐line ART in December 2018 [16]. But DTG also has its share of problems. Peri‐conceptional exposure to DTG is associated with a three times higher risk of neural tube defects in infants as compared to exposure to non‐dolutegravir‐based ART [17]. Risk of neuropsychiatric symptoms (especially insomnia) [18], a significant increase in body weight (especially in female patients), and an increase in truncal fat mass [19, 20] have been associated with DTG use. As a result, an alternative regimen to TLD was needed.
However, WHO guidelines [16] recommended that TLE600 STR should be used only in special circumstances. The main reason for this was the increased prevalence of pretreatment NNRTI resistance in LMICs, which is known to decrease the efficacy of TLE600 STR [16]. In addition, EFV is also linked to neuropsychiatric AEs (abnormal dreams, anxiety, dizziness, somnolence, and depression) [21] and suicidality (suicidal ideation or attempted/completed suicide) [22]. Despite excellent virological potency, the use of PIs in first‐line ART in LMICs is prohibitive because of the excess cost [23].
To search for effective first‐line ART options in LMICs, to combat the problem of ART toxicity and to reduce ART manufacturing costs, ART optimization strategies that aim to reduce the dose of individual drugs within an STR have been recommended to combat the problem of ART toxicity, to reduce ART manufacturing costs and to aid in the search for effective first‐line ART options in LMICs [24]. A reduction in the dose of EFV from 600 mg to 400 mg to replace the popular TLE600 STR with TLE400 STR was one such strategy. The ENCORE1 trial [25] demonstrated the virological noninferiority of the TLE400 regimen in comparison to TLE600. AEs related to EFV and treatment cessation because of serious AEs were significantly more frequent in recipients of 600 mg EFV. Subsequently, the NAMSAL Agence Nationale de Recherche sur le Sida et les Hépatites Virales (ANRS) 12313 trial [26] compared two regimens (TLD and TLE400) for the treatment of ART‐naïve PWH in Cameroon. The trial demonstrated noninferiority of TLE400 to TLD with regard to the primary endpoint of virological suppression [plasma viral load (VL) on ART < 50 HIV‐1 RNA copies/mL]. Both regimens were virologically noninferior in the group of patients with the highest baseline VL (≥ 500 000 copies/mL). Multivariate analyses showed that the presence of primary NNRTI resistance (mutations conferring resistance to EFV) was not associated with an absence of viral suppression (VL < 50 copies/mL) in the TLE400 group. Approximately 6% of participants in the TLE400 group had primary resistance to EFV at baseline; however, DTG was not virologically superior to EFV400. In addition, TLE400 was associated with a lower incidence of obesity compared to TLD (5.4% versus 12.3%, respectively). In the light of the data from these two trials and demonstrations of the safety and efficacy of EFV400 in pregnancy [27] and in HIV/tuberculosis coinfection [28], WHO recommended TLE400 STR as an alternate first‐line ART regimen for LMICs in July 2019 [29]. However, a clinical trial to evaluate the efficacy and safety of TLE400 STR in Indian PWH has not been conducted, despite the availability of TLE400 STR in the country since July 2017 [25, 26, 30, 31]. This represents a major knowledge gap that needs to be addressed before the introduction of TLE400 STR in the Indian National ART Program. As a first step to address this gap, we conducted a retrospective, observational cohort study to evaluate the safety and efficacy of TLE400 STR as a switch regimen in PWH who are virologically suppressed (plasma VL on ART < 1000 copies/mL) [32] on first‐line NNRTI‐containing ART (predominantly TLE600 STR) in Pune, Maharashtra.
Materials and methods
Study setting
This retrospective cohort study was conducted in private sector ART clinics in three tertiary‐level hospitals (Ruby Hall Clinic, Poona Hospital, and Noble Hospital) in Pune, Maharashtra, western India. These three hospitals provide clinical care, diagnostic, and treatment services to PWH in the state of Maharashtra. Patients are referred by primary care physicians, private practitioners of alternative medical systems, antenatal clinics and TB clinics. All individuals pay for diagnostic services and ART from their own pocket. Study data including patient demographics and clinical, laboratory and treatment parameters are entered into an electronic database (LiveHealth Software Solutions, Pune, India).
Data collection, patient characteristics, and ethics statement
All PWH > 12 years of age who were registered in the ART programme between 1 March 2009 and 1 March 2018, initiated first‐line ART, completed ≥ 6 months of follow‐up and were virologically suppressed (VL on ART < 1000 copies/mL) [32] were identified. The most common first‐line ART regimens used by subjects were fixed‐dose combination of TDF 300 mg, 3TC 300 mg, and EFV 600 mg (TLE600 STR); TDF 300 mg, emtricitabine (FTC) 200 mg and EFV 600 mg (TEE600 STR); and zidovudine (ZDV) 300 mg, 3TC 150 mg, and nevirapine (NVP) 200 mg (ZLN) [12, 33]. After the introduction of TLE400 STR in India by Mylan Laboratories Limited, patients who exhibited virological suppression on first‐line ART and had normal renal function [estimated creatinine clearance using the Cockcroft Gault (CG) formula > 50 mL/min] were given an option to switch to TLE400 STR. Those who switched to TLE400 were included in our study as cases and their long‐term follow‐up was traced.
Demographic data, such as age, gender, weight, history of past opportunistic infections, history of past and current addictions (cigarette smoking, chewing tobacco and alcohol), history of previous pregnancies, hepatitis B virus (HBV) coinfection and prevalence of comorbidities, such as diabetes mellitus, were noted. Clinical, immunological and biochemical parameters, such as serial CD4 counts (FACS Count; Becton Dickinson, Franklin Lakes, NJ), serum creatinine and creatinine clearance values, haemoglobin (Hb) levels and pre‐TLE400 plasma VLs (NucliSENSEasyQ real‐time nucleic acid sequence‐based amplification; BioMérieux®, Marcy‐l'Étoile, Lyon, France; lower limit of detection 20 copies/mL) were extracted from the database. Details of ART regimens before starting TLE400 STR and their duration were recorded.
After starting TLE400 STR, patients underwent CD4 count and plasma VL testing at 6 and/or 12 months of follow‐up, details of which were recorded. Follow‐up serum creatinine and creatinine clearance values were also recorded. The total number of AEs, AEs attributable to EFV 400 mg, and their severity were recorded at 6‐ and 12‐month clinic visits as a standard of care. The Depression, Anxiety, and Stress Scale (DASS 21) [34] and EFV‐related symptom questionnaires [21] were used for this purpose. Female subjects who became pregnant while on TLE400 STR were identified. The outcomes of pregnancies and the health and HIV status of the newborn infants were also noted. Individuals who developed WHO clinical stage 3 or 4 illnesses while on TLE400 STR were also identified. The use of the database for clinical research was approved by the institutional review boards of all three hospitals.
Statistical analysis
Continuous variables were summarized using median and interquartile range (IQR), while categorical variables were summarized using frequency and percentage. The primary endpoint was identifying participants who achieved complete virological suppression (VS) (plasma VL ≤ 20 copies/mL) at 6 and 12 month of follow‐up on TLE400 STR. Secondary endpoints included identification of subjects with low‐level viraemia (LLV; plasma VL 21–1000 copies/mL) at follow‐up, change in CD4 count at 6 and 12 months of follow‐up and grade 3/4 AEs on TLE400 STR. Virologic failure (VF) was defined as follow‐up VL > 1000 copies/mL [32]. Treatment failure was defined as discontinuation of TLE400 STR because of VF, grade 3/4 AEs, switch to TLD STR (introduced in India in August 2018), switch to earlier first‐line ART regimen (TLE600 STR) at patient’s request, loss to follow‐up (absence of clinic visits for 6 months) or death of the patient. The duration of TLE400 STR was calculated from the date of TLE400 initiation to regimen discontinuation, loss to follow‐up, death or censoring of observations on 1 September 2019.
Results
Baseline characteristics
A total of 502 individuals (41% female) who were receiving first‐line NNRTI‐containing ART and were virologically suppressed (VL < 1000 copies/mL) were switched to TLE400 STR (Table 1). The median age of patients in the cohort was 40 years (IQR 32, 46 years). Eighty‐nine (18.54%) individuals had pre‐existing comorbidities, such as diabetes (n = 39), hypertension (n = 34), and HBV coinfection (n = 12). Forty‐eight (9.6%), 219 (43.6%), and 160 (31.9%) subjects had a history of cigarette smoking, chewing tobacco and alcohol intake, respectively.
Table 1.
Demographic and baseline characteristics of the enrolled patients (n = 502)
| Baseline characteristics of patients | |
|---|---|
| Male:female (%) | 59:41 |
| Age (years) [median (IQR)] | 40 (32, 46) |
| Weight before TLE400 initiation (kg) [median (IQR)] | 63 (54, 73) |
| Opportunistic infections prior to ART [n (%)] | 183 (37) |
| Addictions (past and present) [n (%)] | |
| Smoking | 48 (9.6) |
| Chewing tobacco | 219 (43.6) |
| Alcohol | 160 (31.9) |
| Comorbidities [n (%)] | |
| Diabetes | 39 (7.77) |
| Hypertension | 34 (6.77) |
| Ischaemic heart disease | 4 (0.8) |
| Hepatitis B virus coinfection | 12 (2.39) |
| Biochemistry and immunology | |
| Pre‐ART CD4 count (cells/μL) [median (IQR)] | 192 (92, 333) |
| CD4 count at TLE400 STR initiation (cells/μL) [median (IQR)] | 564 (386, 791) |
| Duration of ART before TLE400 STR initiation (months) [median (IQR)] | 57.0 (27.3, 83.8) |
| Serum creatinine concentration prior to TLE400 STR initiation (mg/dL) [median (IQR)] | 0.80 (0.70, 0.90) |
| Creatinine clearance prior to TLE400 STR initiation (mL/min) [median (IQR)] | 110.7 (88.0, 131.0) |
| ART regimen type before TLE400 STR initiation [n (%)] | |
| Stavudine/lamivudine/efavirenz 600 mg (SLE) | 7 (1.39) |
| Stavudine/lamivudine/nevirapine (SLN) | 6 (1.20) |
| Tenofovir/emtricitabine/efavirenz 600 mg (TEE) | 41 (8.16) |
| Tenofovir/emtricitabine/nevirapine (TEN) | 12 (2.39) |
| Tenofovir/lamivudine/efavirenz 600 mg (TLE600) | 431 (85.86) |
| Zidovudine/lamivudine/nevirapine (ZLN) | 5 (1) |
ART, antiretroviral therapy; STR, single‐tablet regimen; IQR, interquartile range.
Patients had completed a median of 57.0 (IQR 27.3, 83.8) months of ART before starting TLE400 STR. The CD4 count had recovered from a pre‐ART median of 192 (IQR 92, 333) cells/μL to a median of 564 (IQR 386, 791) cells/μL at the time of initiation of TLE400 STR. Median serum creatinine and creatinine clearance values prior to starting TLE400 STR were 0.8 (IQR 0.70, 0.90) mg/dL and 110.7 (IQR 88, 131) mL/min, respectively. The majority of the patients were on TLE600 STR (431; 85.67%) before switching to TLE400. The median duration of follow‐up on TLE400 STR was 12 (IQR 9, 15) months.
Plasma viral load
Four hundred and seventy‐six (94.8%) and 26 (5.2%) patients had a plasma VL on first‐line ART of ≤ 20 copies/mL and 21–1000 copies/mL (LLV), respectively (Table 2). All these 502 subjects were switched to TLE400 STR. Of these, 217 (43.2%) subjects underwent plasma VL testing at 6 months. In total, 97.7% (212/217) of patients achieved a VL of ≤ 20 copies/mL at 6 months. LLV and VF at 6 months of follow‐up were seen in three (1.4%) and two (0.9%) patients, respectively. Three hundred and two (60%) patients underwent plasma VL measurements at 12 months of follow‐up. Of these, 98.0% (296/302) achieved a VL of ≤ 20 copies/mL. LLV and VF were seen in three (1.0%) and three (1.0%) individuals, respectively. Only six (1.4%) patients had a follow‐up duration on TLE400 STR of > 24 months. CD4 and plasma VL analyses were performed for all six patients. One person had a VL > 1000 copies/mL at this time‐point while the remainder had complete VS. Thus, during the entire course of the study, LLV and VF were each seen in six (1.4%) patients (Fig. 1a, b). All six patients with VF were shifted to second‐line PI‐containing ART. Of the 26 individuals who had LLV before starting TLE400 STR, 21 subjects (80.8%) achieved complete VS, three (11.5%) continued to have LLV and two (7.7%) progressed to VF at 12 months of follow‐up.
Table 2.
Evaluation of plasma viral load (primary endpoint) on a single‐tablet regimen containing tenofovir disoproxil fumarate 300 mg + lamivudine 300 mg + efavirenz 400 mg (TLE400 STR)
| Plasma viral load | At TLE400 STR initiation (n = 502) | At 6 months of follow‐up on TLE400 STR (n = 217) | At 12 months of follow‐up on TLE400 STR (n = 302) |
|---|---|---|---|
| ≤ 20 copies/mL (VS) | 476 (94.8) | 212 (97.7) | 296 (98.0) |
| 21–1000 copies/mL (LLV) | 26 (5.2) | 3 (1.4) | 3 (1.0) |
| > 1000 copies/mL (VF) | 0 (0) | 2 (0.9) | 3 (1.0) |
Values are n (%).
LLV, low‐level viraemia; VF, virological failure; VS, virological suppression.
Fig. 1.
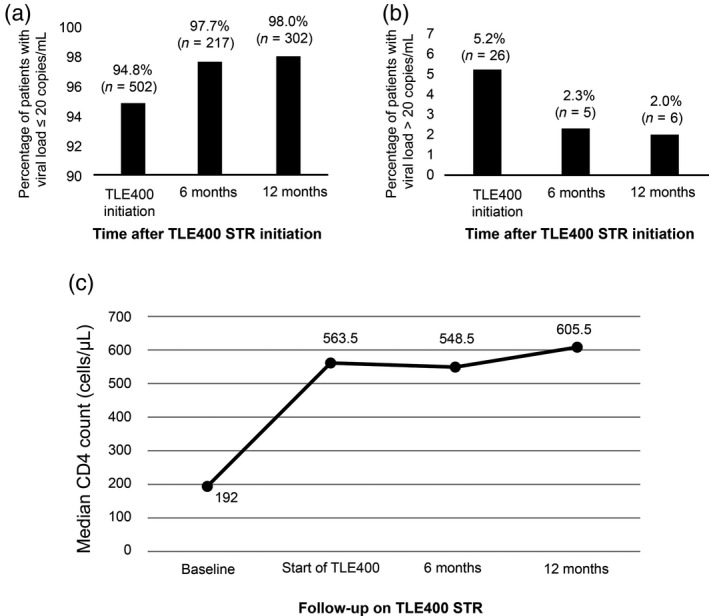
A single‐tablet regimen containing tenofovir disoproxil fumarate 300 mg + lamivudine 300 mg + efavirenz 400 mg (TLE400 STR) was successful in maintaining complete virological suppression [viral load (VL) ≤ 20 copies/mL and CD4 recovery]. (a) Percentage of patients having a plasma VL ≤ 20 copies/mL at initiation of TLE400 STR and at 6 and 12 months of follow‐up. (b) Percentage of patients having VL > 20 copies/mL at initiation of TLE400 STR and at 6 and 12 months of follow‐up. (c) CD4 counts pre‐antiretroviral therapy (ART), at initiation of TLE400 STR, and at 6 and 12 months of follow‐up were recorded and plotted at each evaluation time‐point.
CD4 count
TLE400 STR was successful in maintaining CD4 counts within the range observed at the onset of the study, with a median CD4 count of 548.5 (IQR 386.8, 788.3) cells/μL at 6 months of follow‐up. At 12 months, the median CD4 had increased to 605.5 (IQR 440.3, 818.3) cells/μL (Table 3 and Figure 1c).
Table 3.
Evaluation of CD4 counts at 6 and 12 months after initiation of a single‐tablet regimen containing tenofovir disoproxil fumarate 300 mg + lamivudine 300 mg + efavirenz 400 mg (TLE400 STR)
| Pre‐ART (n = 501) | At TLE400 initiation (n = 502) | At 6 months (n = 217) | At 12 months (n = 302) | |
|---|---|---|---|---|
| CD4 count (cells/μL) | 192.0 (92.0, 333.0) | 563.5 (386.0, 791.0) | 548.5 (386.8, 788.3) | 605.5 (440.3, 818.3) |
Values are median (interquartile range).
ART, antiretroviral therapy.
Renal function
Median creatinine clearance at 6 and 12 months of follow‐up was 102.3 (IQR 87.0, 123.6) and 109.6 (IQR 89.9, 134.9) mL/min, respectively. After starting TLE400 STR, the median decline in kidney function (creatinine clearance) over 1 year was 0.95 (IQR −4.77, 18.94) mL/min. An increase in serum creatinine to > 2 mg/dL and a decline in creatinine clearance to < 50 mL/min was seen in one patient, leading to TLE400 STR discontinuation.
Weight gain
After 12 months of TLE400 STR, there was a median weight gain of 0.5 (IQR 0, 2) kg. Severe weight gain (≥ 10% increase in body weight) was seen in 2% (6/301) of subjects. As height was not measured in all subjects, the change in body mass index (BMI) and the incidence of obesity could not be calculated.
Adverse events and treatment failure
Fifty per cent (250/502) of subjects reported AEs [central nervous system (CNS) or otherwise] while on TLE400 STR. Giddiness (119; 23.7%), depressive symptoms (91; 18.1%), insomnia (73; 14.5%), anxiety (62; 12.4%), and heaviness of head/headache (58; 11.6%) were the five most common CNS AEs attributable to EFV 400 mg (Table 4). The majority (92.4%) of AEs were grade 1 or 2. Grade 3/4 AEs were observed in 19 (3.8%) patients and led to discontinuation of TLE400 STR in 11 (2.2%) patients. Suicidal ideation was observed in four subjects, all of whom discontinued TLE400 STR. Of the 472 patients who switched from TLE600 or TEE600 STR to TLE400 STR, 217 (45.97%) indicated that TLE400 STR was a better regimen a a result of reduction or disappearance of CNS AEs. Two hundred and twenty‐three (47.25%) subjects indicated that the two regimens had similar AE profiles, while 32 (6.78%) believed that the overall experience was better on TLE600 STR.
Table 4.
Adverse events on a single‐tablet regimen containing tenofovir disoproxil fumarate 300 mg + lamivudine 300 mg + efavirenz 400 mg (TLE400 STR)
| Adverse effects | n | % |
|---|---|---|
| Giddiness | 119 | 23.7 |
| Depressive symptoms | 91 | 18.1 |
| Insomnia | 73 | 14.5 |
| Anxiety | 62 | 12.4 |
| Heaviness of head/headache | 58 | 11.6 |
| Somnolence | 56 | 11.2 |
| Abnormal dreams | 53 | 10.6 |
| Nightmares | 5 | 1.0 |
| Suicidal symptoms | 4 | 0.8 |
| Nausea | 3 | 0.6 |
| Hepatitis | 3 | 0.6 |
| Rash/pruritus | 2 | 0.4 |
| Renal | 1 | 0.2 |
| Pancreatitis | 0 | 0.0 |
| IRIS | 0 | 0.0 |
| Death | 0 | 0.0 |
IRIS, immune reconstitution inflammatory syndrome.
Treatment regimen failure was observed in 49 (9.8%) individuals (Table 5). Among these, six patients (12.24%) had VF, 11 (22.47 %) developed grade 3/4 AEs, and four were lost to follow‐up. There were no deaths in the cohort during the duration of the study.
Table 5.
Treatment regimen failure for a single‐tablet regimen containing tenofovir disoproxil fumarate 300 mg + lamivudine 300 mg + efavirenz 400 mg (TLE400 STR)
| Treatment failure | n | % |
|---|---|---|
| Yes | 49 | 100 |
| Virological failure on ART (VL > 1000 copies/mL) | 6 | 12.24 |
| Switch to earlier first‐line ART (TLE600 STR) | 12 | 24.48 |
| Switch to DTG‐based regimen | 16 | 32.65 |
| Discontinuation of TLE400 STR because of CNS Aes | 10 | 20.41 |
| Discontinuation of TLE400 because of non‐CNS Aes | 1 | 2.06 |
| Loss to follow‐up | 4 | 8.16 |
AE, adverse event; ART, antiretroviral therapy; CNS, central nervous system; DTG, dolutegravir; VL, viral load.
Pregnancy
Of the 206 women who were switched to TLE400 STR, two (0.97%) became pregnant during follow‐up. Both the children were born alive, without any reported congenital abnormalities, and tested HIV negative by qualitative DNA polymerase chain reaction (PCR). Both the mothers had complete VS at delivery.
WHO clinical stage 3/4 illness
One individual developed incident TB during follow‐up. He was started on weight‐based four‐drug anti‐tubercular therapy (isoniazid + rifampicin + pyrazinamide + ethambutol) together with TLE400 STR [35]. At the end of the anti‐tubercular therapy, VS on TLE400 STR was maintained.
Discussion
The goal of this retrospective observational study was to evaluate the safety and efficacy of switching from virologically suppressive, first‐line, NNRTI‐containing ART (predominantly TLE600 STR) to TLE400 STR among PWH in Pune, India. It was observed that TLE400 STR had excellent virological and immunological efficacy as a switch regimen. More than 97% of patients in this study who switched to TLE400 STR were able to maintain complete VS (VL ≤ 20 copies/mL). The rate of grade 3 and 4 AEs in this study (19 patients; 3.8%) was comparable to that observed in the ENCORE1 study (4.5%) [25], highlighting the overall safety of TLE400 STR. Almost 46% of patients who switched from TLE600 to TLE400 reported reduction or disappearance of CNS AEs.
The advantages of TLE400 STR over TLD STR (current WHO‐recommended preferred first‐line ART) include noninferior virological and immunological efficacy [26], lower incidence of obesity [19, 20, 26], safety and efficacy in pregnancy [27], and no need for dose adjustment in cases of HIV/TB coinfection (doubling of DTG dose needed when used with rifampicin) [36]. In a study by Lamorde et al., switching PWH who were on various NNRTI‐containing first‐line ART regimens to DTG‐containing first‐line ART led to an increased incidence of new‐onset hyperglycaemia. The majority of the hyperglycaemia events were severe, accompanied by weight loss, and required oral anti‐diabetic agents and substitution of DTG [37]. Patients with such events may benefit from a switch to EFV 400 mg. The drawbacks of the TLE400 STR include a low genetic barrier to resistance [16, 29] and the presence of neuropsychiatric AEs. In this cohort, almost 50% of patients developed AEs, although serious AEs leading to TLE400 STR discontinuation occurred only in 2.2% of patients.
The Joint United Nations Programme on HIV/AIDS (UNAIDS) previously set the 90‐90‐90 target, which envisioned that, by 2020, 90% of PWH would know their HIV status, 90% of people who knew their HIV‐positive status would be accessing ART, and 90% of people on ART would have suppressed VL [38]. However, in India, 79% of PWH knew their status and, of these, 56% were on ART by 2017 [6, 7, 13, 39]. Data concerning the third ‘90’ target are currently unavailable. Thus, despite the availability of single‐tablet ART regimens (TLE600 STR and TEE600 STR) in India and other LMICs for over a decade, the goals of starting all PWH on ART and maintaining VS have faced several challenges. Some of the main challenges include toxicity of current regimens leading to regimen discontinuation, stagnant international and national public health expenditure, finite global ART manufacturing capacity, and increasing demand [40, 41]. In order to meet the UNAIDS 90‐90‐90 target and WHO Treat All recommendations there is going to be an unprecedented demand for ART. Global ART spending will increase from US$1.4 billion in 2014 to $3.8 billion in 2020 [42]. ART optimization through dose reduction of individual drugs can improve the efficacy and reduce the toxicity of ART regimens, lower their manufacturing costs, and help to achieve these goals [23, 42]. It has been evaluated in several phase I–IV clinical trials with varying degrees of success [43, 44, 45]. The biggest success of dose reduction studies has been in the case of EFV. EFV was approved through the US Food and Drug Administration’s (FDA) accelerated review process in 1998. In the pivotal trial where EFV was evaluated in the context of ZLE, VS (VL < 400 or < 40 copies/mL at week 24) was not compromised following dose de‐escalation of EFV from 600 to 200 mg [41]. More importantly, a high level of AE‐related dropout was observed in patients who received 600 mg of EFV[41]. Despite this, the maximum effective dose of 600 mg EFV was licensed by the FDA to avoid the risk of the emergence of genotypic NNRTI resistance. Thirteen years later, the efficacy and safety of EFV 400 was proven once again in the ENCORE1 study [25]. Our study adds to the knowledge about this new regimen by positioning it as a first‐line switch strategy.
In the context of EFV‐related cost, using a 400 mg EFV dose would reduce the treatment cost by $8 per patient per year, which would result in an annual saving of approximately $84 million. Alternatively, this would allow an additional 5 million patients to be treated with the same public health expenditure [41]. There are several upper/middle‐income countries (UMICs) where branded DTG is much more expensive than generic EFV [46]. Reduction of the EFV dose from 600 to 400 mg, leading to reduced manufacturing costs, a better safety profile and the availability of generic TLE400 STR, will further increase the cost‐effectiveness of EFV 400 mg. As a result, branded versions of DTG may no longer be cost‐effective when compared to low‐cost generic EFV 400 mg.
Limitations
This study has several limitations. First, as for all retrospective studies, some subjects switching from NNRTI‐containing first‐line ART to TLE400 STR may have been unreported, leading to measurement bias. Secondly, a control group of PWH who continued TLE600 STR without switching to TLE400 STR would have helped us to compare the efficacies and safeties of the two regimens. However, such a control group was not available for this study. Thirdly, as a consequence of the pre‐existing dominance of the TLE600 STR regimen in the cohort, the efficacy of TLE400 as a stand‐alone agent could not be independently analysed. Fourthly, only six subjects completed 24 months of follow‐up on TLE400 STR. As a result, the long‐term efficacy and safety of the regimen could not be ascertained. Fifthly, although this study reported the overall frequency of AEs in response to EFV 400, it did not compare the AE rates between EFV400 and EFV600. Such a comparison has already been performed in the ENCORE1 study, which demonstrated that TLE400 resulted in fewer drug‐related AEs in comparison to TLE600 [25]. Sixthly, BMI data were not available for all patients in the cohort, and so the incidence of obesity on TLE400 STR could not be calculated. Finally, no attempt was made to evaluate the effects of pharmacogenomic determinants, such as CYP2B6, CYP3A4, CYPD6, CYP2C19 and CYP2A6, on EFV efficacy. These pharmacogenomic determinants are recognized as important influencers of EFV bioavailability and can interfere with the ability of the drug to reduce viral RNA levels [47]. Despite these limitations, the findings of this study are critical in bridging important knowledge gaps and will allow policymakers to advocate the switching of PWH who are virologically suppressed on first‐line NNRTI‐containing ART to TLE400 STR. Given that the 38 clinical sites evaluated in the landmark ENCORE1 study did not include any sites from India, this study setting provides the much‐needed first point of comparison for future studies evaluating the efficacy of TLE400 with respect to TLE600 in an Indian setting.
Conclusions
This retrospective observational cohort study performed in the LMIC setting of Pune, India demonstrates the excellent efficacy and safety of TLE400 STR as a first‐line switch regimen among Indian PWH and allows recommendation of its introduction in the Indian National ART Program as a maintenance regimen in individuals virologically suppressed on first‐line NNRTI‐containing ART.
Author contributions
AD, UM, MK, CG and CS conceived of the study and drafted the manuscript. TB, AS, RG, SK, NR and MD participated in its design and coordination, and read, revised and approved the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
Manisha Ghate MD, PhD [National AIDS Research Institute (NARI), Pune, India] edited the manuscript. Gaurav Arun Joshi MBA (Accenture Strategy Consulting) helped to prepare the figure and tables.
Conflicts of interest: The authors declare that they have no competing interests.
Funding : This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
References
- 1. Mahy M, Marsh K, Sabin K, Wanyeki I, Daher J, Ghys PD. HIV estimates through 2018. AIDS 2019; 2018: S203–S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO | Data and statistics . Available at: http://www.who.int/hiv/data/en/. Accessed on: 13 Nov 2019.
- 3. HIV and AIDS in Asia & the Pacific regional overview, Avert. 2015 . Available at: https://www.avert.org/professionals/hiv‐around‐world/asia‐pacific/overview. Accessed on: 13 Nov 2019 .
- 4. Phanuphak N, Lo Y‐R, Shao Y et al HIV epidemic in Asia: implications for HIV vaccine and other prevention trials. AIDS Res Hum Retroviruses 2015; 31: 1060–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Country comparison: HIV/AIDS ‐ people living with HIV/AIDS — the world factbook ‐ central intelligence agency . Available at: https://www.cia.gov/library/publications/resources/the‐world‐factbook/fields/364rank.html. Accessed on13 Nov 2019.
- 6. HIV and AIDS in India, Avert. 2015 . Available at: https://www.avert.org/professionals/hiv‐around‐world/asia‐pacific/india. Accessed on 13 Nov 2019
- 7. HIV facts & figures, National AIDS Control Organization, Ministry of Health and Family Welfare , Available at: http://naco.gov.in/hiv‐facts‐figures. Accessed on13 Nov 2019.
- 8. Mohite RV, Mohite VR. Performance of the prevention of parent to child transmission program: A decadal trend from rural Maharashtra, India. Indian J Sex Transm Dis AIDS 2016; 37: 52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NHM Health Statistics Information Portal, NHM Health Statistics Information Porta, Health Management Information System (HMIS) . Available at: https://nrhm‐mis.nic.in. Accessed on 13 Nov 2019.
- 10. Bhatti AB, Usman M, Kandi V. Current scenario of HIV/AIDs, treatment options, and major challenges with compliance to antiretroviral therapy. Cureus 2016; 8: e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prokofjeva MM, Kochetkov SN, Prassolov VS. Therapy of HIV infection: current approaches and prospects. Acta Naturae 2016; 8: 23–32. [PMC free article] [PubMed] [Google Scholar]
- 12. WHO, 7.2.1 First‐line ART for adults . Available at: https://www.who.int/hiv/pub/guidelines/arv2013/art/artadults/en/. Accessed on 21 Nov 2019.
- 13. National AIDS Control Organization (NACO) Annual report 2018–19. Available at: https://mohfw.gov.in/sites/default/files/24%20Chapter%20496AN2018‐19.pdf.
- 14. Walmsley SL, Antela A, Clumeck N et al Dolutegravir plus abacavir–lamivudine for the treatment of HIV‐1 infection. N Engl J Med 2013; 369: 1807–18. [DOI] [PubMed] [Google Scholar]
- 15. Clinton Health Access Initiative . ARV market report: the state of the antiretroviral drug market in low‐ and middle‐income countries, 2016–2021. September 2017 (https://clintonhealthaccess.org/content/uploads/2017/09/2017‐ARV‐Market‐Report_Final.pdf).
- 16. Updated recommendations on first‐line and second‐line antiretroviral regimens and post‐exposure prophylaxis and recommendations on early infant diagnosis of HIV: a supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization, December 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/277395/WHO‐CDS‐HIV‐18.51‐eng.pdf?ua=1. [Google Scholar]
- 17. Zash R, Holmes L, Diseko M et al Neural‐tube defects and antiretroviral treatment regimens in botswana. N Engl J Med 2019; 381: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta‐analysis of randomized trials. CurrOpin HIV AIDS 2018; 13: 102–11. [DOI] [PubMed] [Google Scholar]
- 19. Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad 2019; 5: 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Venter WDF, Moorhouse M, Sokhela S et al Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381: 803–815. [DOI] [PubMed] [Google Scholar]
- 21. Apostolova N, Funes HA, Blas‐Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother 2015; 70: 2693–2708. [DOI] [PubMed] [Google Scholar]
- 22. Mollan KR, Smurzynski M, Eron JJ, et al Association between efavirenz as initial therapy for HIV‐1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 2014; 161: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brogan AJ, Davis AE, Goodwin B. Short‐term cost analysis of raltegravir versus atazanavir + ritonavir or darunavir + ritonavir for treatment‐naive adults with HIV‐1 infection in the United States. PLoS One 2018; 13: e0203293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris E, Pérez‐Casas C, Barnhart M et al Accelerating access and scale‐up of optimized ART in low‐income and middle‐income countries: a call for a coordinated end‐to‐end approach. CurrOpin HIV AIDS 2017; 12: 383–9. [DOI] [PubMed] [Google Scholar]
- 25. ENCORE1 Study Group . Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV‐infected, antiretroviral‐naive adults (Encore1): a randomised, double‐blind, placebo‐controlled, non‐inferiority trial. Lancet 2014; 383: 1474–1482. [DOI] [PubMed] [Google Scholar]
- 26. Kouanfack C, Mpoudi‐Etame M, OmgbaBassega P et al Dolutegravir‐based or low‐dose efavirenz‐based regimen for the treatment of HIV‐1. N Engl J Med 2019; 381: 816–826. [DOI] [PubMed] [Google Scholar]
- 27. Cerrone M, Wang X, Neary M et al Pharmacokinetics of efavirenz 400 mg once daily coadministered with isoniazid and rifampicin in human immunodeficiency virus–infected individuals. Clin Infect Dis 2018; 68: 446–52. [DOI] [PubMed] [Google Scholar]
- 28. Lamorde M, Wang X, Neary M et al Pharmacokinetics, pharmacodynamics, and pharmacogenetics of efavirenz 400 mg once daily during pregnancy and post‐partum. Clin Infect Dis 2018; 67: 785–90. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization (WHO) . Update of recommendations on first‐ and second‐line antiretroviral regimens. July 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO‐CDS‐HIV‐19.15‐eng.pdf?ua=1.
- 30. Truong WR, Schafer JJ, Short WR. Once‐daily, single‐tablet regimens for the treatment of HIV‐1 infection. P T 2015; 40: 44–55. [PMC free article] [PubMed] [Google Scholar]
- 31. Dubrocq G, Rakhmanina N. The pharmacokinetics, pharmacodynamics, and clinical role of fixed dose combination of tenofovir disoproxil fumarate, lamivudine and reduced dose efavirenz (TLE‐400) in treating HIV‐1 infection. Expert Opin Drug MetabToxicol 2018; 14: 773–779. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization . WHO guidelines on the use of CD4, Viral load and early infant diagnosis (EID) tests for initiation and monitoring of ART. Available at: https://www.who.int/hiv/amds/102_WHO_Guidelines_on_CD4_and_VL_for_ART_Doherty.pdf.
- 33. National AIDS control organization (NACO) . Antiretroviral therapy guidelines. Available at: http://naco.gov.in/sites/default/files/NACO%20‐%20National%20Technical%20Guidelines%20on%20ART_October%202018%20%281%29.pdf.
- 34. Lovibond SH, Lovibond PF. Manual for the Depression Anxiety & Stress Scales. (2nd Ed.) Sydney: Psychology Foundation; Available at: http://www2.psy.unsw.edu.au/dass/Download%20files/Dass21.pdf. [Google Scholar]
- 35. Nahid P, Dorman S, Alipanah N et al Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug‐Susceptible Tuberculosis. Clin Infect Dis 2016; 63: 853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Modongo C, Wang Q, Dima M, Matsiri O, Kgwaadira B, Rankgoane‐Pono G. Clinical and Virological Outcomes of TB/HIV Coinfected Patients Treated With Dolutegravir‐Based HIV Antiretroviral Regimens: Programmatic Experience From Botswana. J Acquir Immune DeficSyndr 2019;82:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lamorde M, Atwiine M, Owarwo NC et al Dolutegravir‐associated hyperglycaemia in patients with HIV. Lancet HIV 2020;7:e461–e462. 10.1016/S2352-3018(20)30042-4. [DOI] [PubMed] [Google Scholar]
- 38. UNAIDS: 90–90‐90 . An ambitious treatment target to help end the AIDS epidemic. Available at: https://www.unaids.org/sites/default/files/media_asset/90‐90‐90_en.pdf.
- 39. Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90–90‐90 target be achieved? A systematic analysis of national HIV treatment cascades.BMJ. Global Health 2016; 1: e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grinsztejn B, Coelho LE, Luz PM, Veloso VG. Towards an ideal antiretroviral regimen for the global HIV epidemic. J Virus Erad 2017;3:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boffito M, Lamorde M, Watkins M, Pozniak A. Antiretroviral dose optimization: the future of efavirenz 400 mg dosing. Current Opinion in HIV and AIDS 2017; 12: 339–42. [DOI] [PubMed] [Google Scholar]
- 42. Accelerating Access to Simpler, Safer, and More Affordable HIV Treatment through ART Optimization, ICAP at Columbia University, OPTIMIZE. Available at: https://optimize.icap.columbia.edu. Accessed on 21 Nov 2019
- 43. Jackson A, Hill A, Puls R et al Pharmacokinetics of plasma lopinavir/ritonavir following the administration of 400/100 mg, 200/150 mg and 200/50 mg twice daily in HIV‐negative volunteers. J Antimicrob Chemother 2011; 66: 635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bunupuradah T, Kiertiburanakul S, Avihingsanon A et al Low‐dose versus standard‐dose ritonavir‐boosted atazanavir in virologically suppressed Thai adults with HIV (Lasa): a randomised, open‐label, non‐inferiority trial. Lancet HIV 2016; 3: e343–e50. [DOI] [PubMed] [Google Scholar]
- 45. Moltó J, Valle M, Ferrer E et al Reduced darunavir dose is as effective in maintaining HIV suppression as the standard dose in virologically suppressed HIV‐infected patients:a randomized clinical trial. J Antimicrob Chemother 2015; 70: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 46. Sim J, Hill A. Is pricing of dolutegravir equitable?A comparative analysis of price and country income level in 52 countries. J Virus Erad 2018; 4: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Swart M, Evans J, Skelton M et al An expanded analysis of pharmacogenetics determinants of efavirenz response that includes 3′‐utr single nucleotide polymorphisms among black South African HIV/AIDS patients. Front Genet 2016; 7: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]


