Abstract
Urine-derived stem cells (USCs) are autologous stem cells that exhibit self-renewal ability and multi-lineage differentiation potential. These characteristics make USCs an ideal cell source for hepatocellular transplantation. Here, we investigated the biological characteristics of USCs and their potential use for the treatment of chronic liver injury. We characterized the cell-surface marker profile of USCs by flow cytometry and determined the osteogenic, adipogenic, and hepatic differentiation capacities of USCs using histology. We established a chronic liver-injury model by intraperitoneally injecting carbon tetrachloride into nude mice. USCs were then transplanted via tail vein injection. To determine liver function and histopathology following chronic liver injury, we calculated the liver index, measured serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, and performed histological staining. USCs were small, adherent cells expressing mesenchymal but not hematopoietic stem-cell markers. Some induced USCs underwent osteogenic and adipogenic differentiation. When co-cultured with hepatic progenitor cells, about 10% of USCs underwent hepatic differentiation. The ALT and AST levels of the USC-transplanted group were lower than that of the chronic liver-injury model group, and there were no significant differences between the two USC-transplanted groups. However, hepatocyte degeneration and liver fibrosis substantially improved in the hypoxia-pretreated USC-transplanted group compared with the normoxia USC-transplanted group. Taken together, USCs display desirable proliferation and differentiation characteristics, and USC transplantation partially improves abnormal liver function and pathology associated with chronic liver injury. Furthermore, hypoxia pretreatment promotes cell proliferation, migration, and colony formation by inducing autophagy, leading to USC-elicited liver tissue recovery following injury in vivo.
Keywords: Urine-derived stem cells, chronic liver injury, cell transplantation, hypoxia, autophagy
Introduction
Hepatocellular transplantation (HCT) is a cell-based therapy that is widely considered as an alternative to orthotopic liver transplantation. Several animal experiments have indicated that HCT can revert liver fibrosis, repair liver physiology and improve metabolism in many cases of hepatic insufficiency [1-4]. Since its inception, HCT technology has been studied for more than 30 years as a method to treat liver diseases. However, the cell source for HCT is still limiting and imposes a bottleneck that needs to be urgently resolved. Stem cells have proven to be important seed cells for HCT, owing to their multi-lineage differentiation potential, self-renewal ability and immune regulation. Studies have demonstrated that different types of stem cells can be induced into hepatocyte-like cells under certain induction conditions in vitro [5-8]. Transplanted stem cells can additionally activate proliferation and differentiation of endogenous stem cells through paracrine signaling, alleviate local inflammation and protect hepatocytes from apoptosis, thus promoting the regeneration and repair of liver tissue [9,10]. Ideal seed cells for transplantation are autologous stem cells that are derived from the patients themselves, so as to minimize immunological rejection of the transplanted cells. In clinical trials, autologous bone marrow mesenchymal stem cells (BMSCs) transplantation has been used to treat chronic liver injury, resulting in moderate improvement of biochemical indicators and clinical symptoms in patients [11]. However, successful mobilization of BMSCs depends on the overall health of the patient. The method of acquisition of BMSCs is invasive, which increases the risk of potential complications. Therefore, an alternative autologous stem cell source for HCT that circumvents the above shortcomings would be preferable.
A small number of stem cells exists in the urine, known as urine-derived stem cells (USCs), and can be obtained using a non-invasive, facile, safe, and low-cost approach. USCs can be easily cultured and passaged, owing to their high proliferative potential. In addition, USCs express mesenchymal stem cell surface markers and display a self-renewal ability as well as multi-lineage differentiation potential. Studies have performed tissue engineering of the urogenital system using USCs to improve renal, penile erectile, and urethral sphincter functions in multiple animal models [12,13]. The application of USCs in HCT for the repair of liver injury has not been reported.
In this study, we isolated and cultured USCs from the urine of healthy volunteers and determined their morphology, phenotype and differentiation potential. To evaluate the therapeutic effect of USCs, we transplanted USCs in vivo in mouse models of CCl4-induced acute or chronic liver injury. We found that USCs could partially improve hepatic function, repair damaged liver tissue and resolve liver fibrosis. Hypoxia improved cell proliferation, migration and colony formation of USCs by inducing autophagy, and thus improved liver recovery induced by USCs in vivo. Autologous HCT of USCs in patients with chronic liver injury may therefore have potential as a treatment strategy for end-stage liver diseases.
Materials and methods
Isolation and culture of USCs
The USCs culture medium was prepared as described [14]. Institutional review board committee approval for this study was obtained for the collection of urine samples. Urine samples were centrifuged at 1500 rpm for 5 min at room temperature. The cell pellets were re-suspended with 24 mL of USCs culture medium and then plated in one 24-well tissue culture plate. Cells were cultured in the 19% oxygen (normoxia) incubator (37°C, 5% CO2) for 10-14 days and then passaged to a 6-well plate. The cells were cultured in a 3% oxygen (hypoxia) incubator (37°C, 5% CO2) during hypoxia treatment.
The cells were divided into 4 groups: normal oxygen (normoxia) group, hypoxia 48 hours group, hypoxia 48 hours + 3-MA (3-methyladenine) (20 mmol/L) group; hypoxia 48 hours + Baf (Bafilomycin) (50 nmol/L) group. In the last two groups, cells were treated with 3-MA or Baf for 3 hours in advance, then the medium was changed to USCs culture medium. 3-MA and Baf were purchased from Sigma Aldrich (St. Louis, MO, USA).
Flow cytometry analysis
Cells were washed twice and resuspended at a concentration of 1 × 106 cells/mL in phosphate buffer saline (PBS) (Solarbio, Beijing, China). Cells were incubated in the dark at 4°C with antibodies (murine anti-human CD24-FITC, CD29-PE, CD31-FITC, CD34-PE, CD45-FITC, CD73-PE, CD90-PE, CD105-FITC, CD146-PE (Becton, Dickinson and Company, USA). After 30 min, the cell suspensions were washed twice and resuspended in 300 μL PBS for flow cytometry.
Multiple differentiation capability
USCs at P3 were seeded in 24-well plate at approximately 30% cell confluence, followed with osteogenic and adipogenic differentiation induction (Cyagen Biosciences Inc, Guangzhou, China) respectively. The medium was changed every 3 days. Early osteogenic differentiation was stimulated for 7 days, adipogenic and late osteogenic differentiation were stimulated for 14 days. Cells in the 24-well plates were fixed with 4% paraformaldehyde for 30 min for ALP staining (Solarbio, Beijing, China), Alizarin red staining (Solarbio, Beijing, China) and Oil red O staining (Beijing Reagan Biotechnology Co., Ltd.) respectively.
For hepatocyte-induced differentiation, USCs at P3 were individually incubated in 24-well plates or co-incubated with embryonic hepatic progenitor cells (HPCs) [15] in 24-well plate with transwell chambers (Solarbio, Beijing, China) (HPCs in wells of 24-well plate, USCs in transwell chambers) at approximately 30% cell confluence, and induced with hepatic differentiation induction medium as described [16], and the medium was changed once every 3 days. After 10 days of induction Indocyanine green (ICG) (Sigma-Aldrich, USA) uptake release test and PAS staining (Solarbio, Beijing, China) were performed to detect hepatic function as described [17,18].
Cell viability assay
Trypan blue staining was performed to measure cell viability. Cells were digested and plated in the 12-well plates at 5 × 104 cells per well. The whole cell suspensions were collected and mixed with 0.4% trypan blue buffer (Solarbio, Beijing, China) at indicated time points. Cell mixture (10 μL) were counted in the hemocytometer under the microscope (Nikon, Tokyo, Japan), while blue-stained cells were identified as dead cells. Three independent experiments were carried out in duplicate.
Colony formation assay
USCs were planted in 12-well plates at 200 cells per well. After treatment for 7 days, cells were fixed with 4% paraformaldehyde then stained with 0.1% crystal violet. Cell colonies containing more than 50 cells were counted under an inverted microscope (Nikon ECLIPSE Ti, Japan) and the plate clone-forming efficiency was calculated as follows: (number of colonies/number of seeded cells) × 100% [19].
Wound healing assay
Cells were plated in 6-well plates reached a level of 100% confluent monolayer. A consistent gap in the surface of confluent cells was created by a pipette tip across the cell layer. After washed with PBS, cells were incubated with different treatments. Bright-field images of the same wound field were captured at indicated time points [19].
Transwell assay for cell migration
Cells of each group were resuspended with serum-free Dullbecco’s modified Eagle’s medium (DMEM) and then 200 μL cell suspension (3 × 104 per well) was seeded into the upper transwell chambers (8.0 μm, Corning, NY, USA). Medium containing 5% FBS was added into the lower chambers. After 48 h of incubation, cells in the upper chamber were carefully removed, and the remaining cells that have migrated to the lower surface were fixed in 4% paraformaldehyde at room temperature for 30 min and stained with 0.1% crystal violet (Beyotime, Shanghai, China) for 20 min. Five independent fields of view were quantified under a microscope (Nikon, Tokyo, Japan). The stain was dissolved by absolute ethanol and the absorbance was detected at 570 nm with a micro plate reader (Thermo Scientific, MA, USA).
Cell apoptosis assay
An annexin V-FITC apoptosis detection kit (BD, New Jersey, USA) was used in flow cytometry. Cells were resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/mL. Then 5 μL of FITC and 5 μL of PI were added into the solution and incubated for 15 min at room temperature in the dark. Add a 400 μL of 1 × binding buffer into each tube to dilute the solution above and the samples were subsequently analyzed by flow cytometry.
FACS Analysis for cell cycle distribution
The cells were fixed with cold 70% ethanol for 24 h, incubated with RNase A (Solarbio, Beijing, China) and then propidium iodide solution (Solarbio, Beijing, China). Cell cycle distribution was then analysed by flow cytometry using FACS analysis (BD FACS Calibur, BD Biosciences, San Jose, CA, USA). Finally, the percentage of cells in different phases of cell cycle was determined by FlowJo single-cell analysis software (Ashland, OR, USA).
Transmission electron microscopy (TEM) detection
Cells were digested by 0.25% Trypsin, then cell suspension was centrifuged with the rapid of 1500 r/min for 10 min to obtain cell pellet. The pellet samples were subsequently fixed, rinsed, dehydrated, soaked and embedded. The ultrathin sections were observed by TEM in the electron microscope laboratory of the Institute of Life Sciences, Chongqing Medical University.
Western blotting analysis
Total proteins of each group were extracted (Beyotime, Shanghai, China). Equal amounts of protein per group were separated on 15% SDS-PAGE gel (Beyotime, Shanghai, China) and subsequently transferred to PVDF membrane (Millipore, Billerica, MA, USA). After blocked with Quick-Block blocking buffer (Beyotime, Shanghai, China) for 15 min, the membranes were incubated overnight at 4°C with primary antibodies respectively against β-actin (1:2000; Sigma Aldrich, St. Louis, MO, USA), Microtuble-associated protein 1 light chain 3 (LC3) (1:1000; Cell Signaling Technology, MA, USA), Beclin1 (1:1000; Cell Signaling Technology, MA, USA) and P62 (1:1000; Sigma Aldrich, St. Louis, MO, USA), followed by probing with appropriate second antibodies (1:2000, ZSGB-Bio, Beijing, China) for 1 h. Finally, the blots were visualized by using enhanced chemiluminescent substrate (Bio-Rad, CA, USA) and exposed under ChemiDoc Touch Imaging System (Bio-Rad, CA, USA).
Laser scanning confocal microscope to measure the autophagic flux
The dual-fluorescene mRFP-GFP-LC3 plasmid (ptfLC3) were transfected into USCs with Lipofactamine 2000 (Invitrogen, Carlsbad, CA, USA), the laser scanning confocal microscope (Nikon, Tokyo, Japan) was used to dynamically measure the autophagic flux at indicated time points with treatment.
Animal models and cell transplantation
Nude mice (8-week-old males, 20-26 g, qualified number: SCXK (Beijing) 2014-0004) were purchased from Tengxin Institute of Biotechnology (Chongqing, China). All mouse procedures described here were reviewed and approved by the Animal Care and Use Committee of Chongqing Medical University. Nude mice were randomly divided into the acute liver injury group (n=36) and the chronic liver injury group (n=20). In the acute liver injury group (n=36), mice were further divided into the control group (n=6), CCl4 model group (n=6, model group), hepatic progenitor cell (HPC) splenic vein group (n=6), HPC tail vein group (n=6), USC splenic vein group (n=6), and USC tail vein group (n=6). In the chronic liver injury group (n=20), mice were further divided into control group (n=5), CCl4 model group (n=5, model group), USC-transplanted group (n=5, CCl4 + USC group), and hypoxia-pretreated transplanted group (n=5, CCl4 + hypoxia USC group).
To induce acute liver injury in mice, nude mice were injected with 10% (vol/vol) CCl4 (Shanghai Macklin Biochemical Co., Ltd, Shanghai, China) diluted in olive oil at a dose of 20 mL/kg body weight by intraperitoneal injection. Control mice were similarly injected with an equal volume of olive oil alone [20]. After 24 hours of CCl4 treatment, 2 × 106 HPCs or USCs suspended in 0.2 mL of PBS were injected through the tail vein or splenic vein. To induce chronic liver injury, nude mice were injected with 10% (vol/vol) CCl4 at a dose of 10 mL/kg body weight by intraperitoneal injection. After CCl4 treatment for 8 weeks, 2 × 106 USCs or hypoxiapretreated USCs suspended in 0.2 mL of PBS were injected through the tail vein twice a week for 2 weeks. The CCl4 group was administrated with an equal volume (0.2 mL) of PBS only. In each group, two nude mice were transplanted with cells previously labeled with Hoechst 33342 (Beyotime Institute of Biotechnology, Shanghai, China) to detect the extent of colonization by the transplanted cells, while three other mice of each group in chronic liver fibrosis experiment were transplanted using cells previously labeled with PKH26 (Sigma Aldrich, St. Louis, MO, USA).
Assessment of liver function and histopathology
Serum samples were collected for measurements of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Liver tissues were harvested to calculate liver index (=liver wet weight (g)/body weight (g) × 100) and then fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections were cut at a thickness of 4 μm and used for hematoxylin and eosin (H&E) staining (Beijing Reagan Biotechnology Co., Ltd., Beijing, China), Masson staining (Beijing Reagan Biotechnology Co., Ltd., Beijing, China), and immunohistochemistry for detection of α-smooth muscle actin (α-SMA) (1:800, Bioss Beijing, China), myeloperoxidase (MPO) (1:200, Boster, Wuhan, China) and 8-hydroxy-2’-deoxyguanosine ((8-OHdG) 1:400, Bioss, Beijing, China).
For cell tracking, the transplanted USCs were pre-labeled with Hoechst 33342. Liver tissues were embedded in O.C.T. and frozen sections were cut at a thickness of 10 μm and visualized using a fluorescent imaging system.
Statistical analysis
All data were expressed as mean ± standard deviation and analyzed using SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). Statistical analysis was performed by using a two-tailed Student’s t-test to determine significant differences between two groups, while One-Way ANOVA and a post hoc SNK’s test were used to measure significant differences among more than three groups. A P<0.05 was considered statistically significant.
Results
Morphological and molecular characterization of USCs
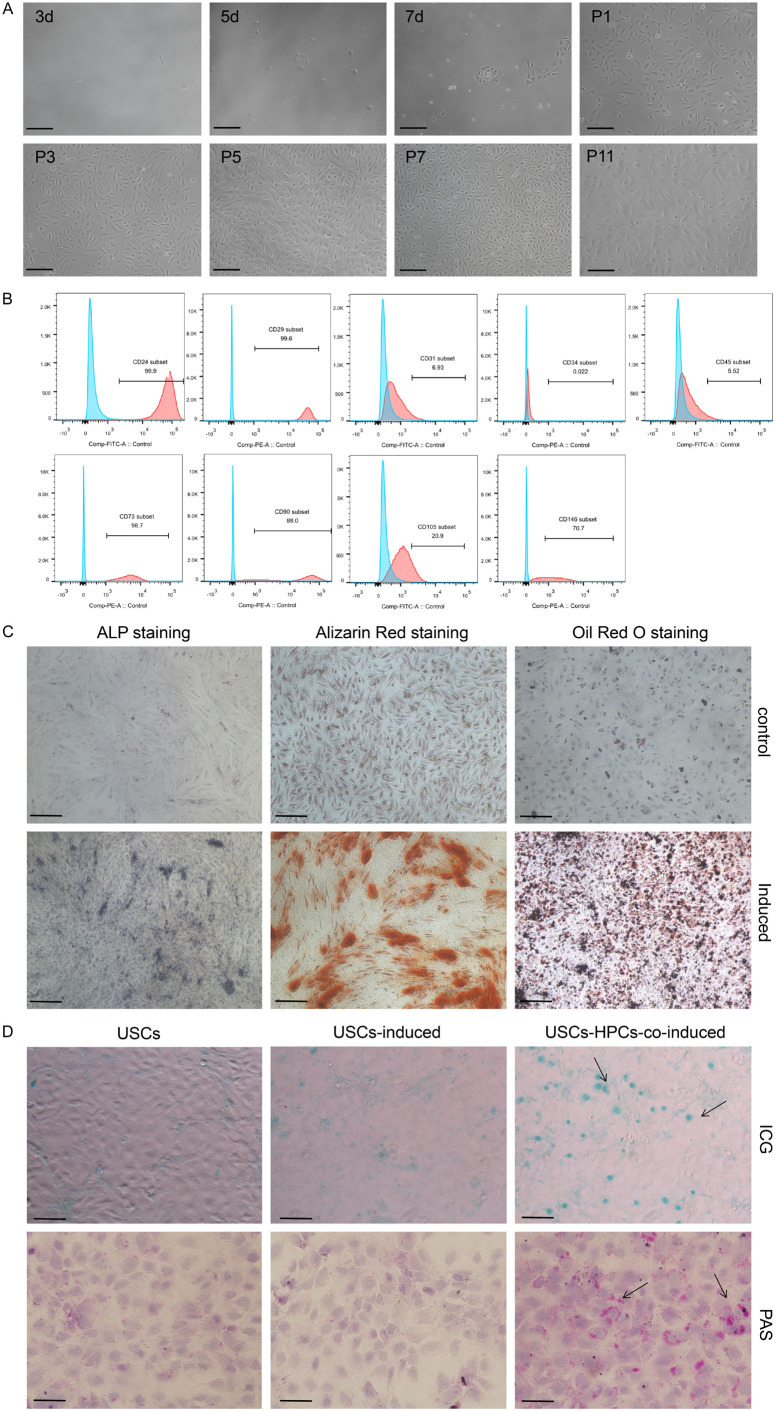
We found that USCs were closely arranged, adherent cells that were granular in shape and uniformly small in size. Primary USCs began to adhere around 3 days after seeding and reached a confluence of 50-60% after 10-14 days. Upon passaging, USCs exhibited accelerated growth and features of stem cells in vitro. The proliferation of cells was significantly reduced after 10 passages (Figure 1A). Flow cytometry analysis showed that USCs expressed mesenchymal stem cell surface markers; CD24 (97.85±2.62%), CD29 (99.95±0.07%), CD73 (95.15±1.06%), CD90 (95.20±6.65), and CD105 (2.22±2.01%); and also the endothelial cell surface marker CD146 (96.45±4.74%). The hematopoietic cell surface markers CD31 (1.69±1.68%), CD34 (0.28±0.03%), and CD45 (0.80±0.42%) were negligibly expressed in USCs (Figure 1B). These results suggest that USCs resemble mesenchymal stem cells.
Figure 1.
Morphological, molecular, and differentiation characteristics of USCs. A. Cell morphology of USCs on different days of primary passage and additional passages. B. FACS analysis for determining surface antigen expression on passage 3 human USCs. C. Alkaline phosphatase (ALP) staining, alizarin red staining and oil red O staining to measure differentiation potential. D. Indocyanine green (ICG) uptake assay: cells with a green-stained nucleus are the positive-stained cells. Periodic acid-Schiff (PAS) staining, purple color in cell plasma indicates glycogen accumulation. Scale bar =100 μm.
Characterization of the differentiation potential of USCs
ALP staining and alizarin red staining have commonly been used for detecting osteogenic differentiation. Cytoplasmic purple-blue staining using ALP is indicative of early osteogenic differentiation, while alizarin red stains for calcium deposits and is indicative of functional osteocytes. Uninduced USCs were apparently unstained, whereas about 20-30% of cells were positively stained for ALP after 7 days of osteogenic induction. After 14 days of osteogenic induction, alizarin red staining significantly increased with about 50% of cells staining red and the appearance of some calcium nodules, which indicates calcium salt deposition and late osteogenic differentiation of USCs. Oil red O staining was applied to examine the ability of USCs to undergo adipogenic differentiation. Uninduced USCs remained unstained. After 14 days of adipogenic induction, approximately 30-40% of cells showed vacuolar, lipid droplets in the cytoplasm and red staining (Figure 1C). Together, these data suggest that USCs might have the potential to differentiate into osteoblasts and adipocytes.
Periodic acid-Schiff (PAS) staining and indocyanine green (ICG) uptake assay were performed to detect glycogen storage and metabolic functioning of mature hepatocytes. To induce hepatic differentiation of USCs, we used a previously described induction method, which could efficiently induce maturation and differentiation of HPCs. After 10 days of hepatic induction, ICG uptake and PAS staining in USCs were undetectable. However, when USCs were co-cultured with HPCs and induced to undergo hepatic differentiation, 8-15% of them exhibited green staining in the nucleus or purple granules in the cytoplasm (Figure 1D). These results indicate that USCs might have the potential to differentiate into functional hepatocytes in the hepatic microenvironment.
USCs can normalize liver function and pathology following acute liver injury in mice
We used previously constructed immortalized HPCs to evaluate their ability to improve the recovery efficiency of acutely injured liver in vivo. Because USCs have the ability to differentiate into functional hepatocytes, they were treated as an additional experimental group. Compared to the control group, the liver index and serum levels of ALT and AST significantly increased in the CCl4-treated acute liver injury mice (Figure 2A-C, P<0.001). This was accompanied by severe hepatic cord disorder and hepatocyte degeneration, hepatocyte steatosis, vacuolar degeneration, and nuclear condensation or disappearance in the liver tissue. With HPC and USC transplantation, the liver index, ALT/AST level and hepatic pathological changes partly recovered, and the recovery efficiency elicited by HPCs was better than that by USCs (Figure 2A-C, 2E). Meanwhile, the number of exogenous cells implanted into the liver of model mice by splenic vein transplantation is much more than that by tail vein transplantation, and the subsequent benefit to liver function and repair is also improved (Figure 2D). These results suggested that USCs could partly recover the liver function following acute liver injury. USCs can therefore be used as a stable cell source for HCT and has the advantage of being an autologous cell source. However, successful colonization and survival of exogenous cells in the liver tissue is key to its therapeutic benefit.
Figure 2.
USCs resolved the functional impairment and pathology associated with acute liver injury in a mouse model. (A) Evaluation of the liver index, serum level of (B) alanine aminotransferase (ALT), and (C) aspartate aminotransferase (AST). (D) Exogenous cells were visualized using fluorescent imaging. The number of cells in splenic vein transplantation group is much greater than that in the tail vein transplantation group. (E) Representative H&E images for the different conditions. *P<0.05, **P<0.01, ***P<0.001 one-way ANOVA. Scale bar =100 μm.
Hypoxia pretreatment affects the biological behavior of USCs in vitro
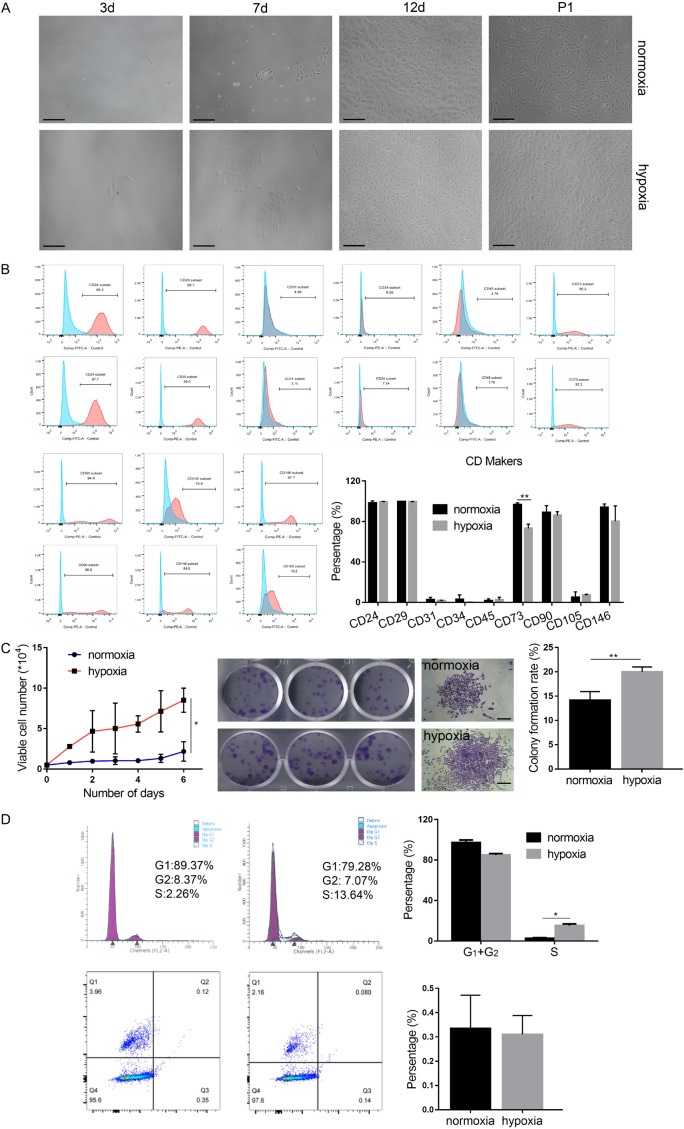
In order to enhance the curative effect of stem cell therapy, in vitro pretreatment of stem cells has previously been used. An appropriate levelof hypoxicpreconditioninghas been reported toimprove survival rate and viability of implanted cells in the relativelyhypoxicenvironment encountered by the cells in vivo. In our study, we found that USCs cultured continuously in hypoxic condition were smaller and more homogeneous compared to those cultured in normoxic conditions (Figure 3A). Flow cytometry analysis showed that a 48-hour hypoxia pretreatment had no significant effect on the expression level of cell surface markers of USCs (Figure 3B). Cell growth in both normoxic and hypoxic USCs remained logarithmic. However, compared to the normoxic environment, cell proliferation was faster in a hypoxic environment and a greater number and size of colonies were observed (Figure 3C). After 48 hours of hypoxic preconditioning, the percentage of cells inSphaseincreasedsignificantly(P<0.05) and theapoptoticrate displayed no noticeable differences (Figure 3D). Additionally,cell migration was promoted after 48 hours of hypoxic preconditioning (Figure 4A, 4B). Differentiation potential was not significantly different between the two groups as assessed by ALP andalizarin red staining following osteogenic induction, and oil red O staining following adipogenic induction (Figure 4C).These results indicate that hypoxia pretreatment could accelerate the cellcycle, promote cell proliferation and colony formation, and improve cell migration but did not affect cell apoptosis and differentiation.
Figure 3.
Hypoxia pretreatment improves cell morphology, promotes cell proliferation, and colony formation, but does not affect apoptosis. A. Morphological comparison of USCs under normoxic and hypoxic culture conditions. B. Statistical analysis histograms and comparison of cell surface marker expression levels in USCs. C. Cell growth curve, colony formation assay, and its statistical analysis. D. Cell cycle and apoptosis measured by flow cytometry. *P<0.05, **P<0.01, ***P<0.001, Student’s t-test. Scale bar =100 μm.
Figure 4.
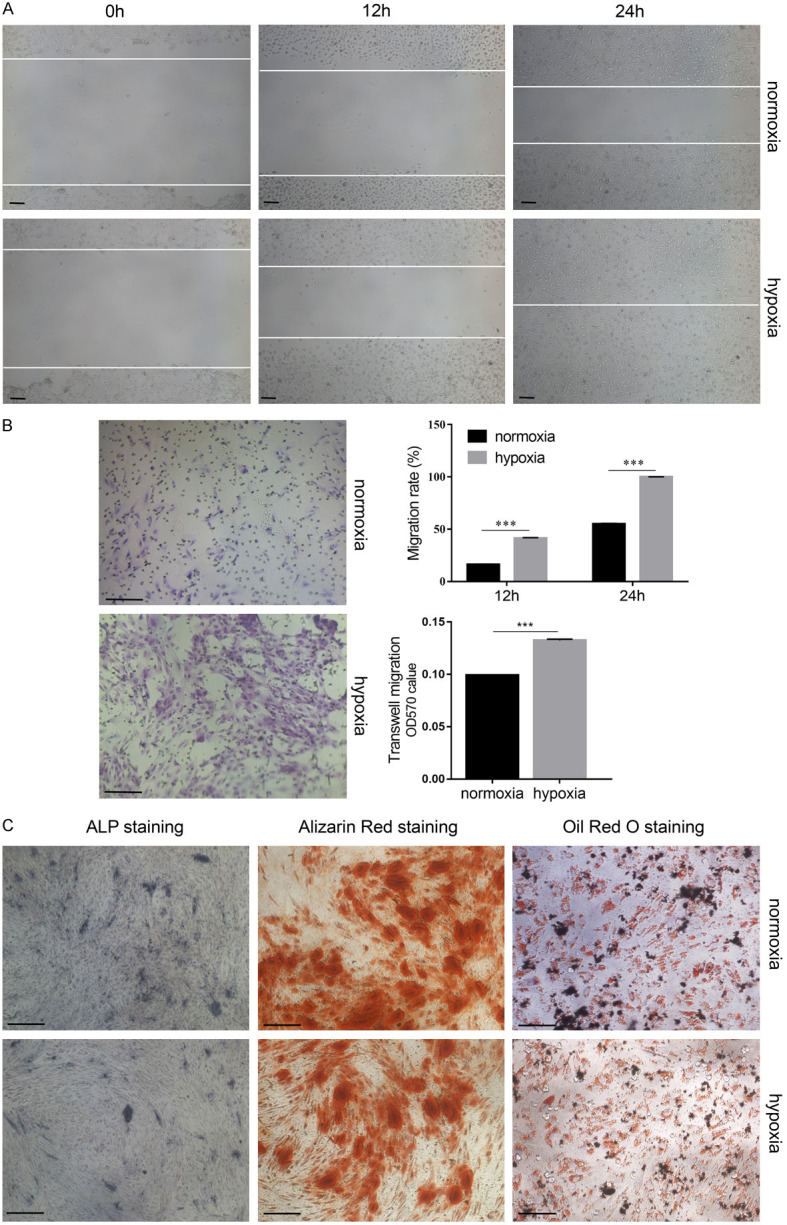
Hypoxia pretreatment promotes cell migration but does not affect cell differentiation. The migration of USCs was determined by (A) wound-healing assay and (B) transwell migration assay, and its statistical analysis. (C) The differentiation potential of USCs was determined by alkaline phosphatase (ALP) staining, alizarin red staining, oil red O staining and Alcian blue staining. ***P<0.001, Student’s t-test. Scale bar =100 μm.
Hypoxia pretreatment of USCs improves recovery efficiency in a chronic liver fibrosis mouse model
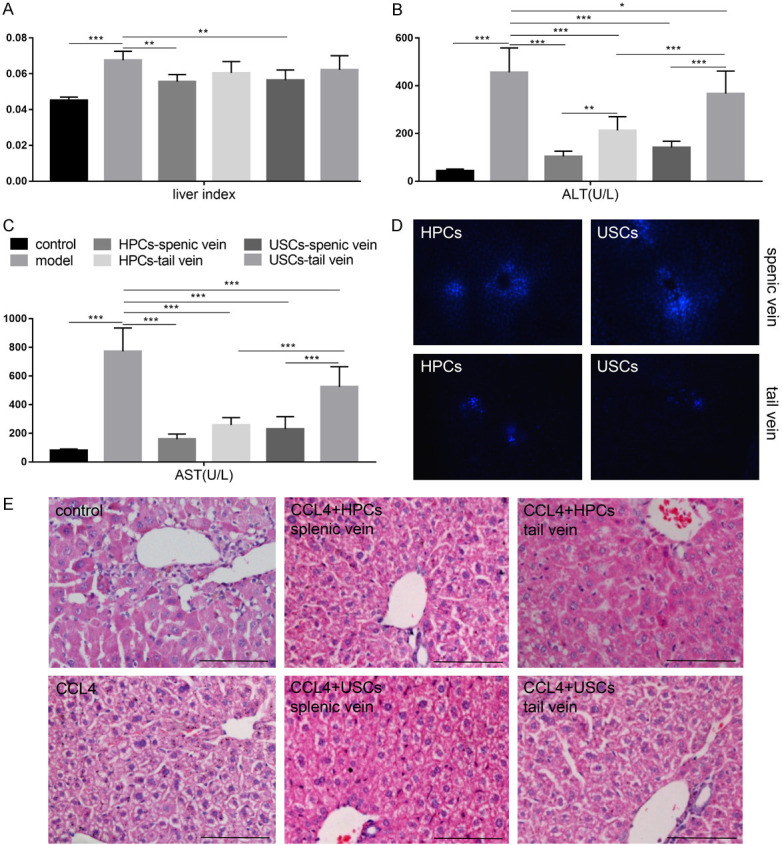
Intraperitoneal injection of 10% CCl4 was performed for 8 weeks to induce chronic liver fibrosis in nude mice. USCs and hypoxia-pretreated USCs were transplanted in vivo through the tail vein twice a week. After four rounds of cell injection, the number of exogenous cells that implanted in liver was greater in hypoxia-pretreated USCs compared to untreated USCs (Figure 5A). Compared to the control group, the liver index and the levels of serum ALT and AST were significantly higher in the chronic CCl4-treated model (Figure 5A, P<0.05). Upon USC transplantation, the liver index and the level of ALT were unchanged, and the level of serum AST partially recovered (Figure 5A, P<0.05). There were no differences in the liver index or levels of ALT and AST between the USC group and the hypoxia-pretreated USC group. H&E staining showed that although the control liver tissue structure was normal and the hepatic lobules intact (Figure 5B), liver tissue of the CCl4 model group displayed obvious alterations of normal structure and composition, including hepatocyte degeneration, disordered hepatic cord structure, nuclear pyknosis or disappearance, island pseudolobules, and infiltration of inflammatory cells in the portal area (Figure 5B). Masson staining showed plenty of blue collagen staining, indicating fibrosis around the portal area in the chronic CCl4-treated group (Figure 5B). Upon USC transplantation, the disorganization of the liver pathological structure significantly recovered (Figure 5B), and blue-stained collagen fibers were obviously reduced (Figure 5B, 5D, P<0.05). The blue-stained area was further decreased upon transplantation of hypoxia-pretreated USCs. The basal expression levels of the fibrosis marker α-smooth muscle actin (α-SMA) and oxidative stress-related genes, MPO and 8-OHdG, were very low in normal liver tissue and were obviously increased in the chronic CCl4-treated group. Upon USC transplantation, the expression levels of α-SMA, MPO, and 8-OHdG were markedly reduced, but no differences were observed between the USC and hypoxia-pretreated USC groups (Figure 5C, 5D). These results indicate that USC transplantation could partially recover liver function and resolve fibrosis following chronic liver injury. Furthermore, hypoxia pretreatment may promote migration/homing rate and cell viability, thus marginally improving the recovery efficiency elicited by USCs following chronic liver fibrosis.
Figure 5.
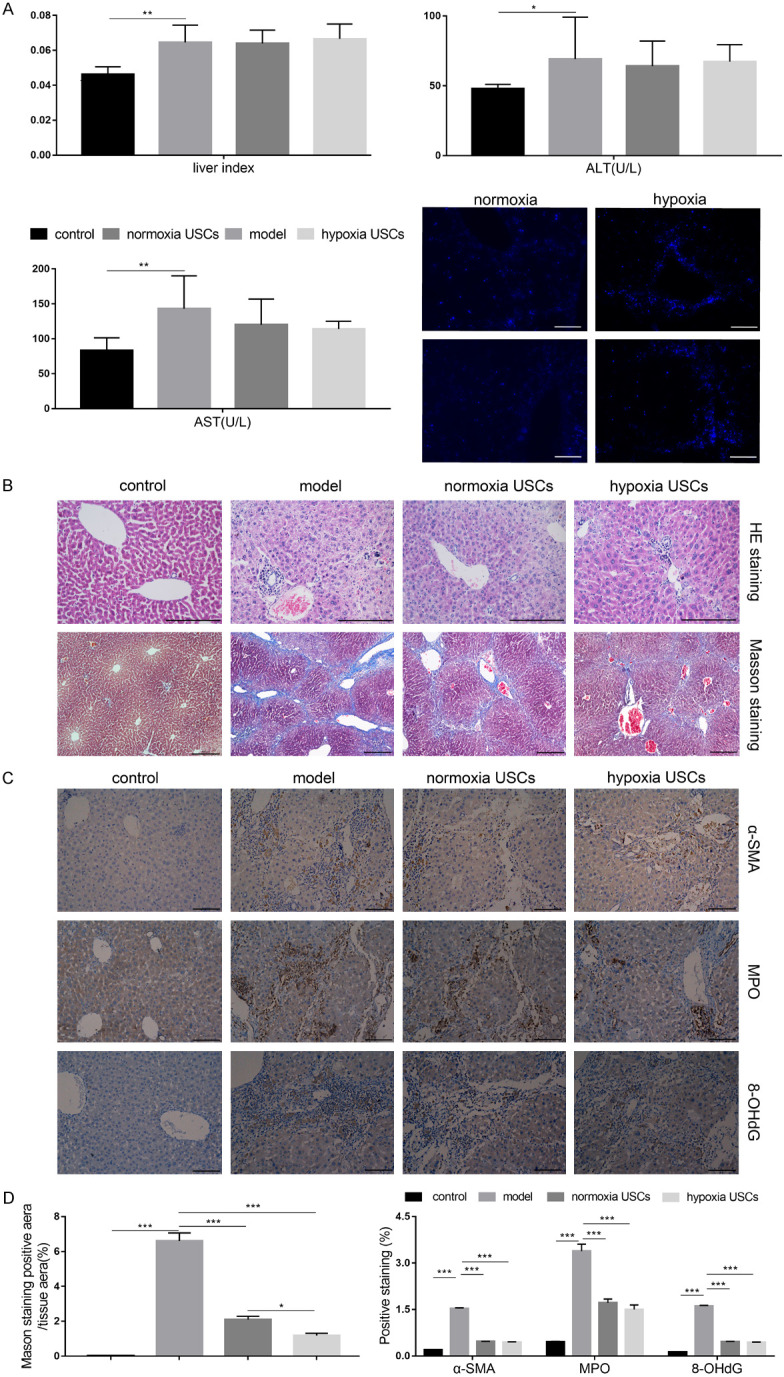
Hypoxia pretreatment can improve the recovery efficiency elicited by USCs in a chronic liver fibrosis mouse model. A. Evaluation of liver index and the serum level of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Exogenous cells were visualized using fluorescent imaging. B. Representative H&E and Masson staining images. C. Representative images of immunohistochemistry for α-SMA, MPO, and 8-OHdG. D. Statistical quantification of Masson staining and immunohistochemistry. *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA. Scale bar =200 μm. (α-SMA - α-smooth muscle actin, MPO - myeloperoxidase, 8-OHdG - 8-hydroxy-2’-deoxyguanosine).
Hypoxia pretreatment promotes cell proliferation, migration, and colony formation by inducing autophagy
Hypoxia is a type of environmental stress, and autophagy is an active regulatory response that enables cells to adapt to this stress. After 48 hours of hypoxia pretreatment, a large number of autophagosomes with bi-layered or multi-layered membrane structures encapsulating organelles could be observed by transmission electron microscopy (TEM) (Figure 6A). At the same time, large autophagic lysosomes were formed by fusion of lysosomes and autophagosomes, which were seldom seen in the normoxia group. Beclin1 and LC3 are typical markers of autophagy. The protein levels of Beclin1 and LC3-II, as well as the LC3-II/LC3-I ratio, were significantly increased after 48 hours of hypoxia treatment in USCs (Figure 6B, P<0.001). USCs were transfected with the LC3 double-fluorescent plasmid, and confocal laser scanning was used to analyze the autophagic state of the cells. Confocal imaging showed only a small amount of autophagosomes (yellow spots) and autophagic lysosomes (red spots) in the normoxic group, whereas more autophagosomes and autophagic lysosomes appeared in the hypoxia pretreatment group (Figure 6C). Therefore, autophagy can be predictably induced by hypoxia treatment in USCs.
Figure 6.
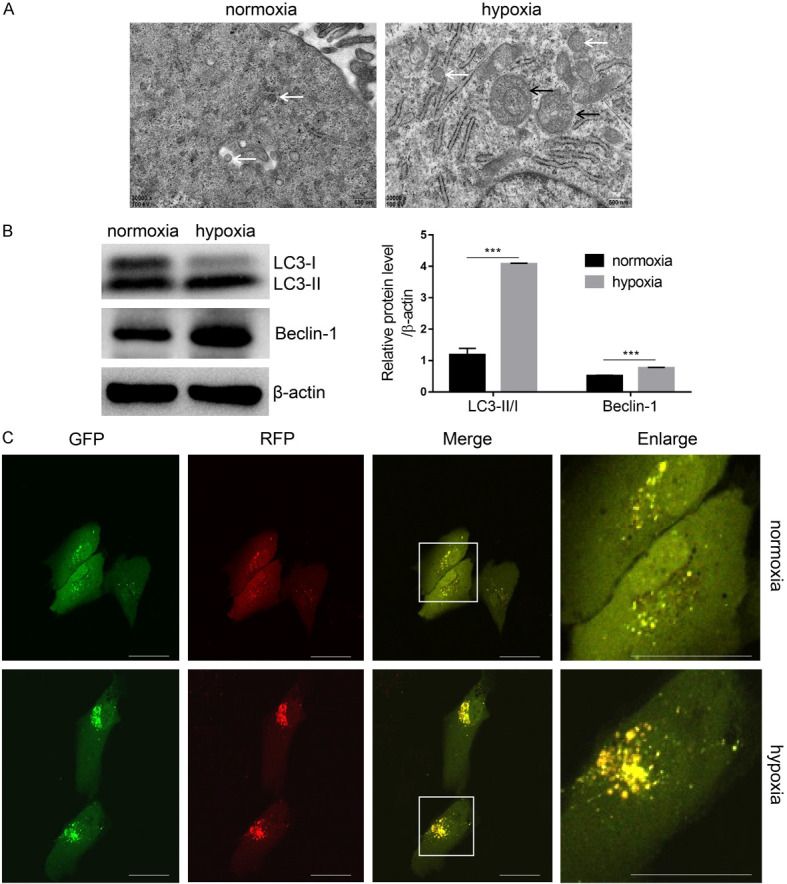
Hypoxia pretreatment promotes cell autophagy. A. Autophagosomes were observed under transmission electron microscopy (TEM). B. Autophagy-related markers LC3 and Beclin1 were analyzed by western blot with β-actin normalization. C. USCs were transfected with ptfLC3 and treated as above described. Autophagic flux was dynamically observed by laser scanning confocal microscope. ***P<0.001, Student’s t-test. Scale bar =200 μm. (White arrow indicates autophagosome, black arrow indicates autophagic lysosome).
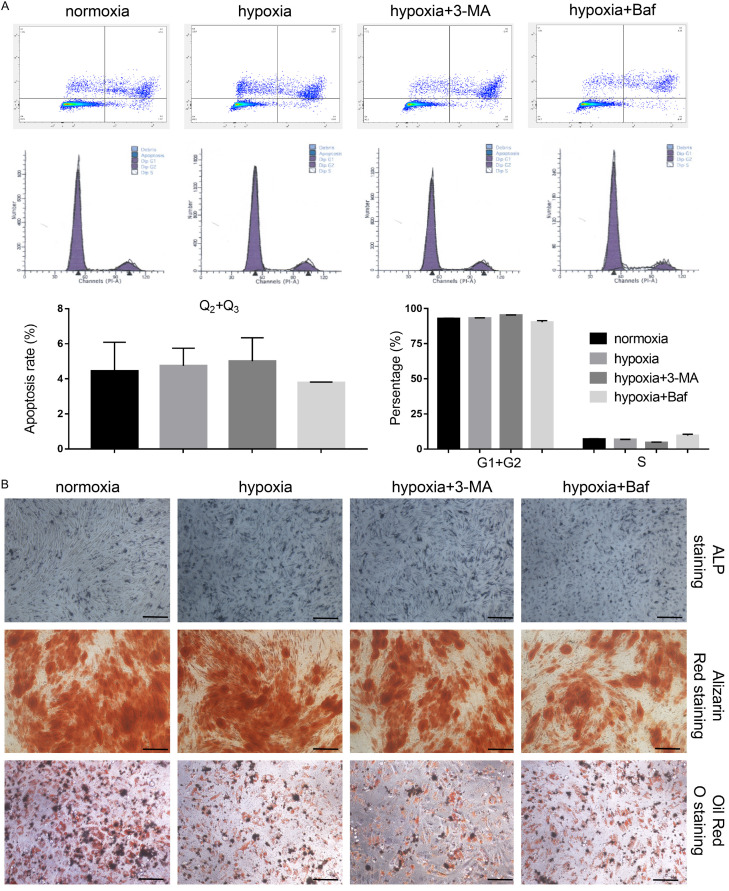
Next, we used 3-Methyladenine (3-MA) and Bafilomycin to inhibit autophagy. Hypoxia induced the expression of Beclin1, P62, and LC3-II, and the LC3-II/LC3-I ratio was significantly de-creased after 3-MA and Bafilomycin treatment (Figure 7A, P<0.001). Upon inhibition of autophagy under hypoxic conditions, cell proliferation and colony size was significantly decreased, especially in the Bafilomycin-treated group (Figure 7B, 7C, P<0.001). Scratch wound in a monolayer of USCs in the hypoxia group almost entirely healed within 48 hours after scratch creation, but the wound-healing rate was significantly decreased in 3-MA and Bafilomycin groups, suggesting that cell migration was also inhibited upon autophagy inhibition (Figure 7D, P<0.001). Similarly, lower cell numbers were observed in a transwell assay in 3-MA and Bafilomycin-treated groups than in the untreated hypoxia group. Apoptosis and cell cycle displayed no significant differences among the treated groups (Figure 8A). Additionally, the differentiation potential of USCs was unaffected by hypoxic culture conditions and cellular autophagy levels (Figure 8B). Therefore, these results suggest that hypoxia pretreatment might promote USC proliferation, migration, and colony formation by inducing cellular autophagy.
Figure 7.
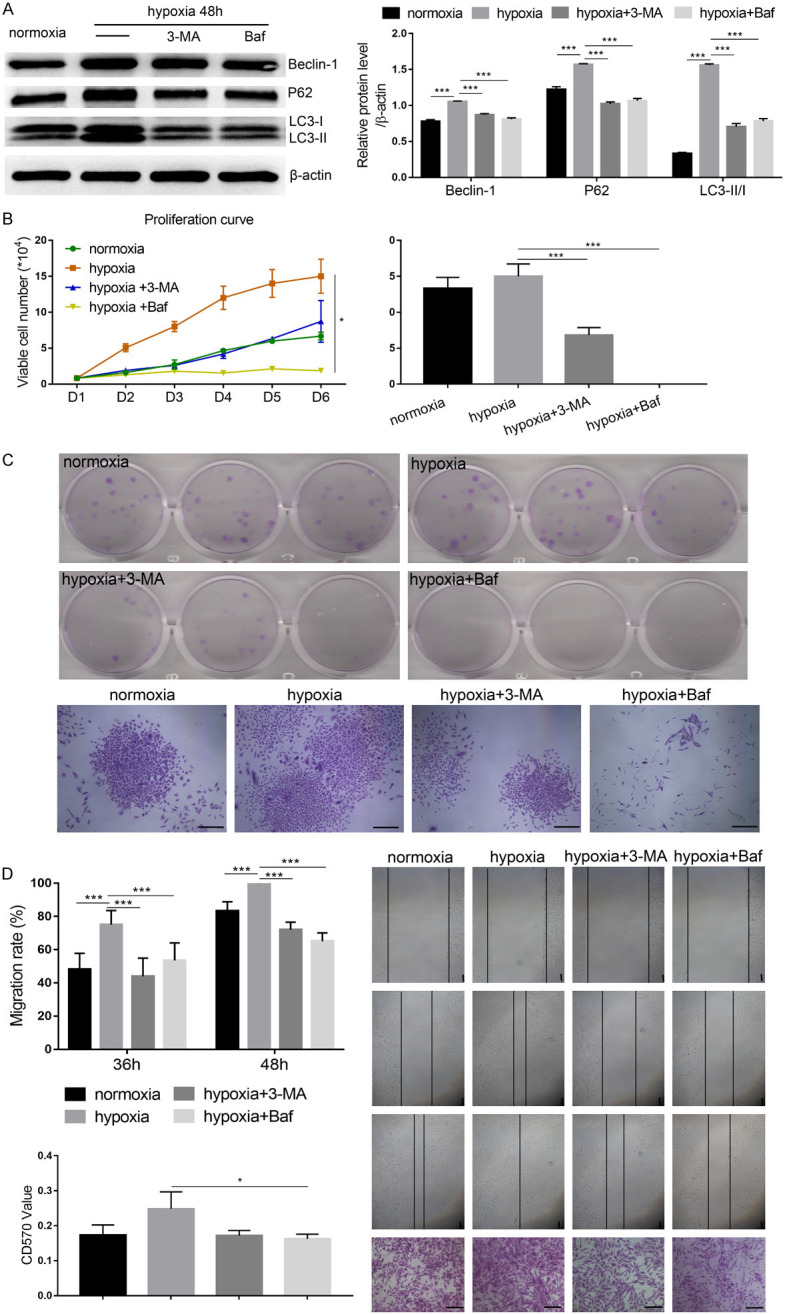
3-Methyladenine (3-MA) and Bafilomycin (Baf) could reverse the effect of hypoxia-induced USC proliferation and migration by inhibiting autophagy. A. Autophagy-related markers were detected by western blot. B. Cell growth was measured by trypan blue staining. C. Cell proliferation was determined by colony formation assay. D. The migration of USCs was determined by a wound-healing assay and transwell migration assay. *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA. Scale bar =100 μm.
Figure 8.
3-Methyladenine (3-MA) and Bafilomycin (Baf) had no significant effect on apoptosis, cell cycle, and differentiation potential of USCs. A. Apoptosis and cell cycle phase were measured by flow cytometry. B. The differentiation potential of USCs was determined by alkaline phosphatase (ALP) staining, alizarin red staining and oil red O staining. Scale bar =100 μm.
Discussion
USCs display a high proliferative capacity and characteristics of mesenchymal stem cells, which have the ability to differentiate into multiple lineages. Furthermore, USCs have the ability to regulate the immune system and induce paracrine effects in the surrounding cells [14,21,22]. In vitro experiments have demonstrated that USCs can differentiate into osteocytes, chondrocytes, adipocytes, and smooth muscle cells (mesoderm), as well as functional epithelial cells (endoderm) and nerve cells (ectoderm) [23,24]. USCs have immunomodulatory functions that can inhibit the proliferation of T and B cells in the peripheral blood. USCs also elicit paracrine effects by secreting a variety of nutrient factors, such as vascular endothelial growth factor, fibroblast growth factor, platelet-derived growth factor, insulin-like growth factor, and hepatic growth factor [25,26]. These factors help promote angiogenesis in the regenerated tissues and improve the survival of transplanted cells. After several generations of culture, the chromosome numbers in USCs remain stable and no tumors form after subcutaneous or subrenal capsule transplantation in nude mice after more than 3 months [27].
In this study, we have confirmed that USCs have multi-lineage differentiation potential. USCs are likely stem cells from the kidney that is mesodermal in origin. Thus, USCs have the ability to differentiate into other mesodermal cells such as osteocytes and adipocytes, but not into endodermal hepatocytes. USCs did not respond to the hepatic differentiation induction medium, which is specifically formulated for HPCs derived from liver tissue. However, a small number of USCs demonstrated anabolism when co-cultured with HPCs, suggesting that USCs may have the potential to differentiate into hepatocytes in a liver-mimetic microenvironment. These data suggest that USCs can serve as seed cells for HCT. In our previous study, we constructed a reversibly immortalized HPC line and verified its transplantation efficiency in an acute liver injury mouse model. Herein, USCs were set as an experimental group, and we found that USC transplantation could partially restore liver function and attenuate the histopathology associated with acute liver injury. The number of exogenous cells that successfully implanted in the liver of model mice by tail vein transplantation was far less than by splenic vein transplantation and the restorative effect on liver function was consequently weaker upon splenic vein transplantation. In the clinic, liver cell transplantation may require multiple rounds of transplantation. Peripheral vein transplantation is minimally invasive and simple to perform, which can minimize the pain experienced by patients. Therefore, devising a means to improve the homing ability of exogenous cells to the liver and enhancing the viability of transplanted cells is key to improving the efficiency of HCT.
We reasoned that the concept of tissue-cell preconditioning tohypoxiacould provide a solution. In recent years, various methods of pretreatment in vitro have been used to improve cell viability in vitro and in vivo, including gene modification, drug pretreatment, and co-culture [28-31]. Hypoxic preconditioning is the simplest, safest and most effective method and has been used for the repair of heart, lung, liver, brain and skeletal muscle injury [32]. Normally, cells experience a physiological oxygen level of 2-5% in tissues. However, most cell culture experiments in vitro are performed in a normal oxygen (20% O2) incubator. When incubation time exceeds 24 hours under the normal oxygen conditions in vitro, the number of directionally homing cells is significantly reduced. It has also been reported that exposure to hypoxia in vitro can enhance the proliferation and directional differentiation of mesenchymal stem cells, adipose stem cells, and germ stem cells, while promoting cell homing, migration, and angiogenesis in vivo [33-35]. In this study, we found that hypoxia can significantly enhance USC proliferation, migration, and colony formation in vitro, but did not affect its differentiation capability.
USCs have a high proliferation capacity-a single USC can generate 3.8 ×108 cells after 4 weeks of culture. The average number of cells generated from a 200 mL urine sample can reach about 5.0 ×109 at 4 weeks after 5 passages. Thus, a sufficient number of cells can be obtained from 24 hours of urine collection. The advantage of using USCs for transplantation is that it can be an autologous cell source, which can completely avoid autoimmune rejection following transplantation. Traditional autologous cell sources require several weeks of collection to obtain sufficient cell numbers. Thus, USCs may be a more suitable alternative for the treatment of chronic liver diseases. Upon CCl4 administration, the liver tissue exhibited obvious degeneration of hepatocytes and fibrosis, disordered liver tissue structure, and false lobules. After stopping CCl4 treatment, the pathological damage in liver tissue persisted for the following 2 weeks, suggesting that the model was stable and can be used to evaluate the effect of USC transplantation. In the CCl4-induced chronic liver fibrosis model, the levels of serum ALT and AST in the model group were higher than in the normal group, but the absolute value was much lower than that in the acute liver injury model. In this study, we simulated peripheral vein transplantation in the clinic by transplanting USCs into the tail (caudal) vein four times within 2 weeks. The liver index in the transplantation group was significantly reduced, while histology showed resolution of liver fibrosis and decreased hepatocyte necrosis, indicating that USC transplantation could partially repair the pathological changes associated with chronic liver injury. Furthermore, hypoxia pretreatment could improve the recovery of injured liver tissue elicited by USCs. We used Hoechst-labeled USCs for in vivo tracing and found that only a few implanted cells persisted in the injured liver tissue of the USC-transplanted group, whereas more labeled cells were found in the hypoxia-pretreated group. These results indicate that hypoxia pretreatment promotes homing, migration, and cell viability of USCs in vivo. Whether exogenous cells differentiate into functional hepatocytes or improve liver function by secreting nutrient factors remains to be further investigated.
Autophagy is a beneficial metabolic pathway, which maintains the stability of the internal cellular environment by digesting damaged, degenerated and aging proteins and organelles, recycling their components, and maintaining cell viability [36-40]. Autophagy has been implicated in the maintenance and proliferation of adult hematopoietic stem cells, BMSCs, and glioblastoma stem cells. Deferoxamine can upregulate reactive oxygen species (ROS)-induced autophagy and promote the migration of dental pulp cells [41]. Autophagy often occurs in cells in response to stress, nutritional deficiency, or hypoxia. Hypoxia triggers autophagy in BMSCs and stimulates the migration of umbilical vein endothelial cells via paracrine effects [42]. In this study, autophagy in USCs was stimulated by hypoxic pretreatment. Inhibition of autophagy by 3-MA and Bafilomycin could reverse hypoxia-induced cell proliferation, migration, and colony formation. Therefore, autophagy may be an important regulatory mechanism that is activated in response to hypoxia, which promotes the migration and homing of USCs in vivo. These data lay a critical theoretical foundation for the application of hypoxic preconditioning of USCs prior to HCT.
This study has confirmed that USCs have a similar cell phenotype to mesenchymal stem cells along with the potential for self-renewal and multi-lineage differentiation. USC transplantation into acute or chronic liver-injury mouse models can effectively improve liver function and recover liver tissue damage. Hypoxia pretreatment might promote cell proliferation, migration, and colony formation by inducing autophagy, which can promote USC-elicited liver tissue recovery following injury in vivo. Autologous USCs thus obtained by a non-invasive method are an ideal cell source for HCT and have a promising potential as treatment for chronic liver diseases in the clinic.
Acknowledgements
The present study was supported by research grants from the Chongqing Science and Technology Commission (CN) (No. cstc2018jcyjAX0111 to Yun He, csct2018jscx-msybX0048 to Yang Bi).
Disclosure of conflict of interest
None.
References
- 1.Wang F, Zhou L, Ma X, Ma W, Wang C, Lu Y, Chen Y, An L, An W, Yang Y. Monitoring of intrasplenic hepatocyte transplantation for acute-on-chronic liver failure: a prospective five-year follow-up study. Transplant Proc. 2014;46:192–8. doi: 10.1016/j.transproceed.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Koblihová E, Lukšan O, Mrázová I, Ryska M, Červenka L. Hepatocyte transplantation attenuates the course of acute liver failure induced by thioacetamide in Lewis rats. Physiol Res. 2015;64:689–700. doi: 10.33549/physiolres.932914. [DOI] [PubMed] [Google Scholar]
- 3.Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–7. doi: 10.1097/TP.0b013e31823b72d6. [DOI] [PubMed] [Google Scholar]
- 4.Pareja E, Cortés M, Gómez-Lechón MJ, Maupoey J, San Juan F, López R, Mir J. Current status and future perspectives of hepatocyte transplantation. Cir Esp. 2014;92:74–81. doi: 10.1016/j.ciresp.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Magner NL, Jung Y, Wu J, Nolta JA, Zern MA, Zhou P. Insulin and IGFs enhance hepatocyte differentiation from human embryonic stem cells via the PI3K/AKT pathway. Stem Cells. 2013;31:2095–2103. doi: 10.1002/stem.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng F, Francis H, Glaser S, Han Y, DeMorrow S, Stokes A, Staloch D, Venter J, White M, Ueno Y, Reid LM, Alpini G. Role of stem cell factor and granulocyte colony-stimulating factor in remodeling during liver regeneration. Hepatology. 2012;55:209–221. doi: 10.1002/hep.24673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin N, Lin J, Bo L, Weidong P, Chen S, Xu R. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells in an alginate scaffold. Cell Prolif. 2010;43:427–434. doi: 10.1111/j.1365-2184.2010.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgkinson CP, Naidoo V, Patti KG, Gomez JA, Schmeckpeper J, Zhang Z, Davis B, Pratt RE, Mirotsou M, Dzau VJ. Abi3bp is a multifunctional autocrine/paracrine factor that regulates mesenchymal stem cell biology. Stem Cells. 2013;31:1669–1682. doi: 10.1002/stem.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Dolado M, Martínez-Losa M. Cell fusion and tissue regeneration. Adv Exp Med Biol. 2011;713:161–175. doi: 10.1007/978-94-007-0763-4_10. [DOI] [PubMed] [Google Scholar]
- 10.Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci. 2010;365:155–163. doi: 10.1098/rstb.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin D, Long T, Deng J, Zhang Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther. 2014;5:69. doi: 10.1186/scrt458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Q, Chen X, Zheng T, Han D, Zhang H, Shi Y, Bian J, Sun X, Xia K, Liang X, Liu G, Zhang Y, Deng C. Transplantation of human urine-derived stem cells transfected with pigment epithelium-derived factor to protect erectile function in a rat model of cavernous nerve injury. Cell Transplant. 2016;25:1987–2001. doi: 10.3727/096368916X691448. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–2233. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC, Luo J, Wang Y, Kang Q, Luo Q, Chen L, Zuo GW, Jiang W, Liu B, Shi Q, Tang M, Zhang BQ, Weng Y, Huang A, Zhou L, Feng T, Luu HH, Haydon RC, He TC, Tang N. Wnt antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. J Cell Biochem. 2009;108:295–303. doi: 10.1002/jcb.22254. [DOI] [PubMed] [Google Scholar]
- 16.Bi Y, He Y, Huang JY, Xu L, Tang N, He TC, Feng T. Induced maturation of hepatic progenitor cells in vitro. Braz J Med Biol Res. 2013;46:559–566. doi: 10.1590/1414-431X20132455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui H, Ma W, Cui J, Gong M, Wang Y, Zhang Y, He T, Bi Y, He Y. Periodic acid-Schiff staining method for function detection of liver cells is affected by 2% horse serum in induction medium. Mol Med Rep. 2017;16:8062–8068. doi: 10.3892/mmr.2017.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Zhou JW, Xu L, Gong MJ, He TC, Bi Y. Comparison of proliferation and differentiation potential between mouse primary hepatocytes and embryonic hepatic progenitor cells in vitro. Int J Mol Med. 2013;32:476–484. doi: 10.3892/ijmm.2013.1413. [DOI] [PubMed] [Google Scholar]
- 19.Cui J, Gong M, He Y, Li Q, He T, Bi Y. All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1-6 cells through reversing EMT in vitro. Int J Oncol. 2016;48:349–357. doi: 10.3892/ijo.2015.3235. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen-Lefebvre AT, Ajith A, Portik-Dobos V, Horuzsko DD, Arbab AS, Dzutsev A, Sadek R, Trinchieri G, Horuzsko A. The innate immune receptor TREM-1 promotes liver injury and fibrosis. J Clin Invest. 2018;128:4870–4883. doi: 10.1172/JCI98156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889–8901. doi: 10.1016/j.biomaterials.2010.07.108. [DOI] [PubMed] [Google Scholar]
- 22.Bharadwaj S, Liu G, Shi Y, Markert C, Andersson KE, Atala A, Zhang Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng Part A. 2011;17:2123–2132. doi: 10.1089/ten.tea.2010.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, Fan Y, Lu X, Zhou X, Liu H, Atala A, Rohozinski J, Zhang Y. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells. 2013;31:1840–1856. doi: 10.1002/stem.1424. [DOI] [PubMed] [Google Scholar]
- 24.Yi H, Xie B, Liu B, Wang X, Xu L, Liu J, Li M, Zhong X, Peng F. Derivation and identification of motor neurons from human urine-derived induced pluripotent stem cells. Stem Cells Int. 2018;2018:3628578. doi: 10.1155/2018/3628578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Wei G, Li P, Zhou X, Zhang Y. Urine-derived stem cells: a novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014;1:8–17. doi: 10.1016/j.gendis.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Pareta RA, Wu R, Shi Y, Zhou X, Liu H, Deng C, Sun X, Atala A, Opara EC, Zhang Y. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials. 2013;34:1311–1326. doi: 10.1016/j.biomaterials.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–1326. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Mortezaee K, Khanlarkhani N, Sabbaghziarani F, Nekoonam S, Majidpoor J, Hosseini A, Pasbakhsh P, Kashani IR, Zendedel A. Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4 . Cell Tissue Res. 2017;369:303–312. doi: 10.1007/s00441-017-2604-1. [DOI] [PubMed] [Google Scholar]
- 29.Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ML, Lee KD, Huang HC, Tsai YL, Wu YC, Kuo TM, Hu CP, Chang C. HNF-4α determines hepatic differentiation of human mesenchymal stem cells from bone marrow. World J Gastroenterol. 2010;16:5092–5103. doi: 10.3748/wjg.v16.i40.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng J, Yao W, Zhang Y, Xiang AP, Yuan D, Hei Z. Intravenous anesthetics enhance the ability of human bone marrow-derived mesenchymal stem cells to alleviate hepatic ischemia-reperfusion injury in a receptor-dependent manner. Cell Physiol Biochem. 2018;47:556–566. doi: 10.1159/000489989. [DOI] [PubMed] [Google Scholar]
- 32.Hu C, Wu Z, Li L. Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med. 2020;24:40–49. doi: 10.1111/jcmm.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Kim HS, Kim SM, Kim DI, Lee CW. Hypoxia upregulates mitotic cyclins which contribute to the multipotency of human mesenchymal stem cells by expanding proliferation lifespan. Mol Cells. 2018;41:207–213. doi: 10.14348/molcells.2018.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitajima S, Lee KL, Hikasa H, Sun W, Huang RY, Yang H, Matsunaga S, Yamaguchi T, Araki M, Kato H, Poellinger L. Hypoxia-inducible factor-1α promotes cell survival during ammonia stress response in ovarian cancer stem-like cells. Oncotarget. 2017;8:114481–114494. doi: 10.18632/oncotarget.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo YC, Au HK, Hsu JL, Wang HF, Lee CJ, Peng SW, Lai SC, Wu YC, Ho HN, Huang YH. IGF-1R promotes symmetric self-renewal and migration of alkaline phosphatase + germ stem cells through HIF-2α-OCT4/CXCR4 loop under hypoxia. Stem Cell Reports. 2018;10:524–537. doi: 10.1016/j.stemcr.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White E, Mehnert JM, Chan CS. Autophagy, metabolism, and cancer. Clin Cancer Res. 2015;21:5037–5046. doi: 10.1158/1078-0432.CCR-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol. 2014;11:508–516. doi: 10.1038/nrurol.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villar VH, Merhi F, Djavaheri-Mergny M, Durán RV. Glutaminolysis and autophagy in cancer. Autophagy. 2015;11:1198–1208. doi: 10.1080/15548627.2015.1053680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hönscheid P, Datta K, Muders MH. Autophagy: detection, regulation and its role in cancer and therapy response. Int J Radiat Biol. 2014;90:628–635. doi: 10.3109/09553002.2014.907932. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Wu TT, Jiang L, Rong D, Zhu YQ. Deferoxamine-Induced Migration and Odontoblast Differentiation via ROS-Dependent Autophagy in Dental Pulp Stem Cells. Cell Physiol Biochem. 2017;43:2535–2547. doi: 10.1159/000484506. [DOI] [PubMed] [Google Scholar]
- 42.Lee SG, Joe YA. Autophagy mediates enhancement of proangiogenic activity by hypoxia in mesenchymal stromal/stem cells. Biochem Biophys Res Commun. 2018;501:941–947. doi: 10.1016/j.bbrc.2018.05.086. [DOI] [PubMed] [Google Scholar]