Abstract
Aims
To evaluate the efficacy and safety of ultra rapid lispro (URLi) versus lispro in adults with type 1 diabetes in a 26‐week, treat‐to‐target, phase 3 trial.
Materials and methods
After an 8‐week lead‐in to optimize basal insulin glargine or degludec, patients were randomized to double‐blind mealtime URLi (n = 451) or lispro (n = 442), or open‐label post‐meal URLi (n = 329). The primary endpoint was change from baseline glycated haemoglobin (HbA1c) to 26 weeks (non‐inferiority margin 0.4%), with multiplicity‐adjusted objectives for postprandial glucose (PPG) excursions after a meal test.
Results
Both mealtime and post‐meal URLi demonstrated non‐inferiority to lispro for HbA1c: estimated treatment difference (ETD) for mealtime URLi −0.08% [95% confidence interval (CI) −0.16, 0.00] and for post‐meal URLi +0.13% (95% CI 0.04, 0.22), with a significantly higher endpoint HbA1c for post‐meal URLi versus lispro (P = 0.003). Mealtime URLi was superior to lispro in reducing 1‐ and 2‐hour PPG excursions during the meal test: ETD −1.55 mmol/L (95% CI −1.96, −1.14) at 1 hour and − 1.73 mmol/L (95% CI −2.28, −1.18) at 2 hours (both P < 0.001). The rate and incidence of severe, documented and postprandial hypoglycaemia (<3.0 mmol/L) was similar between treatments, but mealtime URLi demonstrated a 37% lower rate in the period >4 hours after meals (P = 0.013). Injection site reactions were reported by 2.9% of patients on mealtime URLi, 2.4% on post‐meal URLi, and 0.2% on lispro. Overall, the incidence of treatment‐emergent adverse events was similar between treatments.
Conclusions
The results showed that URLi provided good glycaemic control, with non‐inferiority to lispro confirmed for both mealtime and post‐meal URLi, while superior PPG control was demonstrated with mealtime dosing.
1. INTRODUCTION
Glycated haemoglobin (HbA1c) is a commonly used measure of overall glucose exposure and a predictor of diabetes‐related complications. 1 , 2 It provides an integrated measurement of fasting and postprandial glycaemic control, 3 , 4 both of which must be well controlled in order to meet HbA1c goals. 5 Unfortunately, controlling postprandial glucose (PPG) excursions remains challenging. An Endocrine Society‐sanctioned working group recently published recommendations for optimizing PPG management in insulin‐treated adults that encompassed areas for future research, strategies for self‐management, advances in continuous glucose monitoring (CGM) and digital algorithms, as well as new insulins. 6 For optimal PPG control, an ideal prandial insulin should match carbohydrate intake by demonstrating faster absorption, more rapid onset, and shorter duration of action. 7 Although rapid‐acting insulin analogues were developed to provide better PPG control, 8 , 9 , 10 , 11 , 12 , 13 they still fall short of matching carbohydrate absorption, leaving many patients unable to achieve optimal glycaemic control.
Ultra rapid lispro (URLi) is a novel ultra rapid insulin lispro formulation developed to more closely match physiological insulin secretion. It contains treprostinil, a prostacyclin analogue, which enhances the absorption of insulin lispro through increased local vasodilation, and citrate, which speeds up insulin absorption by enhancing local vascular permeability. 14 , 15 In a euglycaemic clamp study comparing URLi to lispro (Humalog®) in patients with type 1 diabetes, URLi demonstrated earlier insulin action and a shorter duration of action. 16 The onset of appearance of insulin lispro in serum was 6 minutes faster with URLi, leading to a sevenfold higher insulin exposure in the first 15 minutes after injection. In addition, URLi resulted in 41% lower “late” insulin lispro exposure (starting 3 hours after injection). A corresponding shift was observed in the pharmacodynamic profile. The onset of insulin action was 11 minutes earlier with URLi, resulting in a threefold increase in the amount of glucose infused within the first 30 minutes. Late insulin action (amount of glucose infused from 4 hours to the end of the clamp) was reduced by 54% and the duration of action was 44 minutes shorter with URLi compared with lispro.
The aim of the present study was to demonstrate that URLi was non‐inferior to lispro in glycaemic control, as measured by change from baseline to week 26 in HbA1c, when administered at mealtime in a double‐blind manner in patients with type 1 diabetes. We also examined whether administration of URLi 20 minutes after the start of a meal, resulted in non‐inferior glycaemic control versus mealtime lispro.
2. MATERIALS AND METHODS
2.1. Study design
This phase 3 trial was a randomized, multinational, parallel group, treat‐to‐target study in adults with type 1 diabetes on a multiple daily injection (MDI) regimen in which URLi or lispro was used in combination with either insulin degludec or glargine. The study included a 1‐week screening period, an 8‐week lead‐in, and a 26‐week treatment period which was the pre‐specified primary efficacy endpoint reported here (Figure S1). An additional 26‐week treatment phase for the double‐blind mealtime treatment groups evaluated the long‐term safety of URLi (not reported). A 4‐week safety follow‐up occurred after the last study treatment visit.
The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice 17 and the Declaration of Helsinki. 18 All procedures were approved by an ethical review board and all participants provided written, informed consent. The study was registered at ClinicalTrials.gov (NCT03214367).
2.2. Participants
Adults aged ≥18 years, clinically diagnosed with type 1 diabetes (based on the World Health Organization classification) and continuously using insulin for ≥1 year, were eligible for participation if they had been treated with a rapid‐acting insulin analogue ≥90 days and basal insulin ≥30 days prior to screening, had an HbA1c of 53.0–80.3 mmol/mol (7.0–9.5%), and a body mass index (BMI) ≤35 kg/m2. Exclusion criteria included hypoglycaemia unawareness as judged by an investigator, more than one episode of severe hypoglycaemia requiring assistance, or poor glucose control requiring emergency room visit or hospitalization in the 6 months prior to screening (see Table S1 for additional entry criteria). Investigators at 166 study centres and 18 countries participated in the study.
2.3. Interventions and randomization
2.3.1. Basal dose titration
During the lead‐in period, patients were treated with insulin glargine U100 (100 units/mL) once [n = 584 (47.8%)] or twice daily [n = 99 (8.1%)] or insulin degludec U100 once daily [n = 539 (44.1%)], as determined by the investigator. The same basal insulin regimen (type, time of day and frequency) was used throughout lead‐in and treatment periods. Basal insulin dose was titrated to a fasting blood glucose target of 5.6 mmol/L by the end of lead‐in. Dose assessments were made at least weekly and adjustments were made every 3 to 4 days when appropriate (see Table S2 for basal insulin adjustment plan). After randomization, basal insulin dose was only adjusted if needed to facilitate optimal prandial insulin dosing (weeks 0 to 12) or for safety reasons.
2.3.2. Bolus dose titration and randomization
Patients were switched from their pre‐study prandial insulin to lispro (unit for unit) at the start of lead‐in. Prandial insulin doses were not changed during lead‐in unless adjustments were necessary for safety reasons or to facilitate basal insulin optimization. After lead‐in, patients were randomized in a 4:4:3 ratio to one of the following:
52 weeks of double‐blind URLi U100 administered 0 to 2 minutes prior to the start of a meal (mealtime)
52 weeks of double‐blind lispro U100 at mealtime
26 weeks of open‐label URLi U100 administered 20 minutes after the start of a meal (post‐meal URLi).
Randomization was determined by a computer‐generated random sequence using an interactive web‐response system, and stratified by country, baseline HbA1c [≤58.5 mmol/mol (7.5%), >58.5 mmol/mol], basal insulin type (glargine, degludec), and method of prandial insulin adjustment (carbohydrate counting or pattern adjustment).
During the initial 12 weeks of treatment, prandial insulin was adjusted as necessary to meet target self‐monitored blood glucose (SMBG) levels in line with recommendations by the American Association of Clinical Endocrinologists 19 (Tables S3 and S4). During the maintenance period (weeks 12–26), prandial and basal insulin doses were only adjusted if necessary, to maintain glycaemic control or for safety reasons. Recommended insulin titration algorithms were provided in the protocol and the decisions to adjust insulin dose for each patient were the investigator's responsibility.
2.3.3. Standardized meal test and SMBG
A 4‐hour liquid mixed meal tolerance test (MMTT; ~100 g carbohydrate) was performed at baseline (all patients on lispro) and week 26. Patients were required to have a fasting blood glucose of 3.9–10.0 mmol/L and the meal was to be consumed within 15 minutes. Glucose was measured at −15, 0, 15, 30, 60, 120, 180 and 240 minutes relative to meal start. The prandial insulin dose administered during the MMTT was individualized for each patient (Table S4).
Patients measured 10‐point SMBG profiles prior to scheduled visits at premeal, 1 and 2 hours after the start of the morning, midday and evening meals, and at bedtime. Patients were also instructed to perform daily measurements before morning, midday and evening meals, and at bedtime and as needed for glucose self‐management. Patients were encouraged to perform SMBG whenever hypoglycaemia was suspected and record blood glucose values and timing of events relative to meals. They were asked to treat a blood glucose value <3.9 mmol/L as hypoglycaemia.
2.4. Assessments and statistical methods
The primary efficacy measure was non‐inferiority of mealtime URLi to lispro for HbA1c change from baseline to 26 weeks, with a non‐inferiority margin of 4.4 mmol/mol (0.4%). Two primary analysis methods were employed: (a) mixed‐effects model for repeated measurements (MMRM) analysis in the efficacy estimand, which included all on‐investigational product (IP) data from randomization to week 26, and (b) analysis of covariance (ANCOVA) with multiple imputation for missing data in the intention‐to‐treat estimand (as requested by the US Food and Drug Administration), including all data from randomization to week 26 regardless of IP use. Both analysis models included treatment, strata (pooled country, type of basal insulin, and prandial insulin dosing plan), and the covariate of baseline HbA1c. The MMRM model also included visit, treatment‐by‐visit interaction, and an unstructured covariance structure.
A graphical approach for multiple comparisons 20 (Figure S3) was used to strongly control the overall type I error (two‐sided alpha level of 0.05) for testing the treatment effect for the primary and the following multiplicity‐adjusted objectives: superiority of mealtime URLi to lispro for the 1‐ and 2‐hour PPG excursions and the change from baseline to week 26 in HbA1c; and non‐inferiority of post‐meal URLi to lispro for the change from baseline to week 26 in HbA1c. The superiority of URLi to lispro in controlling HbA1c was assessed with the same primary analysis model.
Safety analyses were conducted on all randomized patients who received ≥1 dose of the IP. Anti‐insulin lispro antibodies were measured throughout the study. Patient‐reported adverse events, including serious adverse events (SAEs) and treatment‐emergent adverse events (TEAEs), were summarized by preferred term and/or system organ class using the Medical Dictionary for Drug Regulatory Activities (MedDRA) version 21.0. When statistical comparisons were applied, Fisher's exact test was used. Severe hypoglycaemia (an episode requiring assistance due to neurological impairment as confirmed by the investigator) was reported as an SAE. For other categories of hypoglycaemia, rate and incidence of events were analysed using a negative binomial regression model and a logistic regression model, respectively.
It was estimated that 1199 randomized patients would provide 99% statistical power to demonstrate non‐inferiority of URLi to lispro for change in HbA1c from baseline to 26 weeks, with assumptions of no difference between treatment, an SD of 1.1%, at a two‐sided α‐level 0.05, a 4:4:3 randomization ratio (mealtime URLi, lispro and post‐meal URLi) and a 15% dropout rate.
3. RESULTS
A total of 1222 patients were randomized to mealtime URLi (n = 451), lispro (n = 442) and post‐meal URLi (n = 329), with 94% of patients completing 26 weeks of study treatment (Figure S2). Demographic and baseline characteristics were similar in the three groups (Table 1).
TABLE 1.
Baseline characteristics
| Mealtime URLi | Mealtime lispro | Post‐meal URLi | Overall | |
|---|---|---|---|---|
| N = 451 | N = 442 | N = 329 | N = 1222 | |
| Age, years | 44.1 ± 13.7 | 44.5 ± 13.6 | 44.5 ± 14.3 | 44.4 ± 13.8 |
| Men, % | 55.4 | 57.9 | 55.3 | 56.3 |
| Race, n (%) | ||||
| American Indian or Alaska native | 1 (0.2) | 0 (0.0) | 2 (0.6) | 3 (0.2) |
| Asian | 86 (19.1) | 78 (17.6) | 63 (19.1) | 227 (18.6) |
| Black or African American | 7 (1.6) | 9 (2.0) | 5 (1.5) | 21 (1.7) |
| Multiple | 10 (2.2) | 11 (2.5) | 5 (1.5) | 26 (2.1) |
| Not reported | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| White | 346 (76.7) | 344 (77.8) | 254 (77.2) | 944 (77.3) |
| Hispanic or Latino, n (%) | 35 (7.8) | 33 (7.5) | 35 (10.6) | 103 (8.4) |
| Weight, kg | 77.3 ± 16.2 | 77.3 ± 16.7 | 77.6 ± 17.3 | 77.4 ± 16.7 |
| BMI, kg/m2 | 26.6 ± 4.2 | 26.4 ± 4.3 | 26.7 ± 4.6 | 26.6 ± 4.4 |
| Duration of diabetes, years | 18.8 ± 12.3 | 19.1 ± 12.0 | 18.8 ± 11.7 | 18.9 ± 12.0 |
| Prandial insulin at study entry, n (%) | ||||
| Insulin aspart | 218 (48.3) | 216 (48.9) | 163 (49.5) | 597 (48.9) |
| Insulin glulisine | 51 (11.3) | 65 (14.7) | 40 (12.2) | 156 (12.8) |
| Insulin lispro | 182 (40.4) | 161 (36.4) | 126 (38.3) | 469 (38.4) |
| Basal insulin during study, n (%) | ||||
| Insulin glargine once daily | 212 (47.0) | 219 (49.5) | 153 (46.5) | 584 (47.8) |
| Insulin glargine twice daily | 42 (9.3) | 30 (6.8) | 27 (8.2) | 99 (8.1) |
| Insulin degludec | 197 (43.7) | 193 (43.7) | 149 (45.3) | 539 (44.1) |
| Bolus insulin plan during study, n (%) | ||||
| Carbohydrate counting | 201 (44.6) | 205 (46.4) | 148 (45.0) | 554 (45.3) |
| Pattern‐adjustment algorithm | 250 (55.4) | 237 (53.6) | 181 (55.0) | 668 (54.7) |
| Personal CGM/FGM use, n (%) | 45 (10.0) | 51 (11.5) | 46 (14.0) | 142 (11.6) |
| HbA1c at study entry | ||||
| mmol/mol | 64.4 ± 7.1 | 64.2 ± 7.3 | 64.2 ± 6.7 | 64.3 ± 7.1 |
| % | 8.04 ± 0.65 | 8.02 ± 0.67 | 8.03 ± 0.61 | 8.03 ± 0.65 |
| HbA1c at baseline | ||||
| mmol/mol | 56.7 ± 7.1 | 56.7 ± 7.3 | 56.9 ± 7.0 | 56.7 ± 7.1 |
| % | 7.34 ± 0.65 | 7.33 ± 0.67 | 7.36 ± 0.64 | 7.34 ± 0.65 |
Abbreviations: BMI, body mass index; CGM, continuous glucose monitoring; FGM, flash glucose monitoring; HbA1c, glycated haemoglobin; URLi, ultra rapid lispro.
Data are mean ± SD, unless otherwise stated.
3.1. Efficacy
3.1.1. Glycated haemoglobin
Mean HbA1c decreased in all groups, from 64.3 mmol/mol (8.03%) at screening, to a baseline of 56.7 mmol/mol (7.34%) with mealtime URLi, 56.7 mmol/mol (7.33%) with lispro and 56.9 mmol/mol (7.36%) with post‐meal URLi. By week 26, mean HbA1c had stabilized to 55.3 mmol/mol (7.21%) with mealtime URLi, 56.1 mmol/mol (7.29%) with lispro, and 57.6 mmol/mol (7.42%) with post‐meal URLi (Figure 1), with significantly higher HbA1c observed for post‐meal URLi from week 4 to 26 (Figure 1). The least‐squares mean change from baseline to week 26 was −1.4 mmol/mol (−0.13%) for mealtime URLi, −0.6 mmol/mol (−0.05%) for lispro, and +0.8 mmol/mol (0.08%) for post‐meal URLi. No significant treatment‐subgroup interactions were observed for HbA1c reduction by baseline HbA1c, prandial insulin dosing plan, or basal insulin type (Table S5).
FIGURE 1.
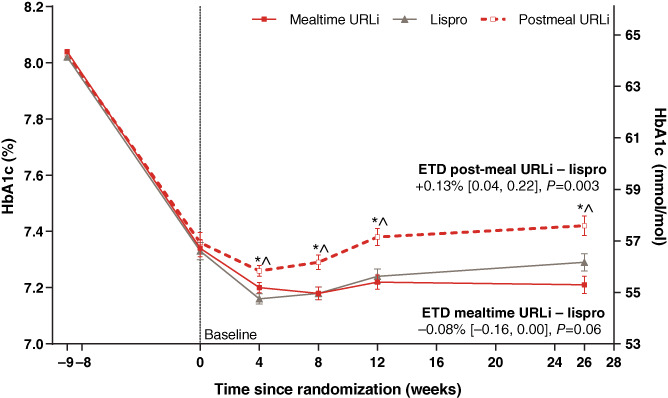
Mean glycated haemoglobin (HbA1c) from study entry to week 26. Data are mean at study entry and least squares mean ± SE at all other time points and based on the mixed‐effects model for repeated measures analysis. *P <0.05 for pairwise comparison of post‐meal ultra rapid lispro (URLi) versus lispro; ^ P <0.05 for pairwise comparison of post‐meal URLi versus mealtime URLi. Estimated treatment difference (ETD) between URLi and lispro (URLi−lispro) was −0.8 mmol/mol (−0.08%) with a two‐sided 95% confidence interval (CI) of −1.7 to 0.0 mmol/mol (−0.16 to 0.00%) for mealtime URLi, and +1.4 mmol/mol (+ 0.13%) with 95% CI 0.5 to 2.4 mmol/mol (0.04 to 0.22%) for post‐meal URLi, demonstrating non‐inferiority of both mealtime and post‐meal URLi to lispro in the change from baseline to week 26 in HbA1c
Non‐inferiority of both mealtime and post‐meal URLi to lispro was confirmed in the change from baseline to week 26 in HbA1c (Figure 1). Mealtime URLi did not reach superiority with the MMRM analysis but was statistically superior to lispro with the ANCOVA analysis: estimated treatment difference (ETD) −0.9 mmol/mol (95% CI −1.8, −0.0); P = 0.041 [−0.08% (95% CI −0.16, −0.00)].
3.1.2. MMTT at week 26
Mealtime URLi demonstrated superiority to lispro in controlling 1‐ and 2‐hour PPG excursions during the MMTT (Figure 2), ETD −1.55 mmol/L and − 1.73 mmol/L (all P < 0.001) at 1‐ and 2‐hours respectively. The incremental area under the serum glucose concentration–time curve (above the glucose level at the start of MMTT) from 0 to 4 hours (iAUC0–4h) was 28% lower in the mealtime URLi versus the lispro group (P < 0.001), not significantly different between the post‐meal URLi and lispro groups (P = 0.352), and 32% higher in the post‐meal versus the mealtime URLi group (P < 0.001). Mean bolus insulin doses for the MMTT were similar in all treatment groups [mealtime URLi 12.9 U (0.17 U/kg); lispro 12.5 U (0.16 U/kg); post‐meal URLi 13.0 U (0.17 U/kg)].
FIGURE 2.
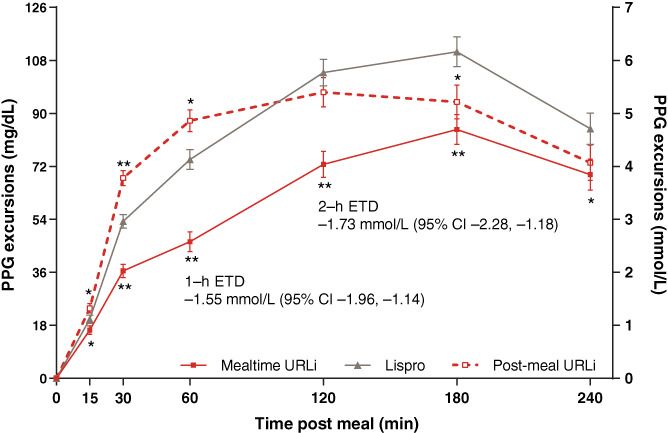
Postprandial glucose (PPG) excursions during a meal test at week 26. Data are least squares mean ± SE. *P <0.05 for pairwise comparison of ultra rapid lispro (URLi) versus lispro; **P <0.001 for pairwise comparison of URLi versus lispro. Prandial insulin dose for the mixed‐meal tolerance test (MMTT) was individualized for each patient based on their prandial insulin dosing plan. For patients using carbohydrate counting, the morning meal insulin‐to‐carbohydrate ratio was used to calculate the prandial insulin dose for the MMTT. For patients not using carbohydrate counting, the prandial insulin dose for MMTT was calculated based on the average total daily insulin dose for the 3 days prior to the MMTT. CI, confidence interval; ETD, estimated treatment difference (mealtime URLi−lispro)
3.1.3. Self‐monitored blood glucose
At week 26, the mealtime URLi group had significantly lower PPG levels at 1 and 2 hours after the morning meal compared to lispro and post‐meal URLi (Figure 3). Overall daily mean glucose values were significantly lower with mealtime URLi compared with post‐meal URLi (8.93 mmol/L versus 9.27 mmol/L; P = 0.012) and not significantly different between mealtime URLi and lispro. However, mealtime URLi resulted in significantly greater improvement from baseline in the mean daily 1‐ and 2‐h PPG values and excursions compared to lispro and post‐meal URLi (Table S6). These improvements were not consistently associated with improvements in measures of between‐ or within‐day glucose variability. Post‐meal URLi resulted in significantly higher daily mean PPG levels and excursions at 1 hour after meals versus lispro (Table S6).
FIGURE 3.
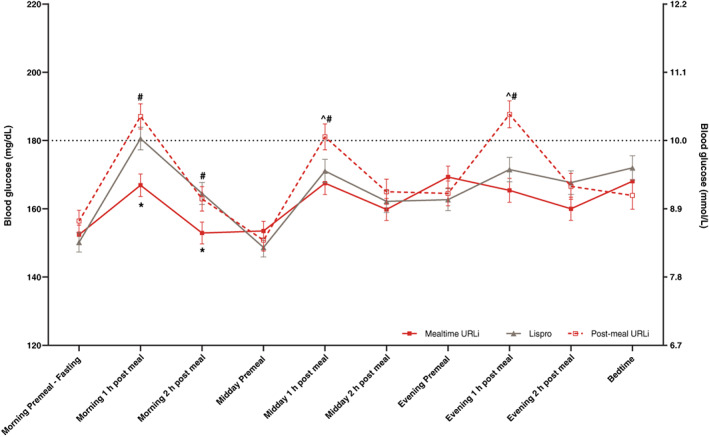
Ten‐point self‐monitored blood glucose profile at week 26. Data are least squares mean ± SE. *P <0.05 for pairwise comparison of mealtime URLi versus lispro; ^ P <0.05 for pairwise comparison of post‐meal URLi versus lispro; #P <0.05 for pairwise comparison of post‐meal URLi versus mealtime URLi
3.1.4. Insulin dosing
There were no significant treatment differences in daily basal and total insulin dose at baseline or week 26 (Table S7). However, a significantly lower daily bolus dose was observed at baseline with mealtime URLi versus post‐meal URLi when adjusted for weight (0.39 versus 0.42 U/kg/d; P = 0.045). No other significant treatment differences were observed in daily bolus insulin dose at baseline or week 26. The ratio of prandial to total insulin dose at week 26 was similar in each group (~52%). No significant treatment‐subgroup interactions were observed for total daily insulin dose or ratio of prandial to total insulin by basal insulin type (Table S5). However, significantly higher total daily insulin dose was observed with URLi treatment when basal insulin degludec was used [ETD 0.04 U/kg/d (95% CI 0.00, 0.08); Table S7].
3.1.5. Other efficacy measures
After 26 weeks of treatment, no significant differences were observed between mealtime URLi and lispro groups in the proportion of patients achieving HbA1c targets <53.0 mmol/mol (7.0%) (URLi, 37.4% vs. lispro, 33.6%) and ≤47.5 mmol/mol (6.5%) (URLi, 16.8% vs. lispro, 15.6%). However, significantly fewer patients achieved HbA1c <53.0 mmol/mol and ≤47.5 mmol/mol in the post‐meal URLi group compared to lispro and mealtime URLi (25.6% and 10.0%, respectively; P <0.05).
3.2. Safety
The incidence and rate of severe hypoglycaemia was similar among the groups, with 25 patients (5.7%) reporting 40 episodes with lispro, 25 (5.5%) reporting 37 episodes with mealtime URLi, and 15 (4.6%) reporting 22 episodes with post‐meal URLi. No significant differences were observed among the groups in the rate or incidence of nocturnal and documented hypoglycaemia (Figure 4). There were no clinically significant treatment differences in the rate and incidence of postprandial hypoglycaemia; however, in the late postprandial period (>4 hours after the meal), the rate of hypoglycaemia was significantly lower for mealtime URLi compared to lispro and post‐meal URLi (Figure 4). No significant treatment‐subgroup interactions were observed for hypoglycaemia risk by baseline HbA1c, basal insulin type or prandial insulin dosing plan (Table S5).
FIGURE 4.
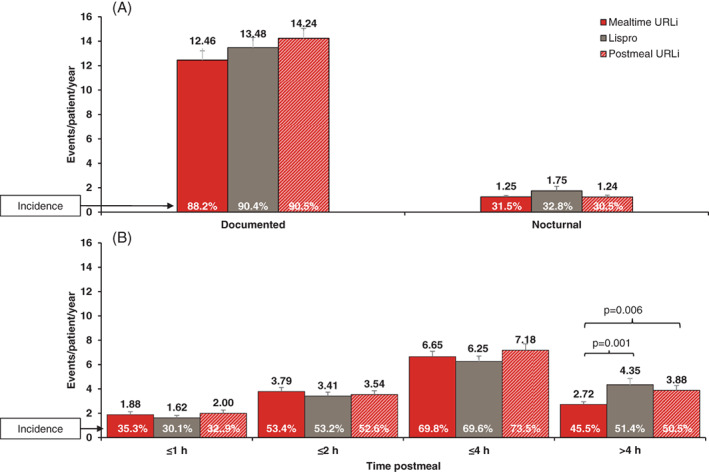
Rate and incidence of hypoglycaemia (with or without symptoms) from randomization to week 26 [blood glucose <3.0 mmol/L (54 mg/dL)]. A, Rate and incidence of documented and nocturnal hypoglycaemia. B, Rate and incidence of post‐meal hypoglycaemia. Data are least squares mean (LSM) + SE for event rate and LSM for incidence. Note: Nocturnal hypoglycaemia was defined as documented hypoglycaemia occurring between bedtime and waking
Two deaths occurred during the study which were not considered related to study treatment: one patient in the mealtime URLi group (colon cancer) and one in the lispro group (aortic stenosis). The incidences of SAEs, discontinuations from the study due to an AE, and incidences of TEAEs were similar across groups (Table S8). One hundred patients (8.2%) experienced ≥1 SAE. Severe hypoglycaemia was the most frequently reported SAE. The numbers of patients who experienced an AE leading to treatment discontinuation (n = 16) were similar among the groups (mealtime URLi, n = 6; lispro, n = 5; post‐meal URLI, n = 5) and included six cases of pregnancy that were reported as AEs for tracking purposes. Overall, 22 patients (1.8%) experienced ≥1 injection site reaction TEAE, with a total of 38 injection site reaction TEAEs documented; all were of mild or moderate severity, and none resulted in discontinuation from study treatment. More patients in the mealtime URLi [n = 13 (2.9%)] and post‐meal URLi [n = 8 (2.4%)] versus the lispro group [n = 1 (0.2%)] experienced ≥1 injection site reaction TEAE, the most common being reported as “injection site reaction” and “injection site pain”.
There were no clinically meaningful changes in any laboratory values from randomization to week 26 in all groups. Overall, no significant treatment differences were observed in number of patients with treatment‐emergent anti‐insulin lispro antibodies. The mean percentage antibody binding values were low throughout the study and no between‐treatment differences were noted after 4 weeks of treatment. No significant treatment differences were observed in change from baseline to week 26 for blood pressure, pulse or BMI. Average weight gain from baseline to week 26 was also similar in the three groups: 0.6 kg mealtime URLi; 0.8 kg lispro; 0.6 kg post‐meal URLi.
4. DISCUSSION
In this trial we confirmed that, in patients with type 1 diabetes using MDI therapy, both mealtime and post‐meal URLi were non‐inferior to lispro for change in HbA1c following 26 weeks of treatment. Mealtime URLi was superior to lispro according to one of the two pre‐specified primary analyses (ANCOVA), but the difference was small [ETD −0.8 mmol/mol (0.08%)]. All three treatment groups showed improved glycaemic control from screening to week 26, with a clinically significant improvement in HbA1c during the lead‐in (basal optimization) period. These improvements are also consistent with those observed in other treat‐to‐target trials conducted in patients with type 1 diabetes using MDI regimens. 21 , 22 , 23 Importantly, the improvement in glycaemic control occurred without increasing the risk of overall, nocturnal or postprandial hypoglycaemia, particularly for the mealtime groups. Postprandial hypoglycaemia during the period >4 hours after meals was lower with mealtime URLi compared with lispro (~37% lower rate) and post‐meal URLi (~30% lower rate). This finding is significant as it reflects the shorter duration of action and lower late insulin exposure observed with URLi compared to lispro as demonstrated in clinical pharmacology studies. 16 , 24
Non‐inferiority of post‐meal URLi to lispro in HbA1c change from baseline to week 26 was also confirmed, although post‐meal administration of URLi resulted in a significantly higher HbA1c at the end of 26 weeks than both lispro and mealtime URLi, and the proportion of patients achieving HbA1c targets ≤47.5 mmol/mol (6.5%) and <53.0 mmol/mol (7.0%) was significantly lower. These results suggest that post‐meal administration of URLi is an acceptable option if needed for dose individualization, for instance, when premeal blood glucose levels are trending low or with unpredictable food intake. However, habitual dosing of URLi 20 minutes after the meal, as required in this registration trial, is not recommended due to its reduced efficacy compared to mealtime administration.
Mealtime URLi demonstrated superiority to lispro in reducing 1‐ and 2‐hour PPG excursions during the MMTT at week 26, meeting the pre‐specified multiplicity‐adjusted objectives. Mealtime URLi treatment resulted in significantly lower PPG excursions compared to lispro treatment at all time points from 15 minutes to 4 hours. Post‐meal URLi resulted in significantly higher PPG excursions within the first hour after the MMTT compared with lispro, but had similar overall PPG control when evaluating the iAUC. Supportive of the MMTT findings, at week 26 the mealtime URLi treatment group had significantly lower SMBG levels at the morning 1‐ and 2‐hour post‐meal time points. Evaluation of mean daily 1‐ and 2‐hour PPG values and excursions also showed that mealtime URLi was associated with improved postprandial control compared with lispro and post‐meal URLi. These findings are further supported by results from a blinded CGM substudy of the patients in the present study. 25 Mealtime URLi reduced post‐meal iAUC0–4h for all meals combined by 75% compared with lispro (P = 0.008) and increased time in target range (3.9–10.0 mmol/L) during the daytime period by 4% versus lispro and 4.5% versus post‐meal URLi (both P <0.05), without increasing time in hypoglycaemia.
The differences observed in HbA1c reduction and PPG excursions were not attributable to differences in prandial, basal or total daily insulin dosing. There were no significant treatment differences in basal, bolus and total insulin dose at baseline and week 26. The ratio of prandial to total insulin dose at week 26 was similar in all treatment groups (~52%) and was consistent with prior treat‐to‐target MDI regimen studies in patients with type 1 diabetes treated with insulin glargine and a rapid‐acting insulin analogue. 26
Treatment with URLi was well tolerated in this 26‐week study. The incidences of SAEs, discontinuations from the study due to an AE, deaths and TEAEs were similar among the groups. As expected in type 1 diabetes, severe hypoglycaemia was the most frequently reported SAE per protocol, and there were no significant differences in the incidence or rate between groups. Although the total number was small, a greater number of patients in the URLi groups (n = 21; 2.7%) compared to the lispro group (n = 1; 0.2%) experienced ≥1 pooled injection site reaction TEAE. Most events were mild, and none led to treatment discontinuation. The incidence of injection site reactions reported in this trial is similar to that for other approved insulins such as fast‐acting insulin aspart (1.6%) 27 and insulin glargine (2.7%). 28
The strengths of the present study include the double‐blinding of participants enrolled in the mealtime treatment groups, which decreases the risk of bias. Limitations are that, although this global study involved a number of countries, the diversity of the study population was limited, with the participants being primarily white. An unexpectedly low number (~12%) of patients reported use of personal CGM or flash glucose monitoring. This may have stemmed from low access to CGM (including availability and affordability) in some participating countries at the time the study was conducted. CGM use was optional and instructions were provided to use the SMBG glucose meter and eDiary system to standardize inputs used for dose assessment.
In conclusion, this 26‐week treat‐to‐target trial demonstrated that URLi, when administered as prandial insulin at mealtime in combination with basal insulin, provided good glycaemic control and superior postprandial glucose control compared to lispro without increasing the risk of hypoglycaemia in patients with type 1 diabetes. Whereas post‐meal dosing of URLi compared to pre‐meal lispro resulted in non‐inferiority of HbA1c, only pre‐meal dosing of URLi at the start of the meal improved postprandial glucose control and thus maximized the benefits of this ultra rapid insulin formulation.
CONFLICTS OF INTEREST
L.K. received research grants from Abbott, Ascensia Diabetes Care, Cnoga Medical, Eli Lilly and Company, Gan & Lee, Medtronic, Novo Nordisk, REMD Biotherapeutics, Sanofi, Senseonics, Xeris and Zealand Pharma. D.C., M.A.D., J.T. and J.M.B‐V. are employees and shareholders of Eli Lilly and Company. J.M. received speaker fees from Abbott, Astellas, Astra Zeneca, Böhringer Ingelheim, Eli Lilly and Company, Johnson & Johnson, Kyowa Kirin, Novartis, Novo Nordisk, MSD, Mylan, Taisho Pharma, Tanabe‐Mitsubishi, Terumo and Sanofi, and consultant fees from Abbott, Astra Zeneca, Kanro, Kowa, and Terumo. D.D. received research grants or speaker fees from Astra Zeneca, Böhringer Ingelheim, Eli Lilly and Company, Lexicon Pharma, Mylan, Novartis, Novo Nordisk, and Zealand Pharma. J.L reports no conflicts of interest. No other potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
L.K., J.M., D.D. and J.L. participated as trial investigators and reviewed and edited the manuscript. D.C. and M.A.D. contributed to the study design, the statistical analyses, the interpretation of the research, and writing the manuscript. J.T. contributed to the study design, medical oversight, interpretation of the research, and writing of the manuscript. J.M.B‐V. was responsible for medical oversight during the trial and contributed to the study design, the data analysis and interpretation of the research, and writing the manuscript. All authors approved the final manuscript to be published. J.M.B‐V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
DATA AVAILABILITY STATEMENT
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data‐sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGMENTS
The authors thank the study participants, and the investigators and study coordinators who provided care for them. The authors also thank Drs Thomas Hardy and Qianyi Zhang (Eli Lilly and Company, Indianapolis, Indiana) for critically reviewing the manuscript and Farai Chigutsa (Eli Lilly and Company) for medical writing and editorial assistance.
Klaff L, Cao D, Dellva MA, et al. Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: Results from the 26‐week PRONTO‐T1D study. Diabetes Obes Metab. 2020;22:1799–1807. 10.1111/dom.14100
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14100.
Funding information Eli Lilly and Company
REFERENCES
- 1. Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643‐2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184‐S190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 6. Leahy JJL, Aleppo G, Fonseca VA, et al. Optimizing postprandial glucose management in adults with insulin‐requiring diabetes: report and recommendations. J Endocr Soc. 2019;3(10):1942‐1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heinemann L, Muchmore DB. Ultrafast‐acting insulins: state of the art. J Diabetes Sci Technol. 2012;6(4):728‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boss AH, Petrucci R, Lorber D. Coverage of prandial insulin requirements by means of an ultra‐rapid‐acting inhaled insulin. J Diabetes Sci Technol. 2012;6(4):773‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heinemann L, Hompesch M, Flacke F, et al. Reduction of postprandial glycemic excursions in patients with type 1 diabetes: a novel human insulin formulation versus a rapid‐acting insulin analog and regular human insulin. J Diabetes Sci Technol. 2011;5(3):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hompesch M, Muchmore DB, Morrow L, Vaughn DE. Accelerated insulin pharmacokinetics and improved postprandial glycemic control in patients with type 1 diabetes after coadministration of prandial insulins with hyaluronidase. Diabetes Care. 2011;34(3):666‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrow L. BIOD‐531 demonstrates superior prandial glucose control, postmeal dosing flexibility, and less insulin “stacking” compared to marketed prandial/basal insulins. Paper presented at: European Association for the Study of Diabetes 2015; Stockholm, Sweden.
- 12. Krasner A, Pohl R, Simms P, Pichotta P, Hauser R, De Souza E. A review of a family of ultra‐rapid‐acting insulins: formulation development. J Diabetes Sci Technol. 2012;6(4):786‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen G, Meiffren G, Lamers D, et al. Ultra‐rapid BioChaperone Lispro improves postprandial blood glucose excursions vs insulin lispro in a 14‐day crossover treatment study in people with type 1 diabetes. Diabetes Obes Metab. 2018;20(11):2627‐2632. [DOI] [PubMed] [Google Scholar]
- 14. Pratt E, Leohr J, Heilmann C, Johnson J, Landschulz W. Treprostinil causes local vasodilation, is well tolerated, and results in faster absorption of insulin lispro. Diabetes. 2017;66:A253. [Google Scholar]
- 15. Michael MD, Zhang C, Siesky AM, et al. Exploration of the mechanism of accelerated absorption for a novel insulin lispro formulation. Diabetes. 2017;66:A250. [Google Scholar]
- 16. Linnebjerg H, Zhang Q, LaBell E, et al. Ultra Rapid Lispro (URLi) accelerates insulin lispro absorption and insulin action vs. Humalog® (Lispro) in patients with T1D. Diabetes. 2019;68:1107. [Google Scholar]
- 17.International Conference on Harmonisation. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice [article online], 2016. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. Accessed October 7, 2019.
- 18. World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects 2018. https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/
- 19. Bailey TS, Grunberger G, Bode BW, et al. American Association of Clinical Endocrinologists and American College of Endocrinology 2016 outpatient glucose monitoring consensus statement. Endocr Pract. 2016;22(2):231‐261. [DOI] [PubMed] [Google Scholar]
- 20. Bretz F, Posch M, Glimm E, Klinglmueller F, Maurer W, Rohmeyer K. Graphical approaches for multiple comparison procedures using weighted Bonferroni, Simes, or parametric tests. Biom J. 2011;53(6):894‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vora J, Christensen T, Rana A, Bain SC. Insulin degludec versus insulin glargine in type 1 and type 2 diabetes mellitus: a meta‐analysis of endpoints in phase 3a trials. Diabetes Ther. 2014;5(2):435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garber AJ. Treat‐to‐target trials: uses, interpretation and review of concepts. Diabetes Obes Metab. 2014;16(3):193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Russell‐Jones D, Bode BW, De Block C, et al. Fast‐acting insulin aspart improves glycemic control in basal‐bolus treatment for type 1 diabetes: results of a 26‐week multicenter, active‐controlled, treat‐to‐target, randomized, parallel‐group trial (onset 1). Diabetes Care. 2017;40(7):943‐950. [DOI] [PubMed] [Google Scholar]
- 24. Leohr J, Dellva M, Coutant D, et al. Ultra Rapid Lispro (URLi) accelerates insulin lispro absorption and insulin action vs. Humalog (Lispro) in patients with T2D. Diabetes. 2019;68:1100. [Google Scholar]
- 25. Bode BW, Cao D, Liu R, Hardy T, Bue‐Valleskey J. Ultra Rapid Lispro (URLi) improves postprandial glucose (PPG) control and time in range (TIR) in T1D compared with humalog (Lispro): PRONTO‐T1D continuous glucose monitoring (CGM) substudy. Diabetes. 2019;68:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Candido R, Wyne K, Romoli E. A review of basal‐bolus therapy using insulin glargine and insulin lispro in the management of diabetes mellitus. Diabetes Ther. 2018;9(3):927‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FIASP (insulin aspart) [package insert]. Bagsvaerd, Denmark, Novo Nordisk, 2017.
- 28.Lantus (insulin glargine injection) [package insert]. Bridgewater, NJ, Sanofi‐Aventis U.S. LLC, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Data Availability Statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data‐sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.


