Abstract
Introduction
Better sensation in the reconstructed breast improves the quality of life. Sensory nerve coaptation is a valuable addition to autologous breast reconstruction. There are few publications concerning the sensory nerves of the breast and the nipple‐areola complex and reports are contradictory, so it is unknown which nerve is best suited as a recipient for coaptation. The current study serves as a proof of concept.
Materials and Methods
The areas innervated by the anterior cutaneous branches (ACBs) of the intercostal nerves (ICNs) were studied on two separate occasions in two healthy women. First, the ACBs of ICNs 2–5 were individually blocked using ultrasound. Next, the ACBs of all levels were blocked simultaneously. Sensation was measured using Semmes‐Weinstein monofilaments. The numbed areas corresponding to the ICNs were drawn in a raster of 2 × 2 cm.
Results
The largest area was supplied by the ACB of the 4th ICN, located in the upper (UIQ) and the lower (LIQ) inner quadrants of the breast. The 2nd‐largest area was supplied by the ACB of the 3rd ICN. Blockage of ACBs 2–5 affected sensation in the nipple and the areola.
Conclusions
Blockage of all levels 2–5 partially affected sensation in the nipple‐areola complex, suggesting innervation by a nerve plexus consisting of both ACBs and lateral cutaneous branches (LCBs). ACB4 supplied the largest area of the breast in the UIQ and LIQ and could be best suited for sensory nerve coaptation to optimize sensation in the autologously reconstructed breast.
Keywords: breast cancer, breast reconstruction, intercostal nerve, nipple‐areola complex, sensation
1. INTRODUCTION
The primary goal of reconstructive breast surgery is to improve the quality of life (QoL) for breast cancer patients. Patient satisfaction after autologous breast reconstruction is high (Damen et al., 2010; Eltahir et al., 2013), mainly because of the cosmetic appearance of the breasts (Isern, Tengrup, Loman, Olsson, & Ringberg, 2008; Koslow et al., 2013). Autologously reconstructed breasts look and age like normal breasts, and just like normal breasts they feel soft and warm from the outside. However, the most widely recounted unexpected experience for patients is that their reconstructed breasts feel numb or completely different from their former breast(s), and this negatively affects their physical wellbeing and QoL (de Boer, van der Hulst, & Slatman, 2015; Rabin, 2017).
Owing to advances in microsurgical techniques, sensory nerve coaptation during autologous breast reconstruction is now possible. This improves the recovery of sensation in the reconstructed breast (Beugels et al., 2019). Since this is associated with improved QoL (Cornelissen et al., 2018), preservation or recovery of sensation in the reconstructed breast and the nipple‐areola complex (NAC) is becoming increasingly important.
The anatomy of the intercostal nerves (ICNs) has been thoroughly studied and documented. The ICNs are the anterior rami of the upper 11 thoracic spinal nerves. They enter the intercostal spaces between the internal intercostal and the innermost intercostal muscles and run together with the intercostal arteries and veins. The segmental neurovascular bundles continue in or just inferior to the costal grooves (Figure 1). The ICN motor branches innervate the external, internal, and innermost intercostal muscles. The sensory components of the ICN consist of two cutaneous systems: (a) the lateral cutaneous branches (LCBs), and (b) the anterior cutaneous branches (ACBs). Among other areas, these two systems innervate the breast (Moore, Dalley, & Agur, 2010). However, the exact subcutaneous course of the cutaneous branches in the breast remains unclear, as they arise from under the ribs and enter the glandular tissue. Available information about the subcutaneous course and distribution of the nerves supplying the breast skin and NAC is scarce and contradictory. There is consensus about the anterior and lateral cutaneous branches of the 4th ICN supplying the NAC (Cooper, 1840; Craig & Sykes, 1970; Edwards, 1976; Gabka & Bohmert, 1997; Goldwyn, 1985; Jaspars, Posma, van Immerseel, & Gittenberger‐de Groot, 1997; Moore et al., 2010; Pandya & Moore, 2011; Sarhadi, Shaw Dunn, Lee, & Soutar, 1996; Schlenz, Kuzbari, Gruber, & Holle, 2000; Schünke, Schulte, Schumacher, Voll, & Wesker, 2010). However, this agreement is largely based on cadaver studies without magnification loupes dating back to 1840 (Cooper, 1840) and other studies lacking histological validation (Craig & Sykes, 1970; Eckhard, 1851; Farina, Newby, & Alani, 1980). In addition, some studies made conflicting statements about the existence of a subdermal neural plexus (Craig & Sykes, 1970; Farina et al., 1980; Jaspars et al., 1997; le Roux, Pan, Matousek, & Ashton, 2011; Pandya & Moore, 2011; Sarhadi et al., 1996).
Figure 1.
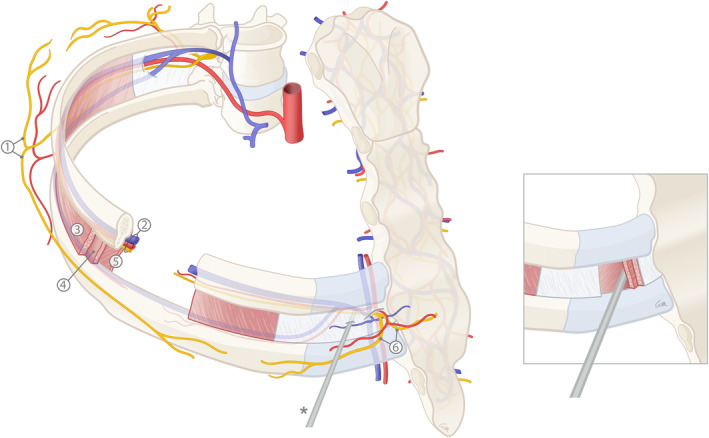
A schematic illustration of the anatomy of the intercostal arteries, veins, and nerves. (1) Lateral cutaneous branches (LCBs) of the intercostal nerve (ICN); (2) Neurovascular bundle running in the intercostal groove (consisting of the intercostal vein, artery and nerve from cranial to caudal); (3) External intercostal muscles; (4) Internal intercostal muscles; (5) Innermost intercostal muscles (imm); (6) Anterior cutaneous branches (ACBs) of the intercostal nerve (ICN)
The aim of this study was to explore the nerves involved in innervation of the female breast by specifically blocking the ACBs of the 2nd to 5th ICNs with a depot of local anesthetics. This study helps to determine the nerve that can best be used as a recipient for sensory nerve coaptation in autologous breast reconstruction.
2. MATERIALS AND METHODS
The study was performed on healthy female volunteers in Maastricht University Medical Center. Ethical approval was obtained from the Medical Ethical Committee (METC) of Maastricht University. Written informed consent was obtained from the participants. The study was conducted in accordance with STROBE guidelines and the World Medical Association Declaration of Helsinki (General Assembly of the World Medical Association, 2014; von Elm et al., 2014).
Inclusion criteria were: healthy women aged 18 years or older. Exclusion criteria were: the history of breast surgery, scars or other distinctive markings on the breasts, and neurological conditions such as diabetes or neuropathy that could affect sensation.
The ACBs of the 2nd to 5th ICNs were blocked in order to determine which nerves are involved in innervating the female breast, the nipple, and areola. First, all levels were blocked individually, which required two separate investigations to preclude overlap between adjacent areas. Thus, the exact skin area supplied by each individual ICN could be identified. During the first measurement session, the ACBs of the 2nd and 5th ICNs were blocked on the right side and those of the 3rd ICN on the left side. During the second measurement session, Level 4 on the right side was investigated. The individual contributions of the ACBs of the 2nd, 3rd, 4th, and 5th ICNs to breast innervation were thus defined. In the same measurement session, the left side was used to determine the contribution of all levels together, ICNs 2–5, to innervation of the female breast.
The targeted intercostal spaces were identified using a Philips Corp. iU22 ultrasound system and a linear transducer 12–5. The volunteer was placed supine and was asked to remain in that position until the end of the procedure to prevent the distribution of the injectate by movement or postural change (Figure 2).
Figure 2.

Schematic overview of the setup. Lateral view from the left side of the participant; injection of lidocaine for sonographically‐guided nerve block of the anterior cutaneous branch (ACB) of an intercostal nerve (ICN) of the right breast. The probe was moved from cephaled to caudad to identify the targeted intercostal space. Once this was identified, the probe was only rotated from medial to lateral without replacement [Color figure can be viewed at wileyonlinelibrary.com]
The transducer was placed longitudinally and parallel to the lateral sternal border and the clavicle was identified. While the transducer was moved caudad, the first hyperechogenic structure to appear was identified as the second rib. Tracing caudad, all further ribs and the corresponding intercostal spaces were identified. Then the skin was disinfected twice over the whole trajectory from above the clavicle to the lower sternal border, and from the sternum medially to the anterior axillary line laterally. The transducer was wrapped in a sterile cover and the procedure was repeated sterile, starting at the clavicle, identifying the second rib, and counting downwards. The intercostal target nerve at the level of interest was identified, being the layer between the internal intercostal muscle (IIM) and the innermost intercostal muscle (imm). When possible, the accompanying artery in the neurovascular bundle was identified with a Doppler signal (Figure 3). The transducer was held in this longitudinal position and a skin wheel was applied distal to it. A sterile 22‐gauge needle was introduced and the needle tip was identified subcutaneously. The needle was then guided sonographically from caudal to cranial, producing an in‐plane image of it throughout the procedure. After the needle tip reached the interfacial intercostal plane, 2 ml of lidocaine 2% was injected.
Figure 3.
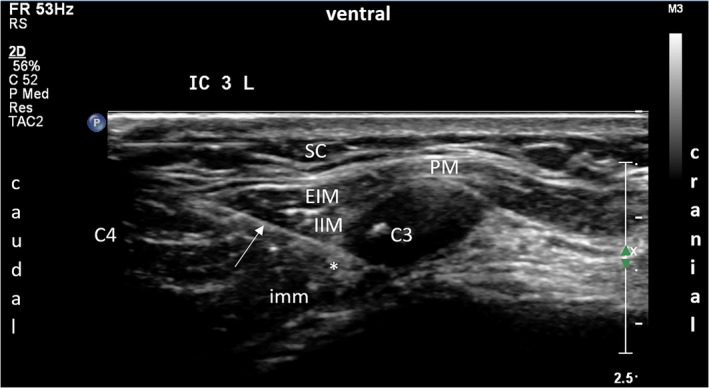
Ultrasound image of the intercostal plane block of the anterior cutaneous branch (ACB) of the third intercostal nerve (ICN) on the left side. Local anesthetic spread (*) between the internal intercostal muscle (IIM) and the innermost intercostal muscle (imm). White arrow: needle. PM, pectoralis major muscle; SC, subcutis; EIM, external intercostal muscle. C3: 3rd rib. C4: 4th rib [Color figure can be viewed at wileyonlinelibrary.com]
After injection, a raster was drawn on the thorax from the sternum to the lateral border of the breast and from the jugular notch to the xiphoid process, using a skin marker. The raster was divided into 2 × 2 cm fields. The measurements were performed in a quiet and temperature‐controlled room.
Sensory measurements started 20–30 min after injection of the lidocaine, and the eyes of the volunteer were closed during examination. A Semmes‐Weinstein monofilament was placed on the skin at an angle of 40–45°, but to preclude deep pressure sensation of the surrounding tissue, no pressure was applied. The monofilament was then moved in a stroking manner over the breast to locate the borders of the numbed area. All measurements were performed by one investigator. The sensation was noted by the participant as “normal” or “abnormal” as the monofilament was moved from the midline to the lateral border of the breast. Every time the participant indicated a change in the feeling, a line was drawn at that point. To ensure validity, each measurement was repeated until the participant identified the change of sensation at the same location she did before. Thus, the numbed area on the breast could be marked on the skin. Photographs of the skin‐marked numb areas of both participants were taken after each measurement. Participant 2 preferred the photos to be converted into drawings before publication.
3. RESULTS
Two healthy participants were included. One participant had a BMI of 23.0 and wore a C‐cup (European size) and the other participant had a BMI of 20.6 and wore an A‐cup. The size and location of the areas are summarized in Table 1, including whether sensation in the NAC was affected.
Table 1.
The size and location of the areas supplied by the 2nd to 5th ICNs and whether sensation in the NAC was affected
| Participant 1 | Participant 2 | |
|---|---|---|
| ICN 2 | ||
| Size | 6 × 6 cm | 9 × 7 cm |
| Location | UIQ | UIQ |
| NAC | No | No |
| ICN 3 | ||
| Size | 8 × 7 cm | 9.5 × 9 cm |
| Location | UIQ and LIQ | UIQ and LIQ |
| NAC | No | Doubtful |
| ICN 4 | ||
| Size | 9 × 7.5 cm | 12 × 8 cm |
| Location | UIQ and LIQ | UIQ and LIQ |
| NAC | No | Doubtful |
| ICN 5 | ||
| Size | 6 × 4 cm | 10 × 6 cm |
| Location | LIQ | LIQ and abdomen |
| NAC | No | No |
| ICN 2–5 | ||
| Size | 11.5 × 16 cm | 21.5 × 13 cm |
| Location | UIQ and LIQ | UIQ, LIQ, UOQ, and LOQ |
| NAC | Nipple and medial half areola | Yes |
Abbreviations: ICN, intercostal nerve; NAC, nipple‐areola‐complex; UIQ, upper‐inner‐quadrant; LIQ, lower‐inner‐quadrant; UOQ, upper‐outer‐quadrant; LOQ, lower‐outer‐quadrant.
The largest individually‐supplied area was supplied by the fourth ICN in both participants and was located in the upper and lower inner quadrants. The sizes were 9 × 7.5 cm (Figure 4) and 12 × 8 cm (Figure 5). In Participant 1, sensation in the NAC was not affected after blockage of the 4th ICN, but Participant 2 found it difficult to distinguish between the altering sensation of the nipple and the hypoesthesia caused by the anesthetic.
Figure 4.
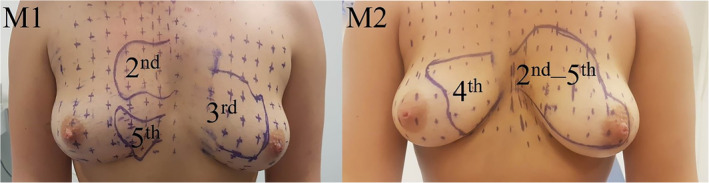
The areas corresponding to the 2nd to 5th ICNs, measured in participant 1. The first measurements (M1) show the areas supplied by the 2nd, 3rd, and 5th ICNs. The second measurements (M2) show the areas supplied by the 4th ICN and all levels together [Color figure can be viewed at wileyonlinelibrary.com]
Figure 5.
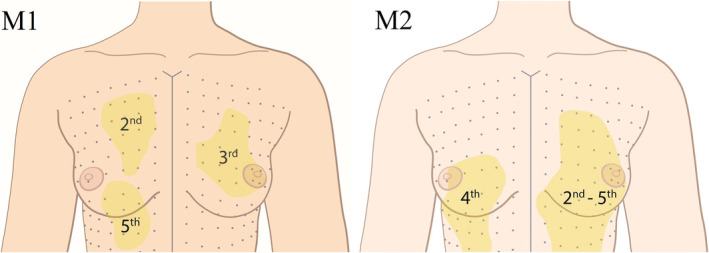
Drawings of the photos of the areas supplied by the 2nd to 5th ICNs, measured in participant 2. The first measurements (M1) show the areas supplied by the 2nd, 3rd, and 5th ICNs. The second measurements (M2) show the areas supplied by the 4th ICN and all levels together [Color figure can be viewed at wileyonlinelibrary.com]
The second‐largest individually‐supplied area was supplied by the 3rd ICN. This area was also located in the upper and lower inner quadrantsand measured 8 × 7 cm and 9.5 × 9 cm. Participant 1 stated that sensation in the NAC was not affected. However, Participant 2 said sensation in her NAC was altered.
3.1. Complications
No major complications were observed. Participant 1 noted irritation of the skin at one of the injection points after the first measurement; it lasted for 5 days and then disappeared. Participant 2 developed a small hematoma after the first measurement, which resorbed spontaneously. No adverse events were observed during or after the second intervention.
4. DISCUSSION
The current study showed that the biggest area of the breast is supplied by the ACB of the 4th ICN. However, no individual contribution of the 4th or any other ICN to sensation in the NAC was found. This confirmed the accounts in the available literature: the nipple is innervated by a multiplicity of nerves. Sir Astley Cooper first described the supply to the nipple by the ACBs of the 3rd and 4th and the LCBs of the 4th and 5th ICNs (Cooper, 1840). Subsequent studies have implicated a wide variety of nerves in nipple innervation, almost always including both the ACBs and LCBs of the 3rd to 5th ICNs (Cooper, 1840; Craig & Sykes, 1970; Edwards, 1976; Gabka & Bohmert, 1997; Goldwyn, 1985; Jaspars et al., 1997; Moore et al., 2010; Pandya & Moore, 2011; Sarhadi et al., 1996; Schlenz et al., 2000; Schünke et al., 2010). All studies have mentioned dominant nipple innervation by the LCB of the 4th ICN. Some state that the NAC is innervated only by the LCBs of the ICNs (Eckhard, 1851; Edgerton, Meyer, & Jacobson, 1961; Farina et al., 1980). However, most of those studies are dated and involved macroscopic cadaver dissections with no histological evidence that the dissected tissue is, in fact, a nerve (Cooper, 1840; Craig & Sykes, 1970; Eckhard, 1851; Edgerton et al., 1961; Farina et al., 1980; Jaspars et al., 1997; Sykes, 1969).
Although most available studies suggest that multiple sensory nerves are involved in innervating the nipple, there is still very little information on the possible existence of a subdermal neural plexus. Some authors explicitly mention that they found no subdermal plexus of sensory nerves under the NAC (Craig & Sykes, 1970; Farina et al., 1980; Jaspars et al., 1997). A subdermal plexus is often not even mentioned. Farina et al. (1980) found only one nerve to be involved in nipple innervation, which would explain the absence of a subdermal plexus. However, Craig and Sykes (1970) (Craig & Sykes, 1970) and Jaspars et al. (1997) (Jaspars et al., 1997) also failed to observe communication of the LCBs and ACBs. The dissection by Craig and Sykes (1970) was macroscopic, with no loupe magnification. Jaspars et al. (1997) did use loupe magnification during their dissection, but they could not identify the outermost nerve endings either. The absence of a subdermal plexus is most likely explained by the very small diameter of the peripheral nerve endings, which makes it difficult to differentiate nervous tissue from subcutaneous or glandular tissue. Only a few authors mention the presence of a subdermal nerve plexus (Cooper, 1840; Michelle le Roux, Kiil, Pan, Rozen, & Ashton, 2010; Pandya & Moore, 2011; Sarhadi et al., 1996). All of them except Cooper mention the use of magnification loupes during dissection, but often fail to state the level of magnification. It is questionable whether Cooper would have been able to identify a nervous plexus without a magnification loupe, but the results of the current study support his statements. Individual blockage of the ACBs of the ICNs did not evidently affect sensation in the NAC. The authors hypothesize that the communicating nerves are responsible for this, and only blockage of all the nerves involved will result in hypesthesia of the NAC.
Most studies describe involvement of the LBCs of the ICNs in innervating the NAC (Cooper, 1840; Craig & Sykes, 1970; Edwards, 1976; Gabka & Bohmert, 1997; Goldwyn, 1985; Jaspars et al., 1997; Moore et al., 2010; Pandya & Moore, 2011; Sarhadi et al., 1996; Schlenz et al., 2000; Schünke et al., 2010), but in the current study, it was decided to limit measurements to the ACBs only. The ACBs are located in the surgical field of autologous breast reconstruction and are therefore directly accessible during surgery, in contrast to the midaxillary‐located LCBs. In addition, the latter is associated with +a higher risk of pneumothorax (Holzer, Kapral, Hellwagner, Eisenmenger‐Pelucha, & Preis, 1998; Shanti, Carlin, & Tyburski, 2001). Therefore, it was decided not to assess the LCBs in the current study.
Some authors describe the involvement of the supraclavicular nerves in the skin innervation of the upper part of the female breast (Pandya & Moore, 2011; Sarhadi et al., 1996; Schünke et al., 2010). The involvement of the supraclavicular nerve was not evaluated in the current study, as it was not expected to supply the largest skin area of the breast. Furthermore, no evidence was found in the literature that the supraclavicular nerve is involved specifically in nipple innervation or contributes to sensation in the areola.
It appeared rather difficult to measure sensation in the female breast after blocking the ACBs of the ICNs. Semmes‐Weinstein monofilaments are generally considered adequate for assessing the cutaneous sensitivity of the breast (Gonzalez, Brown, Gold, Walton, & Shafer, 1993; Hamdi, Greuse, De Mey, & Webster, 2001; Slezak & Dellon, 1993; Tairych et al., 1998; Temple & Hurst, 1999; Terzis, Vincent, Wilkins, Rutledge, & Deane, 1987). They are designed for discriminating static single‐point pressure sensitivity. Of course, the overall sensation is much more complex than pressure sensitivity alone. It also includes temperature discrimination, pain, itch, and erogenous sensation in the case of a breast/nipple. Each type of stimulus is detected by a specific sensory neuron subtype, for example, nociceptors sense painful stimuli. In the current study, the largest Semmes‐Weinstein monofilament (index 6.65) was used without static stimulation. Instead, for dynamic stimulation, it was stroked over the skin without applying pressure to determine the areas correlating with the ICNs. The sensory neurons detecting nonpainful mechanical stimuli or touch are low threshold mechanoreceptors (LTMRs). The LTMRs in glabrous skin consists of four types of sensory neurons: the Aβ slowly‐adapting (SA)1‐LTMRs (Merkel cells), the Aβ SA2‐LTMRs (Ruffini corpuscles), the Aβ rapidly‐adapting (RA)1‐LTMRs (Meissner's corpuscles) and the Aβ RA2‐LTMRs (Pacinian corpuscles). The Merkel cells and Meissner corpuscles are located superficially. Both of them detect low‐frequency stimuli such as static mechanical indentation and low‐frequency vibration (< 40 Hz). Low‐frequency vibration occurs when an object slips in the hand or with motion across the skin.
Pacinian corpuscles are involved in detecting high‐frequency vibration (>200 Hz), which happens when an object vibrates while being held (Zimmerman, Bai, & Ginty, 2014). They are also said to be involved in light touch stimuli (Schünke et al., 2010). However, most literature available on LTMRs is based on studies performed on the extremities, such as hands and feet (McGlone & Reilly, 2010; McGlone & Spence, 2010). Zimmerman et al. studied the LTMRs in the mammalian skin in 2014 and described Pacinian corpuscles as being involved in detecting motion across the skin (Zimmerman et al., 2014). The stimuli of crude touch that are detected by these mechanoreceptors are led through the spinothalamic tract, continuing centrally, and ending in the somatosensory cortex of the postcentral gyrus. Pacinian corpuscles lead the stimuli via the posterior column system, more specifically via the cuneate fasciculus and the gracile fasciculus, to the inferior part of the medulla oblongata, continuing centrally and ending in the somatosensory cortex.
It could be questioned whether the Semmes‐Weinstein monofilament is the best method for measuring breast sensation since it was developed for static sensory testing. Another method, known as dermatomal somatosensory evoked potentials (dSSEPs), has been discussed (DelVecchyo, Caloca Jr., Caloca, & Gomez‐Jauregui, 2004). However, dSSEPs were also developed for static sensory testing, and they were not available at our institution. Both participants underwent each measurement at least twice and always responded at the same level. The current technique using a Semmes‐Weinstein monofilament was therefore considered adequate and reliable. Other techniques should be explored in the future to optimize dynamic sensory testing of the breast.
The course of the ICNs remains unclear. The current study did not examine the course of the ACBs of the ICNs, which could be important during a mastectomy. Mastectomy is known to change the sensitivity of the breast; some sensory nerves of the skin are sacrificed. However, the level of impairment of sensitivity differs between the medial and the lateral parts of the breast (Bijkerk et al., 2019). This suggests that some sensory nerves can be spared during a mastectomy, perhaps because they follow a superficial subcutaneous course (Cooper, 1840; Riccio et al., 2015; Schulz et al., 2017), which can sometimes be noticed during the procedure. However, other studies describe a deep course of the sensory nerves going over the pectoral fascia and then through the mammary gland (Craig & Sykes, 1970; Farina et al., 1980; Jaspars et al., 1997; le Roux et al., 2011; Sarhadi et al., 1996). These contradictory statements could be explained in terms of the wide variety of anatomical courses of the sensory nerves of the breast. This has to be considered when the recipient nerve for sensory nerve coaptation is selected. If a nerve is visibly intact and running towards the skin, it arguably supplies an area of the skin envelope after mastectomy. It would be unfavorable to use such an intact nerve as recipient for nerve coaptation, since this could affect any preserved sensation. Hence, it is best to use a nerve that has already been sacrificed during mastectomy.
Another notable criterion for selecting the recipient nerve is its accessibility during the surgery. The 3rd ICN is most accessible since vascular anastomosis during autologous breast reconstruction is mostly performed in the 3rd intercostal space, which could be advantageous during an already‐challenging surgery. However, we believe that extension of the area innervated by the chosen recipient nerve must dominate the surgical challenge to access that recipient nerve. A nerve that previously supplied a larger area of the skin would stimulate a larger area in the somatosensory cortex of the brain after sensory nerve coaptation. Therefore, if the ACB of the 4th ICN supplies the largest area of the healthy female breast, it would be favorable to use this nerve for sensory nerve coaptation. If the 4th ICN is sacrificed during the mastectomy, the 3rd ICN should be considered second choice since it supplies the second largest area according to the current results and is easy to access during autologous breast reconstruction.
5. CONCLUSIONS
This study shows that the anterior cutaneous branch of the 4th intercostal nerve supplies the largest area of skin in a healthy breast. Hence, the 4th ICN would be the best nerve to use as recipient nerve. However, other aspects, such as accessibility during surgery and whether a nerve is still intact after mastectomy, should be considered. Moreover, anatomical and functional knowledge should be taken into consideration during breast surgery, in general, to preserve as much breast sensation as possible.
CONFLICT OF INTEREST
The authors state that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
E.B. worked in designing of the study, data collection and analysis, interpretation of data, drafting the manuscript, approval of manuscript. A.C. worked in designing of the study, data collection, drafting the manuscript, approval of manuscript. M.S. worked in designing of the study, data collection, interpretation of data, anatomical considerations, revising the manuscript, approval of manuscript. R.H. worked in designing of the study, interpretation of data, revising the manuscript, approval of manuscript. A.L. worked in the medical illustrations, revising the manuscript, approval of the manuscript. S.T. worked in designing of the study, interpretation of data, drafting and revising the manuscript, approval of manuscript.
ETHICAL STATEMENT
All procedures performed during studies involving human participants accorded with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from healthy participants.
ACKNOWLEDGMENTS
We would like to thank the healthy participants for their voluntary contribution to this publication. We would also like to acknowledge the contribution of medical illustrator Greet Mommen of the Department of Anatomy and Embryology of Maastricht University for the figures in this publication (http://www.greetmommen.be).
Bijkerk E, Cornelissen AJM, Sommer M, Van Der Hulst René R. W. J., Lataster A, Tuinder SMH. Intercostal nerve block of the anterior cutaneous branches and the sensibility of the female breast. Clin Anat. 2020;33:1025–1032. 10.1002/ca.23532
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in the paper as it contains a proof of concept and is of descriptive nature.
REFERENCES
- Beugels, J. , Cornelissen, A. J. M. , van Kuijk, S. M. J. , Lataster, A. , Heuts, E. M. , Piatkowski, A. , … Tuinder, S. M. H. (2019). Sensory recovery of the breast following innervated and noninnervated DIEP flap breast reconstruction. Plastic and Reconstructive Surgery, 144, 178e–188e. [DOI] [PubMed] [Google Scholar]
- Bijkerk, E. , van Kuijk, S. M. J. , Beugels, J. , Cornelissen, A. J. M. , Heuts, E. M. , van der Hulst, R. , & Tuinder, S. M. H. (2019). Breast sensibility after mastectomy and implant‐based breast reconstruction. Breast Cancer Research and Treatment, 175, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, A. (1840). The anatomy of the breast. London: Longman. [Google Scholar]
- Cornelissen, A. J. M. , Beugels, J. , van Kuijk, S. M. J. , Heuts, E. M. , Rozen, S. M. , Spiegel, A. J. , … Tuinder, S. M. H. (2018). Sensation of the autologous reconstructed breast improves quality of life: A pilot study. Breast Cancer Research and Treatment, 167, 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, R. D. , & Sykes, P. A. (1970). Nipple sensitivity following reduction mammaplasty. British Journal of Plastic Surgery, 23, 165–172. [DOI] [PubMed] [Google Scholar]
- Damen, T. H. , Timman, R. , Kunst, E. H. , Gopie, J. P. , Bresser, P. J. , Seynaeve, C. , … Tibben, A. (2010). High satisfaction rates in women after DIEP flap breast reconstruction. Journal of Plastic, Reconstructive & Aesthetic Surgery, 63, 93–100. [DOI] [PubMed] [Google Scholar]
- de Boer, M. , van der Hulst, R. , & Slatman, J. (2015). The surprise of a breast reconstruction: A longitudinal phenomenological study to women's expectations about reconstructive surgery. Human Studies, 38, 409–430. [Google Scholar]
- DelVecchyo, C. , Caloca, J., Jr. , Caloca, J. , & Gomez‐Jauregui, J. (2004). Evaluation of breast sensibility using dermatomal somatosensory evoked potentials. Plastic and Reconstructive Surgery, 113, 1975–1983. [DOI] [PubMed] [Google Scholar]
- Eckhard, C. (1851). Die Nerven der weiblichen Brustdrüse und ihr Einfluss auf die Milchsecretion. Betr Anat Physiol, 1, 1–9. [Google Scholar]
- Edgerton, M. T. , Meyer, E. , & Jacobson, W. E. (1961). Augmentation mammaplasty. II. Further surgical and psychiatric evaluation. Plastic and Reconstructive Surgery and the Transplantation Bulletin, 27, 279–302. [DOI] [PubMed] [Google Scholar]
- Edwards, E. (1976). Surgical anatomy of the breast ‐ plastic and reconstructive surgery of the breast. Boston: Little Brown & Co. [Google Scholar]
- Eltahir, Y. , Werners, L. L. , Dreise, M. M. , van Emmichoven, I. A. , Jansen, L. , Werker, P. M. , & de Bock, G. H. (2013). Quality‐of‐life outcomes between mastectomy alone and breast reconstruction: Comparison of patient‐reported BREAST‐Q and other health‐related quality‐of‐life measures. Plastic and Reconstructive Surgery, 132, 201e–209e. [DOI] [PubMed] [Google Scholar]
- Farina, M. A. , Newby, B. G. , & Alani, H. M. (1980). Innervation of the nipple‐areola complex. Plastic and Reconstructive Surgery, 66, 497–501. [DOI] [PubMed] [Google Scholar]
- Gabka, C. J. , & Bohmert, H. (1997). Plastic and reconstructive surgery of the breast. Stuttgart, New York: Thieme. [Google Scholar]
- General Assembly of the World Medical A . (2014). World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. The Journal of the American College of Dentists, 81, 14–18. [PubMed] [Google Scholar]
- Goldwyn, R. M. (1985). Gray's anatomy. Plastic and Reconstructive Surgery, 76, 147–148. [DOI] [PubMed] [Google Scholar]
- Gonzalez, F. , Brown, F. E. , Gold, M. E. , Walton, R. L. , & Shafer, B. (1993). Preoperative and postoperative nipple‐areola sensibility in patients undergoing reduction mammaplasty. Plastic and Reconstructive Surgery, 92, 809–814 discussion 815‐808. [PubMed] [Google Scholar]
- Hamdi, M. , Greuse, M. , De Mey, A. , & Webster, M. H. (2001). A prospective quantitative comparison of breast sensation after superior and inferior pedicle mammaplasty. British Journal of Plastic Surgery, 54, 39–42. [DOI] [PubMed] [Google Scholar]
- Holzer, A. , Kapral, S. , Hellwagner, K. , Eisenmenger‐Pelucha, A. , & Preis, C. (1998). Severe pneumothorax after intercostal nerve blockade. A case report. Acta Anaesthesiologica Scandinavica, 42, 1124–1126. [DOI] [PubMed] [Google Scholar]
- Isern, A. E. , Tengrup, I. , Loman, N. , Olsson, H. , & Ringberg, A. (2008). Aesthetic outcome, patient satisfaction, and health‐related quality of life in women at high risk undergoing prophylactic mastectomy and immediate breast reconstruction. Journal of Plastic, Reconstructive & Aesthetic Surgery, 61, 1177–1187. [DOI] [PubMed] [Google Scholar]
- Jaspars, J. J. , Posma, A. N. , van Immerseel, A. A. , & Gittenberger‐de Groot, A. C. (1997). The cutaneous innervation of the female breast and nipple‐areola complex: Implications for surgery. British Journal of Plastic Surgery, 50, 249–259. [DOI] [PubMed] [Google Scholar]
- Koslow, S. , Pharmer, L. A. , Scott, A. M. , Stempel, M. , Morrow, M. , Pusic, A. L. , & King, T. A. (2013). Long‐term patient‐reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Annals of Surgical Oncology, 20, 3422–3429. [DOI] [PubMed] [Google Scholar]
- le Roux, C. M. , Pan, W. R. , Matousek, S. A. , & Ashton, M. W. (2011). Preventing venous congestion of the nipple‐areola complex: An anatomical guide to preserving essential venous drainage networks. Plastic and Reconstructive Surgery, 127, 1073–1079. [DOI] [PubMed] [Google Scholar]
- McGlone, F. , & Reilly, D. (2010). The cutaneous sensory system. Neuroscience and Biobehavioral Reviews, 34, 148–159. [DOI] [PubMed] [Google Scholar]
- McGlone, F. , & Spence, C. (2010). The cutaneous senses: Touch, temperature, pain/itch, and pleasure. Neuroscience and Biobehavioral Reviews, 34, 145–147. [DOI] [PubMed] [Google Scholar]
- Michelle le Roux, C. , Kiil, B. J. , Pan, W. R. , Rozen, W. M. , & Ashton, M. W. (2010). Preserving the neurovascular supply in the Hall‐Findlay superomedial pedicle breast reduction: An anatomical study. Journal of Plastic, Reconstructive & Aesthetic Surgery, 63, 655–662. [DOI] [PubMed] [Google Scholar]
- Moore, K. , Dalley, A. , & Agur, A. (2010). Clinically oriented anatomy (6th ed., pp. 98–99). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Pandya, S. , & Moore, R. G. (2011). Breast development and anatomy. Clinical Obstetrics and Gynecology, 54, 91–95. [DOI] [PubMed] [Google Scholar]
- Rabin, R. (2017). After mastectomies, an unexpected blow: Numb new breasts. The New York Times; www.nytimes.com/2017/01/29/well/live/after‐mastectomies‐an‐unexpected‐blow‐numb‐new‐breasts.html. Accessed December 2018. [Google Scholar]
- Riccio, C. A. , Zeiderman, M. R. , Chowdhry, S. , Brooks, R. M. , Kelishadi, S. S. , Tutela, J. P. , … Wilhelmi, B. J. (2015). Plastic surgery of the breast: Keeping the nipple sensitive. Eplasty, 15, e28. [PMC free article] [PubMed] [Google Scholar]
- Sarhadi, N. S. , Shaw Dunn, J. , Lee, F. D. , & Soutar, D. S. (1996). An anatomical study of the nerve supply of the breast, including the nipple and areola. British Journal of Plastic Surgery, 49, 156–164. [DOI] [PubMed] [Google Scholar]
- Schlenz, I. , Kuzbari, R. , Gruber, H. , & Holle, J. (2000). The sensitivity of the nipple‐areola complex: An anatomic study. Plastic and Reconstructive Surgery, 105, 905–909. [DOI] [PubMed] [Google Scholar]
- Schulz, S. , Zeiderman, M. R. , Gunn, J. S. , Riccio, C. A. , Chowdhry, S. , Brooks, R. , … Wilhelmi, B. J. (2017). Safe plastic surgery of the breast II: Saving nipple sensation. Eplasty, 17, e33. [PMC free article] [PubMed] [Google Scholar]
- Schünke, M. , Schulte, E. , Schumacher, U. , Voll, M. , & Wesker, K. (2010). Atlas of anatomy Prometheus: General anatomy and musculoskeletal system (Dutch) (2nd ed.). Houten: Bohn Stafleu van Loghum.207. [Google Scholar]
- Shanti, C. M. , Carlin, A. M. , & Tyburski, J. G. (2001). Incidence of pneumothorax from intercostal nerve block for analgesia in rib fractures. The Journal of Trauma, 51, 536–539. [DOI] [PubMed] [Google Scholar]
- Slezak, S. , & Dellon, A. L. (1993). Quantitation of sensibility in gigantomastia and alteration following reduction mammaplasty. Plastic and Reconstructive Surgery, 91, 1265–1269. [DOI] [PubMed] [Google Scholar]
- Sykes, P. A. (1969). The nerve supply of the human nipple. Journal of Anatomy, 105, 201. [PubMed] [Google Scholar]
- Tairych, G. V. , Kuzbari, R. , Rigel, S. , Todoroff, B. P. , Schneider, B. , & Deutinger, M. (1998). Normal cutaneous sensibility of the breast. Plastic and Reconstructive Surgery, 102, 701–704. [DOI] [PubMed] [Google Scholar]
- Temple, C. L. , & Hurst, L. N. (1999). Reduction mammaplasty improves breast sensibility. Plastic and Reconstructive Surgery, 104, 72–76. [PubMed] [Google Scholar]
- Terzis, J. K. , Vincent, M. P. , Wilkins, L. M. , Rutledge, K. , & Deane, L. M. (1987). Breast sensibility: A neurophysiological appraisal in the normal breast. Annals of Plastic Surgery, 19, 318–322. [PubMed] [Google Scholar]
- von Elm, E. , Altman, D. G. , Egger, M. , Pocock, S. J. , Gotzsche, P. C. , Vandenbroucke, J. P. , & Initiative, S. (2014). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. International Journal of Surgery, 12, 1495–1499.25046131 [Google Scholar]
- Zimmerman, A. , Bai, L. , & Ginty, D. D. (2014). The gentle touch receptors of mammalian skin. Science, 346, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the paper as it contains a proof of concept and is of descriptive nature.


