Abstract
Problem
Preeclampsia is a major cause of fetal and maternal mortality and morbidity. Disturbed fetal‐maternal immune tolerance, and therewith memory T cells, might be involved in its etiology. This study aims to give insight into memory T‐cell populations and its associated cytokines in the decidual layers in early‐onset preeclampsia (EO‐PE) and late‐onset preeclampsia (LO‐PE).
Method of Study
Lymphocytes were isolated from the decidua parietalis and basalis from EO‐PE (n = 6), LO‐PE (n = 8) and healthy (n = 15) pregnancies. CD4+ and CD8+ central‐ (CCR7+), effector‐ (CCR7−), tissue resident‐ (CD103+), and regulatory‐ (Foxp3+) memory cell (CD45RO+) populations and their activation status (CD69+) were analyzed using flow cytometry. qRT‐PCR analysis was performed on decidua parietalis and basalis biopsies to detect mRNA expression of interferon‐gamma, interleukin‐1B, IL2, IL6, IL7, IL8, IL10, IL15, and IL23.
Results
CD4+ central‐memory (CM) cell proportions were lower in the decidua parietalis in LO‐PE (P < .0001) and EO‐PE (P < .01) compared to healthy pregnancies. CD8+ memory (P < .05) and CD8+ CM (P < .01) cell proportions were also lower in the decidua parietalis in EO‐PE compared to healthy pregnancies. This was accompanied by higher IL15 (P < .05) and IL23 (P < .05) and lower IL7 (P < .05) mRNA expression in decidua basalis biopsies from EO‐PE compared to healthy pregnancies, analyzed by qPCR.
Conclusion
In conclusion, decidual memory T‐cell proportions, their activation status, and associated cytokines are altered in preeclampsia and might therefore be involved in fetal‐maternal immune tolerance and the pathophysiology of preeclampsia.
Keywords: decidua, early‐onset preeclampsia, late‐onset preeclampsia, memory T cell, pregnancy
1. INTRODUCTION
Preeclampsia affects 5%‐8% of pregnancies and is a major cause of fetal and maternal mortality and morbidity. 1 , 2 Preeclampsia has a multifactorial etiology and is characterized by a poorly established or poorly perfused placenta, endothelial cell activation and a disturbed immune balance leading to hypertension and multi‐organ failure. 3 Early‐onset preeclampsia (EO‐PE) and late‐onset preeclampsia (LO‐PE) are distinguished by onset before or after 34 weeks of gestation. 4 For research purposes, this distinction is often used, since the pathophysiology and immunopathology differs. 5 , 6 EO‐PE is associated with disturbed trophoblast invasion, modification of the uterine spiral arteries and fetal growth restriction, whereas LO‐PE seems associated with trophoblast overload with aging of the placenta causing reduced intervillous perfusion. 5 , 6 , 7
During pregnancy, immune tolerance is required toward the semi‐allogeneic fetus. 8 , 9 , 10 Insufficient immune tolerance is associated with pregnancy complications as preeclampsia, 8 , 9 , 11 skewing away from immune tolerance toward more pregnancy threatening immunity. 12 , 13 The decidua is the maternal tissue that interacts with fetal cells, being the uterine lining in which the fetal trophoblast anchor. 14 Two distinct decidua subtypes are identified, characterized by a different immunologic response and different distribution of T‐cell populations. 15 The decidua parietalis is the maternal tissue around the chorionic sac and is in direct contact with the non‐invading chorionic trophoblast. 14 The decidua basalis is the maternal side of the placenta and is invaded by the extravillous trophoblast. 16 Many different maternal immune cells are present in the decidua basalis and parietalis, including memory T cells. 17 , 18 , 19 , 20 , 21 , 22
Multiple studies provide clues for development of immunologic memory to generate appropriate fetal‐maternal tolerance and prevent immune‐associated pregnancy disorders as preeclampsia. 22 , 23 , 24 Memory T cells are a subset of T cells that have developed after a previous encounter with an antigen. 25 They are long‐living and provide immediate protection upon re‐exposure to the same antigen. 26 Memory T‐cell differentiation, survival, and cytokine secretion are under the influence of cytokines such as interleukin‐7 (IL7), IL15, and IL23. 27 , 28 , 29 Different memory T‐cell populations can be distinguished based on migration pattern, protein expression profile, and cytokine secretion abilities. 26 , 30 , 31 In both the CD4+ and CD8+ cell lineages, central‐memory (CM) and effector‐memory (EM) cells are identified. 25 , 32 , 33 , 34 CM cells circulate in the blood and migrate preferentially to secondary lymphoid organs. 34 Upon activation, CM cells can proliferate into EM cells, which circulate throughout the body and generate an aggressive response upon a secondary encounter with the memorized antigen. 25 , 32 Within the CD8+ memory T‐cell lineage, tissue‐resident memory (TRM) cells can be distinguished. 31 , 35 TRM cells do not circulate, but reside in tissue. 31 In the CD4+ cell lineage, TRM cells are detectable at very low levels. 35 Finally, there is a population of Treg memory cells, identified by Foxp3 expression and executing immune regulatory and immunosuppressive function. 30 CD4+ (general) Treg cells are considered key players in fetal tolerance. 19 In mice, CD4+ Treg memory cells have been found to be recruited to the reproductive tract and are important for pregnancy success, 36 , 37 , 38 in humans their importance has not been proven yet. CD4+ Treg memory cells are found at the fetal‐maternal interface and in the decidua. 39 , 40 Interestingly, in contrast to the lower proportions of (general) Tregs found in preeclampsia, 9 , 19 higher proportions of Treg memory cells are found in the peripheral blood in pregnancy complications as preeclampsia compared to healthy controls. 41 , 42
In contrast to their normal function, memory cells may have a function in fetal tolerance. In murine models, it has been shown that fetal antigen‐specific memory T cells are generated during pregnancy that persist postpartum and decrease fetal resorption rates in a subsequent pregnancy. 38 , 43 , 44 In human decidua, memory T cells, mostly CD8+ EM cells, were found with a different phenotype and function compared to memory T cells in the peripheral blood. 45 , 46 , 47 With reduced granzyme B and perforin expression, and high PD‐1 expression, they appear to be silenced, and however, they remain responsive upon stimulation and have high IFN‐γ expression. 45 , 46 Some studies found that the memory T‐cell populations in the peripheral blood are affected in preeclamptic pregnancies. 39 , 48 , 49 Decidual memory T cells in preeclampsia are not well studied and clear insight into memory T cells, and the memory T‐cell cytokine milieu is lacking. The aim of this study is to evaluate differences in memory T‐cell populations and memory T cell–associated cytokines in decidual tissue between uncomplicated pregnancies and pregnancies complicated with preeclampsia.
2. METHODS
2.1. Samples
To compare immune cells in healthy women and women with preeclampsia, placentas were obtained from 15 healthy women, eight women with LO‐PE, and six women with EO‐PE (Table 1). To compare immune cells in healthy women with spontaneous labor and healthy women with cesarean section, placentas were obtained from 15 healthy women after vaginal delivery, and 12 healthy women after scheduled cesarean section (Table S3). All women had singleton pregnancies, a body mass index <35, no diabetes, no immune abnormalities, and no history of smoking, alcohol, or drug use during the current pregnancy. Preeclampsia was defined as gestational hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure >90 mmHg measured in two different measurements after 20 weeks of gestation) accompanied with either proteinuria (>300 mg/24 hours), uteroplacental dysfunction, or other maternal organ dysfunction following the International Society for the Study of Hypertension (ISSHP) Guideline. 50 Diagnosis of preeclampsia before 34 weeks of gestation was considered early‐onset preeclampsia, and diagnosis after 34 weeks was considered late‐onset preeclampsia following the classification of the ISSHP. 4 Placental tissue was used for this study according to the code of conduct for responsible use following the guideline from the Federation of Medical Scientific Associations. 51 This study, including the consent procedure, was approved by the Medical Ethics Review Board of the University Medical Center Groningen (UMCG; protocol number METc2018/516).
Table 1.
Patient characteristics
| Healthy (n = 15) | Late‐onset preeclampsia (n = 8) | Early‐onset preeclampsia (n = 6) | |
|---|---|---|---|
| Maternal age (y) | 28 (24‐30) | 29 (26.25‐28.75) | 26.0 ± (25.0‐2875) |
| Ethnicity (%) | |||
| White | 86.67 | 75 | 100 |
| Assyrian | 13.33 | 25 | 0 |
| Gestational age (wks) | 39.29 (39.0‐39.86) | 38.14 (37.14‐38.79) a (P = .04) | 30.65 (29.29‐31.32) a (P < .0001) b (P < .0001) |
| Parity | 1 (1‐2) | 1 (1‐2) | 1 (1‐3) |
| Body mass index (kg/m2) | 24.30 (20.35‐26.07) | 26.25 (21.65‐34.96) | 23.85 (21.03‐26.85) |
| Gestational age at onset of preeclampsia (wks) | 37.21 (35.97‐38.21) | 30.0 (28.4‐30.71) b (P < .0001) | |
| Diastolic blood pressure (mmHg) | 80.0 (72.0‐81.0) | 94.0 (86.75‐99.63) a (P = .0038) | 98.50 (89.0‐102.5) a (P = .0009) |
| Systolic blood pressure (mmHg) | 120.0 (110.0‐122.1) | 138.8 (131.3‐155.0) a (P = .0079) | 152.5 (140.0‐172.8) a (P = .0002) |
| Duration of hypertension (days) | 4.5 (1.0‐14.0) | 4.5 (3.0‐7.5) | |
| Random urine protein:creatinine ratio | 0.80 (0.60‐0.93) | 9.35 (1.84‐17.85) b (P = .007) | |
| Corticosteroid therapy for fetal lung maturation (% of patients) | 0 | 0 | 100 a (P < .0001) b (P < .0001) |
| Number of days between last dose of corticosteroids and childbirth (days) | 2.5 (2.0‐5.25) | ||
| Mode of delivery (%) | |||
| Vaginal | 66.67 | 75.0 | 0 a (P = .011) b (P = .021) |
| Spontaneous | 93.33 | 37.50 | 0 |
| Induction on maternal indication | 0 | 62.50 | 0 |
| Induction on fetal indication | 6.67 | 0 | 0 |
| CS | 33.33 | 25.0 | 100 a (P = .011) b (P = .021) |
| Breech presentation | 33.33 | 0 | 0 |
| Previous CS | 66.67 | 50.0 | 0 |
| Fetal indication | 0 | 50.0 | 16.67 |
| Maternal indication | 0 | 0 | 83.33 |
| Birth weight (g) | 3410 (3080‐3680) | 3115 (2698‐3361) | 1235 (990‐1459) a (P < .0001) b (P < .0001) |
| % Below 10th percentile birthweight | 0 | 25 | 66.67 a (P = .0008) |
| Fetal sex (% female/male) | 40/60 | 12.5/87.5 | 66.7/33.3 |
| Time between delivery and start of placental processing (hours) | 6.50 (0‐17.50) | 10.5 (1.38‐17.75) | 17.50 (0.38‐19.25) |
| Amount of cells isolated per mL decidual tissue (106) | |||
| Decidua parietalis | 0.17 (0.063‐0.21) | 0.62 (0.34‐0.90) | 0.11 (0.052‐0.39) |
| Decidua basalis | 0.20 (0.076‐0.45) | 0.63 (0.23‐1.0) | 0.12 (0.10‐0.24) |
Data as median with interquartile range; CS, cesarean section; blood pressure, highest blood pressure measured; duration of hypertension, time from first notice of hypertension until birth; statistical analysis by Kolmogorov‐Smirnov test, one‐way ANOVA and Tukey's post hoc test, Kruskal‐Wallis and Dunnett's test, or Fisher's exact test.
Shows comparisons to healthy pregnancies.
Shows comparisons to late‐onset preeclampsia.
2.2. Lymphocyte isolation
Placentas and membranes were stored in phosphate‐buffered saline (PBS) at 4°C directly after delivery until analysis for a maximum of 24 hours. 52 The amnion was torn off manually after which the decidua parietalis was scraped off the chorion using a cell scraper (Corning Incorporated). All visible blood vessels were removed. The villi were cut from the decidua basalis. The entire decidua parietalis and decidua basalis were digested in a 1:2 dilution in StemPro Accutase (Lifetechnologies) using a GentleMACS Dissociator (Miltenyi). Lymphocytes were isolated by centrifugation over a Percoll (Sigma‐Aldrich) gradient using three different density layers: 68% Percoll (ρ = 1.084 g/mL), 45% Percoll (ρ = 1.056 g/mL) and 40% Percoll (ρ = 1.050 g/mL). After centrifugation (30 minutes, 822 g, 20°C) without deceleration, the lymphocyte enriched layer was isolated between the 68% and 45% Percoll layer. Lymphocytes were washed in PBS and centrifuged (5 minutes, 460 g, 20°C). Cells were counted using a Beckman coulter counter.
2.3. Flow cytometry
For viability staining, 106 cells were incubated with 0.001% PercP‐Cy5.5 Fixable Viability Stain 620 (BD Biosciences) in PBS for 15 minutes at room temperature in the dark and thereafter washed in PBS. To prevent non‐specific binding, cells were incubated in 1% Fc‐Block (BD Biosciences) and 10% mouse serum (Sanquin) in PBS at temperature in the dark for 10 minutes. Cells were incubated with APC‐H7 anti‐CD4 (SK3; BD Biosciences), PE‐Cy7 anti‐CD8 (RPA‐T8, BD Biosciences), BV510 anti‐CD45RO (UCHL‐1; BD Biosciences), APC anti‐CD69 (FN50; BD Biosciences), BV421 anti‐CD103 (BER‐ACT8; BD Biosciences), and PE anti‐CCR7 (150503; BD Biosciences) in PBS at 4°C in the dark for 30 minutes. After surface staining, cells were washed with PBS, permeabilized, and fixed using Transcription Factor buffer set (BD Biosciences). Intracellular staining was performed by incubation with Alexa Fluor 488 anti‐Foxp3 (236A/E7; BD Biosciences) in PBS at 4°C in the dark for 30 minutes. Cells were washed with wash buffer (BD Biosciences) and were analyzed with a FACSVerse™ flow cytometer (BD Biosciences) using BD FACS Suite™ software (BD Biosciences). FlowJo V10 software (LLC) was used to analyze flow cytometry data. An overview of the gating strategy is displayed in Figure S1. After doublet exclusion, lymphocytes were gated in a forward sideward scatterplot of the single cells (Figure 1A). Lymphocytes that appeared negative for Fixable Viability Stain were gated as viable lymphocytes (Figure 1A). Within the viable lymphocyte population, CD4+ and CD8+ cells were distinguished (Figure 1A). Within the CD4+ cell population, the total proportion of CD4+ memory cells (CD45RO+CD4+), CD4+ CM cells (CCR7+CD45RO+CD4+), and CD4+ EM cells (CCR7−CD45RO+CD4+) were identified (Figure 1B). CD4+ TRM cells (CD103+CD45RO+CD4+) were identified by their expression of CD103 (Figure 1C). Treg CD4+ memory cell proportions were determined by expression of Foxp3 (Figure 1D, Figure S1G‐J). CD69‐positive cells were gated in all CD4+ memory cell subsets to analyze activated cells (Figure 2A). CD8+ cells were gated following a similar strategy. Within the CD8+ cell population, the total proportion of CD8+ memory cells (CM + EM; CD45RO+CD8+), CD8+ CM cells (CCR7+CD45RO+CD8+), and CD8+ EM cells (CCR7−CD45RO+CD8+) were identified (Figure 3A). Within the CD8+ cell population, CD8+ TRM cells (CD103+CD45RO+CD8+) were identified by expression of CD103 (Figure 3B). No Foxp3+CD8+ memory cells were observed. CD69‐positive cells were gated in all memory CD8+ cell subsets (Figure 4A). Table 2 lists the markers used to define the different memory T‐cell subsets.
Figure 1.
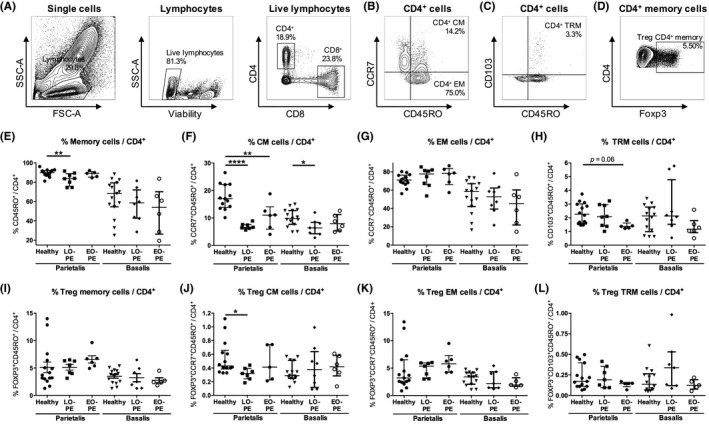
CD4+ memory cells in the decidua parietalis and the decidua basalis. Representative dot plots showing flow cytometric analysis of decidua parietalis live CD4+ lymphocytes using forward/sideward (FSC/SSC) scatterplots, viability stain and CD4 and CD8 expression (A). Within the CD4+ cell population, CD4+ central‐memory (CM) cells (CCR7+CD45RO+CD4+) (B), CD4+ effector‐memory (EM) cells (CCR7−CD45RO+CD4+) (B), general CD4+ memory cells (CM + EM; CD45RO+CD4+) (B), and CD4+ tissue‐resident memory (TRM) cells (CD103+CD45RO+CD4+) (C) were identified. Within the general CD4+ memory cell population, T regulatory (Treg) CD4+ memory cells (Foxp3+CD45RO+CD4+) were identified (D). Proportions of general CD4+ memory cells (E), CD4+ CM cells (F), CD4+ EM cells (G), CD4+ TRM cells (H), Treg CD4+ memory cells (I), Treg CD4+ CM (Foxp3+CCR7+CD45RO+CD4+) cells (J), Treg CD4+ EM (Foxp3+CCR7−CD45RO+CD4+) cells (K), and Treg CD4+ TRM (Foxp3+CD103+CD45RO+CD4+) cells (L), in the decidua parietalis and the decidua basalis from healthy pregnancies and pregnancies complicated by late‐onset preeclampsia (LO‐PE) or early‐onset preeclampsia (EO‐PE), shown as proportion of the CD4+ cell population. Symbols represent individual values per decidua with data as median with interquartile range. Analysis by Kolmogorov‐Smirnov test, one‐way ANOVA and Tukey's post hoc test or Kruskal‐Wallis and Dunn's post hoc test comparing the different groups within the decidua parietalis or the decidua basalis; *P < .05, **P < .01, ****P < .0001
Figure 2.
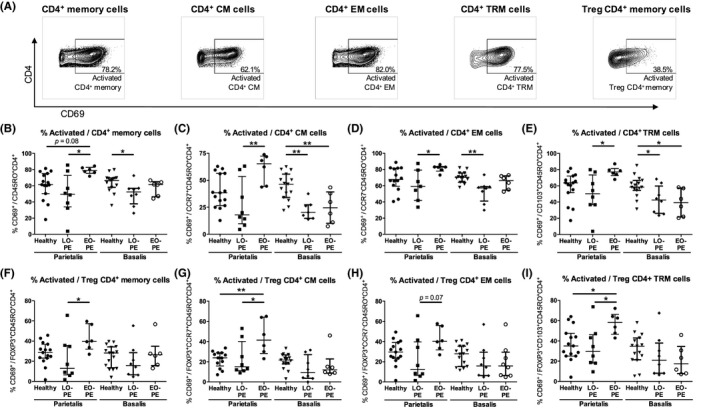
Activated CD4+ memory cells in the decidua parietalis and the decidua basalis. Representative dot plots showing flow cytometric analysis of decidua parietalis activated (CD69+) general CD4+ memory cells (CM + EM; CD69+CD45RO+CD4+), CD4+ central‐memory (CM) cells (CD69+CCR7+CD45RO+CD4+), CD4+ effector‐memory (EM) cells (CD69+CCR7−CD45RO+CD4+), CD4+ tissue‐resident memory (TRM) cells (CD69+CD103+CD45RO+CD4+), and T regulatory (Treg) CD4+ memory cells (CD69+Foxp3+CD45RO+CD4+) (A). Proportions of activated cells of the CD4+ memory cell population (B), the CD4+ CM cell population (C), the CD4+ EM cell population (D), the CD4+ TRM cell population (E), the Treg CD4+ memory cell population (F), the Treg CD4+ CM (Foxp3+CCR7+CD45RO+CD4+) cell population (G), the Treg CD4+ EM (Foxp3+CCR7−CD45RO+CD4+) cell population (H), and the Treg CD4+ TRM (Foxp3+CD103+CD45RO+CD4+) cell population (I), in the decidua parietalis and the decidua basalis from healthy pregnancies and pregnancies complicated by late‐onset preeclampsia (LO‐PE) or early‐onset preeclampsia (EO‐PE). Symbols represent individual values per decidua with data as median with interquartile range. Analysis by Kolmogorov‐Smirnov test, one‐way ANOVA and Tukey's post hoc test or Kruskal‐Wallis and Dunn's post hoc test comparing the different groups within the decidua parietalis or the decidua basalis; *P < .05, **P < .01
Figure 3.
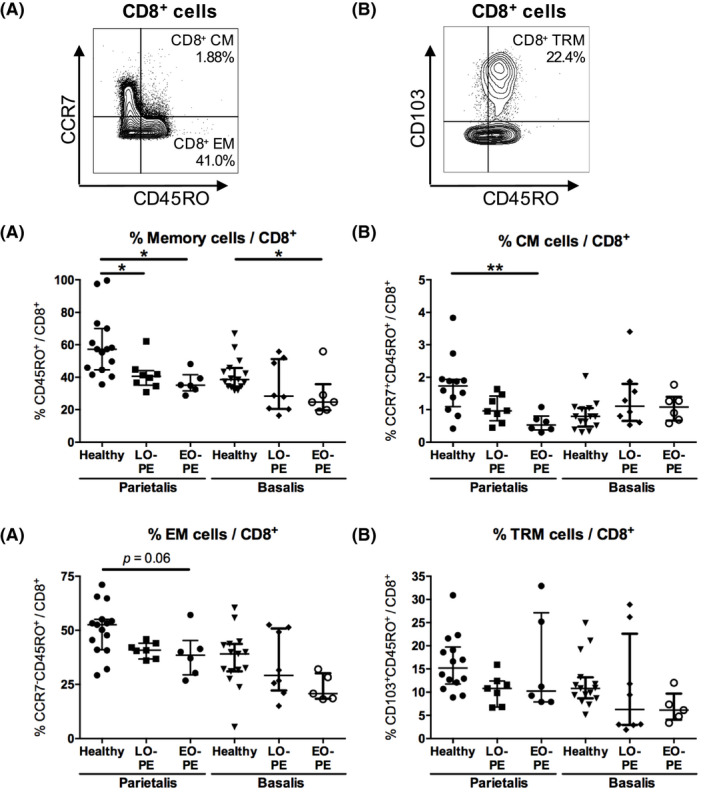
CD8+ memory cells in the decidua parietalis and the decidua basalis. Representative dot plots showing flow cytometric analysis of decidua parietalis CD8+ central‐memory (CM) cells (CCR7+CD45RO+CD8+) (A), CD8+ effector‐memory (EM) cells (CCR7−CD45RO+CD8+) (A), general CD8+ memory cells (CM + EM; CD45RO+CD8+) (A), and CD8+ tissue‐resident memory (TRM) cells (CD103+CD45RO+CD8+) (B), identified in the CD8+ cell population. Proportions of general CD8+ memory cells (C), CD8+ CM cells (D), CD8+ EM cells (E), and CD8+ TRM cells (F), in the decidua parietalis and the decidua basalis from healthy pregnancies and pregnancies complicated by late‐onset preeclampsia (LO‐PE) or early‐onset preeclampsia (EO‐PE), shown as proportion of the CD8+ cell population. Symbols represent individual values per decidua with data as median with interquartile range. Analysis by Kolmogorov‐Smirnov test, one‐way ANOVA and Tukey's post hoc test or Kruskal‐Wallis and Dunn's post hoc test comparing the different groups within the decidua parietalis or the decidua basalis; *P < .05, **P < .01
Figure 4.
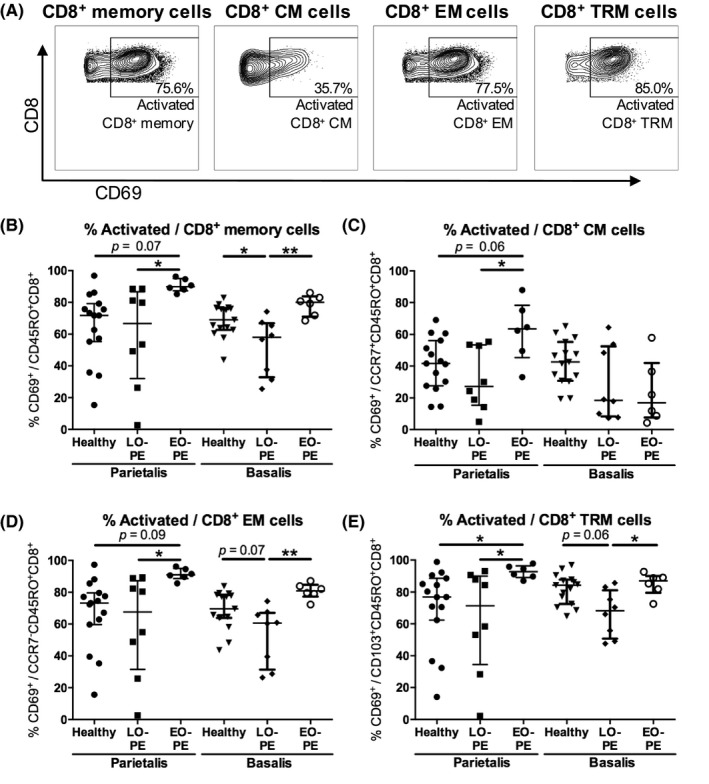
Activated CD8+ memory cells in the decidua parietalis and the decidua basalis. Representative dot plots showing flow cytometric analysis of decidua parietalis activated (CD69+) general CD8+ memory cells (CM + EM; CD69+CD45RO+CD8+), CD8+ central‐memory (CM) cells (CD69+CCR7+CD45RO+CD8+), CD8+ effector‐memory (EM) cells (CD69+CCR7−CD45RO+CD8+), and CD8+ tissue‐resident memory (TRM) cells (CD69+CD103+CD45RO+CD8+) (A). Proportions of activated cells of the CD8+ memory cell population (B), the CD8+ CM cell population (C), the CD8+ EM cell population (D), the CD8+ TRM cell population (E), in the decidua parietalis and the decidua basalis from healthy pregnancies and pregnancies complicated by late‐onset preeclampsia (LO‐PE) or early‐onset preeclampsia (EO‐PE). Symbols represent individual values per decidua with data as median with interquartile range. Analysis by Kolmogorov‐Smirnov test, one‐way ANOVA and Tukey's post hoc test or Kruskal‐Wallis and Dunn's post hoc test comparing the different groups within the decidua parietalis or the decidua basalis; *P < .05, **P < .01
Table 2.
Memory T‐cell subsets and identification markers
| Memory T‐cell subset | Markers used for identification | |
|---|---|---|
| CD4+ | Memory cells (CD4+) | CD45RO+CD4+ |
| Treg CD4+ memory | Foxp3+CD45RO+CD4+ | |
| Central‐memory (CD4+ CM) | CCR7+CD45RO+CD4+ | |
| Treg CD4+ CM cells | Foxp3+CCR7+CD45RO+CD4+ | |
| Effector‐memory (CD4+ EM) | CCR7−CD45RO+CD4+ | |
| Treg CD4+ EM cells | Foxp3+CCR7−CD45RO+CD4+ | |
| Tissue‐resident memory (CD4+ TRM) | CD103+CD45RO+CD4+ | |
| Treg CD4+ TRM | Foxp3+CD103+CD45RO+CD4+ | |
| Activated CD4+ cell | CD69+CD4+ | |
| CD8+ | Memory cells (CD8+) | CD45RO+CD8+ |
| Central‐memory (CD8+ CM) | CCR7+CD45RO+CD8+ | |
| Effector‐memory (CD8+ EM) | CCR7−CD45RO+CD8+ | |
| Tissue‐resident memory (CD8+ TRM) | CD103+CD45RO+CD8+ | |
| Activated CD8+ cell | CD69+CD8+ |
Abbreviations: CCR7, chemokine receptor 7; CM, central‐memory; EM, effector‐memory; Foxp3, forkhead box P3; Treg, T regulatory; TRM, tissue‐resident memory.
2.4. qRT‐PCR
After mechanical separation of the decidua basalis and parietalis, biopsies of 1 × 1 cm were taken from the central region of both the decidua basalis and parietalis. Biopsies were put in RNA Later (eBioscience) and immediately stored at 4°C for 6 hours and were then put at −20°C until analysis. Tissue was disrupted with a TissueLyser (Qiagen; 50 Hz, 2 minutes) in 1% β‐mercapto‐ethanol (Sigma‐Aldrich) in RLT buffer (Qiagen). RNA was isolated with an Allprep DNA/RNA mini kit (Qiagen). Absorbance‐based quantification of each RNA sample was performed using a NanoDrop Spectrophotometer (ND‐1000; NanoDrop Technologies Inc). Intactness of RNA samples was confirmed in random samples from both the decidua basalis and the decidua parietalis by electrophoresis in 2% agarose gel with ethidium bromide (0.2 µg). cDNA was transcribed with Superscript‐II Reverse Transcriptase kit (Invitrogen). Housekeeping genes hypoxanthine‐guanine phosphoribosyltransferase (HPRT; Hs02800695_m1) and β‐actin (ACTB; Hs99999903_m1) were analyzed. mRNA expression of HPRT was normally distributed and had a lower standard deviation compared to mRNA expression of ACTB. Therefore, HPRT was used as reference housekeeping gene. 53 mRNA expression of memory T cell–associated cytokines IL7 (Hs00174202_m1), IL15 (Hs01003716_m1), and IL23 (Hs00372324_m1), and preeclampsia‐associated cytokines interferon‐γ (IFN‐γ; Hs00989291_m1), interleukin‐1β (IL1β; Hs00174097_m1), IL2 (Hs00174114_m1), IL6 (Hs00985639_m1), IL8 (Hs00174103_m1), and IL10 (Hs00961622_m1) was analyzed using TaqMan Gene Expression Assays (Thermo Fisher). PCR reactions were performed in triplicates with 15 ng cDNA. Cycles were performed on a ViiA7‐Real‐Time PCR System (Thermo Fisher). Data were normalized to HPRT using .
2.5. Statistics
Data were tested for normality using Kolmogorov‐Smirnov test (P < .05). When data were normally distributed, all groups were compared using one‐way ANOVA followed by Tukey's post hoc test. When data were distributed non‐normally, the Kruskal‐Wallis test followed by Dunn's post hoc test was used. Fisher's exact test was used for categorical variables. Depending on parametric or nonparametric distribution, Pearson or Spearman correlation coefficient tests were used for correlation of mRNA expression with memory T‐cell proportions and for post hoc analyses to identify possible confounders. Correlation coefficients of r ≥ .5 or r ≤ .5 were considered relevant. Linear regression analyses were performed to assess the possible correlation between gestational age and memory T‐cell proportions. Outliers were excluded following the ROUT method. 54 Prism 7 software for Mac and Windows (GraphPad software Inc; Microsoft) and SPSS for Windows, version 23.0 software (SPSS Inc) was used. Differences were considered statistically significant if P < .05. A P‐value < .10 was considered a statistical trend.
3. RESULTS
3.1. Patient characteristics
An overview of the patient characteristics is provided in Table 1. Pregnancies complicated with LO‐PE (P = .04) or EO‐PE (P < .0001) had a lower gestational age compared to healthy pregnancies. The birthweight of babies after pregnancies complicated with EO‐PE was lower compared to LO‐PE (P < .0001) and healthy pregnancies (P < .0001). Systolic and diastolic blood pressure were significantly higher in EO‐PE (P = .0002 and P = .0009) and LO‐PE (P = .0079 and P = .0038) pregnancies compared to healthy pregnancies. In addition, more babies from EO‐PE pregnancies were delivered through cesarean section compared to LO‐PE pregnancies (P = .021).
3.2. Decidual CD4+ memory cell subsets in LO‐PE, EO‐PE, and healthy pregnancies
To investigate CD4+ memory T‐cell proportions at the fetal‐maternal interface in preeclampsia, flow cytometric analyses were performed on lymphocytes isolated from the decidua parietalis and decidua basalis from pregnancies with LO‐PE, EO‐PE, and healthy pregnancies (Figure 1A‐D; Figure S2).
In the decidua parietalis, the total CD4+ cell proportion of live lymphocytes did not significantly differ between the groups (Figure S3). Lower proportions of the general CD4+ memory cell (P = .0096) population were found in LO‐PE compared to healthy pregnancies in the decidua parietalis (Figure 1E). Lower CD4+ CM cell proportions were found in the decidua parietalis in EO‐PE (P = .0073) and even more in LO‐PE (P < .0001) compared to healthy pregnancies (Figure 1F). A trend toward lower (P = .059) CD4+ TRM cells was found in EO‐PE compared to healthy pregnancies in the decidua parietalis (Figure 1H). Lower Treg CD4+ CM cell proportions were found in the decidua parietalis from LO‐PE compared to healthy pregnancies (P = .035; Figure 1J). There were no significant differences in any of the CD4+ memory cell subsets between EO‐PE and LO‐PE.
Similar to the decidua parietalis, in the decidua basalis, the total CD4+ cell proportion did not differ between the groups (not shown) and lower CD4+ CM cells were observed in LO‐PE pregnancies compared to healthy pregnancies (P = .0407; Figure 1F). Apart from this CD4+ CM cell difference, in the decidua basalis no differences between the groups were found in any of the other CD4+ memory cell subsets (Figure 1). There were no significant differences in any of the CD4+ memory cell subsets between EO‐PE and LO‐PE.
3.3. Activated decidual CD4+ memory cell subsets in LO‐PE, EO‐PE, and healthy pregnancies
In the decidua parietalis, a trend toward higher activated proportions of the general CD4+ memory cell population was found in EO‐PE compared to healthy pregnancies (P = .083; Figure 2B). Higher activated proportions of the Treg CD4+ CM (P = .0091), and the Treg CD4+ TRM (P = .047) cell populations were found in EO‐PE pregnancies compared to healthy pregnancies (Figure 2G,I). The total CD4+ cell (P = .017; not shown), the general CD4+ memory cell (P = .019), CD4+ CM cell (P = .0045), CD4+ EM cell (P = .039), Treg CD4+ memory cell (P = .047), Treg CD4+ CM cell (P = .021), and Treg CD4+ TRM cell (P = .041) populations, showed a higher activated proportion in EO‐PE pregnancies compared to LO‐PE pregnancies (Figure 2B‐G,I).
In the decidua basalis, lower activated proportions of the CD4+CD45RO+ memory cell population (P = .030) and the CD4+ EM cell population (P = .0014) were found in LO‐PE compared to healthy pregnancies (Figure 2B,D). In the CD4+ CM cell population (P = .0018 and P = .0078) and the TRM CD4+ memory cell population (P = .048 and P = .028), lower activated proportions were found in the decidua basalis from both preeclamptic groups (LO‐PE and EO‐PE, respectively) compared to the control group (Figure 2C,E). No differences in activation in the total CD4+ cell population and in any of the memory T‐cell subsets were found between the preeclampsia subtypes in the decidua basalis.
Comparing the decidua parietalis and basalis, lower activated proportions of all CD4+ memory cell subsets were found in EO‐PE in the decidua basalis compared to the decidua parietalis (P < .05; Figure 2).
3.4. Decidual CD8+ memory cell subsets in LO‐PE, EO‐PE, and healthy pregnancies
The general CD8+ memory cell population, the CD8+ CM cell population, the CD8+ EM cell population, and the CD8+ TRM cell population were analyzed by flow cytometry in the decidua basalis and decidua parietalis from LO‐PE, EO‐PE, and healthy pregnancies (Figure 3A,B, Figure S4).
In the decidua parietalis, the total CD8+ cell proportion of live lymphocytes did not significantly differ between the groups (Figure S3). The general CD8+ memory cell population (P = .013) and the CD8+ CM cell population (P = .0064) were lower in EO‐PE compared to healthy pregnancies in the decidua parietalis (Figure 3C,D). The general CD8+ memory cell population was also lower in LO‐PE compared to healthy pregnancies in the decidua parietalis (P = .034; Figure 3C). CD8+ EM cells showed a trend toward lower proportions in EO‐PE compared to healthy pregnancies in the decidua parietalis (P = .063; Figure 3E). No differences were observed between the groups in CD8+ TRM cell proportions in the decidua parietalis (Figure 3F). The preeclampsia subtypes had no effect on CD8+ memory cell proportions in the decidua parietalis.
In the decidua basalis, the general CD8+ memory cell proportion was lower in EO‐PE compared to healthy pregnancies (P = .042; Figure 3C). No differences in the total CD8+ cell population and in any of the other CD8+ memory cell subsets were found between the groups in the decidua basalis.
3.5. Activated decidual CD8+ memory cell subsets in LO‐PE, EO‐PE, and healthy pregnancies
In the decidua parietalis, a trend toward higher activated (CD69+) proportions of the general CD8+ memory cell population (P = .076), the CD8+ CM cell population (P = .062), the CD8+ EM cell population (P = .095), and a significantly higher proportion of the CD8+ TRM cell population (P = .036) was observed in EO‐PE compared to healthy pregnancies (Figure 4B‐E). When comparing the preeclampsia subtypes, higher activated proportions of the total CD8+ cell population (not shown), and all CD8+ memory cell subsets were found in EO‐PE compared to LO‐PE in the decidua parietalis (P < .05; Figure 4B‐E). In the decidua basalis, lower activated proportions of the general CD8+ memory cell population (P = .015), and a trend toward lower activated proportions of the CD8+ EM cell population (P = .073) and CD8+ TRM cell population (P = .066) were found in LO‐PE compared to healthy pregnancies (Figure 4B,D,E). Activated proportions of the CD8+ CM cell population did not differ between the groups in the decidua basalis (Figure 4C). Comparison of the preeclampsia subtypes showed higher activated proportions of general CD8+ memory cells (P = .0018), CD8+ EM cells (P = .0018) and CD8+ TRM cells (P = .011) in EO‐PE pregnancies compared to LO‐PE pregnancies (Figure 4B,D,E). Lower activated proportions of all CD8+ memory cell subsets were found in EO‐PE in the decidua basalis compared to the decidua parietalis (P < .05; Figure 4).
3.6. mRNA expression of memory T cell‐associated cytokines in biopsies from the decidua parietalis and basalis in LO‐PE, EO‐PE, and healthy pregnancies
In the decidua parietalis, a trend toward higher IL15 mRNA expression was found in EO‐PE pregnancies compared to healthy pregnancies (P = .072; Figure 5B). In the decidua parietalis, no differences were found between the groups for IL7 and IL23 mRNA expression (Figure 5A,C
Figure 5.
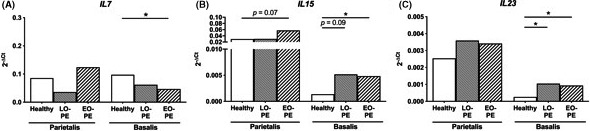
mRNA expression of memory T cell–associated cytokines in the decidua. mRNA expression of IL7 (A), IL15 (B), and IL23 (C), in biopsies from the decidua parietalis and the decidua basalis from healthy pregnancies and pregnancies complicated by late‐onset preeclampsia (LO‐PE) or early‐onset preeclampsia (EO‐PE). Data are shown as median with interquartile range mRNA target gene expression normalized to housekeeper gene HPRT. Analysis by Kolmogorov‐Smirnov test, one‐way ANOVA and Tukey's post hoc test or Kruskal‐Wallis and Dunn's post hoc test comparing the different groups within the decidua parietalis or the decidua basalis; *P < .05
).
In the decidua basalis, IL7 mRNA expression was lower in EO‐PE pregnancies as compared to healthy pregnancies (P = .044; Figure 5A). mRNA expression of IL15 (P = .013) and IL23 (P = .030) was higher in the decidua basalis from EO‐PE pregnancies compared to healthy pregnancies (Figure 5B,C). Also in the decidua basalis, a trend toward higher mRNA expression of IL15 (P = .091) and significantly higher mRNA expression of IL23 (P = .011) was found in LO‐PE compared to healthy pregnancies (Figure 5B,C). Correlation analyses in the decidua parietalis showed that mRNA expression of IL15 was associated with activated proportions of CD8+ memory cells (P = .014, r = .50) and CD8+ CM cells (P = .0062, r = .54; Tables S1 and S2). mRNA expression of IL7 and IL23 were not associated with any of the memory T‐cell proportions in either the decidua parietalis or basalis.
3.7. mRNA expression of preeclampsia‐associated cytokines in biopsies from the decidua parietalis and basalis in LO‐PE, EO‐PE, and healthy pregnancies
In the decidua parietalis, IFN‐γ showed a trend toward higher mRNA expression in EO‐PE compared to healthy pregnancies (P = .065; Figure 6A). Higher mRNA expression of IL2 (P = .0064) was found in the decidua parietalis from EO‐PE compared to healthy pregnancies (Figure 6C). No differences in mRNA expression of IL1β were found between the groups in the decidua parietalis (Figure 6B). Comparison of the preeclampsia subtypes showed a trend toward higher IFN‐γ (P = .090) and IL2 (P = .070) mRNA expression and a trend toward lower IL6 (P = .10) and IL8 (P = .10) mRNA expression in EO‐PE compared to LO‐PE in the decidua parietalis (Figure 6A,C‐E).
Figure 6.
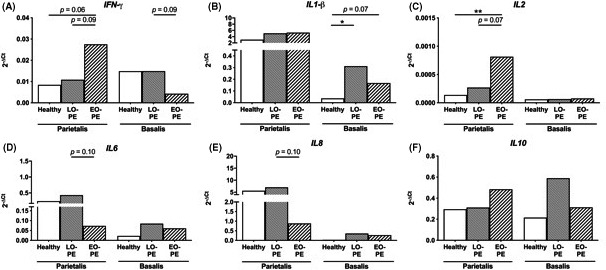
mRNA expression of preeclampsia‐associated cytokines in the decidua. mRNA expression of interferon‐γ (IFN‐γ) (A), IL1β (B), IL2 (C) IL6 (D), IL8 (E), and IL10 (F), in biopsies from the decidua parietalis and the decidua basalis from healthy pregnancies and pregnancies complicated by late‐onset preeclampsia (LO‐PE) or early‐onset preeclampsia (EO‐PE). Data are shown as median mRNA target gene expression normalized to housekeeper gene HPRT. Analysis by Kolmogorov‐Smirnov test, one‐way ANOVA and Tukey's post hoc test or Kruskal‐Wallis and Dunn's post hoc test comparing the different groups within the decidua parietalis or the decidua basalis; *P < .05, **P < .01
In the decidua basalis, higher mRNA expression of IL1β in LO‐PE (P = .011) and a trend toward higher expression in EO‐PE (P = .078) was found compared to healthy pregnancies (Figure 6B). Analysis of IL2, IL6, IL8, and IL10 mRNA expression did not show any significant differences between the groups in the decidua basalis (Figure 6C‐F). When comparing the effect of the preeclampsia subtypes on mRNA expression of preeclampsia‐associated cytokines in the decidua basalis, a trend toward lower IFN‐γ was observed in EO‐PE compared to LO‐PE (P = .091; Figure 6A).
Correlation analyses in the decidua parietalis showed that mRNA expression of IFN‐γ was associated with activated proportions of CD8+ memory cells (P = .0018, r = .62), CD8+ CM cells (P = .0054, r = .59), and CD8+ TRM cells (P = .0004, r = .67), and that mRNA expression of IL1β was associated with CD4+ CM cells (P = .020, r = −.51). mRNA expression of IL2 was associated with activated proportions of all CD8+ memory cell subsets (P < .01‐.05, r = .51‐.58), and IL8 was associated with activated proportions of CD4+ memory cells (P = .011, r = −.52), CD4+ EM cells (P = .0005, r = −.67), CD8+ memory cells (P = .0084, r = −.54), and CD8+ TRM cells (P = .0080, r = −.54; Table S1). In the decidua basalis, IFN‐γ mRNA expression was associated with CD4+ EM cells (P = .0090, r = .51), CD4+ TRM cells (P = .0036, r = .56), CD8+ TRM cells (P = .0068, r = .53), and activated proportions of CD8+ CM cells (P = .011, r = .50; Table S2).
4. DISCUSSION
In this study, we showed fewer CD4+ CM cells and CD8+ memory cells in the decidua parietalis from EO‐PE and LO‐PE compared to healthy pregnancies. All CD4+ and CD8+ memory cell subsets analyzed in this study had higher activated proportions in the decidua parietalis from EO‐PE compared to LO‐PE. These findings were accompanied by lower IL7 mRNA expression and higher mRNA expression of IL15 and IL23 in the decidua basalis of EO‐PE compared to healthy pregnancies.
In accordance with previous studies, our study showed that various memory T‐cell subpopulations are present at the fetal‐maternal interface. 15 , 20 Since our methods were designed to minimize contamination of decidual T cells with fetal T cells and T cells from maternal blood (Figure S5 and Supplementary Methods), we consider the memory T cells analyzed in this study, a good representation of the decidual memory T‐cell populations. Previously, Tilburgs et al showed that CD8+ memory cells at the fetal‐maternal interface have decreased cytotoxic activity, as they showed reduced levels of perforin and granzyme B, but increased levels of programmed cell death‐1 (PD‐1). 17 Mice studies suggest that the function of memory T cells during pregnancy has changed. 38 , 43 , 44 It may be suggested that memory T cells are differently regulated at the fetal‐maternal interface, which affects their function and keeps them quiescent throughout pregnancy to generate tolerance to fetal antigens, however, remain capable of eliciting an immune response toward pathogens.
Our findings suggest that CM and EM cells are differently maintained in decidua tissue. CM and EM cells have different homing capacities and homing receptors. 25 , 33 , 34 Contrary to EM cells, CM cells express homing receptors CCR7 and CD62L which enables central immune surveillance by patrolling the lymph nodes draining peripheral tissues. 22 , 33 However, our study and various other studies have shown that the distinction between CM and EM cells may not be so clear, since CM cells are also found in non‐lymphoid tissue and in these tissues may have effector functions similar to EM cells. 17 , 46 , 55 CD4+ CM cells are long‐living cells and are able to persist in the circulation for a long time. 56 They can also transform into different CD4+ cell subsets, including EM cells. 56 , 57 It may be that CD4+ CM cells persist in the circulation after pregnancy. This suggestion is in line with our previous study in parous women after uncomplicated pregnancies, which showed persisting higher levels of CD4+ CM cells in women postpartum compared to nulligravid women. 58 Recently, we found lower activated proportions of CD4+ CM cells in women after a preeclamptic pregnancy compared to women after a healthy pregnancy. 59
In the present study, we did find Foxp3+ CD4+ memory cells in the decidua parietalis and the decidua basalis in both healthy and preeclamptic pregnancies. It is suggested that Treg CD8+ memory cells are distinguished by other markers. 60 , 61 Within the CD4+ cell population, we were able to identify Treg CM, Treg EM, and Treg TRM cells. Studies using transgenic mouse models have shown a Treg CD4+ memory cell population with fetal antigen specificity which is generated during gestation, remains postpartum and contributes to lower resorption rates in subsequent pregnancies. 38 We observed lower Treg CD4+ CM cells in the decidua parietalis from LO‐PE compared to healthy pregnancies. Other Treg CD4+ memory cell populations did not differ between healthy and preeclamptic pregnancies. Our study may thus indicate that Treg CD4+ memory cells in the decidua are not so much affected in preeclampsia. As it is known that Treg cells are increased at mid‐gestation and decrease toward the end of pregnancy, 62 , 63 the lack of a decrease of Treg CD4+ memory cells in preeclampsia may also be due to differences in gestational age between the groups. The difference in gestational age between our groups is a limitation of our study, however, the optimal “healthy” preterm cohort which matches the median gestational age of 30.65 weeks of gestation similar to that of the patients in the EO‐PE group is most likely also pathologic, which could therefore result in false‐negative or false‐positive results. To analyze a possible effect of gestational age on our results, we performed linear regression analyses in the healthy pregnancy group separately and in all groups together. These analyses did not show any significant effect of gestational age on memory T‐cell proportions and also no effect on mRNA expression of preeclampsia‐associated cytokines was observed. It was shown in previous studies that IL15 mRNA expression in decidual and placental tissues increases with gestational age. 64 , 65 We found a trend toward higher IL15 mRNA expression in the decidua parietalis and significantly higher IL15 mRNA expression in the decidua basalis of the EO‐PE group compared to the healthy group. This is the opposite of what was expected when looking at the gestational age of these groups since the EO‐PE group has a significantly lower gestational age compared to the healthy group. Therefore, the changes found in IL15 mRNA expression are most likely due to preeclampsia and not gestational age.
Although the sample size of our study is limited, the very homogenous patient groups are a strength. To exclude an effect of possible confounders, we performed several post hoc analyses. We performed correlation analyses to detect possible effects of sample processing time on memory cell subsets. As R 2 was <.3 for all analyses, we consider the processing time not as a relevant confounder. To detect whether mode of delivery might interfere with our results, we analyzed an additional cohort of patients and found no significant differences in CD4+ and CD8+ memory T‐cell populations and their activated proportions in the decidua basalis and parietalis between healthy women who delivered through cesarean section and healthy women who delivered vaginally (Figure S5). A trend toward lower CD4+ and CD8+ memory cells was observed in the decidua basalis of women who delivered through cesarean section, and a trend toward lower activated proportions of CD8+ memory cells was also observed in the decidua basalis of these women. These differences were not seen in the cell populations when comparing the preeclamptic groups to the healthy group except for the CD8+ memory cell population. Therefore, we conclude that the difference in mode of delivery between our preeclamptic and control groups does not affect our main findings. Only women in the EO‐PE group received corticosteroid therapy for fetal lung maturation. Using correlation analysis, we found a relevant correlation regarding time since steroid use and levels of activated CD4+ CM cells (R 2 = .70) in the decidua parietalis in the EO‐PE group. This indicates that the higher activated CD4+ CM cell proportions that we found in the EO‐PE compared to the LO‐PE group might be due to steroid use. Interestingly, the activated CD4+ CM cell population did not differ between the EO‐PE and the healthy control group, who also did not receive steroids. In addition, all other memory T‐cell subsets did not show any correlation with time since steroid use which suggests only very minimal effects on the results. Effects of steroids on memory T cells in pregnancy are a relevant subject for future studies, especially since the interference of steroids in human immunological studies of preeclampsia is unavoidable since it is standard clinical care.
To explore potential mechanisms related to decreased memory T‐cell populations in the preeclamptic decidua, we measured mRNA expression of memory T‐cell associated cytokines in decidua parietalis and basalis biopsies. We showed slightly lower IL7 mRNA expression in the decidua basalis in LO‐PE and significantly lower IL7 mRNA expression in EO‐PE compared to healthy pregnancies. IL7 promotes survival and self‐renewal of both CD4+ and CD8+ memory cell lineages, 66 , 67 which could indicate that the decreased proportions of CD8+ memory T‐cell populations in the preeclamptic decidua basalis are due to decreased levels of IL7, and however, correlation analyses did not show a relevant association. IL15 and IL23 are pro‐inflammatory cytokines that have stimulatory effects on CD4+ and CD8+ memory cells, promote long term survival, and induce IFN‐γ, tumour necrosis factor B, and IL17 secretion. 27 , 68 , 69 , 70 Therefore, the higher levels of IL15 and IL23 mRNA expression in the decidua basalis from preeclamptic pregnancies and their known stimulatory effects on memory T cells 27 , 68 , 69 , 70 indicate that these cytokines may not be involved in the decreased proportions of memory T cells in the decidua. The levels of IL15 in the decidua parietalis might be involved in the higher activated proportions of CD8+ memory cell subsets, since the IL15 mRNA expression was associated with the higher activated proportions of CD8+ memory cells, CD8+ CM cells, and CD8+ TRM cells. Various papers have shown that the decidua parietalis and basalis have different functional characteristics and consist of a different immune cell repertoire which might be due to different blood supply, cell recruitment, differential HLA expression on trophoblasts and prostaglandin secretion in the different anatomic locations. 14 , 15 , 16 , 18 , 71 These earlier studies showed differences in activation and proportions of macrophage subsets and T cells. 14 , 15 , 16 , 18 , 71 The most prominent difference we found between the two decidual compartments in this study are the lower activated proportions of all CD4+ and CD8+ memory cell subsets in EO‐PE in the decidua basalis compared to the decidua parietalis. An explanation for this might be the cytokines present in the decidua parietalis since correlation analyses showed that activated proportions of the memory T cells were associated with IL8, IL15, and IFN‐γ mRNA expression in the decidua parietalis and not in the decidua basalis. When we compared EO‐PE and LO‐PE, we found higher activated proportions in almost every memory T‐cell subset analyzed in the decidua parietalis from EO‐PE compared to LO‐PE. Since EO‐PE is associated with higher oxidative stress compared to LO‐PE, 5 , 6 , 72 and oxidative stress is associated with higher CD69 expression, 73 the higher activated proportions of memory T cells in EO‐PE compared to LO‐PE in this study may be due to higher oxidative stress. Also, excess syncytiotrophoblast microparticle shedding into the maternal circulation in EO‐PE and not LO‐PE could cause increased activated proportions and higher IFN‐γ and IL2 mRNA expression in EO‐PE compared to LO‐PE. 74
In conclusion, this study showed that memory T‐cell subsets in the decidua basalis and parietalis are altered in EO‐PE and LO‐PE compared to healthy pregnancies. In addition, higher activated proportions of memory T cells were found in EO‐PE compared to LO‐PE in the decidua parietalis. These findings were accompanied by lower mRNA expression of the memory T cell–stimulating cytokine IL7 and higher IL15 and IL23 mRNA expression in EO‐PE compared to healthy pregnancies. We hypothesize that memory T cells are involved in fetal‐maternal tolerance and that the decreased presence of specific memory T‐cell subsets at the fetal‐maternal interface in preeclampsia may play a role in the pathophysiology of preeclampsia, that is, decreased fetal tolerance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all patients that contributed to the study and RN Verkaik‐Schakel and MH Schoots for their technical support. We thank the University Medical Center Groningen, the University of Groningen and a Mandema Stipendium (awarded to JR Prins MD PhD) for their financial support.
Kieffer TEC, Laskewitz A, Vledder A, Scherjon SA, Faas MM, Prins JR. Decidual memory T‐cell subsets and memory T‐cell stimulatory cytokines in early‐ and late‐onset preeclampsia. Am J Reprod Immunol. 2020;84:e13293 10.1111/aji.13293
REFERENCES
- 1. Saftlas AF, Olson DR, Franks AL, et al. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163:460‐465. [DOI] [PubMed] [Google Scholar]
- 2. Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1‐7. [DOI] [PubMed] [Google Scholar]
- 3. Steegers EAP, von Dadelszen P, Duvekot JJ, et al. Pre‐eclampsia. Lancet. 2010;376:631‐644. [DOI] [PubMed] [Google Scholar]
- 4. Tranquilli AL, Brown MA, Zeeman GG, et al. The definition of severe and early‐onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens An Int J Women’s Cardiovasc Heal. 2013;3:44‐47. [DOI] [PubMed] [Google Scholar]
- 5. Staff AC, Redman CWG. The differences between early‐ and late‐onset pre‐eclampsia In: Saito S, ed. Preeclampsia, Comprehensive Gynecology and Obstetrics. Singapore: Springer Nature; 2018:157‐172. [Google Scholar]
- 6. Raymond D, Peterson E. A critical review of early‐onset and late‐onset preeclampsia. Obstet Gynecol Surv. 2011;66:497‐506. [DOI] [PubMed] [Google Scholar]
- 7. Robillard P‐Y, Dekker G, Chaouat G, et al. Historical evolution of ideas on eclampsia/preeclampsia: a proposed optimistic view of preeclampsia. J Reprod Immunol. 2017;123:72‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zenclussen AC, Schumacher A, Zenclussen ML, et al. Immunology of pregnancy: cellular mechanisms allowing fetal survival within the maternal uterus. Expert Rev Mol Med. 2007;9:1‐14. [DOI] [PubMed] [Google Scholar]
- 9. Prins JR, Boelens HM, Heimweg J, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300‐311. [DOI] [PubMed] [Google Scholar]
- 10. Cudihy D, Lee R. The pathophysiology of pre‐eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576‐582. [DOI] [PubMed] [Google Scholar]
- 11. Clark DA, Rahmati M, Gohner C, et al. Seminal plasma peptides may determine maternal immune response that alters success or failure of pregnancy in the abortion‐prone CBAxDBA/2 model. J Reprod Immunol. 2013;99:46‐53. [DOI] [PubMed] [Google Scholar]
- 12. de Groot CJM, van der Mast BJ, Visser W, et al. Preeclampsia is associated with increased cytotoxic T‐cell capacity to paternal antigens. Am J Obstet Gynecol. 2010;203:496 .e1 ‐ 6. [DOI] [PubMed] [Google Scholar]
- 13. Rolfo A, Giuffrida D, Nuzzo AM, et al. Pro‐inflammatory profile of preeclamptic placental mesenchymal stromal cells: new insights into the etiopathogenesis of preeclampsia. PLoS One. 2013;8:e59403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baergen RN. Histology of the chorionic villi, fetal membranes, and umbilical cord In: Manual of Benirschke and Kaufmann’s. New York, NY: Springer‐Verlag; 2005: 80‐95. [Google Scholar]
- 15. Tilburgs T, Roelen D, Vandermast B, et al. Differential distribution of CD4+CD25bright and CD8+CD28‐ T‐cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27:47‐53. [DOI] [PubMed] [Google Scholar]
- 16. Baergen RN. Development and histology of the nonvillous portions of the placenta In: Manual of Benirschke and Kaufmann’s. New York, NY: Springer‐Verlag; 2005: 107‐134. [Google Scholar]
- 17. Tilburgs T, Schonkeren D, Eikmans M, et al. Human decidual tissue contains differentiated CD8+ effector‐memory T cells with unique properties. J Immunol. 2010;185:4470‐4477. [DOI] [PubMed] [Google Scholar]
- 18. Schonkeren D, van der Hoorn M‐L, Khedoe P, et al. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol. 2011;178:709‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guerin LR, Prins JR, Robertson SA. Regulatory T‐cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nancy P, Erlebacher A. T cell behavior at the maternal‐fetal interface. Int J Dev Biol. 2014;58:189‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vento‐Tormo R, Efremova M, Botting RA, et al. Single‐cell reconstruction of the early maternal–fetal interface in humans. Nature. 2018;563:347‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kieffer TEC, Laskewitz A, Scherjon SA, et al. Memory T cells in pregnancy. Front Immunol. 2019;10:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saftlas AF, Rubenstein L, Prater K, et al. Cumulative exposure to paternal seminal fluid prior to conception and subsequent risk of preeclampsia. J Reprod Immunol. 2014;101–102:104‐110. [DOI] [PubMed] [Google Scholar]
- 24. Robillard P‐Y, Périanin J, Janky E, et al. Association of pregnancy‐induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344:973‐975. [DOI] [PubMed] [Google Scholar]
- 25. Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708‐712. [DOI] [PubMed] [Google Scholar]
- 26. Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326‐332. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Wang H, Lu H, et al. Regulation of memory T cells by interleukin‐23. Int Arch Allergy Immunol. 2016;169:157‐162. [DOI] [PubMed] [Google Scholar]
- 28. Kondrack RM, Harbertson J, Tan JT, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richer MJ, Pewe LL, Hancox LS, et al. Inflammatory IL‐15 is required for optimal memory T cell responses. J Clin Invest. 2015;125:3477‐3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol. 2015;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Topham DJ, Reilly EC. Tissue‐resident memory CD8+ T cells: from phenotype to function. Front Immunol. 2018;9:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sallusto F, Kremmer E, Palermo B, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037‐2045. [DOI] [PubMed] [Google Scholar]
- 33. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745‐763. [DOI] [PubMed] [Google Scholar]
- 34. Förster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23‐33. [DOI] [PubMed] [Google Scholar]
- 35. Kumar BV, Ma W, Miron M, et al. Human tissue‐resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20:2921‐2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen T, Darrasse‐Jèze G, Bergot A‐S, et al. Self‐specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191:2273‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kalekar LA, Schmiel SE, Nandiwada SL, et al. CD4+ T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat Immunol. 2016;17:304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rowe JH, Ertelt JM, Xin L, et al. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen TA, Kahn DA, Loewendorf AI. Maternal—fetal rejection reactions are unconstrained in preeclamptic women. PLoS One. 2017;12:e0188250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tilburgs T, Roelen D, van der Mast B, et al. Evidence for a selective migration of fetus‐specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737‐5745. [DOI] [PubMed] [Google Scholar]
- 41. Kälble F, Mai C, Wagner M, et al. Aberrant ICOS+‐T cell differentiation in women with spontaneous preterm labor. Am J Reprod Immunol. 2016;76:415‐425. [DOI] [PubMed] [Google Scholar]
- 42. Schober L, Radnai D, Spratte J, et al. The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus. Clin Exp Immunol. 2014;177:76‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barton BM, Xu R, Wherry EJ, et al. Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long‐lived maternal T cells. J Leukoc Biol. 2017;101:975‐987. [DOI] [PubMed] [Google Scholar]
- 44. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen‐specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. 2010;107:9299‐9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Zwan A, Bi K, Norwitz ER, et al. Mixed signature of activation and dysfunction allows human decidual CD8+ T cells to provide both tolerance and immunity. Proc Natl Acad Sci USA. 2018;115:385‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Powell RM, Lissauer D, Tamblyn J, et al. Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J Immunol. 2017;199:3406‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeng W, Liu Z, Liu X, et al. Distinct transcriptional and alternative splicing signatures of decidual CD4+ T cells in early human pregnancy. Front Immunol. 2017;8:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wagner MI, Jöst M, Spratte J, et al. Differentiation of ICOS+ and ICOS‐ recent thymic emigrant regulatory T cells (RTE T regs) during normal pregnancy, pre‐eclampsia and HELLP syndrome. Clin Exp Immunol. 2016;183:129‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wagner MI, Mai C, Schmitt E, et al. The role of recent thymic emigrant‐regulatory T‐cell (RTE‐Treg) differentiation during pregnancy. Immunol Cell Biol. 2015;93:858‐867. [DOI] [PubMed] [Google Scholar]
- 50. Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24‐43. [DOI] [PubMed] [Google Scholar]
- 51. Committee for Guidelines in Research (COREON) . Human tissue and medical research: Code of conduct for responsible use; 2011. www.federa.org. Accessed November 10, 2018
- 52. van Lochem A, van Beelen E, van der Keur C, et al. Preservation of human placenta is feasible and facilitates studies on the local immune regulation in normal and aberrant pregnancies. J Reprod Immunol. 2012;94:45. [DOI] [PubMed] [Google Scholar]
- 53. Mane VP, Heuer MA, Hillyer P, et al. Systematic method for determining an ideal housekeeping gene for real‐time PCR analysis. J Biomol Tech. 2008;19:342‐347. [PMC free article] [PubMed] [Google Scholar]
- 54. Motulsky HJ, Brown RE, Barnett V, et al. Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gehad A, Teague JE, Matos TR, et al. A primary role for human central memory cells in tissue immunosurveillance. Blood Adv. 2018;2:292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zaph C, Uzonna J, Beverley SM, et al. Central memory T cells mediate long‐term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104‐1110. [DOI] [PubMed] [Google Scholar]
- 57. Gray JI, Westerhof LM, MacLeod MKL. The roles of resident, central and effector memory CD4 T‐cells in protective immunity following infection or vaccination. Immunology. 2018;154:574‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kieffer TEC, Faas MM, Scherjon SA, et al. Pregnancy persistently affects memory T cell populations. J Reprod Immunol. 2017;119:1‐8. [DOI] [PubMed] [Google Scholar]
- 59. Kieffer TEC, Scherjon SA, Faas MM, et al. Lower activation of CD4+ memory T cells in preeclampsia compared to healthy pregnancies persists postpartum. J Reprod Immunol. 2019;136:165‐378. [DOI] [PubMed] [Google Scholar]
- 60. Wang S‐C, Li Y‐H, Piao H‐L, et al. PD‐1 and Tim‐3 pathways are associated with regulatory CD8+ T‐cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015;6:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Machicote A, Belén S, Baz P, et al. Human CD8+HLA‐DR+ regulatory T cells, similarly to classical CD4+Foxp3+ cells, suppress immune responses via PD‐1/PD‐L1 axis. Front Immunol. 2018;9:2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T‐cell subset. Immunology. 2004;112:38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Santner‐Nanan B, Peek MJ, Khanam R, et al. Systemic increase in the ratio between Foxp3+ and IL‐17‐producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023‐7030. [DOI] [PubMed] [Google Scholar]
- 64. Okada S, Okada H, Sanezumi M, et al. Expression of interleukin‐15 in human endometrium and decidua. Mol Hum Reprod. 2000;6:75‐80. [DOI] [PubMed] [Google Scholar]
- 65. Agarwal R, Loganath A, Roy AC, et al. Expression profiles of interleukin‐15 in early and late gestational human placenta and in pre‐eclamptic placenta. Mol Hum Reprod. 2001;7:97‐101. [DOI] [PubMed] [Google Scholar]
- 66. Cui G, Staron M, Gray S, et al. IL‐7‐induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell. 2015;161:750‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chetoui N, Boisvert M, Gendron S, et al. Interleukin‐7 promotes the survival of human CD4+ effector/memory T cells by up‐regulating Bcl‐2 proteins and activating the JAK/STAT signalling pathway. Immunology. 2010;130:418‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Berard M, Brandt K, Bulfone‐Paus S, et al. IL‐15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018‐5026. [DOI] [PubMed] [Google Scholar]
- 69. Weng N‐P, Liu K, Catalfamo M, et al. IL‐15 is a growth factor and an activator of CD8 memory T cells. Ann N Y Acad Sci. 2002;975:46‐56. [DOI] [PubMed] [Google Scholar]
- 70. Duvallet E, Semerano L, Assier E, et al. Interleukin‐23: a key cytokine in inflammatory diseases. Ann Med. 2011;43:503‐511. [DOI] [PubMed] [Google Scholar]
- 71. Nakabayashi Y, Nakashima A, Yoshino O, et al. Impairment of the accumulation of decidual T cells, NK cells, and monocytes, and the poor vascular remodeling of spiral arteries, were observed in oocyte donation cases, regardless of the presence or absence of preeclampsia. J Reprod Immunol. 2015;114:65‐74. [DOI] [PubMed] [Google Scholar]
- 72. Raijmakers M, Peters W, Steegers E, et al. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre‐eclamptic women. Trophobl Res. 2004;25:85‐89. [DOI] [PubMed] [Google Scholar]
- 73. Grassi F, Tell G, Robbie‐Ryan M, et al. Oxidative stress causes bone loss in estrogen‐deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci USA. 2007;104:15087‐15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goswami D, Tannetta DS, Magee LA, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early‐onset pre‐eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56‐61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


