ABSTRACT
Background
Severe iodine deficiency or excess during pregnancy can cause congenital hypothyroidism (CH). Iodine deficiency is common in pregnant women in the United States.
Objectives
We conducted a nested case–control study in a cohort of ∼2.5 million births in California to determine whether iodine status is related to CH in a US population.
Methods
Dried blood spots from 907 newborns with CH identified by newborn screening and 909 unaffected controls matched by month of birth were obtained from the California Newborn Screening Program to measure whole-blood iodine concentration. Iodine status was compared between cases and controls, and logistic regression was used to assess the association between CH status and blood iodine concentrations. Iodine status was also compared between cases and controls among infants treated in a neonatal intensive care unit (NICU) because CH has been reported in infants exposed to high levels of iodine in the NICU.
Results
Blood iodine concentrations did not differ significantly between cases (median: 20.0 ng/mL; IQR: 12.1–29.8 ng/mL) and controls (median: 20.3 ng/mL; IQR: 12.5–30.9 ng/mL; P = 0.59). Neither extremely high nor extremely low blood iodine concentrations (1st, 5th, 95th, and 99th percentiles of the distribution) were more common in cases. Among infants treated in NICUs, however, cases had significantly (P = 0.01) higher iodine (median: 22.7 ng/mL; IQR: 16.4–32.1 ng/mL) compared with controls (median: 17.3 ng/mL; IQR: 8.3–26.6 ng/mL).
Conclusions
CH cases did not have significantly higher or lower iodine in this population, which is reassuring given that maternal iodine deficiency is common in the United States. Among newborns in the NICU, CH cases had higher blood iodine concentrations compared with controls, suggesting that excess iodine exposure in the NICU could be causing CH. It may be beneficial to monitor iodine exposure from surgical procedures, imaging, and iodine-containing disinfectants and to consider non-iodine alternatives.
Keywords: congenital hypothyroidism, iodine, pregnancy, newborn screening, iodine deficiency, iodine excess, Wolff–Chaikoff effect, NICU iodine exposure
Introduction
The human fetus is completely dependent on thyroid hormone from the mother until at least week 11, when fetal thyroid hormone production begins (1). Fetuses are vulnerable to fluctuations in maternal iodine status because until birth, the fetus is completely reliant on maternal iodine intake. More than 50% of US pregnant women have urinary iodine concentrations in the deficient range (2), and pregnant women in the US as a group are considered iodine insufficient by the Iodine Global Network as of 2017 (3). Iodine deficiency during pregnancy can lead to many detrimental effects on the fetus, including intellectual disability, stunted growth, and increased mortality (4). There is no way for a fetus to compensate for deficient maternal iodine intake, and thus hypothyroidism can result. Severe maternal iodine deficiency is a well-known cause of congenital hypothyroidism (CH) (5), but less is known about the risk of CH in women who are mildly or moderately iodine-deficient (5).
It has also been shown that excessive maternal iodine intake during pregnancy can inhibit fetal thyroid hormone synthesis (6–9). This is known as the Wolff–Chaikoff effect, which prevents iodine-induced hyperthyroidism by temporarily blocking thyroid hormone production. This effect is transient in adults and children, but it persists until 36 weeks of gestation in fetuses (10). Thus, thyroid hormone production does not resume until near term. Excessive iodine intake may come from dietary supplements, topical antiseptics, and some medications, as well as iodine-rich foods such as seaweed (8). Thus, both inadequate and excessive iodine exposure in utero may increase the risk of CH in the newborn.
We conducted a nested case–control study in a large cohort of newborns to determine whether in utero iodine exposure is related to CH risk in a US population.
Methods
The California Newborn Screening Program (NBS) screens all live-born infants in California for CH. Details of the screening procedure have been published previously (11). Blood from each infant is spotted onto a piece of filter paper, typically between 12 and 48 h after birth, and used to measure concentrations of thyroid-stimulating hormone (TSH) using a fluorescent immunoassay followed by HPLC. Abnormal results (defined as a TSH concentration >25 μIU/mL) are reported to the newborn's primary care provider, with the recommendation that TSH and thyroxine (T4) should be measured for confirmation by the provider (12).
We identified all CH cases recognized by the screening program between January 2010 and March 14, 2015, excluding stillbirths and infants who were confirmed as transient CH cases by physicians. Approximately 2.5 million live births occurred in California during this time period. As controls, we identified infants who had normal TSH screening results, excluding stillbirths and infants with any California NBS registry disorder. Cases and controls were matched by birth month and year, such that for each case there was a control that was born in the same month of the same year.
Demographic and medical information regarding the pregnancies for cases and controls was obtained from vital statistics and screening records. To ensure protection of patient privacy and confidentiality, data were de-identified, and personally identifiable information was not included.
This study was approved by the California Department of Public Health Institutional Review Board (IRB) 17–02-2899 and exempted from IRB approval by the Human Subjects Research Protection Office NIH OHSRP 17-NICHD-00050.
Laboratory methods
We measured iodine concentrations from the residual blood left on filter paper spots after newborn screening, which included TSH testing. Iodine assays were performed using HPLC tandem MS as previously described (13). Briefly, a full-circle 16-mm-diameter dried blood spot disc that we assumed based on the literature contains 50 μL of whole blood was cut into small pieces and placed in a 15-mL polypropylene tube. HPLC-grade reagent water (0.5 mL) and Cl18O4− (50 ng/mL; internal standard) and 1.5 mL methanol were added and kept at room temperature for 10 min. Then, acetic acid (5%, 40 μL), ascorbic acid (2.5 mg/mL, 20 μL), and tetramethylammonium hydroxide (2.5%, 100 μL) were added in sequence and mechanically shaken for 20 min in an ultrasonic bath (1 h, 30°C). The extract was then loaded onto an Oasis WAX SPE cartridge (60 mg, 3 cc) that was conditioned with 0.1% formic acid (3 mL) and methanol (3 mL). After loading the sample, the cartridge was dried for 1 min under vacuum and then eluted with 3 mL of ammonium hydroxide [0.3% NH3 basis, dissolved in acetonitrile and water mixture (4:1)] and 3 mL of methanol. The extracts (6 mL) were then evaporated to 250 μL under a gentle nitrogen stream at 37°C, transferred into nylon microcentrifuge filters (0.2 μm, 2 mL), and centrifuged at 13,000 × g for 3 min. The extracts were analyzed by HPLC (Agilent 1100 series HPLC; Agilent Technologies) coupled with electrospray triple quadrupole MS (API 2000; Applied Biosystems). The chromatographic separation was accomplished by an IonPac AS-21 column (250 mm × 2 mm; Dionex) that was serially connected to a guard filter (2 mm; Agilent Technologies). The mobile phase consisted of 40 mM methylamine in reagent water (pH adjusted to 10 with 0.1% acetic acid) that was eluted isocratically at a flow rate of 300 μL/min at 35°C for a total run time of 20 min. A total of 20 μL of the final extract was injected. Electrospray negative ionization and multiple-reaction monitoring modes were used for the identification and quantification of iodide (127 [127I−] > 127 [127I−]) and 18O-labeled perchlorate (107 [Cl18O4−] > 89 [Cl18O3−]). The limit of detection was 0.05 ng/mL. Iodine measurements from filter paper in this laboratory have been shown to be significantly correlated with whole-blood samples (13).
Statistical methods
A total of 124 infants (66 cases and 58 controls) had blood iodine concentrations that were below the limit of detection of the instrumentation used for measurement. Their iodine concentrations were recorded as 0.0354 ng/mL, a value calculated by dividing the limit of detection by the square root of 2.
Descriptive analysis comparing sociodemographic variables by CH status was conducted using chi-square tests or Fisher's exact tests. Because the iodine distribution was skewed, appropriate nonparametric tests were used for non-normal distributions and results were presented as medians, IQRs, and percentiles. Wilcoxon's signed-rank tests were used to compare the median blood iodine concentrations between cases and controls, stratified by pregnancy characteristics and sociodemographic characteristics. We compared the overall distribution of iodine levels graphically between the cases and controls, as well as the top 10%, 5%, and 1%. The Kolmogorov–Smirnov test was used to compare the distribution of blood iodine concentrations between cases and controls. To investigate a possible nonlinear relation between iodine and CH risk, we also examined the data by quartiles of iodine concentration. P values ≤0.05 were considered significant.
Iodine status was compared between cases and controls among infants treated in a neonatal intensive care unit (NICU) using the Wilcoxon's signed-rank test. The same test was used to compare blood iodine concentrations between NICU cases and non-NICU controls, between NICU controls and non-NICU controls, between NICU cases and non-NICU cases, and between non-NICU cases and non-NICU controls. P values ≤0.05 were considered significant.
Finally, logistic regression analyses were conducted to assess the association between CH status and blood iodine concentrations. The regression models were adjusted for an a priori defined set of covariates, namely race/ethnicity, sex, gestational age, nursery type, birth weight, age at screening, and mother's age. We conducted multiple different regression analyses examining the impact of extreme high and low values of iodine on CH risk.
All analyses described previously were conducted using R statistical software, version 3.6.1 (R Foundation for Statistical Computing).
Results
We identified 911 infants with CH and 909 controls, for a total of 1820 samples. Four of the samples from infants with CH were missing identifying information and thus had to be excluded from the data set. For 13 infant samples (10 cases and 3 controls), blood iodine concentrations could not be determined, but these individuals were kept in the data set for descriptive analyses and counted as missing for iodine comparisons. The final study population included 907 cases and 909 controls, for a total sample size of 1816.
The cases and the controls are compared in Table 1. Infants with CH were significantly more likely to be female, Hispanic, and post-term compared with controls, and they were significantly more likely to have been treated in an NICU. They also had significantly higher TSH concentrations, which is to be expected because TSH levels are used to determine whether an infant has CH.
TABLE 1.
Sociodemographic and pregnancy data by CH case–control status1
| Cases | Controls | P value | |
|---|---|---|---|
| Race/ethnicity | 5.0 × 10−4 | ||
| Asian | 106 (11.7) | 101 (11.1) | |
| Black | 18 (1.99) | 64 (7.04) | |
| Hispanic | 592 (65.3) | 492 (54.1) | |
| Non-Hispanic white | 169 (18.6) | 230 (25.3) | |
| Other | 22 (2.43) | 22 (2.42) | |
| Nursery type | 0.003 | ||
| Regular nursery | 748 (82.5) | 803 (88.3) | |
| NICU | 114 (12.6) | 69 (7.59) | |
| Other | 30 (3.31) | 25 (2.75) | |
| Home | 1 (0.110) | 0 (0.00) | |
| Not provided | 14 (1.54) | 12(1.32) | |
| Sex | 1.2 × 10−14 | ||
| Female | 595 (65.6) | 434 (47.8) | |
| Male | 308 (34.0) | 473 (52.0) | |
| Not provided | 4 (0.441) | 2 (0.220) | |
| Birth weight, g | 0.040 | ||
| <1500 | 11 (1.21) | 8 (0.880) | |
| 1500–2499 | 69 (7.61) | 42 (4.62) | |
| 2500–3000 | 141 (15.6) | 159 (17.5) | |
| >3000 | 686 (75.6) | 700 (77.0) | |
| Gestational age at birth | 0.043 | ||
| Preterm (<37 wk) | 72 (7.94) | 63 (6.93) | |
| Term (37–41 wk) | 813 (89.6) | 836 (92.0) | |
| Post-term (≥42 wk) | 17 (1.87) | 6 (0.660) | |
| Not provided | 5 (0.551) | 4 (0.440) | |
| Mother's age, y | 28.0 (23.0–33.0) | 29.0 (24.0–33.0) | 0.202 |
| Infant's age at screening, h | 27.0 (24.0–36.0) | 26.0 (24.0–36.0) | 0.816 |
| Blood TSH2, μIU/mL | 140 (57.7–235) | 4.48 (2.87–6.57) | <2.2 × 10−16 |
Values are median (IQR) or frequency (%). CH, congenital hypothyroidism; NICU, neonatal intensive care unit; TSH, thyroid-stimulating hormone.
From newborn screening results.
The median iodine concentration was 20.0 ng/mL in cases (IQR: 12.1–29.8 ng/mL) and 20.3 ng/mL in controls (IQR: 12.5–30.9 ng/mL). There was no significant difference between iodine concentrations in cases and controls (P = 0.59). In addition, the distribution of iodine concentrations did not differ significantly between cases and controls (Figure 1). We postulated that hypothyroidism caused by insufficient or excess iodine exposure in utero would be seen as an excess of cases at the lower or upper tail of the distribution, respectively. When we examined the distribution's tails, the top and bottom 1% and 5% of blood iodine concentrations were not significantly different between cases and controls. The lower and higher extremes of the iodine distribution are shown in Figure 2 and Table 2. There was no significant relation between CH status and iodine quartile (P = 0.83).
FIGURE 1.
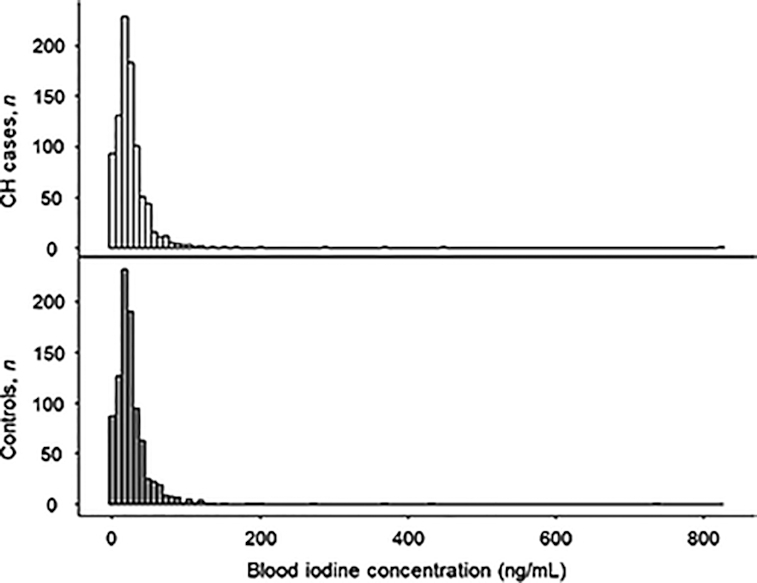
Distributions of blood iodine concentration in infants with and without CH. No significant difference in distribution was found between cases and controls (P = 0.96). CH, congenital hypothyroidism.
FIGURE 2.
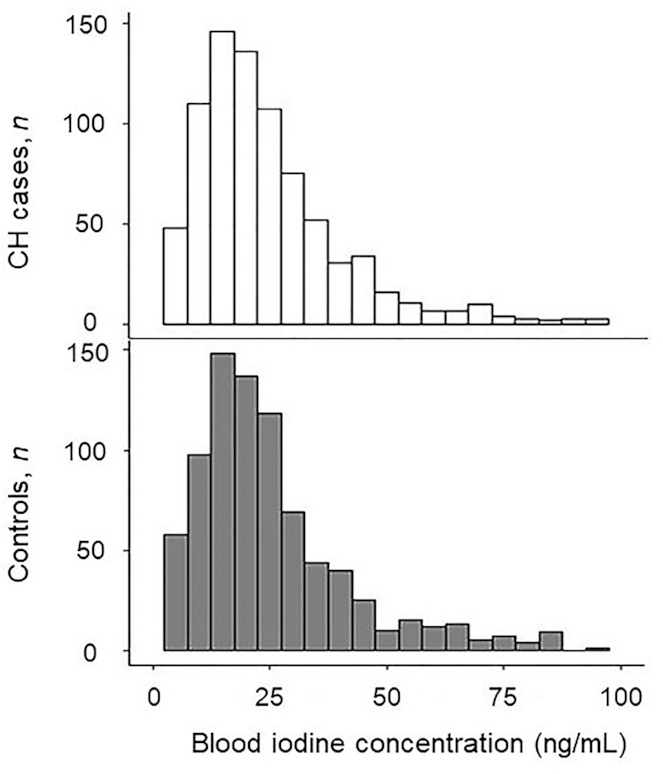
Lower extremes of the iodine distributions in infants with and without CH. The bottom 10%, 6.8%, and 1% of blood iodine concentrations do not differ significantly between cases and controls. CH, congenital hypothyroidism.
TABLE 2.
Distribution of CH cases and controls with the highest (top 0.44%) blood iodine concentrations1
| Blood iodine, ng/mL | Cases, n | Controls, n |
|---|---|---|
| 200–299 | 1 | 1 |
| 300–399 | 1 | 1 |
| 400–499 | 1 | 1 |
| 500–599 | 0 | 0 |
| 600–699 | 0 | 0 |
| 700–799 | 0 | 1 |
| ≥800 | 1 | 0 |
CH, congenital hypothyroidism.
Blood iodine concentrations in cases and controls, stratified by sociodemographic and pregnancy-related variables, are shown in Table 3. It is noteworthy that among cases and controls treated in the NICU, cases had significantly higher iodine concentrations compared with controls (Figure 3). This result suggests that iodine exposure in the NICU may have caused hypothyroidism at the time samples were collected. NICU cases had borderline (P = 0.077) significantly higher iodine compared to non-NICU controls, despite the fact that the non-NICU controls had significantly longer gestations (38 wk compared with 39 wk median gestations in cases and controls, respectively; P < 0.001) and, therefore, more time to obtain iodine from their mothers. In addition, NICU controls, as expected, had lower blood iodine concentrations compared with non-NICU controls, although the difference did not quite reach statistical significance (P = 0.059). Blood iodine concentrations in NICU cases were also significantly higher than blood iodine concentrations in non-NICU cases (P = 0.012). It is noteworthy that NICU cases and controls did not differ in length of gestation (P = 0.24). Furthermore, elevated TSH values due to illness would be unlikely to differ between NICU cases and NICU controls. Infants treated in an NICU were significantly older at the time of screening (median age: 50 h) compared with infants not treated in an NICU (median age: 26 h; P < 0.001).
TABLE 3.
Blood iodine concentrations in CH cases and controls stratified by pregnancy and sociodemographic characteristics1
| Cases, ng/mL | Controls, ng/mL | P value | |
|---|---|---|---|
| Race/ethnicity | |||
| Asian | 20.1 (10.7–29.9) | 23.7 (13.0–32.9) | 0.08 |
| Black | 28.3 (19.2–36.8) | 21.3 (11.9–31.2) | 0.17 |
| Hispanic | 19.9 (12.6–28.8) | 19.8 (12.8–29.4) | 0.80 |
| Non-Hispanic white | 20.8 (11.2–33.4) | 20.1 (12.4–32.0) | 0.83 |
| Other | 19.4 (12.9–24.2) | 23.1 (10.5–37.2) | 0.42 |
| Nursery type2 | |||
| Regular nursery | 19.4 (11.8–28.8) | 20.6 (12.8–31.1) | 0.15 |
| NICU | 22.7 (16.4–32.1) | 17.3 (8.30–26.6) | 0.01 |
| Other | 20.8 (12.4–31.9) | 22.1 (15.9–28.1) | 0.84 |
| Home | 29.9 (29.9–29.9) | NA3 | NA3 |
| Sex2 | |||
| Female | 19.2 (12.0–28.6) | 20.0 (12.4–29.0) | 0.63 |
| Male | 21.6 (12.2–33.2) | 20.6 (12.8–32.7) | 0.71 |
| Birth weight, g | |||
| <1500 | 29.9 (22.0–37.3) | 48.0 (24.5–95.8) | 0.40 |
| 1500–2499 | 16.6 (10.7–28.5) | 15.7 (8.25–26.1) | 0.34 |
| 2500–3000 | 20.8 (11.7–29.8) | 20.6 (13.1–32.3) | 0.59 |
| >3000 | 19.9 (12.1–29.5) | 20.4 (12.6–30.6) | 0.69 |
| Gestational age at birth2 | |||
| Preterm (<37 wk) | 19.9 (13.3–33.4) | 17.3 (8.50–28.8) | 0.30 |
| Term (37–41 wk) | 19.9 (11.9–29.0) | 20.5 (12.7–31.0) | 0.28 |
| Post-term (≥42 wk) | 27.6 (17.2–31.5) | 21.6 (11.7–29.1) | 0.60 |
Values are median (IQR). CH, congenital hypothyroidism; NA, not applicable; NICU, neonatal intensive care unit.
Infants with unavailable data were excluded from analysis.
There were no home births in the control group.
FIGURE 3.
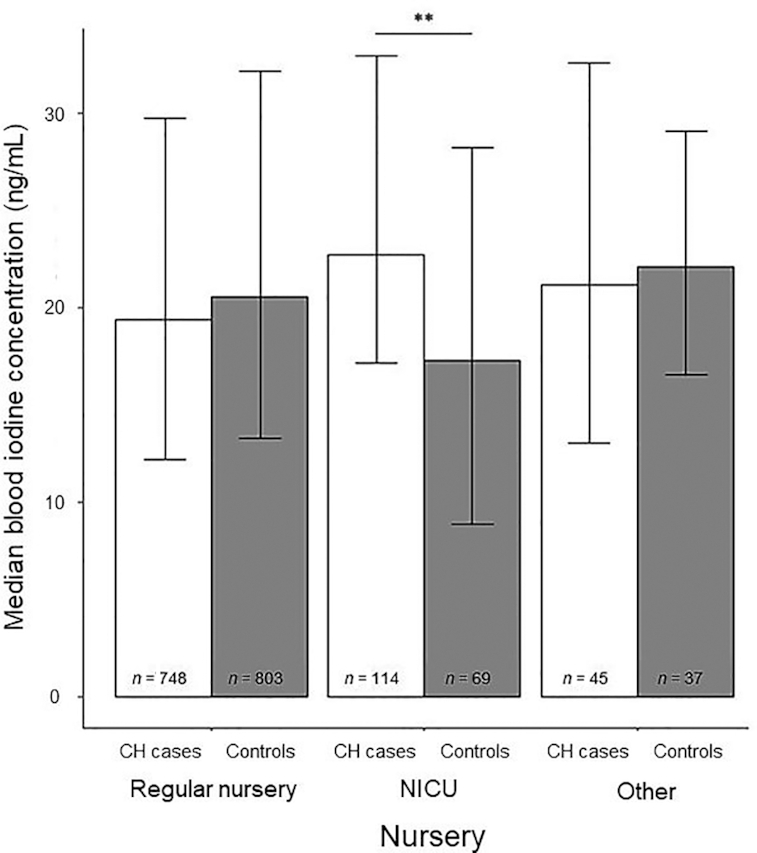
Median blood iodine concentrations in infants with and without CH by nursery type. Error bars represent IQR. **Case and control means differ, P = 0.01. CH, congenital hypothyroidism; NICU, neonatal intensive care unit.
Results from logistic regression (Table 4) showed that having blood iodine concentrations at the tails of the distribution did not significantly increase risk for CH. This remained true when adjusting for race/ethnicity, sex, gestational age, nursery type, age at screening, mother's age, and birth weight.
TABLE 4.
OR of being a CH case at the extremes of the blood iodine distribution1
| Extreme iodine values2 | OR (95% CI) | Adjusted OR (95% CI)3 |
|---|---|---|
| Lowest 6.8% (0–0.0354 ng/mL, n = 124) | 1.16 (0.81, 1.68) | 1.38 (0.89, 2.16) |
| Lowest 10% (0–4.10 ng/mL, n = 182) | 1.09 (0.80, 1.48) | 1.24 (0.86, 1.81) |
| Lowest 25% (0–12.3 ng/mL, n = 455) | 1.08 (0.87, 1.34) | 1.10 (0.85, 1.42) |
| Highest 25% (30.3–820 ng/mL, n = 452) | 0.92 (0.74, 1.14) | 1.04 (0.81, 1.34) |
| Highest 10% (47.1–820 ng/mL, n = 181) | 0.84 (0.62, 1.14) | 0.94 (0.65, 1.36) |
| Highest 5% (66.0–820 ng/mL, n = 91) | 0.75 (0.49, 1.14) | 0.82 (0.49, 1.34) |
| Highest 3% (80.4–820 ng/mL, n = 55) | 0.67 (0.38, 1.14) | 0.82 (0.43, 1.56) |
CH, congenital hypothyroidism; NICU, neonatal intensive care unit.
Percentiles <6.8 could not be calculated due to the presence of samples below the limit of detection.
Adjusted for race/ethnicity (Asian, Black, Hispanic, Non-Hispanic white, other), sex (male, female), nursery type (regular nursery, NICU, other), birthweight (<1500 g, 1500–2499 g, 2500–3000 g, >3000 g), gestational age, age at screening, and mother's age. Infants with unavailable data for sex or nursery were excluded from analysis.
Discussion
We found that CH case infants did not differ significantly from control infants in measured blood iodine concentrations soon after birth. Neither was there any evidence that CH cases were more likely to have very low or very high blood iodine concentrations.
In the United States, in which maternal iodine deficiency is common during pregnancy (2), it is reassuring that newborns with CH were not more likely than controls to have low blood iodine concentrations. It is also reassuring that exposure to high concentrations of iodine was not associated with CH in our study.
Interestingly, we found that within the group of infants treated in the NICU, CH cases had significantly higher blood iodine concentrations compared with controls. Infants may be exposed to high levels of iodine in the NICU in the form of topical antiseptics and imaging contrast agents, which can lead to the development of hypothyroidism (14–17). Thus, it is likely that some of the NICU infants in our study were exposed to excess iodine, although we cannot be certain because data on exposures were not available. There is likely to be variation in the amount of iodine to which infants are exposed in the NICU based on whether they had surgery or were exposed to iodine-containing antiseptics or contrast media, and infants who are exposed to higher levels of iodine would be more likely to develop hypothyroidism in response. This may explain why we found significantly higher blood iodine concentrations in cases than controls in infants admitted to the NICU, and in NICU cases compared with non-NICU cases, whereas there was no such trend in the non-NICU population. Moreover, comparing NICU cases with NICU controls is a good way of controlling for other risk factors that could lead to NICU admission. Blood iodine concentrations increased with gestational age, but despite the shorter gestations of the NICU groups, NICU cases had higher iodine compared with non-NICU controls, whereas NICU controls had the expected lower blood iodine concentrations compared with non-NICU controls.
Past studies have shown that it may take several days for an increase in TSH to occur after excess iodine exposure (16, 17). The median age at screening for NICU infants in our study was 50 h compared with 26 h for non-NICU infants, which might have been sufficient time for TSH to increase in response to high iodine exposure in the NICU. It is also possible that fetal problems could have led to iodine exposure earlier during a cesarean section and to subsequent NICU admission. Finally, pre- or postnatal iodine exposure might have blocked or diminished the decline in TSH that normally occurs after birth (10).
Ours is the first report of an effect of NICU treatment on risk for hypothyroidism in a large, unselected population. This effect may be mediated by iodine, as suggested by our results, or by another unknown factor. If it is iodine that is causing this increased risk, it may be necessary to re-examine nursery protocols and consider other options for topical antiseptics. The British National Formulary for Children suggests that topical iodine antiseptics should not be used in newborns with gestational ages <32 wk or birth weights <1500 g (16). Although the effect of excess iodine in the NICU may be transient, it can still lead to diagnosis of CH and potentially unnecessary medical interventions in these infants.
The effects of inadequate iodine supply to the fetus are well known and can be devastating. Thus, it is important to document that iodine is most likely not contributing to CH in the general birth population. It is worth noting that there are many causes of CH in addition to iodine deficiency. In another study performed in a population of California infants, it was found that 32% of CH cases were due to agenesis of the thyroid, 25% resulted from ectopic thyroid, and 43% had a normal-appearing thyroid, indicating that in these cases there may have been a problem with the biochemical pathways involved in thyroid hormone synthesis (18) or lack of iodine. In our population, some cases almost certainly resulted from agenesis of the thyroid gland, a condition unrelated to iodine. In others, genetic variants may have caused complete blockage of the pathway that produces thyroid hormones. These cases too are unlikely to be influenced by iodine. On the other hand, partial enzyme defects may be ameliorated or worsened depending on iodine status. Similarly, some dysplastic thyroid gland problems may be worsened by lack of iodine (19).
CH cases included disproportionately large numbers of Hispanic and female infants compared with controls, which is consistent with results of previous studies (20). There was a higher proportion of cases in the lower birth weight categories than controls. Previous studies have found that infants with CH are more likely to have low birth weights compared with infants without CH, but there is also evidence that CH increases risk for abnormally high birth weight, which our results did not demonstrate (20).
Our study has some notable strengths. We evaluated what we believe is the largest number of cases including virtually all cases within a geographically defined population of ∼2.5 million births. The size of our study population gave us excellent statistical power and allowed us to identify small differences that might not have been detectable in a smaller study population. In addition, we acquired our iodine measurements from dried blood spots, and studies have suggested that blood iodine may be a better marker than urinary iodine (21–23).
Our study had limitations as well. Medical and demographic information was limited to protect confidentiality, including restriction of birth weight data to wide ranges rather than discrete values. We were unable to obtain long-term follow-up data or history of exposure to drugs or dietary factors that might have confirmed high iodine values. Therefore, we did not have access to data on whether cases had thyroid aplasia, ectopic glands, or eutopic glands. It is noteworthy that even if ∼30–40% of cases had aplasia (18, 24–26), we had at least 560 cases that were potentially iodine-related, more than any other study. As in all iodine studies, the iodine data had to be analyzed in the aggregate rather than at the individual level because single measures are not sufficient to determine iodine status in an individual due to their high temporal variability (27). In addition, because whole-blood iodine is a new and less common method of measuring iodine, there are no external standards to which to compare our findings, so it is unclear whether the 6 ng/mL difference we found in iodine concentration between cases and controls in the NICU is clinically significant. Although we excluded cases determined by the NBS to be transient, cases due to high iodine exposure frequently resolve when the iodine exposure is removed. However, these cases persisted long enough to require medical intervention, and to our knowledge, no studies have examined the effect of Wolff–Chaikoff-induced hypothyroidism on neurodevelopment.
In conclusion, our study did not find a significant difference in blood iodine concentrations between newborns with CH and those without CH except in those treated in the NICU, which indicates that deficient or excessive iodine consumption during pregnancy is not an important factor in CH in this large and diverse study population. This is a reassuring result given that iodine deficiency is on the rise among American women of childbearing age (2). We confirmed previous studies showing that Hispanic and female infants are at increased risk for CH. In addition, we expanded on previous reports suggesting that infants treated in an NICU may be at greater risk for CH, showing for the first time that CH cases in the NICU had significantly higher blood iodine concentrations compared with controls. Our results provide some assurance that neither iodine deficiency nor excess during pregnancy is an important contributor to congenital hypothyroidism in this large US population and emphasize the need to avoid unnecessary iodine exposure whenever possible in intensive care settings.
Acknowledgments
The authors’ responsibilities were as follows—JLM: designed the research; JLM, KK, CG, and RS: conducted the research and provided essential materials; ECR and RS: analyzed the data; JLM: wrote the paper; GMS: reviewed the manuscript for important content; JLM: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This study was funded by the Intramural Research Program, National Institute of Child Health and Human Development, NIH (contract HHSN2752016000011) to Wadsworth Center, New York State Department of Health for the analysis of samples.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: CH, congenital hypothyroidism; NBS, newborn screening; NICU, neonatal intensive care unit; TSH, thyroid-stimulating hormone; T4, thyroxine.
Contributor Information
James L Mills, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Elijah C Reische, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Kurunthachalam Kannan, Wadsworth Center, New York State Department of Health, Albany, NY, USA.
Chongjing Gao, Wadsworth Center, New York State Department of Health, Albany, NY, USA.
Gary M Shaw, Department of Pediatrics, Stanford University, Stanford, CA, USA.
Rajeshwari Sundaram, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
References
- 1. Trueba SS, Augé J, Mattei G, Etchevers H, Martinovic J, Czernichow P, Vekemans M, Polak M, Attié-Bitach T. PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. J Clin Endocrinol Metab. 2005;90(1):455–62. [DOI] [PubMed] [Google Scholar]
- 2. Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid. 2011;21(4):419–27. [DOI] [PubMed] [Google Scholar]
- 3. Iodine Global Network. Global scorecard of iodine nutrition in 2017 in the general population and in pregnant women (PW). [Internet]. 2017; [cited 2019 Nov 7]. Available from: https://www.ign.org/cm_data/IGN_Global_Scorecard_AllPop_and_PW_May2017.pdf. [Google Scholar]
- 4. Zimmermann MB. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol. 2012;26(suppl 1):108–17. [DOI] [PubMed] [Google Scholar]
- 5. Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB. Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr. 2016;104(suppl 3):918S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connelly KJ, Boston BA, Pearce EN, Sesser D, Snyder D, Braverman LE, Pino S, LaFranchi SH. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161(4):760–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee SY, Pearce EN. Reproductive endocrinology: iodine intake in pregnancy—even a little excess is too much. Nat Rev Endocrinol. 2015;11(5):260–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10(3):136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas Jd V, Collett-Solberg PF. Perinatal goiter with increased iodine uptake and hypothyroidism due to excess maternal iodine ingestion. Horm Res. 2009;72(6):344–7. [DOI] [PubMed] [Google Scholar]
- 10. Polak M. Human fetal thyroid function. Endocr Dev. 2014;26:17–25. [DOI] [PubMed] [Google Scholar]
- 11. Feuchtbaum L, Carter J, Dowray S, Currier RJ, Lorey F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet Med. 2012;14(11):937–45. [DOI] [PubMed] [Google Scholar]
- 12. Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, Varma SK. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117(6):2290–303. [DOI] [PubMed] [Google Scholar]
- 13. Kim UJ, Kannan K. Method for the determination of iodide in dried blood spots from newborns by high performance liquid chromatography tandem mass spectrometry. Anal Chem. 2018;90(5):3291–8. [DOI] [PubMed] [Google Scholar]
- 14. Linder N, Davidovitch N, Reichman B, Kuint J, Lubin D, Meyerovitch J, Sela BA, Dolfin Z, Sack J. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J Pediatr. 1997;131(3):434–9. [DOI] [PubMed] [Google Scholar]
- 15. Thaker VV, Leung AM, Braverman LE, Brown RS, Levine B. Iodine-induced hypothyroidism in full-term infants with congenital heart disease: more common than currently appreciated?. J Clin Endocrinol Metab. 2014;99(10):3521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aitken J, Williams FL. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Arch Dis Child Fetal Neonatal Ed. 2014;99(1):F21–8. [DOI] [PubMed] [Google Scholar]
- 17. Smerdely P, Lim A, Boyages SC, Waite K, Wu D, Roberts V, Leslie G, Arnold J, John E, Eastman CJ. Topical iodine-containing antiseptics and neonatal hypothyroidism in very-low-birthweight infants. Lancet. 1989;334(8664):661–4. [DOI] [PubMed] [Google Scholar]
- 18. Schoen EJ, Clapp W, To TT, Fireman BH. The key role of newborn thyroid scintigraphy with isotopic iodide (123I) in defining and managing congenital hypothyroidism. Pediatrics. 2004;114(6):e683. [DOI] [PubMed] [Google Scholar]
- 19. Kwak MJ. Clinical genetics of defects in thyroid hormone synthesis. Ann Pediatr Endocrinol Metab. 2018;23(4):169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waller DK, Anderson JL, Lorey F, Cunningham GC. Risk factors for congenital hypothyroidism: an investigation of infant's birth weight, ethnicity, and gender in California, 1990–1998. Teratology. 2000;62(1):36–41. [DOI] [PubMed] [Google Scholar]
- 21. Yu S, Yin Y, Cheng Q, Han J, Cheng X, Guo Y, Sun D, Xie S, Qiu L. Validation of a simple inductively coupled plasma mass spectrometry method for detecting urine and serum iodine and evaluation of iodine status of pregnant women in Beijing. Scand J Clin Lab Invest. 2018;78(6):501–7. [DOI] [PubMed] [Google Scholar]
- 22. Michalke B, Witte H. Characterization of a rapid and reliable method for iodide biomonitoring in serum and urine based on ion chromatography–ICP–mass spectrometry. J Trace Elem Med Biol. 2015;29:63–68. [DOI] [PubMed] [Google Scholar]
- 23. Michalke B, Schramel P, Hasse S. Separation of free iodide from other I-species in human serum: quantification in serum pools and individual samples. Anal Bioanal Chem. 1996;354(5–6):576–9. [DOI] [PubMed] [Google Scholar]
- 24. Connelly JF, Coakley JC, Gold H, Francis I, Mathur KS, Rickards AL, Price GJ, Halliday JL, Wolfe R. Newborn screening for congenital hypothyroidism, Victoria, Australia, 1977–1997. Part 1: the screening programme, demography, baseline perinatal data and diagnostic classification. J Pediatr Endocrinol Metab. 2001;14(9):1597–610. [DOI] [PubMed] [Google Scholar]
- 25. Grant DB, Smith I, Fuggle PW, Tokar S, Chapple J. Congenital hypothyroidism detected by neonatal screening: relationship between biochemical severity and early clinical features. Arch Dis Child. 1992;67(1):87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Law WY, Bradley DM, Lazarus JH, John R, Gregory JW. Congenital hypothyroidism in Wales (1982–1993): demographic features, clinical presentation and effects on early neurodevelopment. Clin Endocrinol. 1998;48(2):201–7. [DOI] [PubMed] [Google Scholar]
- 27. Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53(5):401–7. [DOI] [PubMed] [Google Scholar]


