Abstract
Enzyme replacement therapy (ERT) with recombinant α‐galactosidase A (r‐αGAL A) for the treatment of Fabry disease has been available for over 15 years. Long‐term treatment may slow down disease progression, but cardiac, renal, and cerebral complications still develop in most patients. In addition, lifelong intravenous treatment is burdensome. Therefore, several new treatment approaches have been explored over the past decade. Chaperone therapy (Migalastat; 1‐deoxygalactonojirimycin) is the only other currently approved therapy for Fabry disease. This oral small molecule aims to improve enzyme activity of mutated α‐galactosidase A and can only be used in patients with specific mutations. Treatments currently under evaluation in (pre)clinical trials are second generation enzyme replacement therapies (Pegunigalsidase‐alfa, Moss‐aGal), substrate reduction therapies (Venglustat and Lucerastat), mRNA‐ and gene‐based therapy. This review summarises the knowledge on currently available and potential future options for the treatment of Fabry disease.
Keywords: chaperone therapy, enzyme replacement therapy (ERT), Fabry disease, gene therapy, substrate reduction therapy (SRT), treatment
Abbreviations
- ADAs
anti‐drug antibodies
- CVA
cerebrovascular accident
- enzyme: αGAL A, gene: GLA
alpha‐galactosidase A
- ERT
enzyme replacement therapy
- FD
Fabry disease
- Gb3
globotriaosylceramide
- GCS
glucosylceramide synthase
- GSL
glycosphingolipids
- LDL
low‐density lipoprotein
- LSDs
lysosomal storage disorders
- lysoGb3
globotriaosylsphingosine
- M6P
mannose 6‐phosphate
- PEG
polyethylene glycol
- PRX‐102
Pegunigalsidase alfa
- r‐αGAL A
recombinant alpha‐galactosidase A
- SRT
substrate reduction therapy
- TIA
transient ischemic attack
1. INTRODUCTION
To date, more than 50 genetic lysosomal storage disorders (LSDs) have been identified, of which Fabry disease (FD) (OMIM number: 301500) is probably the most prevalent. FD is caused by the presence of a deleterious mutation in the GLA gene coding for the enzyme alpha‐galactosidase A (αGAL A) on the X chromosome, resulting in progressive accumulation of the enzyme's substrate. Accumulation of, predominantly, globotriaosylceramide (Gb3) and its derivatives such as globotriaosylsphingosine (lysoGb3) in many different cell types is thought to be responsible for the pathology observed in FD. The lysosomal Gb3 inclusions are most prominent in the endothelium, cardiomyocytes, peripheral neurons, and various renal cell types.1, 2 The disease is characterised by a broad phenotypic spectrum. Variety in disease expression is largely determined by the type of mutation in the GLA gene and sex of the patient. Due to the X‐linked inheritance pattern, males are generally more severely affected than females and develop disease symptoms and complications earlier in life.3 Male patients can be classified as having the classical‐ or non‐classical form of FD based on residual enzyme activity (if appropriately measured in preferably leucocytes) and the presence of characteristic classical symptoms. In females, enzyme activity does not distinguish between the classical and non‐classical phenotype and classification is based on mutation, family history, and clinical as well as biochemical characteristics.4, 5 Symptoms of classical FD are angiokeratomas, cornea verticillata, and heat and exercise induced neuropathic pain (acroparesthesia).6 These symptoms often become apparent in childhood. At a later age, proteinuria, renal function loss, white matter lesions in the brain, electrocardiographic changes and left ventricular hypertrophy may occur. In the absence of treatment, life expectancy of Fabry patients with classic disease is approximately 60 years in males and 75 years in females, with the most common causes of death being sudden cardiac death, renal failure, and stroke.7 For over 15 years, two recombinant enzyme preparations have been available to treat patients with FD. The effectiveness of these two recombinant enzyme preparations is variable, probably depending on timing of treatment initiation and phenotype.8 This emphasises the need to improve our understanding of disease course, as well as develop alternative therapies. In the past years, several new treatment modalities for FD have been developed, of which only Migalastat (chaperone therapy) is currently approved. Several other new approaches are being explored in clinical and preclinical studies. Amongst these are second generation enzyme replacement therapies, substrate reduction therapy (SRT), gene‐ and mRNA based therapy (Figure 1). In the end we briefly elaborate on the potential future approach to stimulate the egress of storage material from the cell (Figure 4). The goal of this review is to provide an overview of the current status of the various therapeutic approaches for the treatment of FD.
Figure 1.
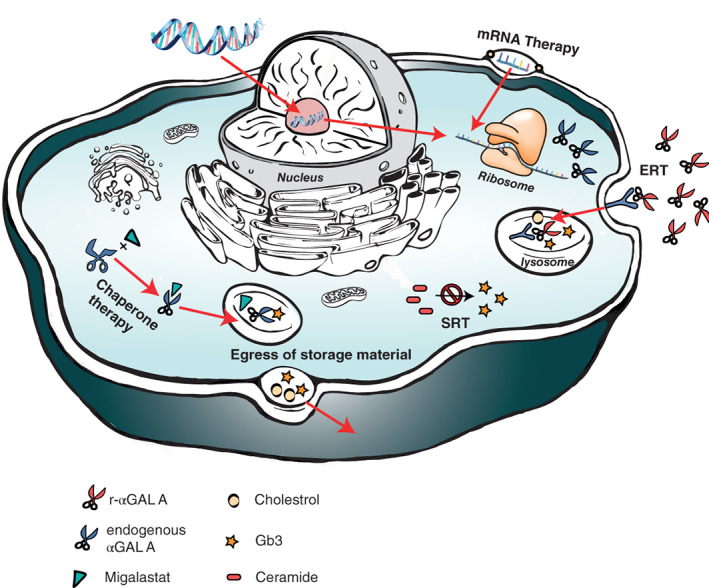
Overview of different approaches in treating Fabry disease; Enzyme replacement therapy (ERT) aims to restitute defective αGAL A. Chaperones bind to the active site of the unstable αGAL A to aid proper folding. Substrate reduction therapy targets the glycosphingolipid synthesis to reduce formation of Gb3 and its derivatives. Gene therapy aims to correct the underlying genetic defect of FD. MRNA therapy induces transient endogenous αGAL A production. The egress of Gb3 can potentially be stimulated by enhancing cholesterol efflux (Figure 4). FD, Fabry disease
2. CURRENTLY AVAILABLE TREATMENT OPTIONS
2.1. Enzyme replacement therapy, developments, and challenges
Currently, two different forms of enzyme replacement therapy (ERT) are available; agalsidase‐alfa (Replagal, Takeda), produced in human fibroblasts and registered at a dose of 0.2 mg/kg biweekly, and agalsidase‐beta (Fabrazyme, Sanofi Genzyme), produced in Chinese hamster ovary cells and registered at a dose of 1.0 mg/kg biweekly. Short term pathological studies on the effect of agalsidase‐beta treatment mainly focused on renal biopsies and showed that treatment resulted in clearance of Gb3 from endothelial cells, mesangial cells and podocytes.9, 10 In a study with agalsidase‐alfa, studying different cell types, decreased Gb3 accumulation in liver and in tubular epithelial cells was observed, as well as a reduction of Gb3 excretion in urine.11 Tøndel et al showed that 5 years of treatment with either preparation resulted in complete clearance of glomerular endothelial as well as mesangial inclusions. Patients treated with the highest dose also had a notable reduction in podocytes inclusions.12 Long‐term clinical studies showed a small but significant effect of ERT on cardiovascular and renal complication rate, with some superiority of the higher dosed agalsidase‐beta compared to agalsidase‐alfa.13, 14 Especially loss of renal function, occurring in the vast majority of male patients with classic FD, is attenuated by ERT.8 These clinical benefits were mainly observed in patients who started ERT before the presence of irreversible organ damage.12, 15, 16, 17 The presence of decreased renal function, proteinuria and/or cardiac fibrosis at the time of treatment initiation was associated with disease progression despite treatment with ERT.8 In 2008 the deacylated form of Gb3, lysoGb3, was discovered as FD biomarker. Plasma lysoGb3 levels are strongly related to disease phenotype, with high levels in classical patients and lower levels in non‐classical patients.4, 18, 19 In non‐classical FD patients and in female patients with classical FD, plasma lysoGb3 levels correlate with disease severity.20, 21, 22, 23, 24, 25 This relation is not present in the most severely affected group with the highest lysoGb3 levels (male patients with classical FD),13, 18 probably due to a non‐linear relationship or a plateaued response. Plasma levels of lysoGb3 decrease substantially during treatment with ERT in male patients with classic FD,18, 19 often into the ranges of non‐classical or female patients.13 During treatment, lysoGb3 levels were lower in patients who started treatment before the age of 25 years, compared to those who started later in life.15 In light of these findings, starting treatment early, before signs of organ damage become apparent, especially in male patients with classical FD seems a logical approach. However, the exact timing of treatment initiation is unclear, since no randomised controlled studies have so far been performed and the follow‐up of early treated classically affected boys is still too short to draw firm conclusions.26, 27 For the other patient groups (females with classical FD and patients with non‐classical FD), timing of treatment initiation is even more complex given the great variability in both disease severity and age of onset of disease manifestations. This also makes evaluation of treatment effectiveness in these groups much more difficult. In patients with non‐classical FD, as well as in female patients with classical FD, the heart is often the most prominently affected organ. The fact that in cardiac biopsies of male and female FD patients Gb3 was not cleared from cardiomyocytes during treatment with agalsidase‐beta,28 is worrying. Several studies reported an initial reduction in cardiac mass in response to ERT in women.29 However, most included studies were of short duration (2‐36 months) and used echocardiography to measure cardiac mass. Unfortunately, echocardiography is a suboptimal technique to assess changes in cardiac mass because of the large inter‐ and intra‐observer variability in the measurements.30, 31 Cardiac fibrosis is a well‐known consequence of FD and predisposes for arrhythmias and cardiac dysfunction. It is unknown whether ERT slows progression of fibrosis development and assessment of development of fibrosis is especially difficult in women, where this complication can occur even in the absence of left ventricular hypertrophy.32, 33 In addition to gender, phenotype and timing of ERT, another factor influencing the response to ERT is the formation of anti‐drug antibodies (ADAs) against recombinant alpha‐galactosidase A (r‐αGAL A). Depending on the used assay, estimations on the prevalence of ADAs against r‐αGAL A may vary.34, 35 ADAs can negatively influence treatment efficacy by changing distribution, cellular uptake, cellular localization and/or catalytic activity of the administered enzyme.36 ADAs that inhibit the catalytic activity of r‐αGAL A in vitro (iADAs), occur exclusively in approximately 50% of male patients with the classical phenotype and are associated with a less robust decline in plasma lysoGb3 levels13, 37, 38, 39 as well as with increased urinary Gb3 levels.40, 41 We recently showed that there was an antibody dose effect: higher iADA titers were associated with less reduction in plasma lysoGb3.42 A reduction in lysoGb3 does not guarantee a clinical response, however, a loss of the lysoGb3 response suggests a concomitant loss of therapeutic effectiveness. In fact, the presence of iADAs also resulted in an accelarated decline in renal function.42 Increasing the ERT dose in patients with established iADAs may attenuate the negative effect of those antibodies by saturation of the present ADAs, leaving excess of enzyme to perform its catalytic function.23, 41 In patients that received a kidney transplant, the immunosuppressive agents administered (most often tacrolimus, prednisolone and/or myophenolate‐mofetil/mycophenolic acid) prevented ADA formation in formerly treatment naive patients and temporarily reduced ADA titers in ERT treated patients.43 Whether ADAs inhibit enzyme uptake in the target cells in FD remains to be investigated.
Another factor that could contribute to the limited effectiveness of ERT is the inefficient bio‐distribution. The majority of administered recombinant enzyme ends up in the liver, whereas cardiomyocytes and podocytes, two of the most severely affected cell types in FD, take up very limited amounts of recombinant enzyme.10, 28, 44 The variation in r‐αGAL A uptake between different cell types is not yet fully understood. Although it is traditionally thought that mannose 6‐phosphate (M6P) mediated endocytosis is the main mechanism of r‐αGAL A uptake, more recent studies show that other pathways are involved as well. Blocking the M6P receptor inhibited r‐αGAL A uptake in fibroblasts, but it did not affect uptake in endothelial cells, indicating a different uptake mechanism of r‐αGAL A in the latter cell type. This observation is further supported by the lack of M6P receptors on the plasma membrane of endothelial cells.45 Currently available forms of ERT do clear storage material from endothelial cells,9, 46 indicating that these non‐M6P dependent endocytic pathways can be adequately utilised. In podocytes, enzyme uptake is in part mediated by M6P receptors, along with two other receptors: megalin and sortilin. However, blocking all three receptors only inhibited recombinant enzyme uptake by 39%,47 again indicating the existance of additional uptake mechanisms. Finally, none of the recombinant enzyme preparations can pass the blood‐brain barrier. Whether or not this is relevant for FD, remains a subject of debate. Although there is some accumulation of Gb3 in the brain of FD patients48, 49 the clinical significance remains unclear, as the main complications like TIAs and CVAs most likely result from vascular pathology.50
2.2. Chaperone therapy
Several missense mutations in FD patients have been shown to result in a mutant protein with normal αGAL A catalytic activity. The reduction in overall αGAL A enzymatic activity in patients carrying these GLA mutations has been attributed to the strongly reduced stability of the mutated protein. This is caused by protein misfolding and subsequent premature degradation.51, 52 The goal of chaperone therapy is to enhance correct folding of the mutated protein to improve its stability. The first in vitro studies on the effect of a chaperone in FD used galactose. Adding galactose to the culture medium of COS‐1 cells with the p.Q279E mutation increased the enzyme activity in vitro.51 The follow‐up study showed that galactose increased enzyme activity in COS‐1 cells and lymphoblasts for several, but not all GLA mutations (eg, no response for the p.G328R mutation).53 To date, the only study on the clinical use of galactose describes a male FD patient with the p.G328R mutation who received 1 g/kg galactose intravenously every other day for 2 years. In apparent contrast with the in vitro results, a 180% increase in enzymatic activity in endo‐myocardial biopsy specimens and reduction in cardiac mass was reported.54 Subsequent chaperone studies mostly used the galactose analogue 1‐deoxygalactonojirimycin (now known as Migalastat, Amicus Therapeutics), in which the oxygen is replaced by a nitrogen atom in the ring, resulting in an iminosugar (Figure 2). Migalastat is a potent inhibitor of αGAL A, but at lower doses increases enzymatic activity for some GLA mutations (Figure 3).55 It is believed that due to binding of the iminosugar to the catalytic domain of αGAL A, the enzyme is properly folded and after transportation to the lysosome the competitive inhibitor is replaced by the natural substrate of αGAL A. Patients eligibility for treatment with Migalastat is determined using an in vitro enzyme activity assay. In short, wild type HEK‐293 cells are transiently transfected with plasmids containing mutant GLA DNA and incubated with Migalastat. Empty vector‐transfected cells are used to determine endogenous enzyme activity and this is subtracted from the total enzyme activity of cells transfected with mutant DNA. If the corrected αGAL A activity increases at least 1.2‐fold, with an absolute increase in activity of >3%, patients with such mutations in the GLA gene are deemed eligible for treatment with Migalastat. Within the eligible group, the increase in αGAL A activity ranges from 1.2 up to 30.4‐fold56 and is positively related to baseline enzyme activity. The broad range of αGAL A activity increase in response to treatment with Migalastat may, at least partially, explain the highly variable (biochemical) response to Migalastat treatment in the clinical studies described below. Furthermore, the fact that the analyses were done in wild‐type cells instead of GLA‐knock out cells may warrant some caution in the interpretation of the results as endogenous αGAL A activity may vary per cell and cell count may vary per plate.
Figure 2.
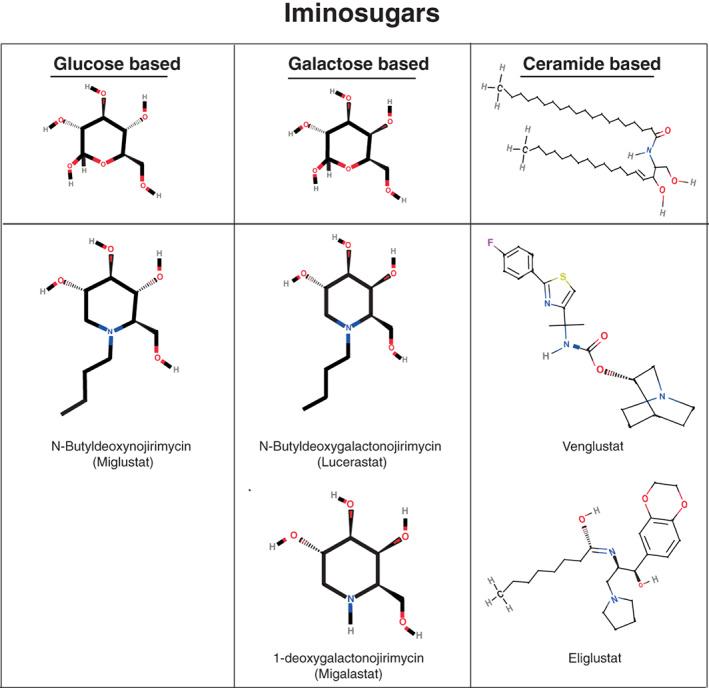
Similarities and differences in chemical structures of different iminosugars (both chaperone and substrate reduction therapy)
Figure 3.
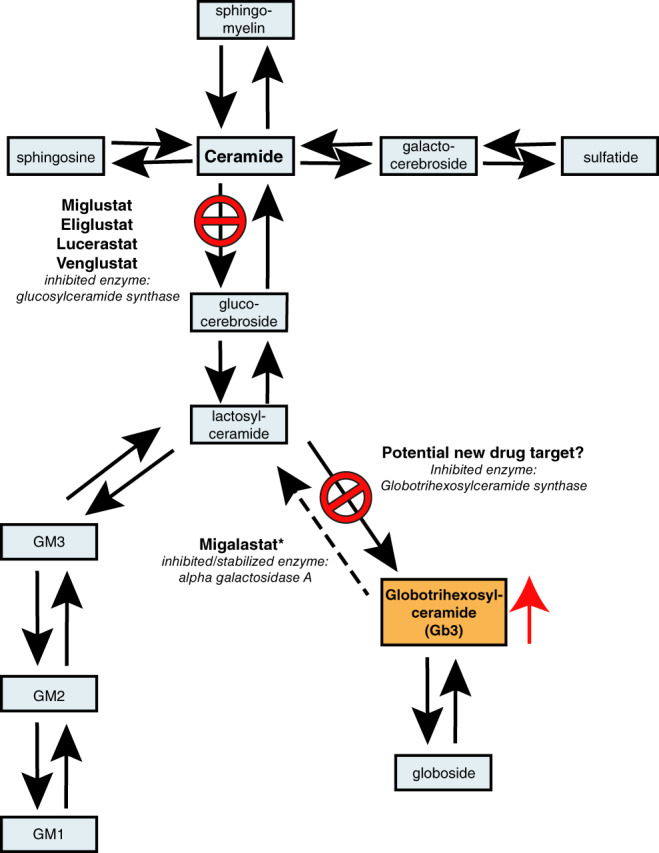
Glycosphingolipids pathways and location of intervention of substrate reduction therapy and chaperone therapy preparations. Dashed arrow indicates the mutated enzyme in FD. GM 1, 2, and 3 are gangliosides. Arrows indicate specific enzymatic reactions. Stop signs indicate point of intervention for each iminosugar. Red arrow indicates accumulation. *Migalastat inhibits αGAL A in high doses but aids proper folding of unstable αGAL A, resulting from certain amenable mutations, thus increasing enzymatic activity. FD, Fabry disease
Migalastat is currently the only oral treatment for FD approved by the US Food Drug Administration (FDA) and European Medicines Agency (EMA).56, 57, 58 Countries outside the USA and EU that have also approved Migalastat are Israel, Australia and Canada. A recent study showed that Migalastat may reduce the accumulation of Gb3 in podocytes after 6 months of treatment.59 In the same study, gastrointestinal symptoms improved in Migalastat treated patients compared to placebo treated patients.60 In an 18‐month open label study in 57 FD patients, comparing a switch from Fabrazyme or Replagal to Migalastat, no significant differences in kidney function decline (primary endpoint of the study) were observed.58 A recent publication about Migalastat described a reduction of cardiac mass of 5% on echocardiography in FD patients, a mean increase in αGAL A activity in patient leucocytes and a 45% reduction of plasma lysoGb3 in the previously untreated group.61 However, as previously pointed out,62 echocardiographic measurements of cardiac mass are highly variable (inter‐observer variability 15%‐19%, de Simone et al 1999), making it very difficult to draw any conclusion in only 14 patients. For a more precise evaluation of cardiac mass,63 cMRI (inter‐observer variability 4%‐10%) should be considered for future studies. In addition, native T1 values, late gadolinium enhancement and extracellular volume fractions (ECV) could be measured to assess the development and progress of cardiac fibrosis, although the latter should only be performed in patients with an eGFR >30 mL/min.64 Furthermore the increase in enzymatic activity as well as the reduction in plasma lysoGb3 did not occur in all patients, in fact one patient showed an increase in lysoGb3 after switch from ERT. Although the rate of reduction of lysoGb3 may differ between treatments, as they differ in bio‐distribution and working mechanism, a rise in lysoGb3 after switch from ERT to chaperone therapy does suggests re‐accumulation of the substrate. Lenders et al. recently reported that patients with the p.N215S mutation had a significant reduction of plasma lysoGb3 levels, as well as an increase in leucocyte αGAL A activity upon treatment with Migalastat. In contrast, enzyme activity did not increase in FD patients with the p.L294S mutation and lysoGb3 levels rose significantly in these patients after switch from ERT. Further investigation showed that when amenability was tested in GLA‐knockout HEK‐293 cells and patient derived cell lines, there was indeed no response to Migalastat in cells carrying the p.L294S mutation, strengthening the clinical observations.65 Careful monitoring of the in‐vivo response, including leukocyte αGAL A activity, plasma lysoGb3 changes and detailed clinical evaluation, will help to select patients that are most likely to benefit from treatment with Migalastat.
3. POTENTIAL FUTURE TREATMENT OPTIONS
3.1. Second generation enzyme therapies
Recently, two new forms of ERT for the treatment of FD have been developed; Pegunigalsidase‐alfa (Protalix Biotherapeutics, Israel) and moss‐aGal (Greenovation biopharmaceuticals, Germany). Being plant‐derived, these enzymes do not carry M6P on their surface,66, 67 which may result in a different bio distribution compared to the first generation enzymes.
3.1.1. Pegunigalsidase‐alfa
Pegunigalsidase‐alfa (PRX‐102) is produced in tobacco cells and has been chemically modified with polyethylene glycol (PEG) to reduce clearance and increase the stability of the enzyme. The increased stability of the enzyme was confirmed in vitro in human plasma and under lysosomal conditions (eg, pH 4.6), as well as in vivo in a murine model. Uptake assays in patients fibroblasts showed co‐localization of PRX‐102 with lysosomes, but the concentrations used in vitro (160 mg/L) exceeded the expected concentration of PRX‐102 in plasma several fold (assuming the dose is 1 mg/kg, which is currently used in clinical trials). The fact that uptake of protein into cells is often concentration dependent makes it difficult to draw any conclusions regarding the expected in vivo uptake.66 The murine FD model showed different pharmacokinetics and improved bio distribution of PRX‐102 compared to agalsidase‐alfa and substantial reduction in Gb3 accumulation in heart and kidneys.66 Data from the phase I/II safety and dose‐ranging trial show a mean half‐life of 80 hours for PRX‐102 (compared to <1 hour for agalsidase‐beta) in plasma of patients as well as a reduction of Gb3 in human kidney biopsies in response to treatment.68 PRX‐102 is currently being tested in phase 3 clinical trials (NCT02795676, NCT03018730, NCT03180840). Concerns regarding immunogenicity are that either the altered glycosylation pattern of a plant derived protein69 and/or the PEG group may serve as an epitope, eliciting an immune response. The latter has been described for other pegylated biologicals.70 On the other hand, because of the extended half‐life in plasma, exposure of the immune system to the enzyme will be much greater, which may lead to immunological tolerance.71 In the phase I/II trial, treatment was terminated in one patient because of a hypersensitive reaction during the first infusion. Three out of eleven male patients developed ADAs against PRX‐102, all of which tolerized after 1 year of treatment.68 It should be noted that the increased plasma half‐life of PRX‐102 might interfere with the results of both the Elisa and the inhibitory assays currently used to detect ADAs. Circulating r‐αGAL A in plasma at the moment of sampling could bind the ADAs and thereby prevent their detection, as has been shown for other intravenously administered proteins.72 However, the fact that the profound effect of the ADAs on pharmacokinetics and pharmacodynamics of PRX‐102 in the three ADA positive patients was also transient is promising.68
3.1.2. Moss‐aGal
Another plant derived form of r‐αGAL A is moss‐aGal. In vitro, moss‐aGal was shown to be adequately taken up in endothelial cells but not in fibroblasts. R‐αGAL A enzymatic activity was higher in kidney biopsies from moss‐aGal treated FD mice compared to agalsidase‐alfa treated FD mice, though no differences were found in Gb3 clearance from these cell types. The half‐life of the administered recombinant enzyme in heart and kidney were generally lower for moss‐aGal compared to agalsidase‐alfa.67 A phase I clinical trial, in which a single dose of moss‐aGal (0.2 mg/kg) was administered to 7 females with GLA mutations (4 classical, 2 late‐onset, 1 benign phenotype) (NCT02995993) has recently been completed. No serious adverse events were reported and pharmacokinetic evaluation shows a half‐life of 14 minutes in plasma after a single infusion.73
3.2. Substrate reduction therapy
The rationale behind substrate reduction therapy (SRT), another oral therapy for FD, is to limit the formation of metabolites that cannot be degraded due to the underlying enzymatic defect. Precaution in dosing should be taken, for the complete abrogation of a single enzymatic reaction could potentially disrupt the homeostasis of the cell, affecting processes such as apoptosis, cell growth and differentiation.74 In patients with residual enzyme activity, SRT might be sufficient to reduce the production of the substrate to a level compatible with the remaining enzyme activity. Additional mechanisms to clear accumulated Gb3 in FD patients, such as excretion in bile, may also contribute to the balance between accumulation and degradation of Gb3 in FD patients as has been shown for glucosylceramide in Gaucher disease (Tokoro et al., 1987).74, 75 In patients with minimal to no residual enzyme activity, SRT may not suffice as a single therapy but could still be of added value in addition to ERT.76 One of the additional potential benefits of iminosugars (SRT's and chaperone therapy) is that they are small molecules which, unlike ERT, do not induce ADA development and may be capable of passing the blood‐brain barrier.77
The first SRT used to treat an LSD (eg, Gaucher disease) was the glucose based iminosugar N‐butyldeoxynojirimycin (Figure 2). N‐butyldeoxynojirimycin inhibits glucosylceramide synthase (GCS), the first step in glycosphingolipid syntheses (Figure 3) and the drug was introduced as Miglustat (Actelion Pharmaceuticals) for the treatment of Gaucher disease.78, 79 Later on, the more selective GCS inhibitor Eliglustat (Sanofi Genzyme) was introduced, which is successfully used for the treatment of Gaucher disease,80 but is unsuitable for the treatment of Fabry disease because of its effect on cardiac conduction. Subsequently, novel SRT molecules were developed and tested for FD, such as the ceramide based Venglustat (Sanofi Genzyme) and the galactose derivative Lucerastat (Idorsia Pharmaceuticals, Switzerland), both inhibiting GCS (Figures 2 and 3).81, 82 Differences in inhibitory capacity and specificity are mentioned in the supplemental file. Preliminary data from clinical trials evaluating the effect of Venglustat in treatment‐naïve Fabry patients suggest a slow but gradual clearance of Gb3 from superficial skin capillary endothelium and a gradual decrease of plasma lysoGb3 in most included patients over the course of 3 years of treatment (Deegan et al., book of abstracts SSIEM 2019).83, 84
Lucerastat is the galactose form of Miglustat (Figure 2). In a 12 week open label clinical safety trial in a small group of Fabry patients combining ERT with Lucerastat, Gb3 serum levels and urine Gb3 concentrations were lower in the group treated with both ERT and Lucerastat (N = 10) compared to the group treated with ERT alone (N = 4).84
Because the above mentioned SRTs all inhibit GCS, the formation of a large number of glycosphingolipids is suppressed, whilst for the treatment of F D only the formation of Gb3 needs to be inhibited (Figure 3). The only study examining the inhibition of Gb3 synthase crossed aGAL A deficient (Fabry) mice with mice lacking Gb3 synthase activity. This resulted in a reduction in globosides like Gb3 in, amongst others, cells of the heart and kidney as well as restoration of lysosomal morphology in these organs.85
Finally, some general characteristics of iminosugars are good to keep in mind. I) It has been shown for several iminosugars that they can both function as inhibitor as well as chaperone on the same enzyme, depending on their concentration (lower concentrations tend to stabilise mutant enzyme in cell lines with specific mutations, higher concentrations tend to inhibit enzyme activity).86, 87 Thus, for clinical applications, an estimate of the dose that results in the right intracellular concentration of the iminosugar needs to be made. II) Iminosugars are not fully specific and may also affect other reactions (supplemental file).
3.3. Stem cell, gene, and mRNA based therapies
The first bone‐marrow transplantations in LSD patients were performed in the 1980s in a mucopolysaccharidosis type I (MPS I) and a Gaucher patient.88, 89 Subsequent clinical experience showed that this treatment can partially (MPSI) or fully (type I Gaucher disease) prevent or reverse disease symptoms.89 Though new techniques greatly reduced transplantation related mortality, the slowly progressive nature of FD may not justify the risk of severe complications such as graft vs host disease and infections.90 Especially since the transplantation would preferably be performed in young FD patients, before the onset of organ damage. Furthermore, bone‐marrow transplantation is most likely not the optimal route to target cells of the kidney and the heart. In the last decades, many pre‐clinical gene therapy studies for FD have been reported. Both in vivo approaches as well as ex vivo approaches have been explored with several different vectors (retroviral, lentiviral, adenoviral, adeno‐associated viral, and non‐viral vectors), which have been summarised previously.91 The main challenge of gene therapy in FD is the targeting of all affected cell types and tissues. As this is unlikely to be accomplished, the efficacy of this approach will mostly depend on the ability of transfected cells to release αGal A into the circulation. The fact that heterozygous female FD patients are still symptomatic despite the fact that they express a normal copy of the GLA gene in, on average, half their cells, demonstrates that the enzyme is either not excreted sufficiently or not taken up efficiently enough by neighbouring cells under normal conditions. Whether or not inducing overexpression of αGal A could result in sufficient release of the enzyme into the circulation and adequate uptake by affected tissues in humans remains to be determined.92, 93, 94 Very recently, the first Fabry patients have been treated in phase I and II clinical trials using an ex vivo approach, in which haematopoetic stem cells of the patient are recruited, transfected using lentiviruses (AVR‐RD‐01, Avrobio) and re‐administered to the patient (NCT02800070 and NCT03454893). Next in line are pending clinical trials using adeno‐associated viral (AAV) mediated gene therapy, aiming at enzyme production and secretion by the liver using liver specific promoters. Transfection of mice using FLT190 (Freeline therapeutics, UK) and ST‐920 (Sangamo Therapeutics) indeed resulted in overexpression and measurable increases in plasma αGAL A.95, 96 Amongst others, there are immunological challenges that accompany gene therapy. In AAV mediated gene therapy, AAV‐neutralising antibodies can directly limit transduction and CD8+ T cells may target AAV‐transduced cells, causing loss of transfected cells.97 Finally, for all forms of gene therapy in FD, the question that remains to be answered is whether or not male patients with classical FD will go on to develop antibodies and/or immunological reactions against the expressed enzyme. It is likely that the continuous exposure and endogenous glycosylation will result in tolerance in most, if not all, transfected patients. However, no conclusions can be drawn from gene therapy trials in Fabry or other protein deficiencies to date, since both high titers of pre‐existing ADAs and the use of immunomodulatory drugs under ERT have been an exclusion criterion.98 Ongoing and pending clinical trials in treatment‐naïve male patients with classical FD will give more insight in the risk of antibody development in gene therapy treated patients.
In addition to gene therapy, systemic messenger RNA (mRNA) therapy is currently being developed for FD (Moderna Inc and Translate Bio). mRNA‐based therapy has the advantage over DNA‐based therapy that it is not at risk for insertional mutagenesis. A downside of mRNA‐based therapy is that the effect is transient, and thus requires repeated administration. Potential advantages over ERT could be that the endogenous protein translation system ensures proper folding, glycosylation and intracellular trafficking of αGAL A.99 mRNA therapy, encapsulated in lipid nanoparticles, primarily targets hepatocytes in which the enzyme is produced, secreted into the circulation and taken up by tissues. The steady production of enzyme after a single infusion of mRNA in mice and non‐human primates resulted in a plasma half‐life of αGAL A of 7.5 hours.100, 101 Further murine and non‐human primate studies showed a dose‐dependent elevation of enzyme levels in plasma, kidney and heart with a half‐life of over 100 hours as well as a reduction of Gb3 and lysoGb3 up to 90% and 70% in heart and kidney, respectively, after repeated administration with mRNA.101 mRNA based therapy is relatively new and clinical experience in humans is limited. Within the field of inborn errors of metabolism a phase I/II clinical trial has recently started for methyl malonic acidemia (NCT03810690).
3.4. Removal of storage material
An alternative approach to reduce intra‐lysosomal storage would be to stimulate the egress of storage material from the lysosomal compartment and subsequently the cell. Studies investigating this approach are based on the association between Gb3 and cholesterol homeostasis, which interact in several manners. Gb3, amongst the other glycosphingolipids, is a component of plasma lipoproteins, specifically low‐density lipoprotein (LDL), and can thus be transported into endothelial cells through the LDL receptor.102, 103, 104 Storage of glycosphingolipids (GSL) have been shown to result in intracellular accumulation of cholesterol due to upregulation of the LDL receptor. Thus, Gb3 storage may induce further influx of Gb3, in the context of LDL, into the endothelial cell. The influx of cholesterol results in mistargeting of GSLs to the lysosome instead of the Golgi, further increasing lysosomal storage.105, 106, 107 Furthermore, Gb3 accumulation has been shown to inhibit cholesterol efflux mediated by apolipoprotein A1 (apoA1).108 Therefore, targeting cholesterol metabolism might be a good way to alter glycosphingolipid homeostasis in LSDs such as FD. Incubation of Fabry fibroblasts with HDL or a synthetic replica of apoA1 reduced Gb3 accumulation, by promoting the efflux of both cholesterol and Gb3 from the cell (Figure 4).107
Figure 4.
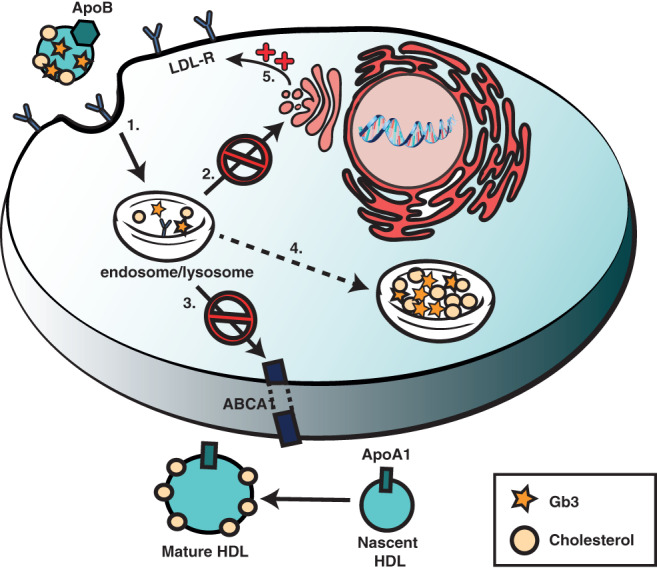
Hypothesized method of altered lipid homeostasis in Fabry disease. 1. Gb3 and cholesterol are taken up through the LDL receptor.102, 103, 104 2. Gb3 is mistargeted to lysosomes instead of Golgi and other membranes.105, 106, 107 3. Gb3 causes inhibition of ApoA1 mediated efflux of cholesterol.108 4. Storage of GSLs causes upregulation of the LDL receptor thus increasing intracellular uptake of cholesterol and additional GSLs.105, 106, 107 5. Increased uptake, mistargeting, and decreased efflux results in lysosomal accumulation of lipids. GSLs, glycosphingolipids; LDL, low‐density lipoprotein
3.5. Non‐Fabry‐specific therapies
Besides the FD specific treatment, many patients require treatment of their FD symptoms with adjuvant medication or interventions. Patients with pain caused by small fibre neuropathy benefit most from treatment with carbamazepine.109 In patients with proteinuria treatment with anti‐proteinuric agents like ACEi or ARBs reduces proteinuria and slows down renal decline.110 Treatment with anti‐platelet drugs like carbasalate calcium or clopidogrel may be considered, specifically in patients with evident white matter lesions, although evidence on their effectiveness in primary prevention of stroke in the general population is still lacking.111 Renal protective and cardiovascular risk management measures should be in place, with particular emphasis on smoking cessation, dietary salt restriction and the treatment of hypertension and dyslipidemia. Some patients require pacemaker implantation because of conductions disorders or sinus node dysfunction. ICD implantation is considered mostly in patients with extensive fibrosis and/or non‐sustained ventricular tachycardia on holter examination. No validated sustained ventricular arrhythmia risk calculation tool exists for Fabry disease. Finally, it should be noted that differences in the use of these additional treatments amongst treatment centres may significantly influence trial outcomes. Furthermore, the start of clinical trials in FD has led to a more intensive follow up in patients and more rigorous symptomatic treatment, as well as an attention to life style interventions such as smoking cessation plans. Therefore, any comparison of currently treated to historical untreated FD patients should be interpreted bearing this in mind.
3.5.1. The whole might be greater than the sum of its parts
In conclusion, although important steps have been taken to improve the treatment of Fabry disease, a cure is not yet in sight. For a small subset of patients with specific mutations, treatment with chaperones might be a suitable approach. For the remaining patients, combining different approaches such as ERT with substrate reduction, might be beneficial, but the high costs of the individual therapies currently form an important barrier for this approach. Gene therapy options have been awaited for some time now and the results of the human trials are highly anticipated. Finally, the first results of mRNA based therapies seem promising. However, the small number of FD patients combined with the heterogeneity and slow progression of the disease, makes it difficult to perform well‐powered trials of sufficient duration to draw valid conclusions regarding the therapeutic efficacy. This emphasises the need for clinically validated biomarkers that predict clinical outcome. With the arrival of new treatment options, care must be taken that we are not left with an overload of underpowered studies of insufficient duration, making it impossible to draw any conclusions regarding relative effectiveness of each treatment modality. To tackle this, independent international registries in which data of patients, both untreated and on different treatments, are systematically collected, are essential.
CONFLICT OF INTEREST
No fees, travel support or grants are obtained from Pharmaceutical Industry by any of the listed authors. M.L. reports to be involved in pre‐marketing studies with Genzyme, Protalix, and Idorsia. Financial arrangements are made through AMC Research BV. C.E.H. reports to be involved in pre‐marketing studies with Genzyme, Protalix, and Idorsia. Financial arrangements are made through AMC Research BV. S.J.V. reports to be involved in a pre‐marketing study with Protalix. Financial arrangements are made through AMC Research BV. A.B.P.K. has nothing to disclose.
AUTHOR CONTRIBUTIONS
S.J.V. devised the topic, wrote the manuscript and designed graphic illustrations. M.L. fine‐tuned the concept, and edited the manuscript accordingly and A.B.P.K. critically reviewed the manuscript and edited where needed. C.E.M.H. oversaw the general direction of the article and critically reviewed the manuscript.
ANIMAL RIGHTS AND/OR INFORMED CONSENT
This article does not contain any studies with human or animal subjects performed by any of the authors.
ACKNOWLEDGMENT
We are grateful to Professor Jan Voorberg for his critical review of the manuscript.
van der Veen SJ, Hollak CEM, van Kuilenburg ABP, Langeveld M. Developments in the treatment of Fabry disease. J Inherit Metab Dis. 2020;43:908–921. 10.1002/jimd.12228
André B. P. van Kuilenburg and Mirjam Langeveld contributed equally to this study.
Communicating Editor: Markus Ries
REFERENCES
- 1. Askari H, Kaneski CR, Semino‐Mora C, et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch. 2007;4:823‐834. [DOI] [PubMed] [Google Scholar]
- 2. Valbuena C, Leitao D, Carneiro F, Oliveira JP. Immunohistochemical diagnosis of Fabry nephropathy and localisation of globotriaosylceramide deposits in paraffin‐embedded kidney tissue sections. Virchows Arch. 2012;2:211‐221. [DOI] [PubMed] [Google Scholar]
- 3. Desnick RJ, Ioannou YA, Eng CM. α‐Galactosidase A deficiency: Fabry disease In: Beaudet AL, Vogelstein B, Kinzler KW, et al., eds. The Online Metabolic and Molecular Bases of Inherited Disease. New York, NY: The McGraw‐Hill Companies, Inc; 2014. [Google Scholar]
- 4. Arends M, Wanner C, Hughes D, et al. Characterization of classical and nonclassical Fabry disease: a multicenter study. J Am Soc Nephrol. 2017;5:1631‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Tol L, Cassiman D, Houge G, et al. Uncertain diagnosis of fabry disease in patients with neuropathic pain, angiokeratoma or cornea verticillata: consensus on the approach to diagnosis and follow‐up. JIMD Rep. 2014;17:83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smid BE, van der Tol L, Cecchi F, et al. Uncertain diagnosis of Fabry disease: consensus recommendation on diagnosis in adults with left ventricular hypertrophy and genetic variants of unknown significance. Int J Cardiol. 2014;2:400‐408. [DOI] [PubMed] [Google Scholar]
- 7. Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry registry. Genet Med. 2009;11:790‐796. [DOI] [PubMed] [Google Scholar]
- 8. Arends M, Biegstraaten M, Hughes DA, et al. Retrospective study of long‐term outcomes of enzyme replacement therapy in Fabry disease: analysis of prognostic factors. PLoS One. 2017;8:e0182379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha‐galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;1:9‐16. [DOI] [PubMed] [Google Scholar]
- 10. Thurberg BL, Rennke H, Colvin RB, et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;6:1933‐1946. [DOI] [PubMed] [Google Scholar]
- 11. Schiffmann R, Kopp JB, Austin HA 3rd, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;21:2743‐2749. [DOI] [PubMed] [Google Scholar]
- 12. Tondel C, Bostad L, Larsen KK, et al. Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol. 2013;1:137‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arends M, Biegstraaten M, Wanner C, et al. Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: an international cohort study. J Med Genet. 2018;5:351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El Dib R, Gomaa H, Ortiz A, Politei J, Kapoor A, Barreto F. Enzyme replacement therapy for Anderson‐Fabry disease: a complementary overview of a Cochrane publication through a linear regression and a pooled analysis of proportions from cohort studies. PLoS One. 2017;3:e0173358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arends M, Wijburg FA, Wanner C, et al. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol Genet Metab. 2017;121:157‐161. [DOI] [PubMed] [Google Scholar]
- 16. Germain DP, Charrow J, Desnick RJ, et al. Ten‐year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet. 2015;5:353‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weidemann F, Niemann M, Breunig F, et al. Long‐term effects of enzyme replacement therapy on fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;4:524‐529. [DOI] [PubMed] [Google Scholar]
- 18. Aerts JM, Groener JE, Kuiper S, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;8:2812‐2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nowak A, Mechtler TP, Hornemann T, et al. Genotype, phenotype and disease severity reflected by serum LysoGb3 levels in patients with Fabry disease. Mol Genet Metab. 2018;2:148‐153. [DOI] [PubMed] [Google Scholar]
- 20. Auray‐Blais C, Lavoie P, Boutin M, et al. Biomarkers associated with clinical manifestations in Fabry disease patients with a late‐onset cardiac variant mutation. Clin Chim Acta. 2017;466:185‐193. [DOI] [PubMed] [Google Scholar]
- 21. Hossain MA, Wu C, Yanagisawa H, et al. Future clinical and biochemical predictions of Fabry disease in females by methylation studies of the GLA gene. Mol Genet Metab Rep. 2019;20:100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavalle L, Thomas AS, Beaton B, et al. Phenotype and biochemical heterogeneity in late onset Fabry disease defined by N215S mutation. PLoS One. 2018;13(4):e0193550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lenders M, Neusser LP, Rudnicki M, et al. Dose‐dependent effect of enzyme replacement therapy on neutralizing antidrug antibody titers and clinical outcome in patients with Fabry disease. J Am Soc Nephrol. 2018;12:2879‐2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rombach SM, Dekker N, Bouwman MG, et al. Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta. 2010;1802(9):741‐748. [DOI] [PubMed] [Google Scholar]
- 25. Yogasundaram H, Nikanj A, Putko BN, et al. Elevated inflammatory plasma biomarkers in patients with Fabry disease: a critical link to heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;21:e009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramaswami U, Bichet DG, Clarke LA, et al. Low‐dose agalsidase beta treatment in male pediatric patients with Fabry disease: a 5‐year randomized controlled trial. Mol Genet Metab. 2019;1:86‐94. [DOI] [PubMed] [Google Scholar]
- 27. Spada M, Baron R, Elliott PM, et al. The effect of enzyme replacement therapy on clinical outcomes in paediatric patients with Fabry disease ‐ a systematic literature review by a European panel of experts. Mol Genet Metab. 2019;3:212‐223. [DOI] [PubMed] [Google Scholar]
- 28. Thurberg BL, Fallon JT, Mitchell R, Aretz T, Gordon RE, O'Callaghan MW. Cardiac microvascular pathology in Fabry disease: evaluation of endomyocardial biopsies before and after enzyme replacement therapy. Circulation. 2009;19:2561‐2567. [DOI] [PubMed] [Google Scholar]
- 29. Germain DP, Arad M, Burlina A, et al. The effect of enzyme replacement therapy on clinical outcomes in female patients with Fabry disease—a systematic literature review by a European panel of experts. Mol Genet Metab. 2018;126(3):224‐235. [DOI] [PubMed] [Google Scholar]
- 30. De Simone G, Muiesan ML, Ganau A, Longini C, et al. Reliability and limitations of echocardiographic measurement of left ventricular mass for risk stratification and follow‐up in single patients: the RES trial. Working group on heart and hypertension of the Italian Society of Hypertension Reliability of M‐mode echocardiographic studies. J Hypertens. 1999;17:1955‐1963. [DOI] [PubMed] [Google Scholar]
- 31. Hazari H, Belenkie I, Kryski A, et al. Comparison of cardiac magnetic resonance imaging and echocardiography in assessment of left ventricular hypertrophy in Fabry disease. Can J Cardiol. 2018;8:1041‐1047. [DOI] [PubMed] [Google Scholar]
- 32. Niemann M, Herrmann S, Hu K, et al. Differences in Fabry cardiomyopathy between female and male patients: consequences for diagnostic assessment. JACC Cardiovasc Imaging. 2011;6:592‐601. [DOI] [PubMed] [Google Scholar]
- 33. Nordin S, Kozor R, Medina‐Menacho K, et al. Proposed stages of myocardial phenotype development in Fabry disease. JACC Cardiovasc Imaging. 2018;12(8 Pt 2):1673‐1683. [DOI] [PubMed] [Google Scholar]
- 34. Benichou B, Goyal S, Sung C, Norfleet AM, O'Brien F. A retrospective analysis of the potential impact of IgG antibodies to agalsidase beta on efficacy during enzyme replacement therapy for Fabry disease. Mol Genet Metab. 2009;1:4‐12. [DOI] [PubMed] [Google Scholar]
- 35. Wilcox WR, Linthorst GE, Germain DP, et al. Anti‐alpha‐galactosidase a antibody response to agalsidase beta treatment: data from the Fabry registry. Mol Genet Metab. 2012;3:443‐449. [DOI] [PubMed] [Google Scholar]
- 36. Bigger BW, Saif M, Linthorst GE. The role of antibodies in enzyme treatments and therapeutic strategies. Best Pract Res Clin Endocrinol Metab. 2015;2:183‐194. [DOI] [PubMed] [Google Scholar]
- 37. Lenders M, Hennermann JB, Kurschat C, et al. Multicenter female Fabry study (MFFS)—clinical survey on current treatment of females with Fabry disease. Orphanet J Rare Dis. 2016;11(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lenders M, Stypmann J, Duning T, Schmitz B, Brand SM, Brand E. Serum‐mediated inhibition of enzyme replacement therapy in Fabry disease. J Am Soc Nephrol. 2016;1:256‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rombach SM, Aerts JM, Poorthuis BJ, et al. Long‐term effect of antibodies against infused alpha‐galactosidase a in Fabry disease on plasma and urinary (lyso)Gb3 reduction and treatment outcome. PLoS One. 2012;10:e47805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Linthorst GE, Hollak CE, Donker‐Koopman WE, Strijland A, Aerts JM. Enzyme therapy for Fabry disease: neutralizing antibodies toward agalsidase alpha and beta. Kidney Int. 2004;4:1589‐1595. [DOI] [PubMed] [Google Scholar]
- 41. Vedder AC, Breunig F, Donker‐Koopman WE, et al. Treatment of Fabry disease with different dosing regimens of agalsidase: effects on antibody formation and GL‐3. Mol Genet Metab. 2008;3:319‐325. [DOI] [PubMed] [Google Scholar]
- 42. van der Veen SJ, van Kuilenburg ABP, Hollak CEM, Kaijen PHP, Voorberg J, Langeveld M. Antibodies against recombinant alpha‐galactosidase a in Fabry disease: subclass analysis and impact on response to treatment. Mol Genet Metab. 2018;126(2):162‐168. [DOI] [PubMed] [Google Scholar]
- 43. Lenders M, Oder D, Nowak A, et al. Impact of immunosuppressive therapy on therapy‐neutralizing antibodies in transplanted patients with Fabry disease. J Intern Med. 2017;282:241‐253. [DOI] [PubMed] [Google Scholar]
- 44. Itier JM, Ret G, Viale S, et al. Effective clearance of GL‐3 in a human iPSC‐derived cardiomyocyte model of Fabry disease. J Inherit Metab Dis. 2014;6:1013‐1022. [DOI] [PubMed] [Google Scholar]
- 45. Marchesan D, Cox TM, Deegan PB. Lysosomal delivery of therapeutic enzymes in cell models of Fabry disease. J Inherit Metab Dis. 2012;6:1107‐1117. [DOI] [PubMed] [Google Scholar]
- 46. Schiffmann R, Murray GJ, Treco D, et al. Infusion of alpha‐galactosidase a reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc Natl Acad Sci U S A. 2000;1:365‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prabakaran T, Nielsen R, Larsen JV, et al. Receptor‐mediated endocytosis of alpha‐galactosidase a in human podocytes in Fabry disease. PLoS One. 2011;9:e25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Okeda R, Nisihara M. An autopsy case of Fabry disease with neuropathological investigation of the pathogenesis of associated dementia. Neuropathology. 2008;5:532‐540. [DOI] [PubMed] [Google Scholar]
- 49. Schiffmann R, Rapkiewicz A, Abu‐Asab M, et al. Pathological findings in a patient with Fabry disease who died after 2.5 years of enzyme replacement. Virchows Arch. 2006;3:337‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Korver S, Vergouwe M, Hollak CEM, van Schaik IN, Langeveld M. Development and clinical consequences of white matter lesions in Fabry disease: a systematic review. Mol Genet Metab. 2018;125:205‐216. [DOI] [PubMed] [Google Scholar]
- 51. Ishii S, Kase R, Sakuraba H, Suzuki Y. Characterization of a mutant alpha‐galactosidase gene product for the late‐onset cardiac form of Fabry disease. Biochem Biophys Res Commun. 1993;3:1585‐1589. [DOI] [PubMed] [Google Scholar]
- 52. Romeo G, D'Urso M, Pisacane A, Blum E, De Falco A, Ruffilli A. Residual activity of alpha‐galactosidase a in Fabry's disease. Biochem Genet. 1975;9‐10:615‐628. [DOI] [PubMed] [Google Scholar]
- 53. Okumiya T, Ishii S, Takenaka T, et al. Galactose stabilizes various missense mutants of alpha‐galactosidase in Fabry disease. Biochem Biophys Res Commun. 1995;3:1219‐1224. [DOI] [PubMed] [Google Scholar]
- 54. Frustaci A, Chimenti C, Ricci R, et al. Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose‐infusion therapy. N Engl J Med. 2001;1:25‐32. [DOI] [PubMed] [Google Scholar]
- 55. Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha‐galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;1:112‐115. [DOI] [PubMed] [Google Scholar]
- 56. Benjamin ER, Della Valle MC, Wu X, et al. The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat. Genet Med. 2017;4:430‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Germain DP, Hughes DA, Nicholls K, et al. Treatment of Fabry's disease with the pharmacologic chaperone Migalastat. N Engl J Med. 2016;6:545‐555. [DOI] [PubMed] [Google Scholar]
- 58. Hughes DA, Nicholls K, Shankar SP, et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18‐month results from the randomised phase III ATTRACT study. J Med Genet. 2017;4:288‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mauer M, Sokolovskiy A, Barth JA, et al. Reduction of podocyte globotriaosylceramide content in adult male patients with Fabry disease with amenable GLA mutations following 6 months of migalastat treatment. J Med Genet. 2017;11:781‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schiffmann R, Bichet DG, Jovanovic A, et al. Migalastat improves diarrhea in patients with Fabry disease: clinical‐biomarker correlations from the phase 3 FACETS trial. Orphanet J Rare Dis. 2018;1:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muntze J, Gensler D, Maniuc O, et al. Oral chaperone therapy Migalastat for treating Fabry disease: enzymatic response and serum biomarker changes after 1 year. Clin Pharmacol Ther. 2018:105(5):1224‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Korver S, Feldt‐Rasmussen U, Svarstad E, Kantola I, Langeveld M. Oral chaperone therapy Migalastat for the treatment of Fabry disease: potentials and pitfalls of real‐world data. Clin Pharmacol Ther. 2019;106:925‐926. [DOI] [PubMed] [Google Scholar]
- 63. Luijnenburg SE, Robbers‐Visser D, Moelker A, Vliegen HW, Mulder BJ, Helbing WA. Intra‐observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging. Int J Cardiovasc Imaging. 2010;26:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium‐based contrast agents in kidney disease: comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can Assoc Radiol J. 2018;69:136‐150. [DOI] [PubMed] [Google Scholar]
- 65. Lenders M, Stappers F, Niemietz C, et al. Mutation‐specific Fabry disease patient‐derived cell model to evaluate the amenability to chaperone therapy. J Med Genet. 2019;8:548‐556. [DOI] [PubMed] [Google Scholar]
- 66. Kizhner T, Azulay Y, Hainrichson M, et al. Characterization of a chemically modified plant cell culture expressed human alpha‐galactosidase‐A enzyme for treatment of Fabry disease. Mol Genet Metab. 2015;2:259‐267. [DOI] [PubMed] [Google Scholar]
- 67. Shen JS, Busch A, Day TS, et al. Mannose receptor‐mediated delivery of moss‐made alpha‐galactosidase a efficiently corrects enzyme deficiency in Fabry mice. J Inherit Metab Dis. 2016;2:293‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schiffmann R, Goker‐Alpan O, Holida M, et al. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: a 1‐year phase 1/2 clinical trial. J Inherit Metab Dis. 2019;3:534‐544. [DOI] [PubMed] [Google Scholar]
- 69. Gomord V, Fitchette AC, Menu‐Bouaouiche L, et al. Plant‐specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J. 2010;5:564‐587. [DOI] [PubMed] [Google Scholar]
- 70. Zhang P, Sun F, Liu S, Jiang S. Anti‐PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release. 2016;244(Pt B):184‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krishna M, Nadler SG. Immunogenicity to biotherapeutics—the role of anti‐drug immune complexes. Front Immunol. 2016;7:21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee JW, Kelley M, King LE, et al. Bioanalytical approaches to quantify "total" and "free" therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J. 2011;1:99‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hennermann JB, Arash‐Kaps L, Fekete G, Schaaf A, Busch A, Frischmuth T. Pharmacokinetics, pharmacodynamics, and safety of moss‐aGalactosidase A in patients with Fabry disease. J Inherit Metab Dis. 2019;3:527‐533. [DOI] [PubMed] [Google Scholar]
- 74. Cox TM. Innovative treatments for lysosomal diseases. Best Pract Res Clin Endocrinol Metab. 2015;2:275‐311. [DOI] [PubMed] [Google Scholar]
- 75. Tokoro T, Gal AE, Gallo LL, Brady RO. Studies of the pathogenesis of Gaucher's disease: tissue distribution and biliary excretion of [14C]L‐glucosylceramide in rats. J Lipid Res. 1987;28(8):968‐972. [PubMed] [Google Scholar]
- 76. Platt FM, Jeyakumar M. Substrate reduction therapy. Acta Paediatr. 2008;457:88‐93. [DOI] [PubMed] [Google Scholar]
- 77. Platt FM, Jeyakumar M, Andersson U, et al. Inhibition of substrate synthesis as a strategy for glycolipid lysosomal storage disease therapy. J Inherit Metab Dis. 2001;2:275‐290. [DOI] [PubMed] [Google Scholar]
- 78. Cox T, Lachmann R, Hollak C, et al. Novel oral treatment of Gaucher's disease with N‐butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;9214:1481‐1485. [DOI] [PubMed] [Google Scholar]
- 79. Platt FM, Neises GR, Karlsson GB, Dwek RA, Butters TD. N‐butyldeoxygalactonojirimycin inhibits glycolipid biosynthesis but does not affect N‐linked oligosaccharide processing. J Biol Chem. 1994;43:27108‐27114. [PubMed] [Google Scholar]
- 80. Mistry PK, Lukina E, Ben Turkia H, et al. Outcomes after 18 months of eliglustat therapy in treatment‐naive adults with Gaucher disease type 1: the phase 3 ENGAGE trial. Am J Hematol. 2017;11:1170‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ashe KM, Budman E, Bangari DS, et al. Efficacy of enzyme and substrate reduction therapy with a novel antagonist of glucosylceramide synthase for Fabry disease. Mol Med. 2015;21:389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marshall J, Ashe KM, Bangari D, et al. Substrate reduction augments the efficacy of enzyme therapy in a mouse model of Fabry disease. PLoS One. 2010;11:e15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Guerard N, Morand O, Dingemanse J. Lucerastat, an iminosugar with potential as substrate reduction therapy for glycolipid storage disorders: safety, tolerability, and pharmacokinetics in healthy subjects. Orphanet J Rare Dis. 2017;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deegan P, Germain DP, Goker‐Alpan O, et al. Three year open label phase 2a investigation of venglustat safety and exploratory efficacy in classic Fabry patients. Book of abstracts SSIEM 2019, JIMD 42 2019; Suppl.1 9:O‐019. [Google Scholar]
- 85. Porubsky S, Jennemann R, Lehmann L, Grone HJ. Depletion of globosides and isoglobosides fully reverts the morphologic phenotype of Fabry disease. Cell Tissue Res. 2014;1:217‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Alfonso P, Pampin S, Estrada J, et al. Miglustat (NB‐DNJ) works as a chaperone for mutated acid beta‐glucosidase in cells transfected with several Gaucher disease mutations. Blood Cells Mol Dis. 2005;2:268‐276. [DOI] [PubMed] [Google Scholar]
- 87. Asano N, Ishii S, Kizu H, et al. In vitro inhibition and intracellular enhancement of lysosomal alpha‐galactosidase a activity in Fabry lymphoblasts by 1‐deoxygalactonojirimycin and its derivatives. Eur J Biochem. 2000;13:4179‐4186. [DOI] [PubMed] [Google Scholar]
- 88. Hobbs JR, Hugh‐Jones K, Barrett AJ, et al. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone‐marrow transplantation. Lancet. 1981;8249:709‐712. [DOI] [PubMed] [Google Scholar]
- 89. Ringden O, Groth CG, Erikson A, et al. Long‐term follow‐up of the first successful bone marrow transplantation in Gaucher disease. Transplantation. 1988;1:66‐70. [DOI] [PubMed] [Google Scholar]
- 90. Henig I, Zuckerman T. Hematopoietic stem cell transplantation‐50 years of evolution and future perspectives. Rambam Maimonides Med J. 2014;4:e0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ruiz de Garibay AP, Solinis MA, Rodriguez‐Gascon A. Gene therapy for fabry disease: a review of the literature. BioDrugs. 2013;3:237‐246. [DOI] [PubMed] [Google Scholar]
- 92. Medin JA, Tudor M, Simovitch R, et al. Correction in trans for Fabry disease: expression, secretion and uptake of alpha‐galactosidase a in patient‐derived cells driven by a high‐titer recombinant retroviral vector. Proc Natl Acad Sci U S A. 1996;15:7917‐7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Takenaka T, Hendrickson CS, Tworek DM, et al. Enzymatic and functional correction along with long‐term enzyme secretion from transduced bone marrow hematopoietic stem/progenitor and stromal cells derived from patients with Fabry disease. Exp Hematol. 1999;7:1149‐1159. [DOI] [PubMed] [Google Scholar]
- 94. Takenaka T, Qin G, Brady RO, Medin JA. Circulating alpha‐galactosidase a derived from transduced bone marrow cells: relevance for corrective gene transfer for Fabry disease. Hum Gene Ther. 1999;12:1931‐1939. [DOI] [PubMed] [Google Scholar]
- 95. Huston M, Yasuda M, Pagant S, et al. Liver‐targeted AAV gene therapy vectors produced by a clinical scale manufacturing process result in high, continuous therapeutic levels of enzyme activity and effective substrate reduction in mouse model of Fabry disease. Mol Genet Metab. 2019;2:s77. [Google Scholar]
- 96. Kia A, McIntosh J, Rosales C, et al. Efficacy evaluation of liver‐directed gene therapy in Fabry mice. Blood. 2018;132(Suppl 1):2209‐2209.30442749 [Google Scholar]
- 97. Vandamme C, Adjali O, Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum Gene Ther. 2017;11:1061‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gollomp KL, Doshi BS, Arruda VR. Gene therapy for hemophilia: Progress to date and challenges moving forward. Transfus Apher Sci. 2019;58:602‐612. [DOI] [PubMed] [Google Scholar]
- 99. Nabhan JF, Wood KM, Rao VP, et al. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich's ataxia. Sci Rep. 2016;17:20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. DeRosa F, Smith L, Shen Y, et al. Improved efficacy in a Fabry disease model using a systemic mRNA liver depot system as compared to enzyme replacement therapy. Mol Ther. 2019;4:878‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhu X, Yin L, Theisen M, et al. Systemic mRNA therapy for the treatment of Fabry disease: preclinical studies in wild‐type mice, Fabry mouse model, and wild‐type non‐human primates. Am J Hum Genet. 2019;4:625‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bodary PF, Shayman JA, Eitzman DT. Alpha‐galactosidase A in vascular disease. Trends Cardiovasc Med. 2007;4:129‐133. [DOI] [PubMed] [Google Scholar]
- 103. Clarke JT. The glycosphingolipids of human plasma lipoproteins. Can J Biochem. 1981;6:412‐417. [DOI] [PubMed] [Google Scholar]
- 104. Slotte JP. Sphingomyelin‐cholesterol interactions in biological and model membranes. Chem Phys Lipids. 1999;1‐2:13‐27. [DOI] [PubMed] [Google Scholar]
- 105. Puri V, Jefferson JR, Singh RD, Wheatley CL, Marks DL, Pagano RE. Sphingolipid storage induces accumulation of intracellular cholesterol by stimulating SREBP‐1 cleavage. J Biol Chem. 2003;23:20961‐20970. [DOI] [PubMed] [Google Scholar]
- 106. Puri V, Watanabe R, Dominguez M, et al. Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid‐storage diseases. Nat Cell Biol. 1999;6:386‐388. [DOI] [PubMed] [Google Scholar]
- 107. Schueler U, Kaneski C, Remaley A, et al. A short synthetic peptide mimetic of apolipoprotein A1 mediates cholesterol and globotriaosylceramide efflux from Fabry fibroblasts. JIMD Rep. 2016;29:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Glaros EN, Kim WS, Quinn CM, et al. Glycosphingolipid accumulation inhibits cholesterol efflux via the ABCA1/apolipoprotein A‐I pathway: 1‐phenyl‐2‐decanoylamino‐3‐morpholino‐1‐propanol is a novel cholesterol efflux accelerator. J Biol Chem. 2005;26:24515‐24523. [DOI] [PubMed] [Google Scholar]
- 109. Schuller Y, Linthorst GE, Hollak CE, Van Schaik IN, Biegstraaten M. Pain management strategies for neuropathic pain in Fabry disease—a systematic review. BMC Neurol. 2016;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tahir H, Jackson LL, Warnock DG. Antiproteinuric therapy and fabry nephropathy: sustained reduction of proteinuria in patients receiving enzyme replacement therapy with agalsidase‐beta. J Am Soc Nephrol. 2007;9:2609‐2617. [DOI] [PubMed] [Google Scholar]
- 111. Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007;1‐2:258‐263. [DOI] [PubMed] [Google Scholar]


