Abstract
Osteonecrosis of the femoral head (ONFH) is a potentially disabling orthopedic condition that, in most late-stage cases, requires total hip arthroplasty. Although direct trauma to the hip (e.g. femoral neck fracture, hip dislocation) that leads to vascular interruption is a strong risk factor for ONFH, there are many non-traumatic risk factors (e.g. use of corticosteroid, alcohol abuse) which molecular mechanisms in ONFH still remain obscured. Long non-coding RNAs (lncRNAs) is a class of regulatory RNAs that play crucial roles in various cellular functions, including cell proliferation, invasion, metabolism, apoptosis and stem cell differentiation. Recent studies also suggested their participation in bone development and regeneration, and a direct involvement in the pathogenesis of numerous of orthopaedic conditions, such as ONFH. LncRNAs are differentially expressed in ONFH tissues as well as bone marrow-mesenchymal stem cells and bone microvascular endothelial cells isolated from ONFH patients. Functional studies further established their critical roles in regulating biological processes, such as osteoblast survival and osteogenic differentiation of bone marrow-mesenchymal stem cells, which are closely related to ONFH. The current review aims at summarizing the recent advancement in this field and discussing the potential diagnostic, prognostic and therapeutic utilities of lncRNAs in the clinical management of ONFH.
Keywords: LncRNA, avascular necrosis, RNA sequencing, steroid
Introduction
Osteonecrosis of the femoral head (ONFH) occurs as a result of decreased blood flow to the bone tissue, leading to ischemia and osteocyte necrosis followed by a complicated repair process with bone resorption, which is predominated over bone formation. ONFH is a multifactorial disease involving both traumatic (e.g. femoral neck fracture, hip dislocation) and non-traumatic (e.g. use of corticosteroid, alcohol abuse, clotting disturbances) risk factors [1]. Patients harbouring certain polymorphisms in genes that related to the coagulation pathway, steroid metabolism, immunity, and the regulation of bone formation are also susceptible to the development of ONFH [2]. The exact mechanism about how most non-traumatic risk factors are linked to ONFH is still largely unknown, in spite of their frequent convergence on pathways, which causes decreased blood flow to the femoral head (e.g. intravascular obstruction due to thrombotic occlusion or fat embolism; intraosseous extravascular compression by lipid deposition and adipocyte hypertrophy in the marrow space) or impaired bone repair (e.g. reduced osteogenesis) [1]. Although efforts such as weight-bearing restriction, utilization of bisphosphonates and statins, core decompression and bone grafting have been adopted to treat or restrict disease progression in early-stage ONFH patients, total hip arthroplasty is frequently unavoidable for those who have progressed to the advanced stage [3].
Long non-coding RNAs (lncRNAs) are a recently identified class of non-protein-coding, regulatory RNAs that are longer than 200 nucleotides in length. Although many lncRNAs are reported to mediate their biological functions through sponging microRNAs (Another class of regulatory RNAs inducing degradation and/or inhibit translation of target mRNAs), some specific lncRNAs were proved to regulate gene expression via other mechanisms, including modulating DNA methylation, recruiting transcriptional factors, and controlling mRNA stability and splicing [4]. Accumulating evidences now support that lncRNAs play essential roles in a variety of physiological and pathological processes, including cell proliferation [5], cell death [6], stem cell pluripotency and lineage commitment [7], metabolic control [8] aging and degeneration [9], as well as inflammation and immunity [10]. Emerging studies also suggested that lncRNAs can regulate bone development and regeneration [11] and contribute to the pathogenesis of different orthopaedic conditions, e.g. osteoporosis [12], osteoarthritis [13], osteosarcoma [14] and intervertebral disc degeneration [15]. Recently, lncRNAs were also revealed to be involved in development of OFNH.
In this review, we summarized dysregulated lncRNAs as well as their functional roles in ONFH. We also discussed their potential possibility as druggable targets and diagnostic markers for ONFH treatment.
LncRNA expression profiling in ONFH
In ONFH, differentially expressed lncRNAs have been identified at the genome-wide level by RNA sequencing or microarray in osteonecrotic tissues, mesenchymal stem cells (MSCs) and bone microvascular endothelial cells (BMECs). Dysregulation of some sort of lncRNAs have also been substantiated by reverse transcription-quantitative PCR (RT-qPCR).
Osteonecrotic tissues
Recently, Luo et al. reported distinct expression profiles of mRNA and lncRNA in three human steroid-associated ONFH samples and three human femoral head fracture samples using third-generation lncRNA microarrays [16]. A total of 1,657 mRNAs (1,092 upregulated and 565 downregulated) and 4,393 lncRNAs (1,179 upregulated and 3,214 downregulated) were found to be differentially expressed in the ONFH group. RT-qPCR was performed to validate selected lncRNAs and confirmed the upregulation of NR 027293 and downregulation of ENST00000565178, NR 038891, and T318776 in the osteonecrotic tissues [16].
Moreover, Huang and colleagues compared the transcriptome difference between ONFH and femoral head fracture samples using RNA sequencing technology [17], revealing a total of 2,965 differentially expressed genes including 1,395 upregulated and 1,570 downregulated genes in the ONFH tissues, among which there were 575 upregulated lncRNAs and 27 downregulated lncRNAs. Co-expression analysis further pointed out a significant correlation of 144 mRNAs with the differentially expressed lncRNAs. These correlated genes were found to be closely related to serine-type endopeptidase, peptidase, and endopeptidase activity. Downregulation of lncRNA FAM201A was confirmed by RT-qPCR [17].
Bone marrow-mesenchymal stem cells
Increased adipogenic and/or decreased osteogenic differentiation of bone marrow-MSCs have been implicated in the pathogenesis of alcohol- and steroid-induced ONFH [18-20]. Wang et al. isolated MSCs from the bone marrow of patients with steroid-associated ONFH and compared their mRNA and lncRNA expression profiles to those with femoral neck fracture using microarray [21]. Resultingly, 2,775 mRNAs and 3,720 lncRNAs were identified to be differentially expressed, among which 838 mRNAs and 1,878 lncRNAs were upregulated, whereas 1937 mRNAs and 1,842 lncRNAs were downregulated in the steroid-associated ONFH group. The top 10 differentially expressed mRNAs and lncRNAs were successfully verified by RT-qPCR, confirming the validity of their microarray results. Reconstruction of the coding-non-coding gene co-expression (CNC) network revealed the critical roles of two lncRNAs, HOTAIR and RP1-193H18.2, in regulating the osteogenic and adipogenic differentiation of bone marrow-MSCs [21].
Similarly, Xiang et al. examined the different expression of lncRNAs in bone marrow-MSCs isolated from patients with steroid-associated ONFH and those from patients with developmental dysplasia of the hip or femoral neck fracture through RNA sequencing [22]. Consequently, they discovered a total of 3,114 differentially expressed mRNAs (1,979 upregulated and 1,135 downregulated) and 572 lncRNAs (181 upregulated and 391 downregulated). The hub function of the lncRNA RP11-154D6 was further predicted by reconstruction of the lncRNA-microRNA-mRNA network [22].
Bone microvascular endothelial cells
Genetic variations of angiogenesis-related genes were associated with altered risks for ONFH [23], whereas stimulation of angiogenesis with vascular endothelial growth factor (VEGF) showed therapeutic effects in a canine model of cryosurgically-induced ONFH [24]. Non-traumatic ONFH is also known to be associated with endothelial cell activation [25]. Therefore, it is of interest to delineate the association between the transcriptomic profile of bone endothelial cells and disease status in ONFH. For such purpose, Yu et al. harvested BMECs from patients who were undergone routine total hip replacement and exposed cells to hydrocortisone (0.1 mg/ml) for 24 h before microarray-based mRNA, microRNA and lncRNA profiling [26,27]. Among 26,646 lncRNAs that were expressed above background in BMECs, 239 lncRNAs (73 upregulated and 166 downregulated), including NAV2-IT1, NAV2-AS5, NAV2-AS1, NTM-IT2, and ARHGEF19-AS1, showed significant dysregulation upon hydrocortisone exposure. RT-qPCR was applied to confirmed the upregulation of ENSG00000259007.1 and downregulation of XLOC_011117. Co-expression analysis of non-coding RNAs and their correlated mRNAs further revealed FoxO signaling as a potential compensatory response of BMECs to hydrocortisone [27]. In this connection, FoxO transcription factors was showed to be closely involved in the regulation of vessel formation in the adult [28].
LncRNA profiling studies on ONFH are summarized as Table 1. It is noteworthy that the top lncRNAs identified by different studies did not overlap. It might occur as a result of the use of different tissues or cell types for expression profiling and the clinical heterogeneity of ONFH.
Table 1.
LncRNAs expression profiles in osteonecrosis of the femoral head
| Num | Method | sample | upregulated | downregulated | Reference |
|---|---|---|---|---|---|
| 1 | Microarray | ONFH patients | 1,179 LncRNAs | 3,214 LncRNAs | [16] |
| RT-PCR | NR 027293 | ENST00000565178 | |||
| NR 038891 | |||||
| T318776 | |||||
| 2 | Microarray | ONFH tissues | 575 LncRNAs | 27 LncRNAs | [17] |
| RT-PCR | FAM201A | ||||
| 3 | Microarray | mesenchymal stem cell from ONFH | 1,878 lncRNAs HOTAIR | 1,842 lncRNAs | [21] |
| RT-PCR | RP1-193H18.2 | ||||
| 4 | Microarray | mesenchymal stem cells from ONFH | 181 lncRNAs | 391 lncRNAs | [22] |
| RT-PCR | |||||
| 5 | Microarray | mesenchymal stem cells from ONFH | 73 lncRNAs | 166 lncRNAs | [26,27] |
| RT-PCR | ENSG00000259007.1 | XLOC_011117 |
Functional roles of specific lncRNAs in ONFH
AWPPH
AWPPH (lncRNA associated with poor prognosis of hepatocellular carcinoma) is a newly discovered lncRNA whose expression was deregulated in a variety types of tumors, including liver cancer [29], osteosarcoma [30], and non-small cell lung cancer [31]. A recent study showed that AWPPH levels were reduced in both serum and mesenchymal stem cells (MSCs) isolated from non-traumatic ONFH patients as compared with those from healthy control [32]. Moreover, low levels of AWPPH in serum and MSCs could differentiate non-traumatic ONFH patients from healthy controls with the area under the curve of 0.8177 and 0.8259, respectively, showing a possibility to be served as a diagnostic marker. Clinicopathological correlation analysis further revealed that reduced AWPPH levels were associated with a shorter course of disease (<5 years) but irrelevant with patients’ age, gender, cigarette smoking or drinking habits. Bone morphogenic proteins (BMPs), for example BMP-2, are known to induce osteoblastic differentiation of MSCs through activating the transcription factor RUNX2 [33]. In this connection, BMP-2 was showed to be induced the expression of AWPPH in human bone marrow-MSCs. Importantly, elevated AWPPH level led to an increased RUNX2 expression, while it was reversed by knockdown of AWPPH. RUNX2 expression levels were also markedly decreased in MSCs, which were isolated from non-traumatic ONFH patients. Thus, these findings collectively suggested that the aberrant downregulation of AWPPH might participate in the progression of ONFH through dampening RUNX2 signaling in MSCs. The reduced circulating levels of AWPPH might also enable this lncRNA to act as a potential biomarker for diagnosing non-traumatic ONFH [32]. Nevertheless, further work is demanded to figure out whether this lncRNA could functionally repress osteoblastic differentiation of MSCs.
RP11-154D6
RP11-154D6 was reported as another differentially expressed lncRNAs in bone marrow-MSCs isolated from patients with steroid-induced ONFH [22]. Bioinformatic analysis further revealed an extensive interaction of RP11-154D6 with microRNAs. Moreover, RP11-154D6 expression was detected to be increased during osteogenic differentiation but decreased during adipogenic differentiation of bone marrow-MSCs. Functionally, enforced expression of RP11-154D6 by a lentiviral vector in bone marrow-MSCs promoted osteogenic differentiation, which was evidenced by increased expression of osteocalcin (OCN) and RUNX2, but diminished adipogenic differentiation and reduced expression of adipogenic markers, such as lipoprotein lipase (LPL) and peroxisome proliferator-activated receptor γ (PPARγ). These data collectively suggested that the downregulation of RP11-154D6 tilts the balance of bone marrow-MSC differentiation from osteogenesis to adipogenesis [22]. Restoration of RP11-154D6 expression might thus represent a potential prophylactic or even therapeutic strategy for steroid-induced ONFH.
MIAT
The lncRNA MIAT (myocardial infarction associated transcript) exhibits dysregulation in various diseases, such as myocardial infarction, ischemic stroke and cancers, which mediates biological functions through sponging miR-22-3p, miR-24 and miR-150 [34]. A recent study revealed that MIAT levels in osteonecrotic tissues were significantly elevated than that in non-necrotic tissues from patients with steroid-associated ONFH [35]. Expression levels of MIAT were found to be gradually decreased during osteogenic differentiation of rat MSC. Silencing of MIAT with small interfering RNAs (siRNAs) facilitated the expression of osteogenic markers ALP, RUNX2 and OCN. Alizarin Red staining further revealed the increased formation of mineralized nodules in MIAT-knockdown rat MSCs. The traditional Chinese medicine HXTL (Huo Xue Tong Luo) used for osteonecrosis treatment promoted histone H3 lysine 27 trimethylation (H3K27me3; a repressive histone mark) and inhibited H3K4me3 (an active histone mark) in the promoter of MIAT to repress its expression [35]. These data suggested that the aberrant overexpression of MIAT might inhibit MSC osteogenic differentiation to lead to steroid-associated ONFH, whereas HXTL might protect against ONFH development through epigenetic silencing of MIAT.
EPIC1
EPIC1 (epigenetically-induced lncRNA1) is a novel Myc-interacting lncRNA showing dysregulation in multiple cancer types [36-38]. A higher expression level of EPIC1 in necrotic femoral head tissues than that in normal tissues surgically isolated from patients with steroid-associated ONFH was ever reported [39]. In this regard, endogenous expression of EPIC1 was proved to protect OB-6 osteoblastic cells and primary human osteoblasts from dexamethasone-induced apoptosis, which was characterized by mitochondria depolarization and cytochrome c release. On the contrary, knockdown of EPIC1 resulted in the opposite effect. Importantly, Myc-knockout OB-6 cells were more susceptible to dexamethasone-induced apoptosis, whereas EPIC1 overexpression protected the wild-type but not the Myc-knockout OB-6 cells from dexamethasone, suggesting that Myc was a target gene of EPIC1 in osteoblasts [39]. Thus, these findings indicated that upregulation of EPIC1 in osteonecrotic tissues might represent a compensatory mechanism to sustain osteoblast survival in response to glucocorticoid treatment (Table 2; Figure 1).
Table 2.
Functional characterization of the lncRNAs in ONFH
| lncRNAs | Expression | Functional role | Related gene or drugs | Role | Reference |
|---|---|---|---|---|---|
| AWPPH | Down | mesenchymal stem cell differentiation | BMP-2 | protect | [32] |
| RP11-154D6 | Down | mesenchymal stem cell differentiation | protect | [22] | |
| LncRNA-Miat | Up | mesenchymal stem cell differentiation | HXTL capsule | Damage | [35] |
| Lnc-EPIC1 | Up | mesenchymal stem cell differentiation | Dex | Damage | [39] |
| Myc | |||||
| HOTAIR | Up | mesenchymal stem cell differentiation | miR-17-5p | Damage | [41] |
| SMAD7 | |||||
| MALAT1 | down | mesenchymal stem cell differentiation | Dex | [43] | |
| apoptosis | Ppm1e | ||||
| AMPK | |||||
| miR-214 |
Figure 1.
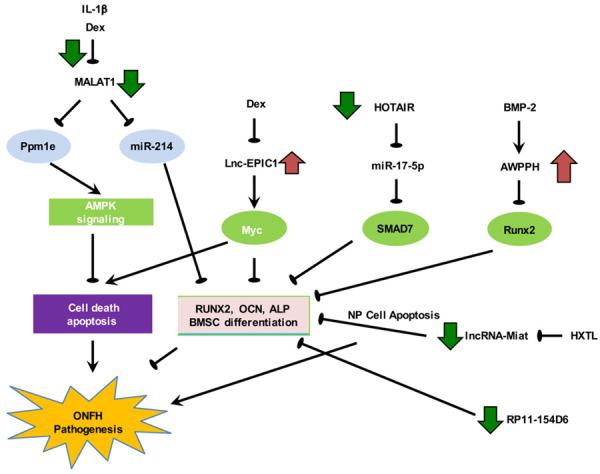
Deregulation of osteoblast survival and differentiation of bone marrow-MSCs by lncRNAs in steroid-associated ONFH. Upregulation of MIAT and HOTAIR and downregulation of AWPPH, RP11-154D6 and MALAT1 contribute directly to the pathogenesis, whereas elevated EPIC1 level represents a compensatory protective response.
HOTAIR
HOTAIR (HOX antisense intergenic RNA) is a recently discovered lncRNA that plays a crucial role in regulating phenotypes pertinent to cancer formation, such as proliferation, survival, migration, and genomic stability [40]. Wei et al. reported that HOTAIR expression in MSCs of patients with non-traumatic ONFH was higher than those from healthy donors or patients with osteoarthritis [41]. Endogenous expression of HOTAIR decreased the level of osteogenic differentiation markers, such as RUNX2, COL1A1 and ALP, in human bone marrow-MSCs, whereas such impact was reversed by knockdown of HOTAIR. Mechanistically, HOTAIR was proved to suppress the osteogenic differentiation markers through the miR-17-5p-SMAD7 (A negative regulator of BMP and transforming growth factor-β signalling) pathway, in which transfection with miR-17-5p mimic or knockdown of SMAD7 nullified the effect of HOTAIR on osteogenesis [41]. These findings indicated that aberrant overexpression of HOTAIR in MSCs contributes to steroid-associated ONFH through sponging miR-17-5p and subsequent derepression of SMAD7 and attenuation of osteogenic BMP signalling. Consistently, by microarray-based profiling of lncRNA expression in BMSCs and validation with RT-qPCR, Wang et al. reported a significant upregulation of HOTAIR in patients with steroid-associated ONFH [21].
MALAT1
Apart from HOTAIR, MALAT1 (metastasis associated lung adenocarcinoma transcript 1) as another highly dysregulated lncRNA in steroid-associated ONFH was identified by Wang et al., which levels were significantly reduced in patients’ BMSCs [21]. Consistently, Fan and colleagues confirmed that MALAT1 levels were substantially lowered in necrotic femoral head tissues than the surrounding normal femoral head tissues from patients with steroid-associated ONFH [42]. The downregulation of MALAT1 could be recapitulated when OB-6 and hFOB1.19 osteoblastic cells and primary human osteoblasts were treated with dexamethasone. Functionally, expression of MALAT1 protected human osteoblasts from dexamethasone-induced cell death, whereas MALAT1 knockdown aggravated the cytotoxicity. The cytoprotective effect of MALAT1 in osteoblasts was associated with the activation of 5’ AMP-activated protein kinase (AMPK) signalling through downregulating PPM1E and the subsequent attenuation of oxidative stress through increasing NRF2 activity [42]. Another recent study showed that dexamethasone impaired osteogenic differentiation of BMSCs, which was paralleled by the downregulation of MALAT1 [43]. Functionally, MALAT1 expression attenuated the inhibitory effect of dexamethasone on osteogenic differentiation, which was mediated through sponging of miR-214 and derepression of its target ATF4 (an osteogenic transcription factor) [43]. These findings suggested that corticosteroid-mediated downregulation of MALAT1 contributes to ONFH through inducing osteoblast cell death and inhibiting osteogenic differentiation of MSCs via regulating the PPM1E-AMPK-NRF2-oxidative stress and miR-214-ATF4 axes, respectively. Restoration of MALAT1 expression or activating its downstream signaling, including AMPK and ATF4, might thus potentially prevent steroid-induced ONFH.
Conclusions and future perspectives
ONFH is a potentially disabling diseases with poorly defined etiology and pathophysiology, which hindered the development of mechanism-driven prophylactic and therapeutic strategies [44]. LncRNA dysregulation (e.g. upregulation of MIAT and HOTAIR and downregulation of AWPPH, RP11-154D6 and MALAT1) was found to play crucial pathogenic roles in steroid-associated ONFH through interacting with signalling pathways pertinent to osteoblast survival and differentiation of bone marrow-MSCs (Figure 1). In this regard, agents that promote osteoblast survival or osteogenic differentiation of MSCs have been shown to protect against disease development in animal models of steroid-induced ONFH [45-48]. Studies scrutinized in this review have also hinted at the potentially therapeutic roles of lncRNAs in steroid-associated ONFH. Nevertheless, in some studies where osteonecrosis tissues were used for lncRNA profiling, the cellular source of the deregulated lncRNAs remains poorly defined as the tissues contained trabecular bone, bone marrow, blood vessels and cartilage. It is also worthwhile to note that a single lncRNA could have divergent functions in different cell types. Future studies involving enrichment of specific cell types before profiling or single-cell transcriptomics could tackle this issue [49].
Pertinent to clinical practice, upregulated lncRNA might be targeted by different approaches, such as genetic ablation by CRISPR/Cas9-mediated, knockdown by antisense oligonucleotides or siRNAs or steric blockade of lncRNA-protein interactions by small molecules [50]. Nevertheless, cell type-specific delivery of lncRNA-directed therapeutics remains a technical challenge. To this end, cell type-specific delivery of siRNAs with aptamer-siRNA chimeras might be hopeful [50], but whether it can be achieved in human still remains to be further demonstrated. From the perspectives of biomarker development, some circulating lncRNAs might differentiate steroid-associated ONFH patients from healthy subjects. Nevertheless, it would be more appropriate to include patients with the same underlying medical conditions receiving corticosteroid treatment but without the development of ONFH as the control group [51]. Importantly, longitudinal profiling of circulating lncRNAs in corticosteroid-treated patients may be helpful to identify lncRNAs the precede the onset of ONFH. Large-cohort, multi-centre validation of the identified lncRNA markers is also required for effective clinical development. Despite these challenges, it is still hopeful that, with more translational studies, the use of lncRNAs as biomarkers and therapeutic targets in ONFH can be realized in the future.
Disclosure of conflict of interest
None.
References
- 1.Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8:201–209. doi: 10.1007/s12178-015-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T, Azeddine B, Mah W, Harvey EJ, Rosenblatt D, Seguin C. Osteonecrosis of the femoral head: genetic basis. Int Orthop. 2019;43:519–530. doi: 10.1007/s00264-018-4172-8. [DOI] [PubMed] [Google Scholar]
- 3.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22:455–464. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 4.Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Liu S, Hu X. Long non-coding RNAs: crucial regulators of gastrointestinal cancer cell proliferation. Cell Death Discov. 2018;4:50. doi: 10.1038/s41420-018-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Y, Wu H, Pavlosky A, Zou LL, Deng X, Zhang ZX, Jevnikar AM. Regulatory non-coding RNA: new instruments in the orchestration of cell death. Cell Death Dis. 2016;7:e2333. doi: 10.1038/cddis.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fico A, Fiorenzano A, Pascale E, Patriarca EJ, Minchiotti G. Long non-coding RNA in stem cell pluripotency and lineage commitment: functions and evolutionary conservation. Cell Mol Life Sci. 2019;76:1459–1471. doi: 10.1007/s00018-018-3000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao XY, Lin JD. Long noncoding RNAs: a new regulatory code in metabolic control. Trends Biochem Sci. 2015;40:586–596. doi: 10.1016/j.tibs.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Tu C, Liu Y. Role of lncRNAs in aging and age-related diseases. Aging Med (Milton) 2018;1:158–175. doi: 10.1002/agm2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Ao L, Yang J. Long non-coding RNAs in diseases related to inflammation and immunity. Ann Transl Med. 2019;7:494. doi: 10.21037/atm.2019.08.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng S, Cao L, He S, Zhong Y, Ma H, Zhang Y, Shuai C. An overview of long noncoding RNAs involved in bone regeneration from mesenchymal stem cells. Stem Cells Int. 2018;2018:8273648. doi: 10.1155/2018/8273648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva AM, Moura SR, Teixeira JH, Barbosa MA, Santos SG, Almeida MI. Long noncoding RNAs: a missing link in osteoporosis. Bone Res. 2019;7:10. doi: 10.1038/s41413-019-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Lu X, Shen B, Zeng Y. The therapeutic potential and role of miRNA, lncRNA, and circRNA in osteoarthritis. Curr Gene Ther. 2019;19:255–263. doi: 10.2174/1566523219666190716092203. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Wang G, Zheng Y, Hua Y, Cai Z. Long non-coding RNAs in osteosarcoma. Oncotarget. 2017;8:20462–20475. doi: 10.18632/oncotarget.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Li X, Chen C, Li S, Shen J, Tse G, Chan MTV, Wu WKK. Long non-coding RNAs in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2018;51:e12483. doi: 10.1111/cpr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo H, Lan W, Li Y, Lian X, Zhang N, Lin X, Chen P. Microarray analysis of long-noncoding RNAs and mRNA expression profiles in human steroid-induced avascular necrosis of the femoral head. J Cell Biochem. 2019;120:15800–15813. doi: 10.1002/jcb.28850. [DOI] [PubMed] [Google Scholar]
- 17.Huang G, Zhao G, Xia J, Wei Y, Chen F, Chen J, Shi J. FGF2 and FAM201A affect the development of osteonecrosis of the femoral head after femoral neck fracture. Gene. 2018;652:39–47. doi: 10.1016/j.gene.2018.01.090. [DOI] [PubMed] [Google Scholar]
- 18.Suh KT, Kim SW, Roh HL, Youn MS, Jung JS. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005:220–225. doi: 10.1097/01.blo.0000150568.16133.3c. [DOI] [PubMed] [Google Scholar]
- 19.Sheng HH, Zhang GG, Cheung WH, Chan CW, Wang YX, Lee KM, Wang HF, Leung KS, Qin LL. Elevated adipogenesis of marrow mesenchymal stem cells during early steroid-associated osteonecrosis development. J Orthop Surg Res. 2007;2:15. doi: 10.1186/1749-799X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han L, Wang B, Wang R, Gong S, Chen G, Xu W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res Ther. 2019;10:377. doi: 10.1186/s13287-019-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Yang Q, Chen G, Du Z, Ren M, Wang A, Zhao H, Li Z, Zhang G, Song Y. LncRNA expression profiling of BMSCs in osteonecrosis of the femoral head associated with increased adipogenic and decreased osteogenic differentiation. Sci Rep. 2018;8:9127. doi: 10.1038/s41598-018-27501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang S, Li Z, Weng X. The role of lncRNA RP11-154D6 in steroid-induced osteonecrosis of the femoral head through BMSC regulation. J Cell Biochem. 2019;120:18435–18445. doi: 10.1002/jcb.29161. [DOI] [PubMed] [Google Scholar]
- 23.Hong JM, Kim TH, Kim HJ, Park EK, Yang EK, Kim SY. Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Mol Med. 2010;42:376–385. doi: 10.3858/emm.2010.42.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dailiana ZH, Stefanou N, Khaldi L, Dimakopoulos G, Bowers JR, Fink C, Urbaniak JR. Vascular endothelial growth factor for the treatment of femoral head osteonecrosis: an experimental study in canines. World J Orthop. 2018;9:120–129. doi: 10.5312/wjo.v9.i9.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seguin C, Kassis J, Busque L, Bestawros A, Theodoropoulos J, Alonso ML, Harvey EJ. Non-traumatic necrosis of bone (osteonecrosis) is associated with endothelial cell activation but not thrombophilia. Rheumatology (Oxford) 2008;47:1151–1155. doi: 10.1093/rheumatology/ken206. [DOI] [PubMed] [Google Scholar]
- 26.Yu Q, Guo W, Shen J, Lv Y. Effect of glucocorticoids on lncRNA and mRNA expression profiles of the bone microcirculatory endothelial cells from femur head of Homo sapiens. Genom Data. 2015;4:140–142. doi: 10.1016/j.gdata.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu QS, Guo WS, Cheng LM, Lu YF, Shen JY, Li P. Glucocorticoids significantly influence the transcriptome of bone microvascular endothelial cells of human femoral head. Chin Med J (Engl) 2015;128:1956–1963. doi: 10.4103/0366-6999.160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X, Liu Y, Yu S. Long noncoding RNA AWPPH promotes hepatocellular carcinoma progression through YBX1 and serves as a prognostic biomarker. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1805–1816. doi: 10.1016/j.bbadis.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Ding W, Wu D, Ji F, Zhang H. Inhibition of long non-coding RNA-AWPPH decreases osteosarcoma cell proliferation, migration and invasion. Oncol Lett. 2019;18:5055–5062. doi: 10.3892/ol.2019.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huo Y, Li A, Wang Z. LncRNA AWPPH participates in the metastasis of non-small cell lung cancer by upregulating TGF-beta1 expression. Oncol Lett. 2019;18:4246–4252. doi: 10.3892/ol.2019.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Li J, Liang D, Zhang L, Wang Q. LncRNA AWPPH participates in the development of non-traumatic osteonecrosis of femoral head by upregulating Runx2. Exp Ther Med. 2020;19:153–159. doi: 10.3892/etm.2019.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–646. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun C, Huang L, Li Z, Leng K, Xu Y, Jiang X, Cui Y. Long non-coding RNA MIAT in development and disease: a new player in an old game. J Biomed Sci. 2018;25:23. doi: 10.1186/s12929-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang B, Li Y, Chen C, Wei Q, Zheng J, Liu Y, He W, Lin D, Li G, Hou Y, Xu L. Huo Xue Tong Luo capsule ameliorates osteonecrosis of femoral head through inhibiting lncRNA-Miat. J Ethnopharmacol. 2019;238:111862. doi: 10.1016/j.jep.2019.111862. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang Y, Jia L, Li S Cancer Genome Atlas Research Network. Xie W, Yang D. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncrna that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell. 2018;33:706–720. e709. doi: 10.1016/j.ccell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Lu HY, Xia YH, Jiang AG, Lv YX. Long non-coding RNA EPIC1 promotes human lung cancer cell growth. Biochem Biophys Res Commun. 2018;503:1342–1348. doi: 10.1016/j.bbrc.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Cai Q, Li W, Feng F, Yang L. Long non-coding RNA EPIC1 promotes cholangiocarcinoma cell growth. Biochem Biophys Res Commun. 2018;504:654–659. doi: 10.1016/j.bbrc.2018.08.174. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XY, Shan HJ, Zhang P, She C, Zhou XZ. LncRNA EPIC1 protects human osteoblasts from dexamethasone-induced cell death. Biochem Biophys Res Commun. 2018;503:2255–2262. doi: 10.1016/j.bbrc.2018.06.146. [DOI] [PubMed] [Google Scholar]
- 40.Tang Q, Hann SS. HOTAIR: an oncogenic long non-coding rna in human cancer. Cell Physiol Biochem. 2018;47:893–913. doi: 10.1159/000490131. [DOI] [PubMed] [Google Scholar]
- 41.Wei B, Wei W, Zhao B, Guo X, Liu S. Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS One. 2017;12:e0169097. doi: 10.1371/journal.pone.0169097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan JB, Zhang Y, Liu W, Zhu XH, Xu DW, Zhao JN, Cui ZM. Long non-coding RNA MALAT1 protects human osteoblasts from dexamethasone-induced injury via activation of PPM1E-AMPK signaling. Cell Physiol Biochem. 2018;51:31–45. doi: 10.1159/000495159. [DOI] [PubMed] [Google Scholar]
- 43.Huang XZ, Huang J, Li WZ, Wang JJ, Song DY, Ni JD. LncRNA-MALAT1 promotes osteogenic differentiation through regulating ATF4 by sponging miR-214: implication of steroid-induced avascular necrosis of the femoral head. Steroids. 2020;154:108533. doi: 10.1016/j.steroids.2019.108533. [DOI] [PubMed] [Google Scholar]
- 44.Qi X, Zeng Y. Biomarkers and pharmaceutical strategies in steroid-induced osteonecrosis of the femoral head: a literature review. J Int Med Res. 2015;43:3–8. doi: 10.1177/0300060514554724. [DOI] [PubMed] [Google Scholar]
- 45.Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, Yang G, Shi X, Levine A, Iqbal J, Yaroslavskiy BB, Isales C, Blair HC. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng S, Zhou JL, Fang HS, Nie ZG, Chen S, Peng H. Sesamin protects the femoral head from osteonecrosis by inhibiting ROS-induced osteoblast apoptosis in rat model. Front Physiol. 2018;9:1787. doi: 10.3389/fphys.2018.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Z, Cheng C, Cao B, Wang J, Wei H, Liu X, Han Y, Yang S, Wang X. Icariin protects against glucocorticoid-induced osteonecrosis of the femoral head in rats. Cell Physiol Biochem. 2018;47:694–706. doi: 10.1159/000490023. [DOI] [PubMed] [Google Scholar]
- 48.Zhang YL, Yin JH, Ding H, Zhang W, Zhang CQ, Gao YS. Vitamin K2 prevents glucocorticoid-induced osteonecrosis of the femoral head in rats. Int J Biol Sci. 2016;12:347–358. doi: 10.7150/ijbs.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50:96. doi: 10.1038/s12276-018-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Jiang C, Li X, Wu WKK, Chen X, Zhu S, Ye C, Chan MTV, Qian W. Circulating microRNA signature of steroid-induced osteonecrosis of the femoral head. Cell Prolif. 2018;51:e12418. doi: 10.1111/cpr.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]


