Abstract
Introduction
Inotersen, an antisense oligonucleotide inhibitor of transthyretin (TTR) protein production, demonstrated significant benefit versus placebo in the modified Neuropathy Impairment Score (NIS) +7 neurophysiologic tests (mNIS+7) in patients with hereditary TTR‐mediated amyloidosis (hATTR) with polyneuropathy. This analysis assessed the mNIS+7 components by anatomic location and the lower limb function (LLF) test.
Methods
Adults with hATTR in the NEURO‐TTR trial (NCT01737398) were randomly assigned to receive weekly doses of subcutaneous inotersen 300 mg or placebo for 65 weeks. The mNIS+7 and LLF were assessed at 35 and 66 weeks.
Results
All major mNIS+7 components (muscle weakness, muscle stretch reflexes, sensation) and the LLF showed significant efficacy in patients receiving inotersen versus placebo; however, NIS‐reflexes (upper limb), touch pressure (upper and lower limbs), and heart rate during deep breathing did not show significant effects.
Discussion
The results of this analysis reinforce the beneficial effect of inotersen on slowing neuropathy progression in patients with hATTR polyneuropathy.
Keywords: amyloidosis, hATTR, inotersen, lower limb function, mNIS+7
Short abstract
Abbreviations
- CI
confidence interval
- CMAP
compound muscle action potential
- Σ5NC
five attributes of nerve conduction
- FAS
full analysis set
- hATTR
hereditary transthyretin‐mediated amyloidosis
- HRDB
heart rate during deep breathing
- LSM
least‐squares mean
- mNIS+7
modified Neuropathy Impairment Score +7 neurophysiological tests
- ND
normal deviate
- NIS
Neuropathy Impairment Score
- Norfolk QoL‐DN
Norfolk Quality of Life–Diabetic Neuropathy questionnaire
- SNAP
sensory nerve action potential
- S ST QSTing
Smart Somatotopic Quantitative Sensation Testing
- TTR
transthyretin
1. INTRODUCTION
Hereditary transthyretin‐mediated amyloidosis (hATTR; also abbreviated as ATTRv; v for variant) is an autosomal‐dominant, rare, progressive, and fatal disease that manifests with a buildup of misfolded transthyretin (TTR) protein in major organ systems, resulting in organ failure. 1 Pathogenic mutations in the TTR gene lead to the development of misfolded TTR and are most often due to a single nucleotide substitution. 2 Most patients experience multi‐systemic clinical manifestations, including cardiac, neurologic, gastrointestinal, and other organ‐related symptoms. 3 , 4 , 5 , 6 The neurologic manifestations typically begin in early or midlife and are progressive, with length‐dependent sensorimotor neuropathy alongside autonomic neuropathy, including gastrointestinal symptoms.
Treatment options for patients with hATTR include gene silencing with inotersen (antisense oligonucleotide therapy) and patisiran (RNA interference therapy), liver transplantation, and TTR stabilization with tafamidis or off‐label diflunisal. 7 , 8 , 9 Inotersen is a transthyretin‐directed antisense oligonucleotide that binds to TTR mRNA to promote the latter's degradation through an RNaseH1 mechanism. Thus, inotersen inhibits the translation of the TTR protein and knocks down circulating TTR protein levels. 10 , 11 In a global, placebo‐controlled pivotal study in patients with hATTR with polyneuropathy (NEURO‐TTR; NCT01737398), inotersen demonstrated significant benefit compared with placebo in the coprimary end points of modified Neuropathy Impairment Score +7 neurophysiologic tests (mNIS+7) and the Norfolk Quality of Life–Diabetic Neuropathy questionnaire (Norfolk QoL‐DN). 7
The mNIS+7 provides an overall measure of polyneuropathy signs and neurophysiologic test abnormalities in hATTR with polyneuropathy. It is a validated functional assessment, and its components are well‐defined, objective, referenced, and quantitative. 12 The mNIS+7 comprises assessments on polyneuropathy signs (24 items on weakness, five on muscle stretch reflex decrease, and eight on sensation loss; all items are assessed bilaterally) and seven neurophysiologic tests (five attributes of nerve conduction, Smart Somatotopic Quantitative Sensation Testing [S ST QSTing] of touch pressure and heat pain, and heart rate during to deep breathing [HRDB]). 15
Lower limb function scoring, an exploratory end point in NEURO‐TTR, is a simple test that can be completed in minutes and is used to assess severity of polyneuropathy in the lower limbs for patients with hATTR with polyneuropathy. 13 Lower limb function scoring consists of assessments of a patient's ability to walk on their toes, walk on their heels, and rise from a kneeling position.
To further understand the applicability and responsiveness of the mNIS+7 and lower limb function in patients with polyneuropathy of hATTR, we evaluated the individual item assessments of these measurement tools and analyzed the component scores by anatomic location (upper and lower limb). Ultimately, this will enable the design of more refined measurement tools for use in future trials and clinical practice. Additionally, this analysis aims to further validate and detail the specific improvements to neuropathy progression that inotersen confers compared with placebo.
2. METHODS
2.1. Patients
Patient inclusion and exclusion criteria were previously reported by Benson et al. 7 Briefly, patients in NEURO‐TTR were 18 to 82 years of age with stage 1 or stage 2 hATTR with polyneuropathy, a positive biopsy for amyloid, a documented TTR mutation, and an NIS score between 10 and 130 points, inclusive.
2.2. Study design
NEURO‐TTR was a phase 3 multicenter, double‐blind, randomized, placebo‐controlled clinical trial in which patients were randomly assigned 2:1 to receive 300 mg inotersen or placebo for 65 weeks. Details of the study design have been previously reported. 7
The trial was approved by the institutional review boards or ethics committees of all participating institutions and was conducted in accordance with the Good Clinical Practice guidelines of the International Conference on Harmonization and the principles of the Declaration of Helsinki. All patients provided written informed consent to participate in the trial.
2.3. Assessments
In this post hoc analysis, the major multi‐item components of the mNIS+7 composite score by anatomic region (upper limb vs lower limb), individual items of the NIS components, and lower limb function were evaluated.
2.3.1. mNIS+7
The mNIS+7 consists of two composite scores: the NIS composite score (244 points) and the modified +7 score (102.3 points). 7 , 12 The NIS composite score has four major components: cranial nerves (40 points), muscle weakness (152 points), decreased muscle stretch reflexes (20 points), and clinical decrease in sensation in toes and fingers (32 points). The modified +7 composite score consists of four major components: HRDB (3.72 points from normal deviates), nerve conduction tests (18.6 points from normal deviates), touch‐pressure sensation (40 points), and heat‐pain sensation (40 points). 15
The nerve conduction tests involve five attributes: fibular compound muscle action potential (CMAP), tibial CMAP, ulnar CMAP, sural sensory nerve action potential (SNAP), and ulnar SNAP amplitudes. Additionally, many of the NIS components and the S ST QSTing assessments (touch‐pressure and heat‐pain) were further analyzed by anatomic location (upper limb vs lower limb). The HRDB and the five attributes of nerve conduction (Σ5NC) results were reported as normal deviates (NDs) from percentiles derived from reference values of healthy study participants. The same reference values were used for these studies across all medical centers because the instruments and peripherals used were sufficiently similar and standardized. Previous studies have shown that amplitudes correlate better with clinical impairments than conduction velocities; therefore, this study reports only amplitude results for the nerve conduction studies. 14 , 15
mNIS+7 assessment was evaluated at weeks 35 and 66. An increase in the mNIS+7 score indicates worsening of polyneuropathy impairments, and a 2‐point change is considered the minimum clinically meaningful change detectable by clinicians. 16 , 17
All assessors of mNIS+7 in NEURO‐TTR were certified neurologists and received specialized training by members of the Mayo Clinic Peripheral Neuropathy Research Laboratory. The NIS component was performed by an independent neurologist who was blinded to treatment assignations and not involved in the conduct of the trial or care of the patient. Additionally, different clinicians performed each separate neuropathy assessment, and duplicate assessments were separated by at least 24 hours. Neurophysiologic tests were performed by certified neurophysiologists or technologists who underwent specialized training and received prepared syllabi. S ST QSTing assessments involved automated and standardized equipment at all clinical sites.
2.3.2. Lower Limb Function
The lower limb function test is an item on the paper case report form, Clinical Neuropathy Assessment, that evaluates the ability of the patient to walk on their toes, walk on their heels, and rise from a kneeling position. 13 Each component is examined by a neurologist who evaluates the component as normal or abnormal, unless the disability is due to age, physical unfitness, or knee disease. The examination assesses each component for the left and right side separately. If the test is normal, it is given a score of 0, and if it is abnormal, a score of 1. Therefore, the maximum score of 6 indicates abnormality in all three components bilaterally and a score of 0 indicates a normal result for all three components bilaterally. The lower limb function test was assessed at baseline and weeks 35 and 66.
2.4. Statistical analysis
Least‐squares mean (LSM) changes from baseline to weeks 35 and 66 were assessed. Outcomes are presented for patients in the full analysis set (FAS), which included all randomized participants who received at least one dose of study drug and who had a baseline and at least one postbaseline assessment for the coprimary end points. The following exploratory post hoc assessments are based on analysis without imputation of missing data. The mixed‐effects model with repeated measures was applied in the current post hoc analyses of the mNIS+7 and lower limb function scores with fixed categorical effects for treatment, time, treatment‐by‐time interaction, and each of the three randomization stratification factors, and fixed covariates for the baseline value and the baseline‐by‐time interaction. Statistical significance was evaluated at a two‐sided alpha of 0.05 for all analyses.
3. RESULTS
3.1. Demographics and disease baseline characteristics
Baseline characteristics of the NEURO‐TTR safety population have been previously reported. 7 The FAS population included a total of 165 of the 172 patients in the safety population (106 inotersen; 59 placebo). Demographics and baseline characteristics were well balanced between the placebo and inotersen groups: mean age was 59.4 and 59.6 years, duration of disease from diagnosis was 39.8 and 43.5 months, and percentage of patients with stage 1/stage 2 disease was 71.2%/67.0%, and 28.8%/33.0%, respectively. Mean baseline mNIS+7 composite score (± SD) was similar between treatment groups (placebo, 74.1 ± 39.0; inotersen, 79.4 ± 37.5), as was the mean baseline lower limb function score (placebo, 2.98 ± 2.86; inotersen, 3.23 ± 2.63). Note that median lower limb function baseline values for placebo and inotersen were 2 and 4, respectively, showing that there was a slight difference between each group in baseline disease severity based on lower limb function.
3.2. mNIS+7 components and individual items
A significantly greater therapeutic benefit was observed in patients treated with inotersen than in those treated with placebo for the NIS components of muscle weakness, reflex decrease, sensation loss, and the modified +7 components of heat‐pain sensation when analyzed by upper and lower limb (Figure 1); reflexes in the upper limb and touch‐pressure (overall and upper and lower limb) were not significantly different between inotersen and placebo. Of note, a large majority of patients (97%) had no impairment in cranial nerves at baseline or at any point postbaseline; therefore, changes in this NIS component were not analyzed. Furthermore, 16 of the 30 individual items in the NIS significantly favored inotersen versus placebo (Supporting Information Table S1, which is available online).
FIGURE 1.
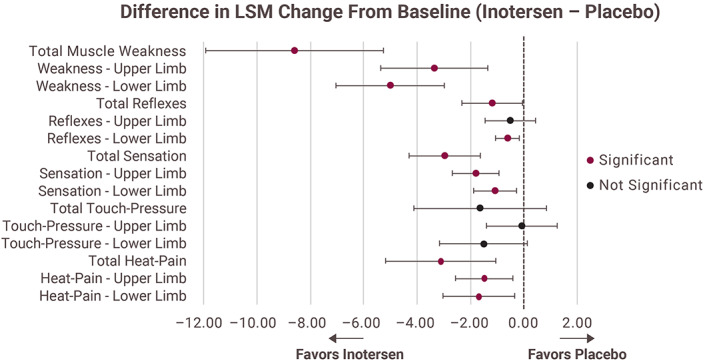
Select mNIS+7 components analyzed as upper limb and lower limb LSM, least‐squares mean; mNIS+7, modified neuropathy impairment scale +7 neurophysiologic tests. Note: Increase in score denotes worse neuropathic deficit [Color figure can be viewed at wileyonlinelibrary.com]
There was no significant difference between the inotersen and placebo groups for the modified +7 component of HRDB (−0.07 NDs; 95% confidence interval [CI], −0.41 to 0.26; P = .661); however, only 58.8% of patients treated with inotersen and 63.5% of patients who received placebo had HRDB data available at week 66.
The modified +7 component of nerve conduction (Σ5NC assessments) significantly favored inotersen compared with placebo at week 66 (−0.53 NDs; 95% CI, −1.00 to −0.06; P = .027). In addition, the individual assessment of ulnar CMAP significantly favored inotersen versus placebo (P = .015); however, the individual assessments of fibular CMAP approached significance (P = .051), whereas the individual assessments of sural SNAP, tibial CMAP, and ulnar SNAP amplitudes did not reach a significant difference (Figure 2).
FIGURE 2.
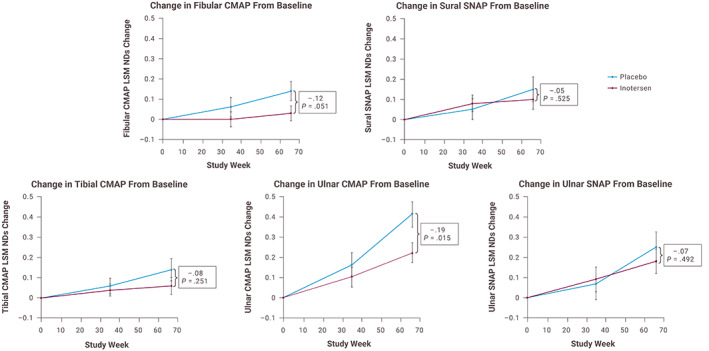
Change in normal deviates of the five attributes of nerve conduction. CMAP, compound muscle action potential; LSM, least‐squares mean; ND, normal deviate; SNAP, sensory nerve action potential. Note: Increase in score denotes worse neuropathic deficit [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Lower limb function and components response
Changes from baseline in the total lower limb function score and each of its three components indicated significantly less neuropathic progression in patients treated with inotersen compared with those receiving placebo. Change in total lower limb function score was significantly lower with inotersen versus placebo (Figure 3). The LSM change from baseline in all three components of the lower limb function test (walk on toes, walk on heels, and rise from a kneeling position) were also significantly lower with inotersen compared with placebo (walk on toes: LSM difference of −0.30; 95% CI, −0.52 to −0.09; P = .006; walk on heels: LSM difference of −0.32; 95% CI, −0.54 to −0.10; P = .005; and rise from a kneeling position: LSM difference of −0.27; 95% CI, 0.49 to −0.04; P = .020).
FIGURE 3.
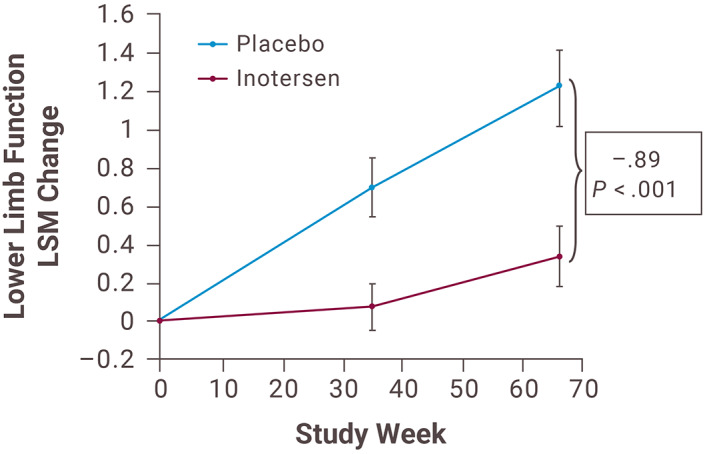
Change in lower limb function score over time. LSM, least‐squares mean [Color figure can be viewed at wileyonlinelibrary.com]
Further analysis based on baseline lower limb function score showed that when patients with a maximum abnormality score at baseline (score of 5–6) were excluded, fewer patients in the inotersen group worsened by week 66 with respect to the total lower limb function score than in the placebo group (22.4% vs 55.2%). In addition, a greater percentage of patients in the inotersen group had improvement in total lower limb function than in the placebo group (8.2% vs 0%). Furthermore, when patients with pre‐existing baseline abnormalities (score >0 at baseline) were excluded for each specific component of the lower limb function test, a larger percentage of patients in the inotersen group remained stabilized by week 66 than in the placebo group (ability to walk on toes: 92.3% of inotersen patients vs 55.6% of placebo patients; ability to walk on heels: 82.8% of inotersen patients vs 50.0% of placebo patients; ability to rise from a kneeling position: 82.9% of inotersen patients vs 59.3% of placebo patients).
4. DISCUSSION
The polyneuropathy of hATTR affects various functional classes of nerve fibers and, thus, causes a variety of symptoms and impairments in peripheral nerves related to this disease. The mNIS+7 is specifically designed to measure these neuropathic impairments. The Neuropathy Impairment Score plus 7 neurophysiological tests (NIS+7) has been shown to adequately assess muscle weakness and muscle stretch reflexes in patients with hATTR amyloidosis, but did not adequately assess sensation loss, autonomic dysfunction, and neurophysiologic test abnormalities. 14
The value of the mNIS+7 comes from its quantitative, reproducible, and referenced nature and was developed to improve characterization and quantification of neuropathic impairment in hATTR with polyneuropathy over the NIS+7. The mNIS+7 and many of its subcomponents described here have previously been shown to have strong correlations with disability and scales of hATTR with polyneuropathy severity, along with other neuropathy end points. 12 The current post hoc analysis of the NEURO‐TTR trial data shows that the mNIS+7 has the potential to detect the benefits of inotersen compared with placebo on several substantial neurologic attributes, including muscle weakness, muscle stretch reflex decrease, sensation loss, attributes of nerve conduction, and somatotopic heat pain and sensation loss.
Most components and many individual items of the mNIS+7 showed significant benefit of inotersen versus placebo when analyzed by upper limb and lower limb responses. The exception to this was the NIS upper limb reflexes assessment, in which a significant difference in disease progression was not demonstrated between inotersen and placebo. On the other hand, the NIS‐reflexes component, which includes the upper and lower limb assessments together, showed significant differences in LSM change from baseline to week 66 between inotersen and placebo.
Smart somatotopic heat‐pain and touch‐pressure sensation loss are two components within the modified +7 that provide further assessment of sensation loss. Both are a part of the S ST QSTing assessment, which has been shown to correlate with measures of neuropathy severity and disability scales for hATTR with polyneuropathy; thus, these tests can prove useful in detecting sensation loss. 18 In the NEURO‐TTR trial, inotersen treatment demonstrated a significant benefit versus placebo in the heat‐pain component of the S ST QSTing assessment (upper limb, lower limb, and both limbs combined), whereas the touch‐pressure component did not detect a difference between inotersen and placebo. 7 This is a surprising finding, as touch‐pressure sensation loss is known to be a major impairment in hATTR with polyneuropathy. The lack of a significant response to touch‐pressure may be due to its complexity in using forced‐choice algorithms of testing. A possible alternative to improve test performance is use of a stepping algorithm. 19
Similar to the S ST QSTing, the Σ5NC are objective, quantitative, and referenced end points of peripheral nerves. Out of the five nerve conduction tests, a significant difference between inotersen and placebo was demonstrated only for ulnar CMAP. Ulnar CMAP may have shown the greatest difference between inotersen and placebo, because for the other Σ5NC most patients may have already been near the maximum detectable impairment at baseline and their scores did not worsen as much.
The total Σ5NC score, which combined all five nerve conduction tests, showed a significant benefit for inotersen compared with placebo. For use in clinical trials, especially those in patients with hATTR with polyneuropathy, any assessment of nerve conduction should involve investigation of the whole body and various nerves. The nerve conduction assessments together are thus valuable end points and should all be used in future clinical trials.
HRDB is a component of the mNIS+7 that assesses autonomic neuropathy; however, it did not reach significance in the NEURO‐TTR trial. This may be because many patients in the NEURO‐TTR trial did not have HRDB data available for analysis because of the presence of active pacing or atrial fibrillation. Given that these conditions are common in patients with hATTR, it is difficult to use HRDB as an end point to assess autonomic dysfunction in this population. Based on these results, future trials should not include HRDB in the mNIS+7 assessments for patients with hATTR with polyneuropathy.
Proper administration of the mNIS+7 requires clinicians to be trained and certified to ensure that they use the same criteria for detection and for scaling neuropathic abnormality. The positive performance of the mNIS+7 in hATTR with polyneuropathy may be attributed to the training of the trial clinicians. This training was informed by previous trials of clinical versus neurophysiological assessment of neuropathic signs that showed that there is great variability among physicians in their assessments of signs and symptoms of polyneuropathy. 20 , 21 , 22 , 23 , 24 Furthermore, the HRDB and Σ5NC assessments include appropriate reference values from healthy participants that were used as part of reporting the normal deviate results to assist with reducing interobserver variability. Additionally, because these measurements are objective, quantitative, and referenced, they are highly confirmative of other evaluations.
In contrast to the mNIS+7, which can take a significant amount of time to complete, the lower limb function test, when performed by a trained neuromuscular physician who can make the requisite judgments, is a relatively quick assessment of a patient's muscle weakness. Treatment with inotersen resulted in a significant difference versus placebo for the overall lower limb function score as well as for its individual components. When patients with abnormal lower limb function results (lower limb function score >0) at baseline were excluded, more patients in the inotersen group experienced stabilization in lower limb function scores than those in the placebo group. These results suggest that a large percentage of patients with early‐stage disease have a disease progression that can be detected by the lower limb function test over a 15‐month study period.
Although the lower limb function test was able to detect a therapeutic effect, it has some limitations, including detecting changes in neuropathy impairments outside of muscle weakness. Also, a ceiling effect was observed with the lower limb function assessment, as roughly 40% of patients already had an abnormality in each lower limb function component at baseline. Thus, it may not be a strong tool to use in assessing patients with more advanced or severe disease. Despite some shortcomings, the lower limb function examination may prove useful in future trials or in the clinic, given its simplicity and ability to discriminate the effects of treatment compared with placebo.
It is important to note that many of the findings herein may not be generalizable to neuropathies other than hATTR polyneuropathy. Hereditary ATTR has multi‐system involvement and exhibits a rapid progressive worsening of neurologic function, which allows treatment effects to be recognized. In other neuropathies with slower progression or limited systemic involvement, the end points may not show therapeutic benefit.
Many individual items and components of the mNIS+7 and lower limb function test showed significant benefit with inotersen treatment because of lack of progression of polyneuropathy. Although many components of the mNIS+7 adequately assessed certain specific impairments, the results of this detailed analysis of components and individual assessments show that further adjustments to the mNIS+7 are necessary. Further analyses of the applicability of the mNIS+7 and the lower limb function test to patients with hATTR with polyneuropathy based on our initial findings are necessary for the optimal design of future studies or development of an assessment instrument for real‐world practice.
DISCLOSURE STATEMENT
P. James B. Dyck has received honoraria/consultancy fees from Akcea Therapeutics. John C. Kincaid was a sub‐investigator at NEURO‐TTR treatment trial site and received honoraria/consultancy fees from Akcea Therapeutics. Janice F. Wiesman was a sub‐investigator at NEURO‐TTR treatment trial site, and served as a consultant to Ionis Pharmaceuticals. Michael J. Polydefkis has received honoraria from Pfizer and Alnylam Pharmaceuticals, Inc. William J. Litchy has received grant/research support from Alnylam Pharmaceuticals. Michelle M. Mauermann has received research support and served as consultant for Ionis Pharmaceuticals, and received research support from Alnylam Pharmaceuticals. Elizabeth J. Ackermann is a consultant for Akcea Therapeutics. Spencer Guthrie is an employee at Aurora Bio and former employee of Akcea Therapeutics. Michael Pollock is an employee and stakeholder of Akcea Therapeutics. Shiangtun Jung is employed by Ionis Pharmaceuticals. Brenda F. Baker is employed by Ionis Pharmaceuticals. Peter J. Dyck has received financial support for the training of investigators for the conduct of therapeutic trials in hATTR PN from Ionis Pharmaceuticals and Alnylam Pharmaceuticals, and served as a consultant for Ionis Pharmaceuticals and Alnylam Pharmaceuticals.
ETHICAL STATEMENT
We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
POTENTIAL COVER ART
Images of a longitudinal paraffin section stained with Congo Red from a sural nerve biopsy showing transthyretin amyloid deposition (L, regular light; R, polarized light). Consequent peripheral nerve function and damage in patients with hATTR assessed by two principal methods in the clinical investigation of inotersen. For details, see Dyck et al: mNIS+7 and Lower Limb Function in Inotersen Treatment of hATTR, pages #‐#; and Dyck et al: Neuropathy Symptom and Change: Inotersen Treatment of Hereditary Transthyretin Amyloidosis, pages #‐#.
Supporting information
Table S1 NIS individual item responses.
ACKNOWLEDGMENTS
Editorial assistance in the preparation of this manuscript was provided by Christopher Pham, PharmD, and Lisa M. Klumpp Callan, PhD (ApotheCom, San Francisco, CA, USA), and was funded by Akcea Therapeutics (Boston, MA, USA).
Dyck PJB, Kincaid JC, Wiesman JF, et al. mNIS+7 and lower limb function in inotersen treatment of hereditary transthyretin‐mediated amyloidosis. Muscle & Nerve. 2020;62:502–508. 10.1002/mus.27022
Data Previously Presented at the 5th Congress of the European Academy of Neurology (EAN); June 29–July 2, 2019; Oslo, Norway; and the Peripheral Nerve Society (PNS) Annual Meeting; June 22–26, 2019; Genoa, Italy; and the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) annual meeting; October 16–19, 2019; Austin, Texas.
REFERENCES
- 1. Conceicao I, Gonzalez‐Duarte A, Obici L, et al. "Red‐flag" symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21(1):5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benson MD, Kincaid JC. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve. 2007;36(4):411‐423. [DOI] [PubMed] [Google Scholar]
- 3. Coelho T, Maurer MS, Suhr OB. THAOS ‐ the Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild‐type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63‐76. [DOI] [PubMed] [Google Scholar]
- 4. Sattianayagam PT, Hahn AF, Whelan CJ, et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J. 2012;33(9):1120‐1127. [DOI] [PubMed] [Google Scholar]
- 5. Benson MD, Uemichi T. Transthyretin amyloidosis. Amyloid. 1996;3(1):44‐56. [Google Scholar]
- 6. Rapezzi C, Quarta CC, Obici L, et al. Disease profile and differential diagnosis of hereditary transthyretin‐related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34(7):520‐528. [DOI] [PubMed] [Google Scholar]
- 7. Benson MD, Waddington‐Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22‐31. [DOI] [PubMed] [Google Scholar]
- 8. Adams D, Gonzalez‐Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 9. Gertz MA. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care. 2017;23(7 suppl):S107‐S112. [PubMed] [Google Scholar]
- 10. TEGSEDI (inotersen) injection, for subcutaneous use [prescribing information] Boston, MA: Akcea Therapeutics; 2018. [Google Scholar]
- 11. Ackermann EJ, Guo S, Benson MD, et al. Suppressing transthyretin production in mice, monkeys and humans using 2nd‐generation antisense oligonucleotides. Amyloid. 2016;23(3):148‐157. [DOI] [PubMed] [Google Scholar]
- 12. Dyck PJ, Kincaid JC, Dyck PJB, et al. Assessing mNIS+7Ionis and international neurologists' proficiency in a familial amyloidotic polyneuropathy trial. Muscle Nerve. 2017;56(5):901‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dyck PJ, Turner DW, Davies JL, et al. Electronic case‐report forms of symptoms and impairments of peripheral neuropathy. Can J Neurol Sci. 2002;29(3):258‐266. [DOI] [PubMed] [Google Scholar]
- 14. Suanprasert N, Berk JL, Benson MD, et al. Retrospective study of a TTR FAP cohort to modify NIS+7 for therapeutic trials. J Neurol Sci. 2014;344(1–2):121‐128. [DOI] [PubMed] [Google Scholar]
- 15. Dyck PJB, Gonzalez‐Duarte A, Obici L, et al. Development of measures of polyneuropathy impairment in hATTR amyloidosis: from NIS to mNIS+7. J Neurol Sci. 2019;405:116424. [DOI] [PubMed] [Google Scholar]
- 16. Peripheral Nerve Society . Diabetic polyneuropathy in controlled clinical trials: consensus report of the peripheral nerve society. Ann Neurol. 1995;38(3):478‐482. [DOI] [PubMed] [Google Scholar]
- 17. Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658‐2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinto MV, Dyck PJB, Gove LE, et al. Kind and distribution of cutaneous sensation loss in hereditary transthyretin amyloidosis with polyneuropathy. J Neurol Sci. 2018;394:78‐83. [DOI] [PubMed] [Google Scholar]
- 19. Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43(8):1508‐1512. [DOI] [PubMed] [Google Scholar]
- 20. Dyck PJ, Overland CJ, Low PA, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. 2010;42(2):157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dyck PJ, Overland CJ, Low PA, et al. "Unequivocally abnormal" vs "usual" signs and symptoms for proficient diagnosis of diabetic polyneuropathy: Cl vs NPhys trial. Arch Neurol. 2012;69(12):1609‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dyck PJ, Argyros B, Russell JW, et al. Multicenter trial of the proficiency of smart quantitative sensation tests. Muscle Nerve. 2014;49(5):645‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Litchy WJ, Wolfe J, et al. Proficiency of nerve conduction using standard methods and reference values (Cl. NPhys trial 4). Muscle Nerve. 2014;50(6):900‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dyck PJ, Albers JW, Wolfe J, et al. A trial of proficiency of nerve conduction: greater standardization still needed. Muscle Nerve. 2013;48(3):369‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 NIS individual item responses.


