Abstract
Aims
To assess insulin therapy, macronutrient intake and glycaemia in professional cyclists with type 1 diabetes (T1D) over a 5‐day Union Cycliste Internationale road‐cycle race.
Material and methods
In this prospective observational study, seven professional cyclists with T1D (age 28 ± 4 years, body mass index 20.9 ± 0.9 kg/m2, glycated haemoglobin concentration 56 ± 7 mmol/mol [7.3% ± 0.6%]) were monitored during a five‐stage professional road cycling race. Real‐time continuous glucose monitoring (rtCGM) data, smart insulin pen dose data and macronutrient intake were assessed by means of repeated‐measure one‐way ANOVA and post hoc testing. Associations between exercise physiological markers and rtCGM data, insulin doses and macronutrient intake were assessed via linear regression modelling (P ≤ 0.05).
Results
Bolus insulin dose was significantly reduced over the 5‐day period (P = 0.03), while carbohydrate intake (P = 0.24) and basal insulin doses remained unchanged (P = 0.64). A higher mean previous‐day race intensity was associated with a lower mean sensor glucose level (P = 0.03), less time above range level 2 (>13.9 mmol/L [250 mg/dL]; P = 0.05) and lower doses of bolus insulin (P = 0.04) on the subsequent day. No significant associations were found for any other glycaemic range and glycaemic variability (P > 0.05).
Conclusions
This is the first study to demonstrate the influence of previous‐day race intensity on subsequent bolus insulin dose requirements in professional cyclists with T1D. These data may help inform therapeutic strategies to ensure safe exercise performance.
1. INTRODUCTION
The positive effects of physical activity and exercise have been described in healthy 1 and chronically ill people, 2 detailing improvements in cardiac and pulmonary diseases, cancer, depression and metabolic‐related disorders. 2 In people with type 1 diabetes (T1D), 3 , 4 the benefits of regular physical activity and exercise on glucose metabolism, therapy management and comorbidities have led to the inclusion of regular exercise in the treatment plan of diabetes. 5 Nevertheless, only approximately 45% of all individuals with T1D achieve current physical activity and exercise recommendations, 6 , 7 the main reason for which is fear of losing glycaemic control. 8 In recreationally active people with T1D, insulin therapy 9 , 10 , 11 as well as carbohydrate intake 12 , 13 must be tightly managed to minimize fluctuations in glucose both below 14 and above 15 the target range. Furthermore, the risk of exercise‐related dysglycaemia persists for many hours subsequent to its performance as a result of relative hyper‐ or hypoinsulinaemia, accompanied by increased levels of insulin‐independent glucose transporter type 4 (GLUT‐4). 5 Therefore, it is recommended that individuals with T1D make adaptations to their basal insulin dose/rate 16 , 17 and/or consume a bedtime carbohydrate‐rich snack without bolus insulin administration after late afternoon exercise sessions. 18
The majority of research pertinent to T1D and exercise has been conducted in recreationally active people, 19 whilst limited evidence exists in athletes. 20 Notably, the physiological characteristics of professional athletes clearly differ from those of recreationally active people, which is specifically observed in cardiopulmonary 21 and metabolic responses 22 to exercise. A recent report assessing real‐time continuous glucose monitoring (rtCGM) sensor glucose responses in a group of professional cyclists with T1D, evidenced a progressive increase in the amount of time spent in hypoglycaemia over 7 days of road‐cycle racing. 23 However, comprehensive assessments of bolus and basal insulin doses and macronutrient intake and their associations with glycaemia over the entire course of a multiple‐stage cycle race are lacking. The aim of the present study, therefore, was to characterize insulin therapy, macronutrient intake and glycaemia in professional cyclists with T1D over a 5‐day Union Cycliste Internationale (UCI) road‐cycle race.
2. MATERIALS AND METHODS
This study was a prospective observational analysis of data collated over 5 days of road‐cycle racing. The study protocol was registered at the German Clinical Trials Register (DRKS.de; DRKS00019928). The study protocol was approved by the College of Engineering Ethics Panel, Research Governance Swansea University, UK (2019–032), and performed in line with Good Clinical Practice guidelines and the Declaration of Helsinki. All participants gave their written informed consent in advance of any trial‐related activities.
2.1. Eligibility criteria
Participants met the following inclusion criteria: diagnosis of T1D ≥12 months ago; age 18 to 65 years; and use of multiple daily injections (MDI) of insulin for 12 months or more as standard therapy. The main exclusion criteria were: presence of a life‐threatening disease; proliferative retinopathy or maculopathy; severe neuropathy; recurrent severe hypoglycaemia (more than one severe hypoglycaemic event during the previous 12 months); hypoglycaemia unawareness as judged by the investigator; hospitalization for diabetic ketoacidosis during the previous 6 months; and any other condition that would interfere with trial participation or evaluation of results, as judged by the investigator.
2.2. Preparation in advance of the UCI tour
Prior to the racing season, participants performed a maximum cardiopulmonary exercise (CPX) test for the assessment of the aerobic, anaerobic and maximum exercise capacity, conducted by the research team and the Team Novo Nordisk staff. 24 These CPX results were used to describe the individual race intensity over the five stages of the tour. The athletes were using an rtCGM device (Dexcom G6; Dexcom Inc., San Diego, California) as their standard glucose measurement device prior to enrolment in the study. The athletes wore the glucose sensor at the back of the upper arm or at the hip. One case was reported in which the sensor was changed during the 5‐day period. One day prior to the start of the race, the cyclists received smart insulin pens (InPen; Companion Medical, San Diego, California) to assess their insulin doses. 25 Insulin doses were downloaded from the smart insulin pens via a mobile phone (Bluetooth), and the type, amount, and time point of insulin dosing was recorded. Priming doses of insulin were automatically detected by the software, however, this was also evaluated by the research team. On an individual basis, some athletes might have been tapering and/or carbohydrate loading prior the race period; however, this was not assessed in detail for the purpose of this study.
2.3. Five‐day race period
From the start of the first race until the end of the last race, rtCGM data, bolus and basal insulin doses and macronutrient intake were collected. rtCGM data and insulin doses were downloaded from the receiver device/smart insulin pen after the last race. Mealtime macronutrient intake outside of the in‐race phase was recorded by the research team. Riders also provided descriptive and photographic accounts of each meal, alongside information on any additional snacks taken outside of set eating times. In‐race macronutrient intake was calculated according to the difference in the quantity of pre‐race versus post‐race food/drink package, with subsequent verification from riders. Additional macronutrient intake received from the Team Novo Nordisk support car was recorded by the research team.
2.4. Statistical analyses
Data were analysed for normal distribution using a Shapiro–Wilk test and are shown as mean ± SD or median (interquartile range) if applicable. Glycaemia between the 2 days prior to the tour (lower amount of training) versus the 5‐day race period was assessed by paired t‐test or Wilcoxon test. Glycaemia, insulin doses and macronutrient intake were analysed via repeated measures one‐way ANOVA or Friedman test with Holm–Sidak's or Dunn's multiple comparison test over the 5 days of the race. Associations for glycaemia were evaluated by means of linear regression modelling, with log‐transformation if required (P ≤ 0.05). All analyses were conducted using an intention‐to‐treat approach. Sensor glucose data obtained from rtCGM were stratified for time below range level 2 (TBR 2; <3.0 mmol/L [<54 mg/dL]), time below range level 1 (TBR 1; 3.0–3.8 mmol/L [54–69 mg/dL]), time in range (TIR; 3.9–10.0 mmol/L [70–180 mg/dL]), time above range level 1 (TAR 1; >10.0–13.9 mmol/L [>180–250 mg/dL]) and time above range level 2 (TAR 2; >13.9 mmol/L [>250 mg/dL]). 26 Data were also stratified for daytime (6:00 am to midnight) and night‐time periods (12:01 am to 5:59 am). Glycaemic variability was calculated based on the coefficient of variation in sensor glucose data.
3. RESULTS
Seven professional cyclists with T1D with a mean ± SD age of 28 ± 4 years, body mass index of 20.9 ± 0.9 kg/m2, glycated haemoglobin concentration of 56 ± 7 mmol/mol (7.3% ± 0.6%), diabetes duration of 10 ± 6 years, who were using MDI and had peak oxygen uptake of 72 ± 5 mL/kg/min, were included in the study. Three athletes were using insulin detemir (Levemir; Novo Nordisk A/S, Bagsværd, Denmark), three were using insulin glargine U‐100 (Lantus; Sanofi, Paris, France) and one insulin glargine U‐300 (Toujeo; Sanofi) as basal insulin. Six athletes were using faster insulin aspart (Fiasp; Novo Nordisk A/S) and one was using insulin Aspart (Novo Rapid; Novo Nordisk A/S).
3.1. Pre‐race versus 5‐day race‐period glycaemia
When comparing the pre‐race (−2 days) versus the 5‐day race periods, significant differences were only found in the coefficient of variation in glycaemia (pre‐race 30% ± 5% vs. 5‐day race period 35% ± 9%; P = 0.03). No significant differences were observed for the mean sensor glucose levels (pre‐race 8.5 ± 2.0 mmol/L [154 ± 26 mg/dL] vs. 5‐day race period 7.9 ± 0.9 mmol/L [142 ± 16 mg/dL]; P = 0.12). When mean sensor glucose levels were stratified for time of day, a significant difference was found for the night‐time period (pre‐race 8.3 ± 1.4 mmol/L [150 ± 25 mg/dL] vs. 5‐day race period 7.2 ± 1.7 mmol/L [129 ± 31 mg/dL]; P = 0.04), while no significant difference was observed for the daytime period (pre‐race 8.7 ± 1.7 mmol/L [156 ± 31 mg/dL] vs. 5‐day race period 8.2 ± 0.8 mmol/L [147 ± 15 mg/dL]; P = 0.26). No significant differences were seen when overall glycaemic ranges were compared (P > 0.05; Figure 1).
FIGURE 1.
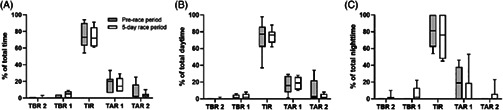
Comparison of glycaemic ranges for the pre‐race period vs. 5‐day race period for overall data (A) and stratified for daytime (B) and night‐time (C). TAR 1, time above range level 1; TAR 2, time above range level 2; TBR 1, time below range level 1; TBR, 2, time below range level 2; TIR, time in range. No significant differences were found for the comparisons (P > 0.05)
3.2. Glycaemia over the 5‐day race period
Over the course of the 5‐day race period, no significant differences were found for overall glycaemic variability (P = 0.75) or mean sensor glucose levels (P = 0.16). No significant differences were found when data were stratified for daytime (P = 0.14) and night‐time glycaemic viability (P = 0.09) or daytime (P = 0.33) and night‐time (P = 0.18) mean sensor glucose levels.
When data were assessed for glycaemic ranges, no significant differences were found for TBR 2, TBR 1, TIR, TAR 1 and TAR 2 over the course of the 5‐day race period, or when data were stratified for time of day (P > 0.05; Table 1).
TABLE 1.
Assessment of glycaemic ranges over the overall 5‐day race period, and stratified by daytime and night‐time period
| % of total time | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | P |
|---|---|---|---|---|---|---|
|
TBR 2 Overall |
0 (0–2) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–3) | 0.81 |
|
TBR 2 Day |
0 (0–2) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0.61 |
|
TBR 2 Night |
N/A a | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.71 |
|
TBR 1 Overall |
0 (0–9) | 0 (0–12) | 4 ± 3 | 0 (0–19) | 5 (0–8) | 0.72 |
|
TBR 1 Day |
0 (0–9) | 0 (0–3) | 0 (0–3) | 0 (0–19) | 0 (0–3) | 0.94 |
|
TBR 1 Night |
N/A* | 0 (0–29) | 0 (0–17) | 0 (0–0) | 0 (0–18) | 0.39 |
|
TIR Overall |
69 ± 15 | 72 ± 18 | 74 ± 17 | 74 ± 16 | 84 ± 14 | 0.67 |
|
TIR Day |
69 ± 15 | 72 ± 15 | 73 ± 16 | 80 ± 12 | 88 (66–94) | 0.75 |
|
TIR Night |
N/A a | 72 ± 30 | 77 (65–100) | 69 (32–100) | 82 ± 22 | 0.53 |
|
TAR 1 Overall |
16 ± 15 | 20 ± 15 | 19 ± 12 | 14 ± 10 | 9 (0–11) | 0.29 |
|
TAR 1 Day |
16 ± 15 | 23 ± 14 | 21 ± 10 | 14 ± 12 | 10 ± 11 | 0.32 |
|
TAR 1 Night |
N/A a | 14 (0–38) | 15 (0–14) | 16 (0–40) | 3 (0–0) | 0.86 |
|
TAR 2 Overall |
4 (0–23) | 0 (0–4) | 0 (0–7) | 0 (0–6) | 0 (0–0) | 0.09 |
|
TAR 2 Day |
4 (0–23) | 0 (0–3) | 0 (0–9) | 0 (0–0) | 0 (0–0) | 0.07 |
|
TAR 2 Night |
N/A a | 0 (0–0) | 0 (0–0) | 0 (0–22) | 0 (0–0) | 0.14 |
Abbreviations: N/A, not applicable; TAR 1/TAR 2, time above range level 1/level 2; TBR 1/TBR 2, time below range level 1/level 2; TIR, time in range.
Data are mean ± SD or median (interquartile range). Glycaemic ranges night‐time day 1 were not available since assessment of data started with the first day in‐ride period. Data analysis started with the in‐race period on day 1 until the end of the in‐race period on day 5.
3.3. In‐ride glycaemia
When comparing in‐ride mean glucose levels (P = 0.34) and glycaemic variability (P = 0.23) over the five stages, no significant differences were found. Overall, no significant differences were found for any of the glycaemic ranges (P > 0.05; Figure 2).
FIGURE 2.
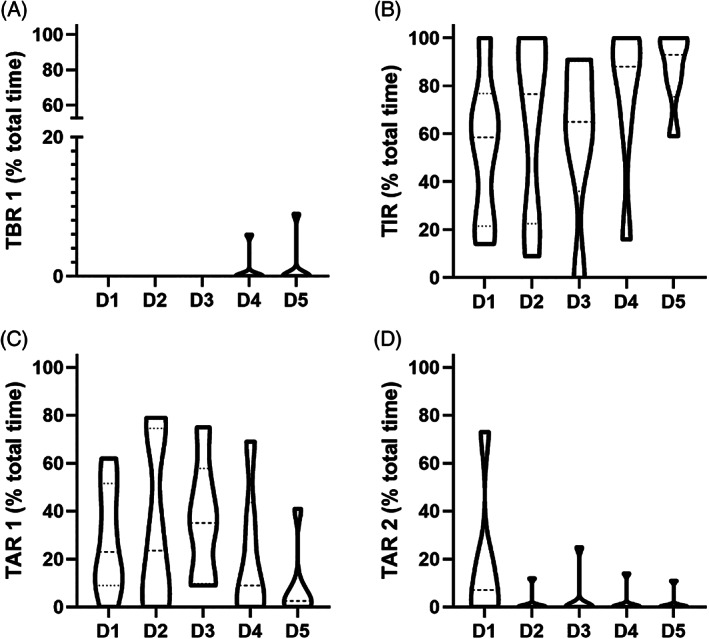
Violin plots for the assessment of in‐ride glycaemic ranges. (A) Time below range level 1 (TBR 1; P = 0.41). (B) Time in range (TIR; P = 0.42). (C) Time above range level 1 (TAR 1; P = 0.47). (D) Time above range level 2 (TAR 2; P = 0.23). TBR 2 is not shown since the athletes did not spend any time in this range
3.4. Insulin administration
In‐ride bolus insulin administration was uncommon and data were only available for four athletes (mean ± SD bolus insulin dose for all in‐ride periods 5 ± 2 IU). Basal insulin dose was not adjusted over the course of the 5 days (P = 0.64), but bolus insulin dose per hour was altered (P = 0.03; Figure 3).
FIGURE 3.
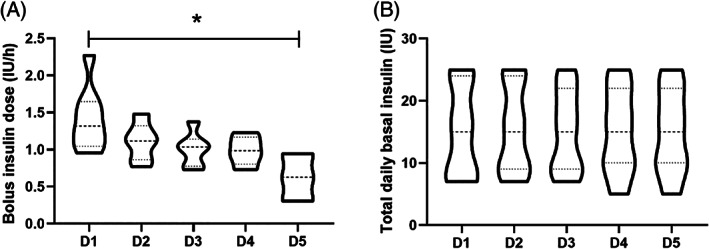
Comparison of (A) bolus and (B) basal insulin doses over the course of the 5 days. Values are given as violin plots. *indicates statistical significance. D, day
3.5. Macronutrient intake
Significant differences were found over the course of the 5 days for fat intake (P = 0.01). No significant differences were found for carbohydrate (P = 0.24) and protein intake (P = 0.08). In‐race intake of fat (P = 0.65), carbohydrates (P = 0.33) and protein (P = 0.70) were not significantly different (Table 2).
TABLE 2.
Macronutrient intake over the 5‐day period
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | P | |
|---|---|---|---|---|---|---|
| Overall, kcal | 3329 ± 268 | 3055 ± 420 | 3896 ± 611 | 3313 ± 811 | 3866 ± 1034 | 0.06 |
| Overall CHO, g | 508 ± 42 | 439 (422–580) | 579 ± 133 | 529 ± 130 | 498 ± 110 | 0.24 |
| Overall fat, g | 73 ± 20 | 56 ± 5 | 91 ± 19 |
77 (33–81) |
139 ± 57 | 0.01 |
|
Overall protein, g |
160 ± 19 | 165 (124–172) | 194 ± 30 | 159 ± 29 | 131 (125–167) | 0.08 |
| Breakfast, kcal | 819 ± 150 | 773 ± 87 | 975 ± 226 | 998 ± 308 | 905 ± 275 | 0.11 |
| Breakfast CHO, g | 131 ± 15 | 122 ± 22 | 157 ± 50 | 158 ± 45 | 128 ± 44 | 0.12 |
| Breakfast fat, g | 20 ± 11 | 19 ± 6 | 24 ± 11 | 24 ± 15 | 28 ± 16 | 0.39 |
| Breakfast protein, g | 30 ± 6 | 29 ± 4 | 34 ± 3 | 37 ± 12 | 36 ± 11 | 0.24 |
| Pre‐race snack, kcal |
0 (0–89) |
60 (0–228) |
0 (0–120) |
0 (0–30) |
126 ± 116 | 0.20 |
| Pre‐race snack CHO, g |
0 (0–18) |
18 ± 22 |
0 (0–25) |
0 (0–5) |
24 ± 24 | 0.29 |
| Pre‐race snack fat, g |
0 (0–2) |
3 ± 3 |
0 (0–2) |
0 (0–1) |
3 ± 3 | 0.52 |
|
Pre‐race snack protein, g |
0 (0–1) |
1 (0–2) |
0 ± 0 |
0 (0–3) |
2 ± 2 | 0.07 |
| In‐race kcal | 878 ± 226 | 778 ± 235 | 1016 ± 368 | 950 ± 559 | 817 ± 410 | 0.39 |
| In‐race CHO, g | 177 ± 45 | 159 ± 53 | 207 ± 89 | 195 ± 97 | 162 ± 69 | 0.33 |
| In‐race fat, g | 16 ± 6 | 12 ± 5 | 17 ± 9 |
6 (4–33) |
8 (4–29) |
0.65 |
| In‐race protein, g | 7 ± 4 | 6 ± 5 | 8 ± 5 | 8 ± 5 | 6 ± 6 | 0.70 |
| Post‐race snack, kcal | 569 ± 0 | 571 ± 102 | 702 (702–728) | 576 (576–651) |
190 (0–811) |
0.07 |
| Post‐race snack CHO, g | 66 ± 0 | 72 ± 27 |
48 (48–55) |
65 (65–82) |
16 (0–91) |
0.25 |
| Post‐race snack fat, g | 5 ± 0 | 9 ± 2 | 18 ± 0 | 7 ± 0 | 10 ± 11 | 0.06 |
| Post‐race snack protein, g | 64 ± 0 |
61 (49–62) |
88 (88–88) |
64 (64–65) |
4 (0–79) |
0.00 |
| Dinner kcal | 1003 ± 230 | 801 ± 119 | 1079 ± 275 | 836 (606–929) | 2016 (1202–2016) | 0.02 |
| Dinner CHO, g | 122 ± 24 | 108 ± 18 | 145 ± 18 | 102 ± 60 | 166 (115–166) | 0.21 |
| Dinner fat, g | 30 ± 11 | 14 ± 5 | 29 ± 18 | 16 ± 11 | 110 (47–110) | 0.00 |
| Dinner protein, g | 59 ± 17 | 59 ± 16 | 59 ± 26 | 50 ± 27 |
86 (66–86) |
0.46 |
Abbreviation: CHO, carbohydrates. Data are mean ± SD or median (interquartile range).
3.6. Effects of exercise intensity and duration on glycaemia and therapy
The overall absolute mean race intensity was 209 ± 17 W, corresponding to 63% ± 5% of the anaerobic threshold (lactate turn point 2 [LTP2]). 27 Stratified per day, race intensities were 188 ± 16 W (58 ± 4% LTP2) on day 1, 211 ± 16 W (63 ± 3% LTP2) on day 2, 201 ± 14 W (62 ± 5% LTP2) on day 3, 234 ± 15 W (71 ± 6% LTP2) on day 4 and 214 ± 40 W (63 ± 7% LTP2) on day 5. A higher mean previous‐day race intensity was associated with a lower mean sensor glucose level (P = 0.03), less TAR 2 (P = 0.05) and lower doses of bolus insulin administration (P = 0.04). No significant associations were found for any other glycaemic range or glycaemic variability (P > 0.05). A longer previous‐day race duration was associated with more TAR 2 (P = 0.05) and higher doses of basal insulin (P = 0.04). No significant associations were found for any other glycaemic range, mean sensor glucose levels or glycaemic variability (P > 0.05). Previous‐day race intensity and duration were not significantly associated with macronutrient intake (P > 0.05).
4. DISCUSSION
This is the first study showing that professional cyclists with T1D reduced their bolus insulin dose over the course of a 5‐day race while macronutrient intake as well as basal insulin dose remained unchanged. In a recent descriptive report, it was shown that athletes with T1D face a higher risk of hypoglycaemia during the nocturnal period, 23 but that these hypoglycaemic episodes were above that deemed clinically relevant 26 ; however, due to issues in data quality, insulin therapy interpretation was unavailable. Our findings demonstrate that professional cyclists with T1D reduce their total daily bolus insulin dose while keeping carbohydrate intake consistent. Of note, basal insulin dose was unaltered, which probably reflects the high training workload of athletes, which does not differ much from race conditions. It can be speculated that a carry‐over effect might exist with respect to basal insulin administration around the races. This finding is contrary to that of a recent study, which showed that people with T1D reduced their basal insulin dose/basal insulin rate around a running event. 28 However, that study was performed in recreationally active people with T1D and the TIR was much lower (∼40%) than observed in the present study. In another study it was found that high intermittent carbohydrate intake during prolonged exercise, combined with proactive use of rtCGM, was linked to good glycaemia during prolonged exercise in recreationally active people with T1D. 29 Nevertheless, the authors concluded that during pre‐exercise/pre‐competition carbohydrate loading, insulin doses should not be drastically increased to avoid an increase in the time spent in hypoglycaemia. Furthermore, since the athletes in the present study were using MDI therapy, insulin adaptations around exercise and competition might be different for athletes using CSII therapy. 19 , 30 Previous studies showed that reducing the basal rate by up to 80% 90 minutes prior the onset of exercise is suitable to improve glycaemia and lower the risk of hypoglycaemia, 10 , 31 but detailed research around competitions and CSII is missing. Although previous studies have shown that basal insulin dose reduction can increase TIR 16 for training over consecutive days and lower the risk of nocturnal hypoglycaemia, 32 the Team Novo Nordisk athletes favour an alternative approach, in which the emphasis is placed on making modifications to the bolus insulin to carbohydrate rate. This finding supports the established advantages of extra carbohydrates 12 and/or bolus insulin dose reductions 14 in avoiding exercise‐induced hypoglycaemia and improving overall glycaemia during exercise. 13 Furthermore, reducing the bolus insulin dose and/or consuming extra carbohydrates might also be easier when compared to basal insulin reductions for recreationally active people with T1D using MDI therapy, and rather allow spontaneous exercise. Nevertheless, the decision for a therapy strategy around exercise/competition should be individualized based on several factors such as experience with exercise, risk of dysglycaemia and volume, type and mode of exercise. The clinical concerns associated with hypoglycaemia during exercise are detrimental to both physical (reduced power output) and psychological (increased worry and anxiety) aspects of exercise performance in people with T1D. 33 Thus, the avoidance of hypoglycaemia during exercise is an integral component of optimizing race performance in elite athletes with T1D.
In a recent study assessing glycaemic responses to exercise training in professional cyclists with T1D, previous‐day exercise intensity was not associated with next‐day glycaemia. 24 Physiological and psychological stress might be much higher around racing conditions, which might explain the divergent results in comparisons of training camp versus racing. However, in the two existing studies that have investigated this group of athletes, 23 , 24 detailed information on insulin administration was missing.
When assessing glycaemia in comparison to non‐race conditions, only glycaemic variability deteriorated. This finding is in slight contrast to a previous study, 28 in which glycaemic variability decreased during a running competition when compared to the pre‐competition period in recreationally active people with T1D. When data were stratified for time of day, night‐time mean sensor glucose improved during the 5‐day race period when compared to pre‐race conditions, which is in contrast to previous studies where glycaemia in general deteriorated. 23 , 24
In‐race glycaemia, as assessed via mean sensor glucose levels, glycaemic ranges and glycaemic variability, was not affected by the exercise accumulating effect (day 1 to day 5), a finding consistent with previous studies. 12 , 16 These results are in support of the effectiveness of current glycaemic management strategies employed by the athletes of the Team Novo Nordisk around a multiple‐stage professional cycling race. Interestingly, longer previous‐day race durations were accompanied by more TAR and higher doses of basal insulin. It is difficult to draw any conclusions from this finding; hence it could be interpreted as a random result.
CONFLICTS OF INTEREST
O.M. has received lecture fees from Medtronic, travel grants from Novo Nordisk A/S, Novo Nordisk AT, Novo Nordisk UK, Medtronic AT, research grants from Sêr Cymru II COFUND fellowship/European Union, Sanofi, Dexcom, Novo Nordisk A/S and Novo Nordisk AT, as well as material funding from Abbott Diabetes Care and Dexcom. R.M.B. reports having received honoraria, travel and educational grant support from Boehringer‐Ingelheim, Eli Lilly and Company, Novo Nordisk and Sanofi‐Aventis. M.L.E. has received a KESS2/European Social Fund scholarship and travel grants from Novo Nordisk A/S. O.Mc. has received a Zienkiewcz scholarship and travel grants from Novo Nordisk UK. The remaining authors have no relevant conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
O.M. is the guarantor and has written the study protocol, performed the measurements, performed statistical analyses and has written the manuscript. M.D. supported with statistical analyses and reviewed/edited the manuscript. O.M., O.Mc., R.M.B. and M.L.E. performed the measurements and reviewed/edited the manuscript. All authors contributed to the writing of the manuscript and reviewed/edited the manuscript.
ACKNOWLEDGMENTS
We thank the athletes and the staff of Team Novo Nordisk.
Moser O, Dietrich M, McCarthy O, Bracken RM, Eckstein ML. Bolus insulin dose depends on previous‐day race intensity during 5 days of professional road‐cycle racing in athletes with type 1 diabetes: A prospective observational study. Diabetes Obes Metab. 2020;22:1714–1721. 10.1111/dom.14083
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14083.
Funding information This study was supported by Team Novo Nordisk. Product supply (smart insulin pens) for study purposes was received from Companion Medical (USA)
REFERENCES
- 1. Leon AS. Physical activity levels and coronary heart disease. Analysis of epidemiologic and supporting studies. Med Clin North Am. 1985;69(1):3‐20. 10.1016/S0025-7125(16)31055-0. [DOI] [PubMed] [Google Scholar]
- 2. Pedersen B, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl 1):3‐63. 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 3. Bohn B, Herbst A, Pfeifer M, et al. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross‐sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38(8):1536‐1543. 10.2337/dc15-0030. [DOI] [PubMed] [Google Scholar]
- 4. Tikkanen‐Dolenc H, Wadén J, Forsblom C, et al. Frequent and intensive physical activity reduces risk of cardiovascular events in type 1 diabetes. Diabetologia. 2016;60:574‐580. 10.1007/s00125-016-4189-8. [DOI] [PubMed] [Google Scholar]
- 5. Moser O, Eckstein ML, West DJ, Goswami N, Sourij H, Hofmann P. Type 1 diabetes and physical exercise: moving (forward) as an adjuvant therapy. Curr Pharm Des. 2020;26:946‐957. 10.2174/1381612826666200108113002. [DOI] [PubMed] [Google Scholar]
- 6. Plotnikoff RC, Taylor LM, Wilson PM, et al. Factors associated with physical activity in Canadian adults with diabetes. Med Sci Sports Exerc. 2006;38(8):1526‐1534. 10.1249/01.mss.0000228937.86539.95. [DOI] [PubMed] [Google Scholar]
- 7. Brazeau AS, Leroux C, Mircescu H, Rabasa‐Lhoret R. Physical activity level and body composition among adults with type 1 diabetes. Diabet Med. 2012;29(11):e402‐e408. 10.1111/j.1464-5491.2012.03757.x. [DOI] [PubMed] [Google Scholar]
- 8. Brazeau A‐S, Strychar I, Rabasa‐Lhoret R, Mirescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31(11):2108‐2109. 10.2337/dc08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bracken RM, West DJ, Stephens JW, Kilduff LP, Luzio S, Bain SC. Impact of pre‐exercise rapid‐acting insulin reductions on ketogenesis following running in type 1 diabetes. Diabet Med. 2011;28(2):218‐222. 10.1111/j.1464-5491.2010.03162.x. [DOI] [PubMed] [Google Scholar]
- 10. Zaharieva DP, McGaugh S, Pooni R, Vienneau T, Ly T, Riddell MC. Improved open‐loop glucose control with basal insulin reduction 90 minutes before aerobic exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Care. 2019;42(5):824‐831. 10.2337/dc18-2204. [DOI] [PubMed] [Google Scholar]
- 11. Moser O, Tschakert G, Mueller A, et al. Effects of high‐intensity interval exercise versus moderate continuous exercise on glucose homeostasis and hormone response in patients with type 1 diabetes mellitus using novel ultra‐long‐acting insulin. Catapano A, PLoS One. 2015;10(8):e0136489. doi: 10.1371/journal.pone.0136489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moser O, Eckstein ML, Mueller A, et al. Pre‐exercise blood glucose levels determine the amount of orally administered carbohydrates during physical exercise in individuals with type 1 diabetes—a randomized cross‐over trial. Nutrients. 2019;11(6):1287 10.3390/nu11061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckstein ML, McCarthy O, Tripolt NJ, et al. Efficacy of carbohydrate supplementation compared to bolus insulin dose reduction around exercise in people with type 1 diabetes: a retrospective controlled analysis. Can J Diabetesournal diabetes. 2020. 10.1016/j.jcjd.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 14. Rabasa‐Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal‐bolus insulin regimen (ultralente‐lispro). Diabetes Care. 2001;24(4):625‐630. 10.2337/diacare.24.4.625. [DOI] [PubMed] [Google Scholar]
- 15. Campbell MD, Walker M, Trenell MI, et al. Metabolic implications when employing heavy pre‐ and post‐exercise rapid‐acting insulin reductions to prevent hypoglycaemia in type 1 diabetes patients: a randomised clinical trial. PLoS One. 2014;9(5):1‐9. 10.1371/journal.pone.0097143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moser O, Eckstein ML, Mueller A, et al. Reduction in insulin degludec dosing for multiple exercise sessions improves time spent in euglycaemia in people with type 1 diabetes: a randomised cross‐over trial. Diabetes Obes Metab. 2019;21(2):349‐356. 10.1111/dom.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsalikian E. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528‐534. 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campbell MD, Walker M, Trenell MI, et al. A low‐glycemic index meal and bedtime snack prevents postprandial hyperglycemia and associated rises in inflammatory markers, providing protection from early but not late nocturnal hypoglycemia following evening exercise in type 1 diabetes. Diabetes Care. 2014;37(7):1845‐1853. 10.2337/dc14-0186. [DOI] [PubMed] [Google Scholar]
- 19. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;8587(17):1‐14. 10.1016/S2213-8587(17)30014-1. [DOI] [PubMed] [Google Scholar]
- 20. Yardley JE, Colberg SR. Update on Management of Type 1 diabetes and type 2 diabetes in athletes. Curr Sports Med Rep. 2017;16(1):38‐44. 10.1249/JSR.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 21. Yamaji K, Miyashita M. Differences in cardio‐respiratory responses to exhaustive exercise between athletes and non‐athletes. Eur J Appl Physiol Occup Physiol. 1978;38(4):233‐238. 10.1007/BF00423106. [DOI] [PubMed] [Google Scholar]
- 22. Johnson RH, Walton JL, Krebs HA, Williamson DH. Metabolic fuels during and after severe exercise in athletes and non‐athletes. Lancet. 1969;294(7618):452‐455. 10.1016/s0140-6736(69)90164-0. [DOI] [PubMed] [Google Scholar]
- 23. Scott SN, Christiansen MP, Fontana FY, et al. Evaluation of factors related to glycemic management in professional cyclists with type 1 diabetes over a 7‐day stage race. Diabetes Care. 2020;43(5):1142‐1145. 10.2337/dc19-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy O, Eckstein ML, Scott SN, et al. Glycemic responses to strenuous training in male professional cyclists with type 1 diabetes: a prospective observational study. BMJ Open Diabetes Res Care. 2020;8:e001245 10.1136/bmjdrc-2020-00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gildon BW. InPen smart insulin pen system: product review and user experience. Diabetes Spectr. 2018;31(4):354‐358. 10.2337/ds18-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593‐1603. 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hofmann P, Bunc V, Leitner H, Pokan R, Gaisl G. Heart rate threshold related to lactate turn point and steady‐state exercise on a cycle ergometer. Eur J Appl Physiol Occup Physiol. 1994;69(2):132‐139. http://www.ncbi.nlm.nih.gov/pubmed/7805667. Accessed October 30, 2017. [DOI] [PubMed] [Google Scholar]
- 28. Moser O, Mueller A, Eckstein ML, et al. Improved glycaemic variability and basal insulin dose reduction during a running competition in recreationally active adults with type 1 diabetes – a single‐Centre, prospective, controlled observational study. Clin J Sport Med. 2020; Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mattsson S, Jendle J, Adolfsson P. Carbohydrate loading followed by high carbohydrate intake during prolonged physical exercise and its impact on glucose control in individuals with diabetes type 1—an exploratory study. Front Endocrinol (Lausanne). 2019;10:1‐8. 10.3389/fendo.2019.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065‐2079. 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McAuley SA, Horsburgh JC, Ward GM, et al. Insulin pump basal adjustment for exercise in type 1 diabetes: a randomised crossover study. Diabetologia. 2016;59(8):1636‐1644. 10.1007/s00125-016-3981-9. [DOI] [PubMed] [Google Scholar]
- 32. Campbell MD, Walker M, Bracken RM, et al. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2015;3(1):e000085 10.1136/bmjdrc-2015-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill NE, Campbell C, Buchanan P, Knight M, Godsland IF, Oliver NS. Biochemical, physiological and psychological changes during endurance exercise in people with type 1 diabetes. J Diabetes Sci Technol. 2017;11(3):529‐536. 10.1177/1932296816671956. [DOI] [PMC free article] [PubMed] [Google Scholar]


