Abstract
TGF‐β induced factor homeobox 1 (TGIF1) and splicing factor 1 (SF1) are important for mammalian reproduction; however, the effects of these genes on litter size in sheep remain unexplored. In this study, we genotyped 768 ewes from seven sheep breeds at two loci: g.37871539C>T, a synonymous mutation of TGIF1; and g.42314637T>C, a 3′UTR variant of SF1. Our analysis of polymorphism revealed only two genotypes at locus g.37871539C>T in TGIF1, with most sheep populations being moderately polymorphic (0.25 < PIC < 0.5) at this site. In contrast, most breeds exhibited low polymorphism (PIC ≤0.25) at the SF1 locus g.42314637T>C. The association analysis revealed that a synonymous mutation at g.37871539C>T in TGIF1 was highly associated with litter size in Small Tail Han sheep, in which it causes a significant decrease in litter size. Conversely, while the SF1 3′UTR variant g.42314637T>C was also highly associated with litter size in sheep, it causes a significant increase in the number of litter size. Combined, these data provide valuable information regarding candidate genetic markers for sheep breeding programs.
Keywords: litter size, SF1, sheep, SNPs, TGIF1
1. INTRODUCTION
Understanding the genetics of reproduction is important for the sheep industry, and recent researches have revealed that the regulation of litter size in sheep and goats is multiallelic and polygenic (Wang et al., 2019; Zhang et al., 2020). For example, FecB is a prominent gene affecting litter size, and ewes with the genotype of FecB BB carry two copies of a FecB mutation. This increases their ovulation rate and litter size by 3 and 1.5, respectively, over ewes with the genotype of FecB++. Likewise, ewes with one copy of the FecB mutation (FecB B+) have an ovulation rate and litter size 1.5 and 1 greater than FecB++ ewes, respectively (Liu et al., 2014). Mutations in a number of other genes have also been demonstrated to be useful genetic markers for sheep breeding. These genes include bone morphogenetic protein 15 (BMP15; Calvo et al., 2020), growth differentiation factor 9 (GDF9; Våge, Husdal, Kent, Klemetsdal, & Boman, 2013), melatonin receptor 1A (MTNR1A; He, Zhang, Liu, & Chu, 2019), BMP7 and BMP2 (Zhang, Liu, et al., 2019). Such findings indicate that the identification of genetic markers can facilitate the progress of molecular marker‐assisted breeding in sheep.
TGF‐β induced factor homeobox 1 (TGIF1) is a member of the three‐amino‐acid loop‐extension (TALE) superfamily that is highly expressed in human (Hu, Yu, Shaw, Renfree, & Pask, 2011) and sheep (Jiang et al., 2014) ovaries, and highly conserved in mammals (Shen & Walsh, 2005). This suggests that TGIF1 plays a critical role in mammalian reproduction. Studies have also revealed that TGF‐β/SMAD signalling is important in reproductive processes, including in follicular activation (Yin, Chang, Yi, Yao, & Leung, 2020), ovarian follicle development (Knight & Glister, 2006) and oocyte maturation (Yin et al., 2020). Indeed, the inhibition or mutation of key members of the TGF‐β/SMAD signalling pathway can cause reproductive problems (Chand, Robertson, Shelling, & Harrison, 2007), including sterility (Li et al., 2017). Significantly, TGIF1 in human and zebra fish functions as a repressor, and reversibly modulates important members of the TGF‐β/SMAD signalling pathways that are activated by TGF‐β, such as SMAD2 and SMAD4 (Hu et al., 2011; Hyman, Bartholin, Newfeld, & Wotton, 2003; Wotton, Lo, Lee, & Massagué, 1999). Therefore, TGIF1 may be a crucial factor in the reproduction of sheep and other mammalian species.
Mammalian splicing factor 1 (SF1) is indispensable for recognizing pre‐mRNA 3′ splice sites during the process of early spliceosome assembly (Selenko et al., 2003). SF1 also participates in the formation of extraspliceosomal complexes (Rino, Desterro, Pacheco, Gadella, & Carmo‐Fonseca, 2008), which may influence the expression of other genes. Further studies have suggested that SF1 plays important functions in alternative splicing (Corioni, Antih, Tanackovic, Zavolan, & Krämer, 2010), and notably, alternative splicing events in genes such as oestrogen receptor alpha (Schoen, Sharbati, Ritter, & Jewgenow, 2013) and kisspeptin‐1 receptor (Mechaly, Viñas, & Piferrer, 2009) were involved in reproduction. Also, Miao, Luo, Zhao, and Qin (2018) revealed that a number of alternative splicing events are associated with ewe fecundity in diverse sheep breeds, by analysing ovarian transcriptomes. Together, these findings suggest that SF1 may be a factor affecting sheep reproduction.
In this study, we examined the association between TGIF1 and SF1 polymorphisms and litter size in Small Tail Han sheep. This is the first analysis of such data, and our results provide useful genetic markers for future sheep breeding programs.
2. MATERIALS AND METHODS
2.1. Animal and sample preparation
All experimental procedures involving animals were approved by the Science Research Department (in charge of animal welfare issues) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS‐CAAS; Beijing, P. R. China). Ethics approval was granted by the animal ethics committee of IAS‐CAAS (No. IASCAAS‐AE‐03; 12 December 2016).
This study began in June, 2016. Total DNA was extracted from blood samples obtained from the jugular veins of 768 ewes representing seven sheep breeds. The higher prolificacy breeds included in this study were the Small Tail Han sheep (n = 384), Hu sheep (n = 83) and Cele Black sheep (n = 68). The remaining four breeds were the Sunite sheep (n = 70), Prairie Tibetan (n = 80) and Suffolk sheep (n = 60) and Tan sheep (n = 23); these are lower prolificacy breeds (Table 1). All ewes used in this study were randomly selected and had been fed in the same regions since they were born, with no sire effects. All sheep were freely mated within their breeds.
TABLE 1.
The numbers and sources of ewes used in this study
| Breed | Number | Type | District |
|---|---|---|---|
| Small Tail Han sheep | 384 | Polytocous type | Southwest Region, Shandong Province, China |
| Hu sheep | 83 | Polytocous type | Xuzhou, Jiangsu Province, China |
| Cele Black sheep | 68 | Polytocous type | Cele, Xinjiang Uygur Autonomous Region, China |
| Sunite sheep | 70 | Monotocous type | Wulatezhongqi, Bayannaoer, Inner Mongolia Autonomous Region, China |
| Prairie Tibetan sheep | 80 | Monotocous type | Dangxiong, Tibet Autonomous Region, China |
| Suffolk sheep | 60 | Monotocous type | Beijing Aoxin Stud Farm Co. Ltd. located in Shunyi District, Beijing, China |
| Tan sheep | 23 | Monotocous type | Yanchi, Ningxia Hui Autonomous Region, China |
2.2. Primer design and genotyping
Primers to genotype the loci g.37871539C>T of TGIF1 and g.42314637T>C of SF1 were designed by MassARRAY Assay Design v.3.1, according to sequences of TGIF1 and SF1 deposited in GenBank (accession numbers: NC_019480.1, TGIF1; NC_019478.1, SF1). These primers were synthesized by Beijing Compass Biotechnology Co., Ltd., and the genotyping process was conducted using the MassARRAY® system. The MassARRAY is a recently developed strategy for the detection of single nucleotide polymorphisms (SNPs). Initially, the amplification of target sequences is conducted (primers are shown in Table 2); subsequently, single‐base extended PCR reactions are performed using specific primers (Table 2). The molecular weight of the amplified products differs after extension because different bases are present at polymorphic sites. These differences are then detected through time‐of‐flight mass spectrometry, which allows the products to be separated based on genotype. The MassARRAY® SNP detection system has been described in further detail elsewhere (He et al., 2019; La, Liu, Zhang, & Chu, 2019; Zhang, Liu, et al., 2019; Zhou et al., 2018).
TABLE 2.
Primer information for genotyping
| Primer | Sequence | Product size | Usage |
|---|---|---|---|
| TGIF1‐F | ACGTTGGATGATACAGCAGATGGCCAACAG | 94 | PCR amplification |
| TGIF1‐R | ACGTTGGATGGGTCCAGATTTACAGGAGTC | ||
| TGIF1‐E | ACAGCAGCTTTACAGA | single‐base extended PCR amplification for g.37871539C>T | |
| SF1‐F | ACGTTGGATGGGGTCTCTCATTCCTCTCAG | 92 | PCR amplification |
| SF1‐R | ACGTTGGATGGTGAGTGGAGTATTTTGGGC | ||
| SF1‐E | GCCCCACCCTCCCAA | single‐base extended PCR amplification for g.42314637T>C |
2.3. Statistical analyses
Based on genotyping data, we calculated allele and genotype frequencies, polymorphism information content (PIC), heterozygosity (HE), and the number of effective alleles (NE) and p values for chi‐square tests. If a particular ewe population had p > .05 (chi‐square test), it was considered to be under Hardy–Weinberg equilibrium. Regarding an association between TGIF1 and SF1 polymorphisms and litter size, chi‐square test was performed to analyse the association of litter size with genotypes in each parity of Small Tail Han sheep.
2.4. Prediction of protein interaction networks via the STRING database
As proteins modulate most physiological activities, identifying the interactions between proteins can enhance our understanding of complex traits such as litter size. To investigate the mechanisms by which TGIF1 and SF1 may modulate ovulation and litter size, we predicted the protein interaction networks of TGIF1 and SF1 using the STRING database (https://string‐db.org).
3. RESULTS
3.1. Genotyping and population genetic analysis of g.37871539C>T in TGIF1 and g.42314637T>C in SF1 in seven sheep breeds
The genotyping results were showed in (Figure 1). The results suggested that only two genotypes were detected at locus g.37871539C>T (TGIF1), while three genotypes were detected at locus g.42314637T>C (SF1. Our population genetic analysis (Table 3) showed that the locus g.37871539C>T in TGIF1 (synonymous mutation) contained only two genotypes across the seven sheep breeds studied. Most of these breeds exhibited moderate polymorphism at this site (0.25 < PIC < 0.5), while the Suffolk sheep exhibited relatively low polymorphism. Only two breeds (Suffolk sheep and Tan sheep) were under Hardy–Weinberg equilibrium at this locus (p > .05). Regarding locus g.42314637T>C in SF1 (3′UTR variant), three sheep breeds (Small Tail Han sheep, Cele Black sheep and Suffolk sheep) possessed three genotypes; the remaining four breeds had two genotypes only. The Cele Black sheep was moderately polymorphic for this locus (0.25 < PIC < 0.5), while the other six breeds exhibited lower polymorphism. With the exception of the Prairie Tibetan sheep, this locus was under Hardy–Weinberg equilibrium in all breeds (p > .05).
FIGURE 1.
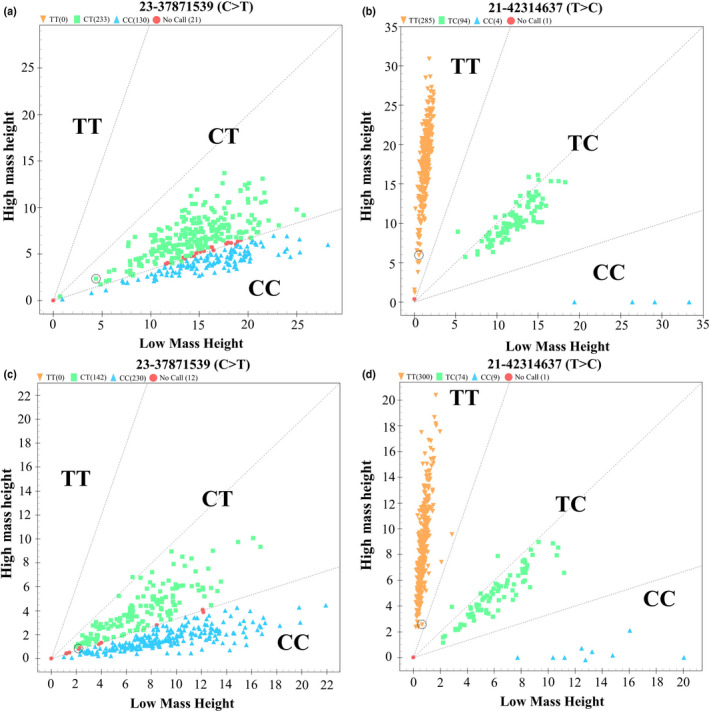
Genotyping of SNPs at reproduction‐related loci in sheep. The genotyping results of g.37871539C>T in TGIF1 (a) and g.42314637T>C in SF1 (b) in six sheep breeds (Hu sheep, Cele Black sheep, Sunite sheep, Prairie Tibetan sheep, Suffolk sheep and Tan sheep). The genotyping results of g.37871539C>T in TGIF1 (c) and g.42314637T>C in SF1 (d), in Small Tail Han sheep
TABLE 3.
Population genetic analysis of g.37871539C>T (TGIF1) and g.42314637T>C (SF1) in seven sheep breeds
| Locus (mutation type) | Breed | Genotype frequency | Allele frequency | PIC | HE | NE | Chi‐square test (p value) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| g.37871539C>T (synonymous mutation) | CC | CT | TT | C | T | |||||
| Small Tail Han Sheep | 0.57 | 0.43 | 0.00 | 0.79 | 0.21 | 0.28 | 0.34 | 1.51 | .00 | |
| Hu sheep | 0.26 | 0.74 | 0.00 | 0.63 | 0.37 | 0.36 | 0.47 | 1.87 | .00 | |
| Cele Black sheep | 0.16 | 0.84 | 0.00 | 0.58 | 0.42 | 0.37 | 0.49 | 1.95 | .00 | |
| Sunite sheep | 0.39 | 0.61 | 0.00 | 0.69 | 0.31 | 0.33 | 0.42 | 1.74 | .00 | |
| Prairie Tibetan sheep | 0.22 | 0.78 | 0.00 | 0.61 | 0.39 | 0.36 | 0.48 | 1.91 | .00 | |
| Suffolk sheep | 0.79 | 0.21 | 0.00 | 0.90 | 0.10 | 0.17 | 0.19 | 1.23 | .38 | |
| Tan sheep | 0.55 | 0.45 | 0.00 | 0.78 | 0.22 | 0.29 | 0.35 | 1.54 | .19 | |
| g.42314637T>C (3′UTR variant) | TT | TC | CC | T | C | |||||
| Small Tail Han Sheep | 0.78 | 0.19 | 0.03 | 0.88 | 0.12 | 0.19 | 0.21 | 1.27 | .09 | |
| Hu sheep | 0.94 | 0.06 | 0.00 | 0.97 | 0.03 | 0.06 | 0.06 | 1.06 | .78 | |
| Cele Black sheep | 0.57 | 0.38 | 0.05 | 0.76 | 0.24 | 0.30 | 0.36 | 1.56 | .61 | |
| Sunite sheep | 0.74 | 0.26 | 0.00 | 0.87 | 0.13 | 0.20 | 0.22 | 1.29 | .22 | |
| Prairie Tibetan sheep | 0.64 | 0.36 | 0.00 | 0.82 | 0.18 | 0.25 | 0.30 | 1.42 | .05 | |
| Suffolk sheep | 0.75 | 0.23 | 0.02 | 0.87 | 0.13 | 0.20 | 0.23 | 1.30 | .94 | |
| Tan sheep | 0.91 | 0.09 | 0.00 | 0.96 | 0.04 | 0.08 | 0.08 | 1.09 | .83 | |
3.2. The association of TGIF1 and SF1 polymorphisms with litter size in Small Tail Han sheep
Across three parities, the litter size of ewes with the wild‐type genotype CC at locus g.37871539C>T (TGIF1) was higher than that of ewes with the genotype CT (p < .05; Table 4), this result means this mutation causes a significant decrease in litter size. Regarding locus g.42314637T>C (SF1), the litter size of ewes with the mutant homozygous genotype CC, over three parities, was higher than that of ewes with the genotype TT (p < .05), this result means this mutation causes a significant increase in litter size.
TABLE 4.
The mean, standard deviations (SD) and p value (chi‐squared test) of litter size in Small Tail Han sheep in the loci g.37871539C>T (TGIF1) and g.42314637T>C (SF1)
| Locus | Genotype | Litter size (mean ± SD) | |||||
|---|---|---|---|---|---|---|---|
| First parity (N) | p value | Second parity (N) | p value | Third parity (N) | p value | ||
| g.37871539C>T | CC | 2.12 (230) ± 0.688 | .045 | 2.32 (121) ± 0.698 | .024 | 2.67 (51) ± 0.817 | .001 |
| CT | 1.80 (142) ± 0.618 | 1.99 (84) ± 0.703 | 2.11 (38) ± 0.727 | ||||
| g.42314637T>C | TT | 1.86 (300) ± 0.648 | .035 | 2.10 (186) ± 0.754 | .042 | 2.35 (66) ± 0.816 | .038 |
| TC | 1.94 (74) ± 0.703 | 2.28 (45) ± 0.815 | 2.59 (17) ± 0.944 | ||||
| CC | 2.43 (9) ± 0.707 | 2.63 (9) ± 0.527 | 3.00 (8) ± 0.000 | ||||
p < .05 in each column indicates a significant difference.
3.3. Protein interaction networks involving TGIF1 and SF1
To investigate the mechanisms by which TGIF1 and SF1 might affect ovulation and litter size in sheep, we predicted protein interaction networks involving TGIF1 and SF1 via the STRING database. This analysis suggested that both TGIF1 and SF1 interact with ten other proteins (Figure S1).
4. DISCUSSION
4.1. TGIF1 and SF1 polymorphism in sheep
TGIF1 and SF1 may be important regulators of reproduction (Corioni et al., 2010; Hu et al., 2011; Hyman et al., 2003; Schoen et al., 2013; Wotton et al., 1999); however, the extent and effects of their polymorphisms in sheep are poorly understood. At the loci studied here, we detected only two TGIF1 genotypes in all ewe populations and two SF1 genotypes in some ewe populations (Table 3); some of these populations also exhibited low polymorphism (PIC≤0.25). These data may be limited by the number of sheep studied, and thus increasing the sample size could enable three genotypes to be captured, which would also increase the PIC value. Importantly, considering the negative effects of mutation on litter size, the lethality is the most likely reason for lack of TT genotype (g.37871539C>T) in all sheep breeds. Furthermore, we found that several sheep breeds were not in Hardy–Weinberg equilibrium (p ≤ .05) at the studied loci (Table 3), which may be a consequence of selection.
4.2. Analysis of the association between TGIF1 and SF1 polymorphisms and litter size
Missense mutations cause the substitution of one amino acid for another and can therefore exert great influence on complex traits. For example, point mutations in BMP15 (Calvo et al., 2020), GDF9 (Våge et al., 2013), MTNR1A (He et al., 2019), BMP7 and BMP2 (Zhang, Liu, et al., 2019) significantly affect litter size in sheep. Recently, synonymous mutations have also received attention for playing key roles in reproductive traits. For example, a synonymous mutation of melatonin receptor 1A was found in Rasa Aragonesa sheep and was highly associated with reproductive seasonality (Martínez‐Royo, Lahoz, Alabart, Folch, & Calvo, 2012); a synonymous mutation of luteinizing hormone beta polypeptide was detected in Small Tail Han sheep and was highly associated with litter size (Wang et al., 2020); and a synonymous mutation in follicle‐stimulating hormone receptor was highly associated with litter size in sheep, including in Small Tail Han and Hu sheep (Pan et al., 2014). These findings indicate that synonymous mutations have the potential to be valuable markers for improving sheep fecundity.
TGIF1 and SF1 act as regulators in reproductive processes (Corioni et al., 2010; Hu et al., 2011; Hyman et al., 2003; Schoen et al., 2013; Wotton et al., 1999); however, their effects on litter size remain to be explored. Here, we conducted an association analysis of TGIF1 and SF1 polymorphisms with litter size in Small Tail Han sheep. Regarding locus g.37871539C>T in TGIF1, only two genotypes were identified, and litter size in ewes with the genotype CT was significantly lower than that of ewes with the wild‐type genotype CC. This suggests that this synonymous mutation may be harmful. Furthermore, we failed to detect the mutant homozygous genotype TT, and the lethality was the most likely reason for lack of TT genotype (g.37871539C>T) in all sheep breeds. TGIF1 can negatively regulate TGF‐β‐activated genes such as SMAD2 and SMAD4 (Hu et al., 2011; Hyman et al., 2003; Wotton et al., 1999), and TGF‐β/SMAD signalling plays crucial roles in mediating reproduction (Knight & Glister, 2006; Liu, Chang, Yi, Yao, & Leung, 2019; Rossetti et al., 2020; Yin et al., 2020). For example, the disruption of TGF‐β/SMAD signalling can cause reproductive problems (Chand et al., 2007), including sterility (Li et al., 2017). Therefore, while on one hand, our failure to detect the third genotype at the g.37871539C>T locus may be due to inadequate ewe numbers; on the other hand, we hypothesize that this synonymous mutation may enhance the negative regulatory effects of TGIF1 on TGF‐β/SMAD signalling, and even cause death in embryos with the homozygous TT genotype.
SF1 is mainly involved in alternative splicing events (Corioni et al., 2010), thus polymorphism in the 3′UTR of SF1 could modulate gene stability (Akdeli et al., 2014) and influence protein abundance. Considering the key roles reported for alternative splicing in sheep reproduction (Miao et al., 2018), we speculate that the SF1 3′UTR mutation g.42314637T>C may increase litter size by regulating alternative splicing events and modifying protein abundance related to reproduction.
4.3. Proteins interacting with TGIF1 and SF1
Most physiological processes are underpinned by multiple proteins; therefore, building interaction networks of proteins can enhance our understanding of complex traits such as reproduction. In our analysis, TGIF1 was predicted to interact with SMAD2, which has been reported to participate in reproductive processes. Specifically, several studies have suggested that SMAD2 may be a candidate gene influencing reproduction in sheep with different fecundity (Zhang, Tang, et al., 2019; Zheng et al., 2019), and the inactivation of SMAD2 in mouse can lead to endometrial dysregulation and infertility (Kriseman et al., 2019). Previous study highlighted that the TGF‐β induced factor homeobox 2 (TGIF2) has the same DNA binding homeodomains as TGIF1, suggesting that these two proteins may bind the same regulatory sequences (Melhuish, Gallo, & Wotton, 2001). As TGIF2 has been detected in tammar granulosa and theca cells, indicating a role in folliculogenesis (Hu et al., 2011), we speculate that TGIF1 may also be involved in this process.
SF1 was predicted to interact with ten proteins, including cell division cycle 5‐like protein (CDC5L) and U2 small nuclear RNA auxiliary factor 2 (U2AF2). While CDC5L is a key factor regulating the cell cycle (Zhang, Kaur, Akhter, & Legerski, 2009), it is also highly abundant in BCB‐positive GV oocytes (Liu et al., 2018) and follicles larger than eight mm‐including prematuration oocytes (Dieleman et al., 2002). CDC5L could therefore act as a regulator promoting oocyte development and maturation. U2AF2 plays an important role in splicing decisions and has been reported to participate in alternative splicing events (Sutandy et al., 2018), such events have also been associated with reproduction (Miao et al., 2018).
5. CONCLUSIONS
This study reported the first analysis of polymorphisms at two loci in TGIF1 and SF1, and suggested that these loci (g.37871539C>T in TGIF1 and g.42314637T>C in SF1) were highly associated with litter size in sheep. All in all, these data provide valuable genetic markers for sheep breeding.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Zhuangbiao Zhang and Mingxing Chu designed this study; Xiaoyun He, Qiuyue Liu and Jishun Tang collected the data used in this study; Zhuangbiao Zhang, Xiaoyun He and Ran Di performed statistical analysis; Zhuangbiao Zhang drafted paper; Mingxing Chu revised this manuscript.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
This research was funded by the National Natural Science Foundation of China (31861143012 and 31772580), the Genetically Modified Organisms Breeding Major Program of China (2016ZX08009‐003‐006), the Earmarked Fund for China Agriculture Research System (CARS‐38), the Central Public‐Interest Scientific Institution Basal Research Fund (Y2017JC24), the Agricultural Science and Technology Innovation Program of China (ASTIP‐IAS13, CAAS‐XTCX2016011‐02‐02), the China Agricultural Scientific Research Outstanding Talents and Their Innovative Teams Program, the China High‐level Talents Special Support Plan Scientific and Technological Innovation Leading Talents Program (W02020274), and the Tianjin Agricultural Science and Technology Achievements Transformation and Popularization Program (201704020). We thank Gemma Richards, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Zhang Z, He X, Liu Q, Tang J, Di R, Chu M. TGIF1 and SF1 polymorphisms are associated with litter size in Small Tail Han sheep. Reprod Dom Anim. 2020;55:1145–1153. 10.1111/rda.13753
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Akdeli, N. , Riemann, K. , Westphal, J. , Hess, J. , Siffert, W. , & Bachmann, H. S. (2014). A 3′UTR polymorphism modulates mRNA stability of the oncogene and drug target Polo‐like Kinase 1. Molecular Cancer, 13, 87 10.1186/1476-4598-13-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo, J. H. , Chantepie, L. , Serrano, M. , Sarto, M. P. , Iguacel, L. P. , Jimenez, M. A. , … Lahoz, B. (2020). A new allele in the BMP15 gene (FecX (RA)) that affects prolificacy co‐segregates with FecX (R) and FecX (GR) in Rasa Aragonesa sheep. Theriogenology, 144, 107–111. 10.1016/j.theriogenology.2020.01.010 [DOI] [PubMed] [Google Scholar]
- Chand, A. L. , Robertson, D. M. , Shelling, A. N. , & Harrison, C. A. (2007). Mutational analysis of betaglycan/TGF‐βRIII in premature ovarian failure. Fertility and Sterility, 87, 210–212. 10.1016/j.fertnstert.2006.05.080 [DOI] [PubMed] [Google Scholar]
- Corioni, M. , Antih, N. , Tanackovic, G. , Zavolan, M. , & Krämer, A. (2010). Analysis of in situ pre‐mRNA targets of human splicing factor SF1 reveals a function in alternative splicing. Nucleic Acids Research, 39, 1868–1879. 10.1093/nar/gkq1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman, S. J. , Hendriksen, P. J. M. , Viuff, D. , Thomsen, P. D. , Hyttel, P. , Knijn, H. M. , … Vos, P. L. (2002). Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre‐implantation embryos. Theriogenology, 57, 5–20. 10.1016/s0093-691x(01)00655-0 [DOI] [PubMed] [Google Scholar]
- He, X. , Zhang, Z. , Liu, Q. , & Chu, M. (2019). Polymorphisms of the melatonin receptor 1A gene that affect the reproductive seasonality and litter size in Small Tail Han sheep. Reproduction in Domestic Animals, 54, 1400–1410. 10.1111/rda.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Yu, H. , Shaw, G. , Renfree, M. B. , & Pask, A. J. (2011). Differential roles of TGIF family genes in mammalian reproduction. BMC Developmental Biology, 11, 58 10.1186/1471-213X-11-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, C. A. , Bartholin, L. , Newfeld, S. J. , & Wotton, D. (2003). Drosophila TGIF proteins are transcriptional activators. Molecular and Cellular Biology, 23, 9262–9274. 10.1128/mcb.23.24.9262-9274.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Xie, M. , Chen, W. , Talbot, R. , Maddox, J. F. , Faraut, T. , … Dalrymple, B. P. (2014). The sheep genome illuminates biology of the rumen and lipid metabolism. Science, 344, 1168–1173. 10.1126/science.1252806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, P. G. , & Glister, C. (2006). TGF‐beta superfamily members and ovarian follicle development. Reproduction, 132, 191–206. 10.1530/rep.1.01074 [DOI] [PubMed] [Google Scholar]
- Kriseman, M. , Monsivais, D. , Agno, J. , Masand, R. P. , Creighton, C. J. , & Matzuk, M. M. (2019). Smad2 uterine double‐conditional inactivation of Smad2 and Smad3 in mice causes endometrial dysregulation, infertility, and uterine cancer. Proceedings of the National Academy of Sciences of the United States of America, 116, 3873–3882. 10.1073/pnas.1806862116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La, Y. , Liu, Q. , Zhang, L. , & Chu, M. (2019). Single nucleotide polymorphisms in SLC5A1, CCNA1, and ABCC1 and the association with litter size in Small‐Tail Han sheep. Animals, 9, 432 10.3390/ani9070432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Schang, G. , Boehm, U. , Deng, C. X. , Graff, J. , & Bernard, D. J. (2017). SMAD3 regulates follicle‐stimulating hormone synthesis by pituitary gonadotrope cells in vivo. Journal of Biological Chemistry, 292, 2301–2314. 10.1074/jbc.M116.759167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Pan, Z. , Wang, X. , Hu, W. , Di, R. , Yao, Y. , & Chu, M. (2014). Progress on major genes for high fecundity in ewes. Frontiers of Agricultural Science and Engineering, 1, 282–290. 10.3724/sp.j.1005.2011.00953 [DOI] [Google Scholar]
- Liu, S. , Chang, H. M. , Yi, Y. , Yao, Y. Q. , & Leung, P. C. K. (2019). SMAD‐dependent signaling mediates morphogenetic protein 6‐induced stimulation of connective tissue growth factor in luteinized human granulosa cells. Biology of Reproduction, 101, 445–456. 10.1093/biolre/ioz108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. M. , Wang, Y. K. , Liu, Y. H. , Yu, X. X. , Wang, P. C. , Li, X. , … Yang, C. X. (2018). Single‐cell transcriptome sequencing reveals that cell division cycle 5‐like protein is essential for porcine oocyte maturation. Journal of Biological Chemistry, 293, 1767–1780. 10.1074/jbc.M117.809608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Royo, A. , Lahoz, B. , Alabart, J. L. , Folch, J. , & Calvo, J. H. (2012). Characterisation of the melatonin receptor 1A (MTNR1A) gene in the Rasa Aragonesa sheep breed: Association with reproductive seasonality. Animal Reproduction Science, 133, 169–175. 10.1016/j.anireprosci.2012.06.018 [DOI] [PubMed] [Google Scholar]
- Mechaly, A. S. , Viñas, J. , & Piferrer, F. (2009). Identification of two isoforms of the kisspeptin‐1 receptor (kiss1r) generated by alternative splicing in a modern teleost, the Senegalese sole (Solea senegalensis). Biology of Reproduction, 80, 60–69. 10.1095/biolreprod.108.072173 [DOI] [PubMed] [Google Scholar]
- Melhuish, T. A. , Gallo, C. M. , & Wotton, D. (2001). TGIF2 interacts with histone deacetylase 1 and represses transcription. Journal of Biological Chemistry, 276, 32109–32114. 10.1074/jbc.M103377200 [DOI] [PubMed] [Google Scholar]
- Miao, X. , Luo, Q. , Zhao, H. , & Qin, X. (2018). Ovarian transcriptomic analysis reveals the alternative splicing events associated with fecundity in different sheep breeds. Animal Reproduction Science, 198, 177–183. 10.1016/j.anireprosci.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Pan, X. , Liu, S. , Li, F. , Wang, W. , Li, C. , Ma, Y. , & Li, T. (2014). Molecular characterization, expression profiles of the ovine FSHR gene and its association with litter size. Molecular Biology Reports, 41, 7749–7754. 10.1007/s11033-014-3666-8 [DOI] [PubMed] [Google Scholar]
- Rino, J. , Desterro, J. M. P. , Pacheco, T. R. , Gadella, T. W. J. , & Carmo‐Fonseca, M. (2008). Splicing factors SF1 and U2AF associate in extraspliceosomal complexes. Molecular and Cellular Biology, 28, 3045–3057. 10.1128/MCB.02015-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti, R. , Ferrari, I. , Bestetti, I. , Moleri, S. , Brancati, F. , Petrone, L. , … Persani, L. (2020). Fundamental role of BMP15 in human ovarian folliculogenesis revealed by null and missense mutations associated with primary ovarian insufficiency. Human Mutation, 41(5), 983–997. 10.1002/humu.23988 [DOI] [PubMed] [Google Scholar]
- Schoen, J. , Sharbati, S. , Ritter, J. , & Jewgenow, K. (2013). Feline gonads exhibit tissue specific alternative splicing of oestrogen receptor alpha (ESR1). Reproduction in Domestic Animals, 47, 30–34. 10.1111/rda.12065 [DOI] [PubMed] [Google Scholar]
- Selenko, P. , Gregorovic, G. , Sprangers, R. , Stier, G. , Rhani, Z. , Krämer, A. , & Sattler, M. (2003). Structural basis for the molecular recognition between human splicing factors U2AF65 and SF1/mBBP. Molecular Cell, 11, 965–976. 10.1111/rda.12065 [DOI] [PubMed] [Google Scholar]
- Shen, J. , & Walsh, C. A. (2005). Targeted disruption of TGIF, the mouse ortholog of a human holoprosencephaly gene, does not result in holoprosencephaly in mice. Molecular and Cellular Biology, 25, 3639–3647. 10.1128/MCB.25.9.3639-3647.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutandy, F. X. R. , Ebersberger, S. , Huang, L. U. , Busch, A. , Bach, M. , Kang, H.‐S. , … König, J. (2018). In vitro iCLIP‐based modeling uncovers how the splicing factor U2AF2 relies on regulation by cofactors. Genome Research, 28, 699–713. 10.1101/gr.229757.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Våge, D. I. , Husdal, M. , Kent, M. P. , Klemetsdal, G. , & Boman, I. A. (2013). A missense mutation in growth differentiation factor 9 (GDF9) is strongly associated with litter size in sheep. BMC Genetics, 14, 1 10.1186/1471-2156-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , La, Y. , Li, F. , Liu, S. , Pan, X. , Li, C. , & Zhang, X. (2020). Molecular characterization and expression profiles of the ovine LHβ gene and its association with litter size in Chinese indigenous Small‐Tailed Han sheep. Animals, 10, 460 10.3390/ani10030460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Yang, Q. , Zhang, S. , Zhang, X. , Pan, C. , Chen, H. , … Lan, X. (2019). Genetic effects of single nucleotide polymorphisms in the goat GDF9 gene on prolificacy: True or false positive? Animals, 9, 886 10.3390/ani9110886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton, D. , Lo, R. S. , Lee, S. , & Massagué, J. (1999). A smad transcriptional corepressor. Cell, 97, 29–39. 10.1016/s0092-8674(00)80712-6 [DOI] [PubMed] [Google Scholar]
- Yin, J. , Chang, H. M. , Yi, Y. , Yao, Y. , & Leung, P. C. K. (2020). TGF‐β1 increases GDNF production by upregulating the expression of GDNF and furin in human granulosa‐lutein cells. Cells, 9, 185 10.3390/cells9010185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N. , Kaur, R. , Akhter, S. , & Legerski, R. J. (2009). CDC5L interacts with ATR and is required for the S‐phase cell‐cycle checkpoint. EMBO Reports, 10, 1029–1035. 10.1038/embor.2009.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Liu, Q. , Di, R. , Hu, W. , Wang, X. , He, X. , … Chu, M. (2019). Single nucleotide polymorphisms in BMP2 and BMP7 and the association with litter size in Small Tail Han sheep. Animal Reproduction Science, 204, 183–192. 10.1016/j.anireprosci.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Tang, J. , Di, R. , Liu, Q. , Wang, X. , Gan, S. , … Hu, W. (2020). Integrated hypothalamic transcriptome profiling reveals the reproductive roles of mRNAs and miRNAs in sheep. Frontiers in Genetics, 10, 1296 10.3389/fgene.2019.01296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Tang, J. , He, X. , Zhu, M. , Gan, S. , Guo, X. , … Chu, M. (2019). Comparative transcriptomics identify key hypothalamic circular RNAs that participate in Sheep (Ovis aries) reproduction. Animals, 9, 557 10.3390/ani9080557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Wang, Z. , Yang, H. , Yao, X. , Yang, P. , Ren, C. , … Zhang, Y. (2019). Pituitary transcriptomic study reveals the differential regulation of lncRNAs and mRNAs related to prolificacy in different FecB genotyping sheep. Genes, 10, 157 10.3390/genes10020157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M. , Pan, Z. , Cao, X. , Guo, X. , He, X. , Sun, Q. , … Chu, M. (2018). Single nucleotide polymorphisms in the HIRA gene affect litter size in Small Tail Han Sheep. Animals, 8, 71 10.3390/ani8050071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


