Abstract
This study aimed to investigate the intercorrelation among long noncoding RNA MALAT1 (lnc-MALAT1), microRNA-125b (miR-125b), FOXQ1, PTGS2 and CDK5, as well as their correlations with disease risk, severity and progression of Alzheimer’s disease (AD). In total, 120 AD patients, 120 Parkinson’s disease (PD) patients and 120 controls were enrolled. Cerebrospinal fluid (CSF) samples were collected from 50 AD patients, 50 PD patients and 50 controls; plasma samples were obtained from all participants. Lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 were detected by RT-qPCR. CSF lnc-MALAT1/FOXQ1 and plasma lnc-MALAT1 were downregulated, while CSF miR-125b/PTGS2/CDK5 and plasma miR-125b/PTGS2 were upregulated in AD patients compared to PD patients and controls, which differentiated AD patients from PD patients and controls, as demonstrated by ROC curve analyses. In AD patients, CSF/plasma lnc-MALAT1 negatively correlated with miR-125b and PTGS2 but positively correlated with FOXQ1; CSF/plasma miR-125b negatively correlated with FOXQ1 but positively correlated with PTGS2/CDK5. In addition, CSF/plasma lnc-MALAT1 and FOXQ1 correlated with alleviated disease severity, while miR-125b, PTGS2 and CDK5 correlated with exacerbated disease severity, which were manifested by their correlations with MMSE score, Aβ42, t-tau and p-tau in AD patients. However, their correlations with MMSE score, Aβ42, t-tau and p-tau were weak in PD patients and controls. Notably, CSF but not plasma lnc-MALAT1 and miR-125b could predict the MMSE score decline at 1 year, 2 years and 3 years in AD patients. In conclusion, lnc-MALAT1 and its target miR-125b are potential biomarkers for AD management via their intercorrelation with FOXQ1, PTGS2 and CDK5.
Keywords: Alzheimer’s disease, lnc-MALAT1, miR-125b, FOXQ1, PTGS2, CDK5
Introduction
Alzheimer’s disease (AD), a complex neurodegenerative disease of elderly people worldwide, is characterized by the sustained formation/deposition of misfolded amyloid-β (Aβ) peptide extracellularly and neurofibrillary tangles intracellularly, which induces neuroinflammation, synaptic abnormalities and neuronal degeneration in wide areas of the cerebral cortex and hippocampus that are involved in learning and memory capacities [1,2]. Prior to the clinical onset of cognitive decline, AD patients generally experience long preclinical and prodromal phases (more than 20 years) with subtle decline in episodic memory deficits [3,4]. The devastating effects of cognitive decline in impaired patient independence places a great burden on patients, their families and society [1]. Despite decades of tremendous progress in basic biology and clinical pathophysiology research on AD, current treatments are still limited to symptomatic relief with short-term efficacy, and drugs that halt AD progression or cure AD remain elusive [5,6]. As no cure is available, finding the efficient biomarkers for predicting AD progression and prognosis is becoming increasingly important.
Long noncoding RNA metastasis-associated lung adenocarcinoma transcript 1 (lnc-MALAT1), a well-studied and highly conserved long noncoding RNA, is closely implicated in the regulation of hyperproliferation, inflammation/immunity and neuroinjury by acting as a competing endogenous RNA (ceRNA) of several target microRNAs (miRNAs) to posttranscriptionally modulate genes, and lnc-MALAT1 is vital in the physiological-pathological processes of various diseases, including neurological disorders [7,8]. For instance, dysregulated lnc-MALAT1 has neuroprotective functions and anti-inflammatory effects in neurological disorders such as traumatic brain injury (lnc-MALAT1 attenuates neuroinflammation by suppressing the polarization of macrophages towards M1 phenotype) and experimental autoimmune encephalomyelitis (lnc-MALAT1 induces anti-apoptosis by downregulating microRNA-204) [9,10]. Meanwhile, microRNA-125b (miR-125b) is identified as a target gene of lnc-MALAT1, which suppresses cell proliferation, promotes tau phosphorylation and apoptosis and facilitates inflammation in AD [7,11-14]. As an example, miR-125b overexpression enhances neuronal apoptosis, neuroinflammation and oxidative stress in an in vitro AD model [11]. Another study demonstrated that miR-125b induces tau phosphorylation by downregulating Bcl-W, DUSP6, and PPP1CA in an in vivo AD model [14]. Furthermore, a collaboration at our institution revealed that lnc-MALAT1 subsequently modulates miR-125b-mediated prostaglandin-endoperoxide synthase 2 (PTGS2), cyclin dependent kinase 5 (CDK5) and forkhead box Q1 (FOXQ1) to suppress neuronal apoptosis and inflammation and enhance neurite outgrowth in AD [12]. Additionally, our preliminary study with small sample size of AD patients revealed an intercorrelation among CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 in AD patients, and they are all dysregulated in AD patients compared to patients with non-neurodegenerative diseases. In light of the above data, a hypothesis was proposed that lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 might have clinical significance as biomarkers for the disease management of AD. However, relevant publications are sparse. Therefore, the focus of the present study was to investigate the intercorrelation among CSF/plasma lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5, and their correlations with disease risk, severity and progression of AD, aiming to offer new prospects for the clinical management of AD.
Materials and methods
Patients
This study consecutively enrolled 120 AD patients, 120 Parkinson’s disease (PD) patients and 120 controls (Ctrls) who were admitted to Zhongshan Hospital Xiamen University between January 2014 and December 2016. The diagnosis of AD was made according to the diagnostic guidelines in the National Institute on Aging-Alzheimer’s association workgroups [15], and all AD patients presented with decreased amyloid β 42 (Aβ42) (< 550 pg/mL), increased total tau (t-tau) (≥ 350 pg/mL) and increased phosphorylated tau (p-tau) (≥ 70 pg/mL) in the cerebrospinal fluid (CSF). The PD patients were confirmed according to the UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria [16]. The Ctrls were patients with neurological diseases that were not neurodegenerative diseases (such as AD, PD, amyotrophic lateral sclerosis, and Huntington’s disease) and were without dementia symptoms (Mini-Mental State Examination (MMSE) score ≥ 27). To match the age of PD patients and Ctrls with that of AD patients, all patients were required to be 60-85 years old. In addition, PD patients and Ctrls were recruited in a sex ratio of 2:3 (male:female) to match the gender of AD patients. In addition, patients with malignancies or hematological diseases, infection, pregnancy or were lactating were excluded from this study.
Ethics
This study was approved by the Ethics Committee of Zhongshan Hospital Xiamen University. All procedures were conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines as defined by the International Conference on Harmonization. Written informed consent was provided by the patients or their family members.
Data collection
The demographics of all patients were documented after enrollment. The cognitive impairment status of patients was assessed using the MMSE score. The total MMSE score ranged from 0 to 30, and a score ≤ 26 was considered to indicate cognitive impairment. All patients received lumbar puncture for diagnosis, and the levels of CSF biomarkers (Aβ42, t-tau and p-tau) were analyzed. For AD diagnosis, the cut-off value of Aβ42 was 550 pg/mL, the cut-off value of t-tau was 350 pg/mL, and the cut-off value of p-tau was 70 pg/mL at Zhongshan Hospital Xiamen University.
Sample collection
For all patients, peripheral blood samples were collected after enrollment, and plasma samples were isolated from peripheral blood by centrifugation. In addition, a total of 150 extra CSF samples not for diagnostic use were acquired by lumbar puncture, which included 50 from AD patients, 50 from PD patients and 50 from Ctrls. The collected plasma samples and CSF samples were stored at -80°C until further detection.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect the relative expression levels of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 CSF and plasma samples. Total RNA was isolated by TRIzol™ Reagent (Thermo Fisher Scientific, USA) and converted to complementary DNA by PrimeScript™ RT reagent Kit (Takara, Japan), followed by amplification using SYBR® Premix DimerEraser™ (Takara, Japan). The qPCR results were computed by the 2-ΔΔCt method with GAPDH as the internal reference for lnc-MALAT1, FOXQ1, PTGS2 and CDK5 mRNAs and U6 as the internal reference for miR-125b. The different internal references for miR-125b and lnc-MALAT1/FOXQ1/PTGS2/CDK5 mRNAs would cause variation, but GAPDH and U6 are widely accepted as internal references with minimum variation. The steps to calculate the relative expression levels of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 (candidate genes) were as follows: (i) ΔCt (test) = Ct (candidate gene, test) - Ct (reference, test); (ii) ΔCt (calibrator) = Ct (candidate gene, calibrator) - Ct (reference, calibrator); (iii) ΔΔCt = ΔCt (test) - ΔCt (calibrator); and (iv) the relative expression of target genes was calculated via the 2-ΔΔCt method. The following primers were used: Lnc-MALAT1, forward primer: 5’-TCCTAAGGTCAAGAGAAGTGTCAG-3’, reverse primer: 5’-GTGGCGATGTGGCAGAGAA-3’; miR-125b, forward primer: 5’-ACACTCCAGCTGGGTCCCTGAGACCCTAACTT-3’, reverse primer: 5’-TGTCGTGGAGTCGGCAATTC-3’; FOXQ1, forward primer: 5’-GCACGCAGCAAGCCATATAC-3’, reverse primer: 5’-GGTTGAGCATCCAGTAGTTGTC-3’; PTGS2, forward primer: 5’-TGACCAGAGCAGGCAGATGA-3’, reverse primer: 5’-CCAGTAGGCAGGAGAACATATAACA-3’; CDK5, forward primer: 5’-GGAAGGCACCTACGGAACTG-3’, reverse primer: 5’-CTCGGCACACCCTCATCATC-3’; GAPDH, forward primer: 5’-TGACCACAGTCCATGCCATCAC-3’, reverse primer: 5’-GCCTGCTTCACCACCTTCTTGA-3’; and U6, forward primer: 5’-CTCGCTTCGGCAGCACATATACTA-3’, reverse primer: 5’-ACGAATTTGCGTGTCATCCTTGC-3’.
Follow-up
All AD patients were followed up to death or 3 years, and during follow-up, the MMSE score was assessed every year. Patients who were lost to follow-up, died at a specific timepoint, or had no available data at a specific timepoint were not included in the analysis of that timepoint. In addition, the 1-year MMSE score decline was calculated using the 1-year MMSE score and subtracting the initial MMSE score; the 2-year MMSE score decline was calculated using the 2-year MMSE score and subtracting the initial MMSE score; and the 3-year MMSE score decline was calculated using the 3-year MMSE score and subtracting the initial MMSE score.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (IBM, USA), and figures were generated using GraphPad Prism 7.00 (GraphPad Software, USA). Data are expressed as the mean ± standard deviation (SD), median (interquartile range, IQR) or count (percentage). Comparisons among AD patients, PD patients and Ctrls were determined by one-way analysis of variance (ANOVA), Kruskal-Wallis H rank sum test or Chi-square test. Multiple comparisons between groups were performed by Dunn’s test. Correlations were analyzed by Spearman’s rank correlation test. The abilities of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 to discriminate AD patients from Ctrls or AD patients from PD patients were determined by receiver operating characteristic (ROC) curves and area under the curve (AUC) with 95% confidence interval (CI). A P value < 0.05 was considered statistically significant.
Results
Characteristics in AD patients, PD patients and Ctrls
No difference in age or gender was observed among AD patients, PD patients and Ctrls (both P > 0.05) (Table 1). The mean age was 70.1±7.1 for AD patients, 68.6±6.6 for PD patients and 69.0±7.2 for Ctrls, and there were 37 (30.8%) males/83 (69.2%) females among AD patients, 49 (40.8%) males/71 (59.2%) females among PD patients, and 47 (39.2%) males/73 (60.8%) females among Ctrls. Education duration, Aβ42, t-tau, p-tau and MMSE score were different among AD patients, PD patients and Ctrls (all P < 0.05). Both education duration and MMSE score were lowest in AD patients, followed by PD patients, and then Ctrls. Aβ42 was lowest in AD patients, followed by Ctrls, and then PD patients. Regarding t-tau and p-tau, they were highest in AD patients, followed by Ctrls, and then PD patients.
Table 1.
Comparison of characteristics among AD patients, PD patients and Ctrls
| Items | AD patients (N = 120) | PD patients (N = 120) | Ctrls (N = 120) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 70.1±7.1 | 68.6±6.6 | 69.0±7.2 | 0.217 |
| Gender, No. (%) | 0.228 | |||
| Male | 37 (30.8) | 49 (40.8) | 47 (39.2) | |
| Female | 83 (69.2) | 71 (59.2) | 73 (60.8) | |
| Education duration (years), median (IQR) | 5.0 (3.0-7.8) | 6.0 (4.0-9.0) | 8.0 (3.0-9.0) | 0.040 |
| CSF biomarkers, median (IQR) | ||||
| Aβ42 (pg/mL) | 346.2 (278.5-417.1) | 860.6 (731.5-1001.7) | 832.5 (727.7-960.7) | < 0.001 |
| t-tau (pg/mL) | 954.6 (766.0-1173.0) | 205.6 (181.1-258.5) | 239.2 (193.8-259.6) | < 0.001 |
| p-tau (pg/mL) | 124.9 (96.0-150.9) | 45.5 (38.2-54.4) | 49.7 (43.9-57.9) | < 0.001 |
| MMSE score, mean ± SD | 17.9±3.8 | 26.9±1.6 | 28.1±1.1 | < 0.001 |
Comparison was determined by one-way analysis of variance (ANOVA), Kruskal-Wallis H rank sum test or Chi-square test. AD, Alzheimer’s disease; PD, Parkinson’s disease; Ctrls, controls; SD, standard deviation; IQR, interquartile range; CSF, cerebrospinal fluid; Aβ42, amyloid β 42; t-tau, total tau; p-tau, phosphorylated tau; MMSE, mini-mental state examination.
Comparisons of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 among AD patients, PD patients and Ctrls
In CSF samples, three-group comparison analyses revealed that lnc-MALAT1 (Figure 1A), miR-125b (Figure 1B), FOXQ1 (Figure 1C), PTGS2 (Figure 1D) and CDK5 (Figure 1E) were all dysregulated among AD patients, PD patients and Ctrls (all P < 0.001). Then, two-group comparison analyses demonstrated that lnc-MALAT1 (Figure 1A) and FOXQ1 (Figure 1C) were reduced, while miR-125b (Figure 1B), PTGS2 (Figure 1D) and CDK5 (Figure 1E) were increased in AD patients compared with PD patients (all P < 0.05) and Ctrls (all P < 0.001).
Figure 1.
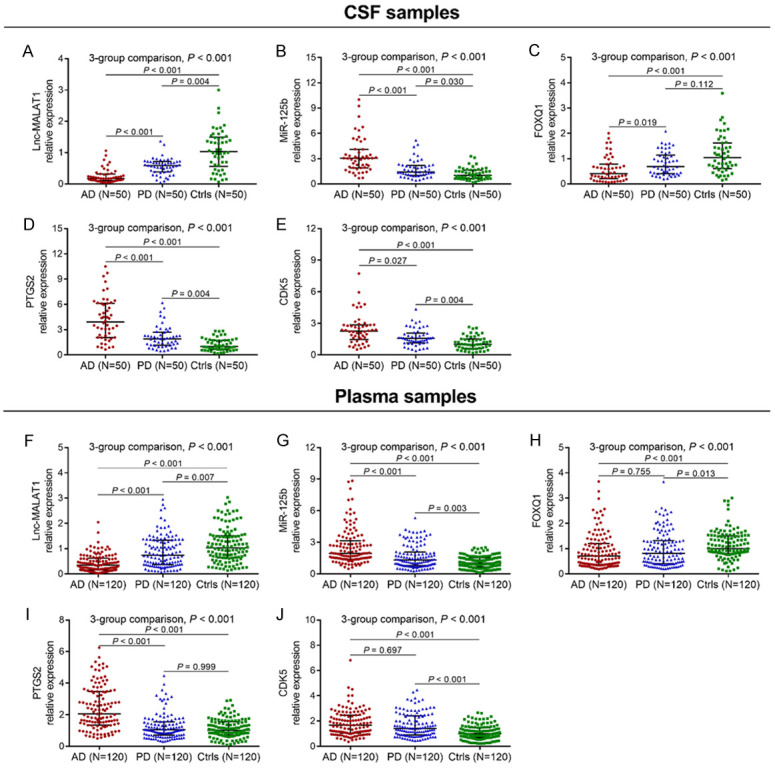
Lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 in AD patients, PD patients and Ctrls. Comparisons of CSF lnc-MALAT1 (A), miR-125b (B), FOXQ1 (C), PTGS2 (D) and CDK5 (E) expressions among AD patients, PD patients and Ctrls. Comparisons of plasma lnc-MALAT1 (F), miR-125b (G), FOXQ1 (H), PTGS2 (I) and CDK5 (J) expressions among AD patients, PD patients and Ctrls. Comparisons among AD patients, PD patients and Ctrls were performed by Kruskal-Wallis H ran sum test. Multiple comparisons between groups were determined by Dunn’s test. P < 0.05 was considered significant. Lnc-MALAT1, long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; AD, Alzheimer’s disease; PD, Parkinson’s disease; Ctrls, controls; CSF, cerebrospinal fluid.
In plasma samples, three-group comparison analyses revealed that lnc-MALAT1 (Figure 1F), miR-125b (Figure 1G), FOXQ1 (Figure 1H), PTGS2 (Figure 1I) and CDK5 (Figure 1J) were all dysregulated among AD patients, PD patients and Ctrls (all P < 0.001). Subsequent two-group comparison analyses showed that lnc-MALAT1 (Figure 1F) was reduced, while miR-125b (Figure 1G) and PTGS2 (Figure 1I) were all elevated in AD patients compared with PD patients (all P < 0.001) and Ctrls (all P < 0.001); FOXQ1 (Figure 1H) was lower in AD patients than in Ctrls (P < 0.001) but similar to that in PD patients (P = 0.755); CDK5 (Figure 1J) was increased in AD patients compared with Ctrls (P < 0.001) but similar to that in PD patients (P = 0.697).
ROC curve analyses
To further assess the value of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 in distinguishing AD patients from Ctrls and PD patients, we performed ROC curve analyses, which showed that in CSF samples, lnc-MALAT1 (AUC: 0.892, 95% CI: 0.827-0.957), miR-125b (AUC: 0.887, 95% CI: 0.824-0.950), FOXQ1 (AUC: 0.765, 95% CI: 0.674-0.857), PTGS2 (AUC: 0.894, 95% CI: 0.833-0.956) and CDK5 (AUC: 0.817, 95% CI: 0.734-0.900) could differentiate AD patients from Ctrls (Figure 2A). Moreover, lnc-MALAT1 (AUC: 0.830, 95% CI: 0.744-0.915), miR-125b (AUC: 0.782, 95% CI: 0.692-0.872), FOXQ1 (AUC: 0.672, 95% CI: 0.566-0.779), PTGS2 (AUC: 0.769, 95% CI: 0.676-0.863) and CDK5 (AUC: 0.671, 95% CI: 0.563-0.779) could also distinguish AD patients from PD patients (Figure 2B).
Figure 2.
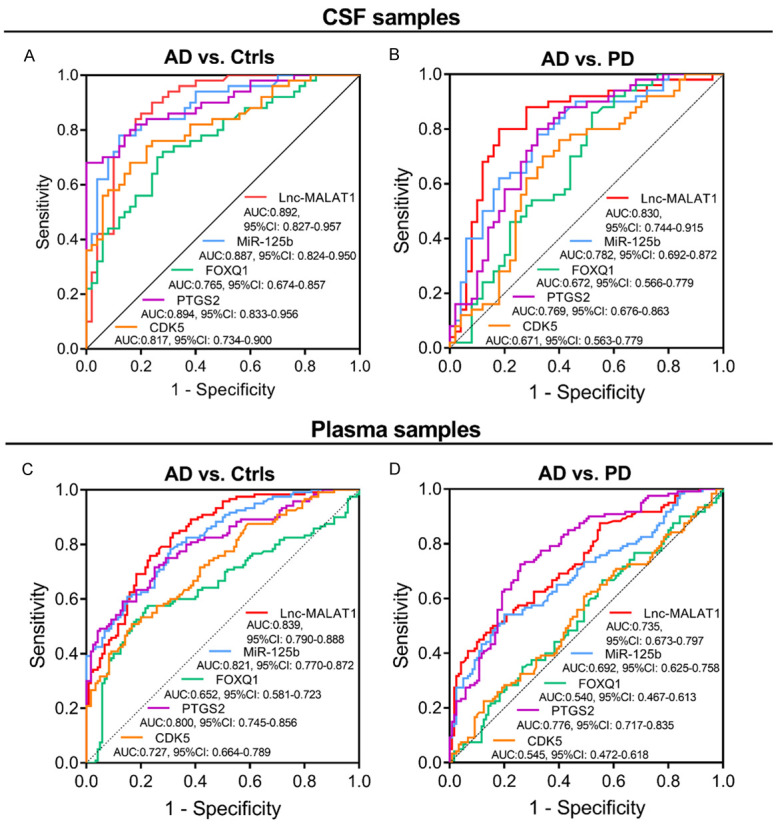
The value of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 for distinguishing AD patients from Ctrls and PD patients. The performance of CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 in differentiating AD patients from Ctrls (A) and PD patients (B). The performance of plasma lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 in discriminating AD patients from Ctrls (C) and PD patients (D). The abilities of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 in distinguishing AD patients from Ctrls or in distinguishing AD patients from PD patients were illuminated by ROC curve and AUC with 95% CI. Lnc-MALAT1, long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; AD, Alzheimer’s disease; Ctrls, controls; PD, Parkinson’s disease; CSF, cerebrospinal fluid; ROC, Receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
In plasma samples, lnc-MALAT1 (AUC: 0.839, 95% CI: 0.790-0.888), miR-125b (AUC: 0.821, 95% CI: 0.770-0.872), FOXQ1 (AUC: 0.652, 95% CI: 0.581-0.723), PTGS2 (AUC: 0.800, 95% CI: 0.745-0.856) and CDK5 (AUC: 0.727, 95% CI: 0.664-0.789) could differentiate AD patients from Ctrls (Figure 2C); In addition, lnc-MALAT1 (AUC: 0.735, 95% CI: 0.673-0.797), miR-125b (AUC: 0.692, 95% CI: 0.625-0.758) and PTGS2 (AUC: 0.776, 95% CI: 0.717-0.835) could differentiate AD patients from PD patients as well, while neither FOXQ1 (AUC: 0.540, 95% CI: 0.467-0.613) nor CDK5 (AUC: 0.545, 95% CI: 0.472-0.618) distinguished AD patients from PD patients (Figure 2D).
Correlation of lnc-MALAT1/miR-125b with FOXQ1, PTGS2 or CDK5
In AD patients, CSF lnc-MALAT1 negatively correlated with miR-125b, PTGS2 and CDK5 but positively correlated with FOXQ1 (all P < 0.05); CSF miR-125b negatively correlated with FOXQ1 but positively correlated with PTGS2 and CDK5 (all P = 0.001). Plasma lnc-MALAT1 negatively correlated with miR-125b and PTGS2 but positively correlated with FOXQ1 (all P < 0.01); and plasma miR-125b negatively correlated with FOXQ1 but positively correlated with PTGS2 and CDK5 (all P < 0.05) (Table 2).
Table 2.
Correlation of lnc-MALAT1/miR-125b with FOXQ1, PTGS2 or CDK5
| Items | Genes | MiR-125b | FOXQ1 | PTGS2 | CDK5 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| P value | r | P value | r | P value | r | P value | r | ||
| AD patients | |||||||||
| CSF samples (N = 50) | Lnc-MALAT1 | < 0.001 | -0.513 | 0.024 | 0.320 | < 0.001 | -0.525 | 0.042 | -0.289 |
| MiR-125b | - | - | 0.001 | -0.470 | 0.001 | 0.472 | 0.001 | 0.473 | |
| Plasma samples (N = 120) | Lnc-MALAT1 | < 0.001 | -0.367 | < 0.001 | 0.451 | 0.002 | -0.282 | 0.099 | -0.151 |
| MiR-125b | - | - | < 0.001 | -0.340 | 0.001 | 0.307 | 0.026 | 0.203 | |
| PD patients | |||||||||
| CSF samples (N = 50) | Lnc-MALAT1 | 0.003 | -0.416 | 0.183 | 0.191 | 0.001 | -0.446 | 0.112 | -0.228 |
| MiR-125b | - | - | 0.425 | -0.115 | < 0.001 | 0.529 | 0.155 | 0.204 | |
| Plasma samples (N = 120) | Lnc-MALAT1 | < 0.001 | -0.358 | 0.205 | 0.116 | 0.558 | -0.054 | 0.773 | -0.027 |
| MiR-125b | - | - | 0.120 | -0.143 | 0.100 | 0.151 | 0.002 | 0.283 | |
| Ctrls | |||||||||
| CSF samples (N = 50) | Lnc-MALAT1 | 0.003 | -0.408 | 0.020 | 0.328 | 0.020 | -0.328 | 0.056 | -0.272 |
| MiR-125b | - | - | 0.016 | -0.339 | 0.109 | 0.229 | 0.019 | 0.330 | |
| Plasma samples (N = 120) | Lnc-MALAT1 | 0.006 | -0.249 | 0.058 | 0.173 | 0.013 | -0.225 | 0.085 | -0.158 |
| MiR-125b | - | - | 0.002 | -0.284 | 0.681 | 0.038 | 0.076 | 0.163 | |
Correlation was determined by Spearman’s rank correlation test. FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; Ctrls, controls; AD, Alzheimer’s disease; CSF, cerebrospinal fluid; Lnc-MALAT1, long noncoding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; PD, Parkinson’s disease.
In PD patients, CSF lnc-MALAT1 negatively correlated with miR-125b and PTGS2 (both P < 0.01); CSF miR-125b positively correlated with PTGS2 (P < 0.001); plasma lnc-MALAT1 negatively correlated with miR-125b (P < 0.001); and plasma miR-125b positively correlated with CDK5 (P = 0.002). In Ctrls, CSF lnc-MALAT1 negatively correlated with miR-125b and PTGS2 but positively correlated with FOXQ1 (all P < 0.05); CSF miR-125b negatively correlated with FOXQ1 but positively correlated with CDK5 (both P < 0.05); plasma lnc-MALAT1 negatively correlated with miR-125b and PTGS2 (both P < 0.05); and plasma miR-125b negatively correlated with FOXQ1 (P = 0.002) (Table 2).
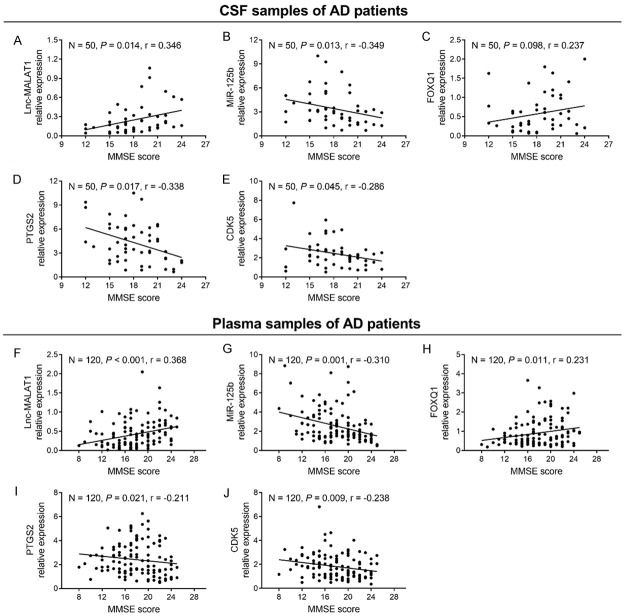
Correlation of candidate genes with MMSE score
In AD patients, CSF lnc-MALAT1 (Figure 3A) positively correlated with MMSE score (P = 0.014); CSF miR-125b (Figure 3B), PTGS2 (Figure 3D) and CDK5 (Figure 3E) negatively correlated with MMSE score (all P < 0.05); and CSF FOXQ1 (Figure 3C) did not correlate with MMSE score (P = 0.098). Moreover, plasma lnc-MALAT1 (Figure 3F) and FOXQ1 (Figure 3H) positively correlated with MMSE score, while plasma miR-125b (Figure 3G), PTGS2 (Figure 3I) and CDK5 (Figure 3J) negatively correlated with MMSE score (all P < 0.05).
Figure 3.
Association of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with MMSE score in AD patients. Correlation of CSF lnc-MALAT1 (A), miR-125b (B), FOXQ1 (C), PTGS2 (D) and CDK5 (E) with MMSE score in AD patients. Correlation of plasma lnc-MALAT1 (F), miR-125b (G), FOXQ1 (H), PTGS2 (I) and CDK5 (J) with MMSE score in AD patients. Correlation was analyzed by Spearman’s rank correlation test. Lnc-MALAT1, long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; AD, Alzheimer’s disease; MMSE, mini-mental state examination; CSF, cerebrospinal fluid.
In PD patients, CSF lnc-MALAT1 positively correlated with MMSE score, while CSF miR-125b and PTGS2 negatively correlated with MMSE score (all P < 0.05) (Table S1). In Ctrls, no correlation of CSF/plasma lnc-MALAT1, miR-125b, FOXQ1, PTGS2 or CDK5 with MMSE score was observed (all P > 0.05).
Correlation of candidate genes with Aβ42, t-tau and p-tau
In AD patients, CSF lnc-MALAT1 negatively correlated with t-tau and p-tau (both P < 0.01); CSF miR-125b positively correlated with t-tau and p-tau (both P = 0.003); CSF FOXQ1 negatively correlated with p-tau (P = 0.002); CSF PTGS2 negatively correlated with Aβ42 but positively correlated with t-tau and p-tau (all P < 0.05); and CSF CDK5 negatively correlated with Aβ42 but positively correlated with p-tau (both P < 0.05) (Table 3). Moreover, plasma lnc-MALAT1 positively correlated with Aβ42 (P = 0.008); plasma miR-125b positively correlated with p-tau (P = 0.039); plasma PTGS2 negatively correlated with Aβ42 but positively correlated with p-tau (both P < 0.05); and plasma CDK5 negatively correlated with Aβ42 (P = 0.023).
Table 3.
Correlation of candidate genes with CSF biomarkers in AD patients
| Samples | Items | Lnc-MALAT1 | MiR-125b | FOXQ1 | PTGS2 | CDK5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| P value | r | P value | r | P value | r | P value | r | P value | r | ||
| CSF (N = 50) | Aβ42 | 0.107 | 0.230 | 0.209 | -0.181 | 0.362 | 0.132 | 0.049 | -0.280 | 0.041 | -0.289 |
| t-tau | 0.001 | -0.466 | 0.003 | 0.408 | 0.053 | -0.275 | 0.034 | 0.300 | 0.083 | 0.248 | |
| p-tau | < 0.001 | -0.494 | 0.003 | 0.418 | 0.002 | -0.419 | 0.019 | 0.331 | 0.041 | 0.290 | |
| Plasma (N = 120) | Aβ42 | 0.008 | 0.241 | 0.110 | -0.147 | 0.162 | 0.129 | 0.003 | -0.271 | 0.023 | -0.207 |
| t-tau | 0.256 | -0.103 | 0.073 | 0.164 | 0.450 | -0.070 | 0.110 | 0.146 | 0.102 | 0.150 | |
| p-tau | 0.053 | -0.177 | 0.039 | 0.189 | 0.373 | -0.080 | 0.034 | 0.194 | 0.111 | 0.146 | |
Correlation was determined by Spearman’s rank correlation test. CSF, cerebrospinal fluid; AD, Alzheimer’s disease; Lnc-MALAT1, long noncoding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; Aβ42, amyloid β 42; t-tau, total tau; p-tau, phosphorylated tau.
In PD patients, CSF miR-125b and PTGS2 positively correlated with t-tau and p-tau (all P < 0.05). Moreover, plasma lnc-MALAT1 negatively correlated with t-tau, and plasma CDK5 negatively correlated with Aβ42 (both P < 0.05) (Table S1). In Ctrls, CSF lnc-MALAT1 positively correlated with Aβ42 (P = 0.036); CSF miR-125b positively correlated with t-tau and p-tau (both P < 0.05); CSF FOXQ1 negatively correlated with t-tau and p-tau (both P < 0.01); and CSF PTGS2 positively correlated with p-tau (P = 0.038). Moreover, plasma lnc-MALAT1 positively correlated with Aβ42, and plasma FOXQ1 negatively correlated with t-tau (both P < 0.05).
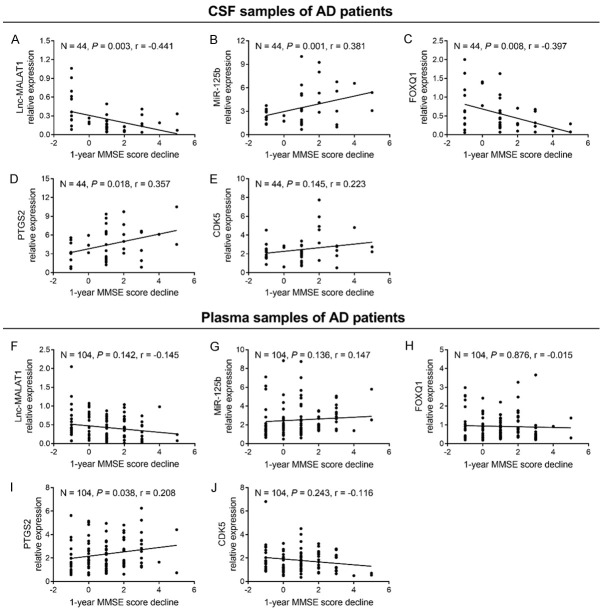
Correlation of candidate genes with 1-year MMSE score decline in AD patients
At the 1-year follow-up, 104 AD patients (44 baseline CSF samples and 104 baseline plasma samples) had 1-year MMSE score data. In CSF samples of AD patients, lnc-MALAT1 (Figure 4A) and FOXQ1 (Figure 4C) negatively correlated with 1-year MMSE score decline (both P < 0.01); miR-125b (Figure 4B) and PTGS2 (Figure 4D) positively correlated with 1-year MMSE score decline (both P < 0.05); while CDK5 (Figure 4E) did not correlate with 1-year MMSE score decline (P = 0.145). In plasma samples of AD patients, only PTGS2 (Figure 4I) positively correlated with 1-year MMSE score decline (P = 0.038), while lnc-MALAT1 (Figure 4F), miR-125b (Figure 4G), FOXQ1 (Figure 4H) and CDK5 (Figure 4J) did not correlate with 1-year MMSE score decline (all P > 0.05).
Figure 4.
Association of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with 1-year MMSE score decline in AD patients. Correlation of CSF lnc-MALAT1 (A), miR-125b (B), FOXQ1 (C), PTGS2 (D) and CDK5 (E) with 1-year MMSE score decline in AD patients. Correlation of plasma lnc-MALAT1 (F), miR-125b (G), FOXQ1 (H), PTGS2 (I) and CDK5 (J) with 1-year MMSE score decline in AD patients. Correlation was analyzed by Spearman’s rank correlation test. Lnc-MALAT1, long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; MMSE, mini-mental state examination; AD, Alzheimer’s disease; CSF, cerebrospinal fluid.
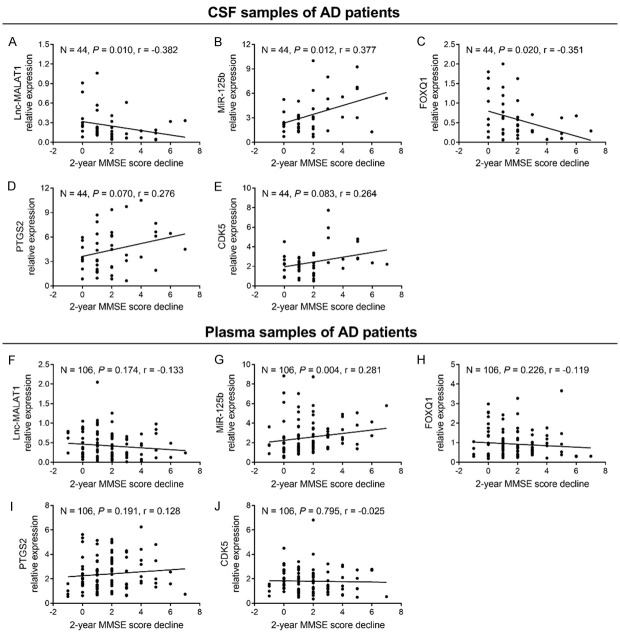
Correlation of candidate genes with 2-year MMSE score decline in AD patients
At the 2-year follow-up, 106 AD patients (44 baseline CSF samples and 106 baseline plasma samples) had 2-year MMSE score data. In CSF samples of AD patients, lnc-MALAT1 (Figure 5A) and FOXQ1 (Figure 5C) negatively correlated with 2-year MMSE score decline (both P < 0.05); miR-125b (Figure 5B) positively correlated with 2-year MMSE score decline (P = 0.012); while PTGS2 (Figure 5D) and CDK5 (Figure 5E) did not correlate with 2-year MMSE score decline (both P > 0.05). In plasma samples of AD patients, only miR-125b (Figure 5G) positively correlated with 2-year MMSE score decline (P = 0.004), whereas lnc-MALAT1 (Figure 5F), FOXQ1 (Figure 5H), PTGS2 (Figure 5I) and CDK5 (Figure 5J) did not correlate with 2-year MMSE score decline (all P > 0.05).
Figure 5.
Association of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with 2-year MMSE score decline in AD patients. Correlation of CSF lnc-MALAT1 (A), miR-125b (B), FOXQ1 (C), PTGS2 (D) and CDK5 (E) with 2-year MMSE score decline in AD patients. Correlation of plasma lnc-MALAT1 (F), miR-125b (G), FOXQ1 (H), PTGS2 (I) and CDK5 (J) with 2-year MMSE score decline in AD patients. Correlation was analyzed by Spearman’s rank correlation test. Lnc-MALAT1, long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; MMSE, mini-mental state examination; CSF, cerebrospinal fluid; AD, Alzheimer’s disease.
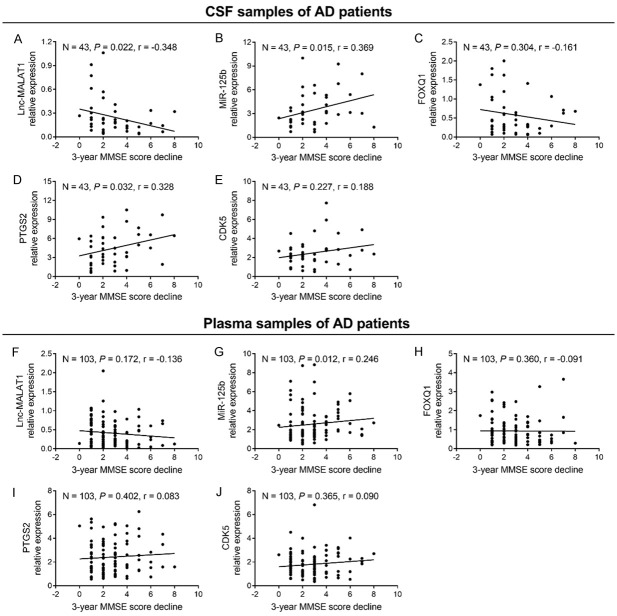
Correlation of candidate genes with 3-year MMSE score decline in AD patients
At the 3-year follow-up, 103 AD patients (43 baseline CSF samples and 103 baseline plasma samples) had 3-year MMSE score data. In CSF samples of AD patients, lnc-MALAT1 (Figure 6A) negatively correlated with 3-year MMSE score decline (P = 0.022); miR-125b (Figure 6B) and PTGS2 (Figure 6D) positively correlated with 3-year MMSE score decline (both P < 0.05); whereas FOXQ1 (Figure 6C) and CDK5 (Figure 6E) did not correlate with 3-year MMSE score decline (all P > 0.05). In plasma samples of AD patients, only miR-125b (Figure 6G) positively correlated with 3-year MMSE score decline (P = 0.012), whereas lnc-MALAT1 (Figure 6F), FOXQ1 (Figure 6H), PTGS2 (Figure 6I) and CDK5 (Figure 6J) did not correlate with 3-year MMSE score decline (all P > 0.05).
Figure 6.
Association of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with 3-year MMSE score decline in AD patients. Correlation of CSF lnc-MALAT1 (A), miR-125b (B), FOXQ1 (C), PTGS2 (D) and CDK5 (E) with 3-year MMSE score decline in AD patients. Correlation of plasma lnc-MALAT1 (F), miR-125b (G), FOXQ1 (H), PTGS2 (I) and CDK5 (J) with 3-year MMSE score decline in AD patients. Correlation was analyzed by Spearman’s rank correlation test. Lnc-MALAT1, long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1; miR-125b, microRNA 125b; FOXQ1, forkhead box Q1; PTGS2, prostaglandin-endoperoxide synthase 2; CDK5, cyclin dependent kinase 5; MMSE, mini-mental state examination; AD, Alzheimer’s disease; CSF, cerebrospinal fluid.
Discussion
Following advances in genome-wide transcriptome studies and the advent of next-generation sequencing techniques in recent years, research on long noncoding RNAs (lncRNAs) has gathered momentum, and a growing body of evidence has implicated lncRNAs in the development and progression of many diseases, including neurological disorders [17,18]. Among the commonly identified lncRNAs, lncRNA-MALAT1, located on chromosome 11q13.1, is abundantly expressed in brain tissues, especially in the high-activity areas of neocortex [17]. Existing data demonstrate that the downregulation of lncRNA-MALAT1 facilitates the polarization of macrophages towards the M1 phenotype and the proliferation of T-cells in experimental autoimmune encephalomyelitis, which implies the potential anti-inflammatory effect of lncRNA-MALAT1 [9]. In spinal cord ischemic injury, lnc-MALAT1 elicits its neuroprotective effect by attenuating interleukin-6, nuclear factor-κB and aquaporin 4 [10]. As a direct target of lncRNA-MALAT1, miR-125b is also highly abundant in the brain and is essential in diverse pathological processes of AD, such as neuronal cell apoptosis, tau phosphorylation, neuroinflammation, and Aβ peptide production [11,13,14,19,20]. For instance, one study demonstrated that miR-125b promotes neuronal cell apoptosis and tau phosphorylation by activating CDK5 and p35/25 in AD [13]. Another study discovered that CSF miR-125b is elevated compared with that in normal participates, and further experiments based on an in vitro AD model showed that miR-125b overexpression suppressed cell proliferation and promoted oxidative stress [11]. A previous exploration by a collaboration at our institution revealed that lncRNA-MALAT1 negatively regulates miR-125b-mediated PTGS2, CDK5 and FOXQ1 to suppress neuronal apoptosis and neuroinflammation and stimulate neurite outgrowth in AD [12]. In addition, our preliminary study showed an intercorrelation among CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5, all of which are dysregulated in AD patients compared to patients with non-neurodegenerative diseases. Although several lines of evidence are available regarding the mechanism of lnc-MALAT1, miR-125b, PTGS2, CDK5 and FOXQ1 underlying neurological disorders, including AD, the clinical implication of lnc-MALAT1, miR-125b, PTGS2, CDK5 and FOXQ1 in AD is still unknown.
The present study detected lnc-MALAT1, miR-125b, PTGS2, CDK5 and FOXQ1 expression in AD patients, PD patients and Ctrls, and the results revealed that CSF lnc-MALAT1 and FOXQ1 were reduced, while miR-125b, PTGS2 and CDK5 were increased in AD patients compared with PD patients and Ctrls. Plasma lnc-MALAT1 was decreased, while miR-125b and PTGS2 were elevated in AD patients compared with PD patients and Ctrls. Subsequent ROC curve analyses showed that CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 could differentiate AD patients from Ctrls and PD patients; plasma lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 could distinguish AD patients from Ctrls, while only plasma lnc-MALAT1, miR-125b and PTGS2 could discriminate AD patients from PD patients. The following explanations are proposed: (1) Lnc-MALAT1 might directly suppress the transcription of target miRNAs directly (such as miR-125b and miR-155) to inhibit downstream pathways, such as suppressor of overexpression of CO 1/Janus kinase-signal transducers and activators of transcription pathway, which subsequently inhibited the release of inflammatory cytokines and neuronal cell apoptosis, thereby, protecting neurons from damage [12,21]. Thus, lnc-MALAT1 expression was lower in AD patients than that in PD patients and Ctrls. (2) MiR-125b might repress the transcription of its target gene FOXQ1, which then stimulated the activation of CDK5 and p35/25, resulting in neuronal apoptosis and tau phosphorylation in AD [13]. Therefore, miR-125b and CDK5 were elevated in AD patients compared with PD patients and Ctrls. (3) PTGS2 might amplify neuroinflammation, facilitate cell apoptosis and inhibit neurite outgrowth by regulating interleukin-1β and Aβ in glial and neuronal cells; thus, PTGS2 was reduced in AD patients compared with PD patients and Ctrls [22]. Of note, the ability of CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 to discriminate AD patients from PD patients and Ctrls was better than that in plasma. A possible explanation is that the regulatory mechanisms of lnc-MALAT1, miR-125b, FOXQ1 and CDK5 were initiated in the brain and reflected by CSF, while in plasma, the expression of these candidate genes might be affected by a variety of unknown factors (such as the blood brain barrier, pulmonary diseases, immune response and inflammation level), which reduced their ability to differentiate AD patients from PD patients and Ctrls [23,24].
In addition, in AD patients, CSF/plasma lnc-MALAT1 negatively correlated with miR-125b and PTGS2 but positively correlated with FOXQ1; CSF/plasma miR-125b positively correlated with PTGS2 and CDK5 but negatively correlated with FOXQ1. These data could be explained by the following. Lnc-MALAT1 was reported to negatively regulate miR-125b, and upregulation of miR-125b was accompanied by increased PTGS2 and CDK5 but attenuated FOXQ1 in AD [12]. Therefore, CSF/plasma lnc-MALAT1 was inversely correlated with miR-125b and PTGS2 but positively correlated with FOXQ1; CSF/plasma miR-125b was negatively correlated with PTGS2 and CDK5 but negatively correlated with FOXQ1 in AD patients.
Furthermore, the present study observed that CSF/plasma lnc-MALAT1 and FOXQ1 correlated with alleviated disease severity, while CSF/plasma miR-125b, PTGS2 and CDK5 correlated with exacerbated disease severity in AD patients, which was manifested by their correlations with MMSE score, Aβ42, t-tau or p-tau. These results could be explained by the following. (1) Lnc-MALAT1 might sponge several neurotoxic miRNAs (e.g. miR-125b and miR-204) and anti-inflammatory miRNAs (e.g. miR-155) to decrease neuronal cell apoptosis, neuroinflammation, synaptic loss and neurodegeneration, resulting in lnc-MALAT1 being associated with alleviated disease severity in AD patients [10,12,21]. (2) MiR-125b might suppress FOXQ1 and sphingosine kinase 1 expression while promoting CDK5 expression to increase Aβ peptide production, suppress cell proliferation, induce neuronal apoptosis, amplify neuroinflammation and accelerate neurodegeneration, resulting in miR-125b and CDK5 being correlated with exacerbated disease severity, but FOXQ1 being correlated with attenuated disease severity in AD patients [11,13]. (3) PTGS2 might exaggerate inflammation and trigger more extensive neuronal cell loss by intensifying the release and production of inflammatory mediators and cytokines, which contributed to disease progression in AD patients [25]. Interestingly, from the perspective of correlation efficiency, the correlation efficiency of CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with disease severity was above 0.3, while the correlation efficiency of plasma lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with disease severity was below 0.3 in AD patients, which indicated that the correlations of CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with disease severity were stronger than those indexes in plasma, which might be explained by lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 being pathologically regulated in the brain, and they were overexpressed in the CSF, while in blood, their expression fluctuated considerably since multiple unknown factors (such as pulmonary diseases, immune response and inflammation level) could affect their expression in blood [23,24].
To further evaluate the impact of candidate genes on AD progression, all AD patients were followed up for 3 years, with MMSE scores assessed at year 1, year 2 and year 3. We found that CSF lnc-MALAT1 and miR-125b could predict MMSE score decline at each time point, while CSF FOXQ1, PTGS2 and CDK5 only predicted MMSE score decline at certain time points. For plasma candidate genes, only plasma PTGS2 and miR-125b could predict MMSE score decline at specific time points. The following are possible explanations. (1) CSF lnc-MALAT1 might suppress neuronal apoptosis and neuroinflammation but facilitate neurite outgrowth by directly targeting miR-125b and miR-155, which subsequently attenuated disease progression [12,21]. Furthermore, CSF miR-125b might promote neuronal apoptosis, tau phosphorylation and neuroinflammation by downregulating FOXQ1, which reduced inflammatory cytokine expression by modulating the SIRT1-NF-κB pathway, and activated CDK5, which induced the hyperphosphorylation of amyloid precursor protein/tau, synaptic damage and neuronal apoptosis, accelerating the disease progression of AD [13,26]. Additionally, CSF PTGS2 might increase inflammation and induce more extensive neuronal apoptosis by stimulating the release of inflammatory cytokines, thereby contributing to the disease progression of AD [25]. Taken together, CSF lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 could predict MMSE score decline in AD patients. (2) Plasma lnc-MALAT1, FOXQ1 and CDK5 could be influenced by a variety of factors (such as pulmonary disease, immune response and inflammation level), which reduced their expression and ability to predict disease progression in AD patients. Plasma miR-125b and PTGS2 are well-known biomarkers for indicating inflammation in blood, and they consistently stimulate and exaggerate inflammation as AD disease manifests [27-29]. Therefore, plasma PTGS2 and miR-125b could predict MMSE scores in AD patients.
Despite the interesting results in the present study, several limitations should be noted when interpreting the findings. First, the sample size was relatively small, which might reduce the statistical power of the analyses. Second, the follow-up duration (3 years) was relatively short since AD is a chronic disease, and further study with extended follow-up time is necessary to explore the value of lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 for long-term disease progression in AD patients. Third, AD patients who were lost to follow-up or died at the last follow-up date were not included in the analysis of MMSE score at that timepoint, which might cause selection bias. Fourth, the value of CSF/plasma lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 for prognosis was not explored in AD patients. Although it was not an aim in our study, it would be of clinical significance to detect the correlation of CSF/plasma lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 with prognosis in AD patients. Last, only baseline lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 expression was measured, and variations in lnc-MALAT1, miR-125b, FOXQ1, PTGS2 and CDK5 were not detected in AD patients during follow-up, which requires further study.
To conclude, lnc-MALAT1 and its target miR-125b display clinical value as biomarkers for disease risk, severity and progression of AD via intercorrelation with FOXQ1, PTGS2 and CDK5, which might offer valuable insights into the optimization of disease management in AD patients. Clinical investigation of larger AD patient cohorts is greatly needed to confirm and validate our findings.
Acknowledgements
This study was supported by Medical Elite Cultivation Program of Fujian, P. R. China (No. 2018-ZQN-85).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Jazvinscak Jembrek M, Slade N, Hof PR, Simic G. The interactions of p53 with tau and ass as potential therapeutic targets for Alzheimer’s disease. Prog Neurobiol. 2018;168:104–127. doi: 10.1016/j.pneurobio.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Osborn LM, Kamphuis W, Wadman WJ, Hol EM. Astrogliosis: an integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol. 2016;144:121–141. doi: 10.1016/j.pneurobio.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 5.Graham WV, Bonito-Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med. 2017;68:413–430. doi: 10.1146/annurev-med-042915-103753. [DOI] [PubMed] [Google Scholar]
- 6.Yuzwa SA, Vocadlo DJ. O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer’s disease and beyond. Chem Soc Rev. 2014;43:6839–6858. doi: 10.1039/c4cs00038b. [DOI] [PubMed] [Google Scholar]
- 7.Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, Zhang MQ, Sedel F, Jourdren L, Coulpier F, Triller A, Spector DL, Bessis A. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–3093. doi: 10.1038/emboj.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei L, Chen J, Huang J, Lu J, Pei S, Ding S, Kang L, Xiao R, Zeng Q. Functions and regulatory mechanisms of metastasis-associated lung adenocarcinoma transcript 1. J Cell Physiol. 2018;234:134–151. doi: 10.1002/jcp.26759. [DOI] [PubMed] [Google Scholar]
- 9.Masoumi F, Ghorbani S, Talebi F, Branton WG, Rajaei S, Power C, Noorbakhsh F. Malat1 long noncoding RNA regulates inflammation and leukocyte differentiation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2019;328:50–59. doi: 10.1016/j.jneuroim.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Qiao Y, Peng C, Li J, Wu D, Wang X. LncRNA MALAT1 is neuroprotective in a rat model of spinal cord ischemia-reperfusion injury through miR-204 regulation. Curr Neurovasc Res. 2018;15:211–219. doi: 10.2174/1567202615666180712153150. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y, Tu Q, Liu M. MicroRNA-125b regulates Alzheimer’s disease through SphK1 regulation. Mol Med Rep. 2018;18:2373–2380. doi: 10.3892/mmr.2018.9156. [DOI] [PubMed] [Google Scholar]
- 12.Ma P, Li Y, Zhang W, Fang F, Sun J, Liu M, Li K, Dong L. Long non-coding RNA MALAT1 inhibits neuron apoptosis and neuroinflammation while stimulates neurite outgrowth and its correlation with MiR-125b mediates PTGS2, CDK5 and FOXQ1 in Alzheimer’s disease. Curr Alzheimer Res. 2019;16:596–612. doi: 10.2174/1567205016666190725130134. [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Liu L, Meng J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci Lett. 2017;661:57–62. doi: 10.1016/j.neulet.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Banzhaf-Strathmann J, Benito E, May S, Arzberger T, Tahirovic S, Kretzschmar H, Fischer A, Edbauer D. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014;33:1667–1680. doi: 10.15252/embj.201387576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel SE, Lees AJ. Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl. 1993;39:165–172. [PubMed] [Google Scholar]
- 17.Zhang X, Hamblin MH, Yin KJ. The long noncoding RNA Malat1: its physiological and pathophysiological functions. RNA Biol. 2017;14:1705–1714. doi: 10.1080/15476286.2017.1358347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenoglio C, Ridolfi E, Galimberti D, Scarpini E. An emerging role for long non-coding RNA dysregulation in neurological disorders. Int J Mol Sci. 2013;14:20427–20442. doi: 10.3390/ijms141020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Liao X, Chen Z, Fang Y, He A, Zhong Y, Gao Q, Xiao H, Li J, Huang W, Liu Y. LncRNA MALAT1 inhibits apoptosis and promotes invasion by antagonizing miR-125b in bladder cancer cells. J Cancer. 2017;8:3803–3811. doi: 10.7150/jca.21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SM, Hu WW. Long non-coding RNA MALAT1 promotes oral squamous cell carcinoma development via microRNA-125b/STAT3 axis. J Cell Physiol. 2018;233:3384–3396. doi: 10.1002/jcp.26185. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Sun Y, Zhong L, Xiao Z, Yang M, Chen M, Wang C, Xie X, Chen X. The suppression of ox-LDL-induced inflammatory cytokine release and apoptosis of HCAECs by long non-coding RNA-MALAT1 via regulating microRNA-155/SOCS1 pathway. Nutr Metab Cardiovasc Dis. 2018;28:1175–1187. doi: 10.1016/j.numecd.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Wang H, Shu Y, Li X. miR-103 promotes neurite outgrowth and suppresses cells apoptosis by targeting Prostaglandin-endoperoxide synthase 2 in cellular models of Alzheimer’s disease. Front Cell Neurosci. 2018;12:91. doi: 10.3389/fncel.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blennow K. A review of fluid biomarkers for Alzheimer’s disease: moving from CSF to blood. Neurol Ther. 2017;6:15–24. doi: 10.1007/s40120-017-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1:226–234. doi: 10.1602/neurorx.1.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma SL, Tang NL, Zhang YP, Ji LD, Tam CW, Lui VW, Chiu HF, Lam LC. Association of prostaglandin-endoperoxide synthase 2 (PTGS2) polymorphisms and Alzheimer’s disease in Chinese. Neurobiol Aging. 2008;29:856–860. doi: 10.1016/j.neurobiolaging.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Liu SL, Wang C, Jiang T, Tan L, Xing A, Yu JT. The role of Cdk5 in Alzheimer’s disease. Mol Neurobiol. 2016;53:4328–4342. doi: 10.1007/s12035-015-9369-x. [DOI] [PubMed] [Google Scholar]
- 27.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner DR, Scherer D, Muir K, Schildkraut J, Boffetta P, Spitz MR, Le Marchand L, Chan AT, Goode EL, Ulrich CM, Hung RJ. A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol Biomarkers Prev. 2014;23:1729–1751. doi: 10.1158/1055-9965.EPI-14-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HM, Kim TS, Jo EK. MiR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep. 2016;49:311–318. doi: 10.5483/BMBRep.2016.49.6.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.