Abstract
Treatment of breast cancer (BC) with overexpression of human epidermal growth factor receptor 2 (HER2) has undergone a prosperous development with the advent of emerging small molecule tyrosine kinase inhibitors (TKIs). However, their efficacy in brain metastases (BMs) requires further investigation in both clinical trials and practice by specifically targeting this population. We herein reported a HER2-positive metastatic bilateral BC case with symptomatic diffusion of parenchymal BM after first-line treatment with trastuzumab-based regimen. She then received pyrotinib (with a disease-free survival of 5.7 months) followed by whole brain radiotherapy. Unexpectedly, under satisfactory control of intracranial parenchymal lesions, the patient based on clinical manifestations, auxiliary examinations and exclusive diagnosis was diagnosed with meningeal progression, and soon died of tumor progression. Thus, pyrotinib response differed from meningeal to parenchymal BM in BC patients, and the need of comprehensive and systematic evaluation of TKIs in some particular clinical scenarios has become a critical issue.
Keywords: Brain parenchymal metastasis, breast cancer, HER2-positive, meningeal metastasis, pyrotinib
Introduction
Breast cancer (BC) patients with overexpression of human epidermal growth factor receptor 2 (HER2) who achieve long-term survival benefits from anti-HER2 systemic treatments are associated with increased incidence of brain metastases (BMs). This has become a hot research topic and complex treatment issue in clinical practice. Current therapy for HER2-positive BC patients with BM mainly involves local brain surgery or radiotherapy, and new-type small molecule tyrosine kinase inhibitors (TKIs) is showing certain prospects in treatment. According to phase II clinical trials LANDSCAPE [1] and TBCRC 022 [2], lapatinib and neratinib have demonstrated a therapeutic efficacy on BM to a certain extent. PHENIX trial [3] enrolled metastatic BC (MBC) patients with controlled BM, and achieved good results. The study confirmed that BC patients with BM have benefitted from pyrotinib treatment, reducing the proportion of progressive BM, and delaying the median time to progressive BM when combined with capecitabine. All these studies have suggested that the investigations of TKIs for treating BC patients with BM are worth further exploration. We herein reported a patient with HER2-positive metastatic bilateral BC during her first diagnosis. She had numerous brain parenchymal metastases (BPMs) following 8-cycle trastuzumab-based treatment, received whole-brain radiation therapy (WBRT), and experienced unusual clinical outcome of meningeal metastasis (MM) progression while had satisfactory control of parenchymal lesions with pyrotinib treatment.
Case presentation
A 47-year-old woman in menopause without any family history of tumors or underlying disease history had a thumb nail size lump in her left breast in September 2017, but was not considered seriously. Two months later, another lump with soybean size was touched in her right breast, but she did not visit the hospital. In January 2018, she had pain in left breast, and had progressive aggravation, and ulceration on the skin surface accompanied with redness, swollen and heat pain. Besides, another painful lump was observed in the left axilla. She then visited local hospital for treatment. The results of physical examination showed no dimpling or discharge in the bilateral nipples, a large-area of yellow color scab on the surface of left breast with no precise noticeability. Also showed slightly red and swollen surface on the right breast, hardened texture of the whole breast; and a 2 cm size of axillary lymph node could be touched, with palpable bilateral supraclavicular lymph nodes (the largest one was about 3 cm). The pathological biopsy results of the right breast mass showed invasive carcinoma (Figure 1A). Immunohistochemistry (IHC) analysis revealed ER (-), PR (-), HER2 (2+~3+), and Ki-67 (+, 20%) (Figure 1A). Fluorescence in situ hybridization (FISH) assay confirmed gene amplification of HER2 (Figure 1A). Imaging assessment of tumor burden including chest and abdominal computed tomography (CT) scan indicated mediastinal lymphatic metastasis (Figure 1B). Emission computed tomography (ECT) of bone scanning suggested multiple bone metastases (Figure 1C). The patient underwent one course of taxotere-epirubicin-cyclophosphamide (TEC) chemotherapy, followed by administration of bisphosphonates to inhibit bone metastases, and opioids to control pain. She was then admitted to our hospital for further medical help. The results of physical examination showed lymph nodes in the left axilla and bilateral supraclavicular were shrunken. No obvious abnormalities were observed in the supplementary cranial magnetic resonance imaging (MRI) examination. Further biopsy of left breast mass revealed an invasive carcinoma, with the status of ER (-), PR (-), HER2 (3+), Ki-67 (+, 50%) by IHC analysis (Figure 1D). Amplification of HER2 gene pertaining to left BC tissues was shown to be positive by FISH assay (Figure 1D). The patient was thus confirmed with the diagnosis of “bilateral BC and metastases of left axillary lymph node, bilateral supraclavicular lymph nodes, mediastinal lymph nodes and bone (cT4N3M1, stage IV, HER2 gene amplification)”. According to HER2 status, the treatment was switched to docetaxel and carboplatin in combination with trastuzumab (TCbH) regimen, which was maintained for 8 cycles. During this treatment, her bilateral breast lumps showed continuous reduction, skin symptoms were recovered, pain disappeared, and the enlarged lymph nodes in the left axilla and bilateral supraclavicular were completely relieved. But the reduction of mediastinal lymph nodes was not satisfactory, and the overall response was evaluated as partial response (PR) with significant improvement. In October, 2018, she had headache and dizziness accompanied with difficulty opening her left eye, vision impairment in the left eye, left limb numbness, and epilepsy symptoms. Head MRI examination found multiple abnormal signals in bilateral cerebral and cerebellar hemispheres, which was considered as numerous metastatic lesions in the brain parenchyma (Figure 2A). Whole brain radiation therapy (WBRT, DT 40 Gy) was subsequently performed, resulting in stable disease with minor improvement (Figure 2A). The patient then received systemic therapy of pyrotinib (400 mg, po, qd) plus capecitabine (1500 mg, po, bid) from November 2018, but showed poor tolerance to capecitabine, and so underwent treatment with etoposide capsules (100 mg, d1-5, q4w) as an alternative partner 1 week later. During this therapy, parenchymal brain metastases exhibited continuous shrinkage in size and decrease in number (Figure 2A), as well as reduction in primary breast lesions (Figure 2B), mediastinal metastatic lymph node (Figure 2C), and the status lasted for nearly half a year. She had pyrotinib-related diarrhea, nausea and loss of appetite, which could be recovered after symptomatic treatment, and maintained a good quality of life.
Figure 1.
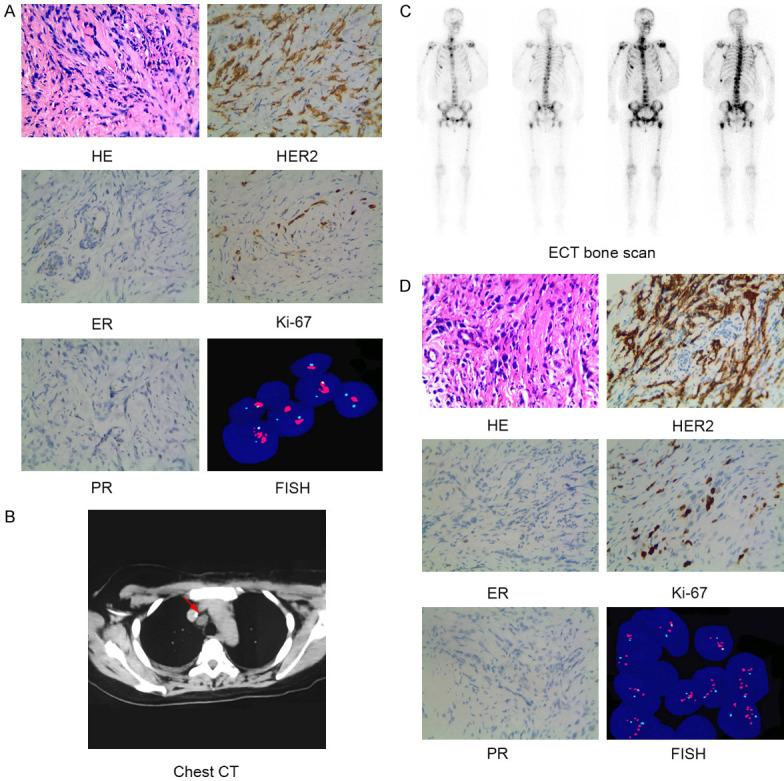
Histopathologic, molecular and imaging characterization identified the patient as HER2-positive bilateral breast cancer with distant metastases. A. HE staining showed infiltrating ductal carcinoma (×400). Immunohistochemical staining (×400) showed the tumor was negative for ER (-) and PR (-), but positive for HER2 (2+~3+) and Ki-67 (+, 20%). FISH assay revealed HER2 gene amplification of infiltrating ductal carcinoma from right breast of the patient. B. Tumor burden of metastatic mediastinal lymph nodes (arrow) as evidenced by chest CT scan. C. Multiple bone metastases as evidenced by ECT bone scanning. D. HE staining showed infiltrating ductal carcinoma (×400). Immunohistochemical staining (×400) showed the tumor was negative for ER (-), PR (-), positive for HER2 (3+), and Ki-67 (+, 50%). FISH assay revealed HER2 gene amplification of infiltrating ductal carcinoma from left breast of the patient. Abbreviations: CT, computed tomography; ECT, emission computed tomography; ER, estrogen receptor; FISH, Fluorescent in situ hybridization; HE, hematoxylin & eosin; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Figure 2.
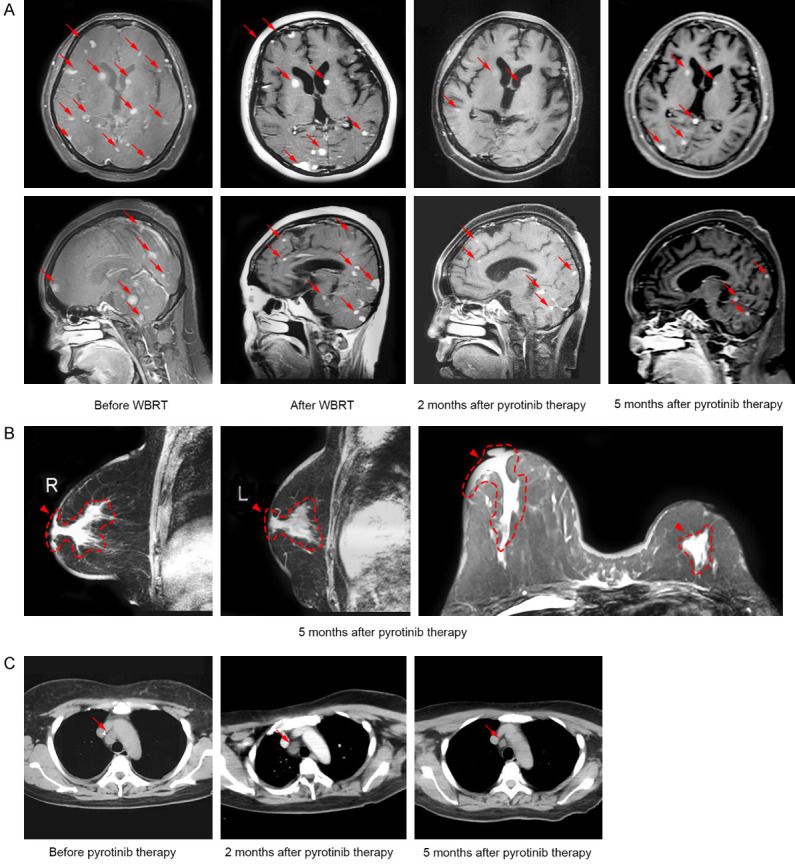
Imaging characterization revealed dynamic changes of the primary and metastatic lesions during the treatment of pyrotinib. A. Cranial MRI revealed continuous shrinkage of brain metastases (arrows) after WBRT and pyrotinib therapy. B. Breast MRI revealed reductions in primary breast lesions (arrowheads) 5 months after pyrotinib therapy. C. Chest CT scan revealed a significant reduction in lesion of mediastinal lymph node (arrows) 5 months after pyrotinib therapy. Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; WBRT, whole brain radiotherapy.
In May 2019, the patient had progressively worsened eye pain again, reduced vision accompanied with obvious headache, dizziness, nausea and vomiting, and frequent epilepsy. Central nervous system examination showed positive signs of slow response, askew mouth (towards left), and reduced left limb strength. MRI examination of the brain indicated continuous reduction of intracranial metastatic parenchymal lesions (Figure 3A and 3B). However, a band-like high signal in left frontotemporal dura (Figure 3B) and obtuse horn of the ventricle (Figure 3C and 3D) were observed. Moreover, a suspected high signal was shown in the left orbital apex (Figure 3E). Ophthalmology consultation concluded no high intraocular pressure, and suggested orbit CT examination. The results did not reveal any obvious abnormalities (Figure 3F), and did not support orbital metastasis. However, hydrocephalus, cranial hypertension and secondary epilepsy have already shown. Sodium valproate was given to prevent epilepsy, phenobarbital was used to control epileptic seizures, and dehydration strategies were used to reduce the intracranial pressure. After alleviation of neurological symptoms, lumbar puncture was performed. Few abnormalities in cerebrospinal fluid (CSF) including elevated opening pressure, and increased total protein concentration (0.65 g/L, normal range: 0.08-0.43 g/L) were observed (Table 1). General laboratory assessments of CSF excluded inflammation and other non-tumor diseases, but no cancerous cells were observed in cytological examination of CSF (Figure 4). Considering the limited sensitivity in initial CSF examination, the patient was diagnosed with MM clinically after exclusive diagnosis of space occupying lesions of cerebral venous sinus and other possible non-tumor diseases. Intrathecal chemotherapy of cytarabine and methotrexate per week for 2 weeks for controlling the symptoms were given. Unfortunately, the disease was still shown to be deteriorating, and the patient died due to tumor progression on June 3, 2019 with an overall survival (OS) time of 20.1 months.
Figure 3.
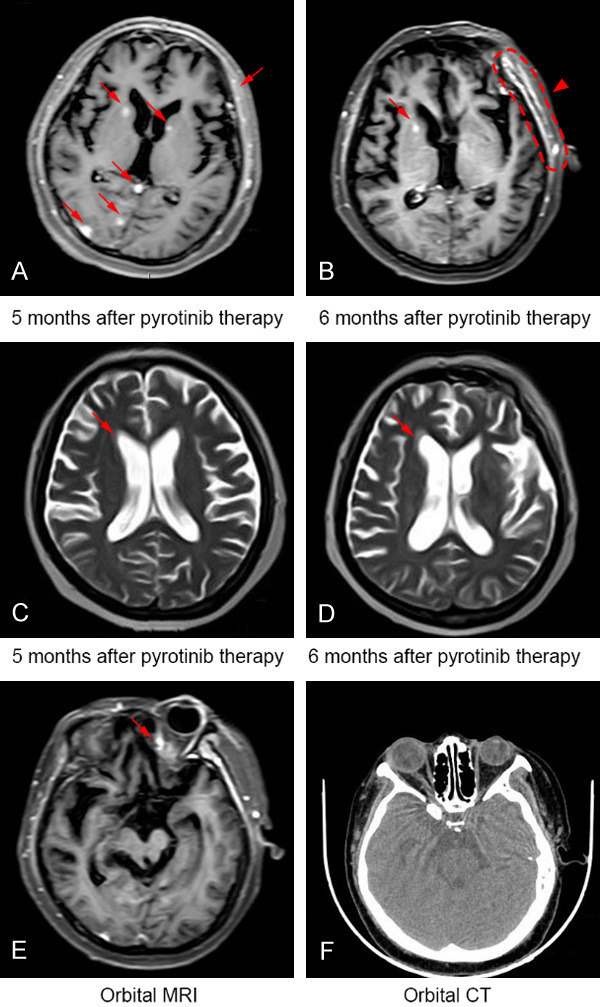
The patient showed tumor response discordance between intracranial parenchymal and meningeal metastatic lesions when receiving further treatment of pyrotinib. A and B. While a sustained relief of intracranial parenchymal lesions (arrows) can be observed by pyrotinib therapy for 6 months, a band-like high signal (arrowhead) in left frontotemporal dura was revealed by cranial MRI. C and D. Obtuse horn of the ventricle (as indicted by arrow) was shown by cranial MRI 6 months after pyrotinib therapy. E. A suspected high signal (arrow) in left orbital apex was shown by cranial MRI. F. Lateral orbital CT scan confirmed no metastasis of the tissue. Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
Table 1.
Biochemical examination of cerebrospinal fluid
| Indicator | Result | Reference Interval | Units |
|---|---|---|---|
| Glucose | 3.80 | 2.5-4.5 | mmol/L |
| Chloride | 122 | 120-132 | mmol/L |
| Total protein | 0.65 | 0.08-0.43 | g/L |
Figure 4.

No cancerous cells were observed in initial cytological examination of cerebrospinal fluid.
Discussion
The current case was preliminary diagnosed with metastatic bilateral BC, and successively discovered bilateral breast lumps within a short time. Whether the latter tumor in the right breast was primary or metastatic from contralateral breast showed no influence on clinical decision making. It is important to note that delayed medical help leads to missing of early treatment in the patient. Although active anti-HER2 targeted therapy was administered later in the course of treatment, the disease still showed continuous progression, proving the vital importance of early diagnosis and treatment, especially for highly invasive HER2-positive BC patients. Due to limited permeability into the blood brain barrier, the macromolecular anti-HER2 targeted drugs, such as trastuzumab, pertuzumab and T-DM1, exhibited restricted efficacy for intracranial lesions. However, HER2-positive BC patients have achieved long-term survival by controlling systemic lesions, especially extracranial lesions, which thus led to the occurrence of more BM. Risk factors regarding the occurrence of BM from BC have included high expression of HER2, negative hormone receptor, and multiple regional lymph node metastases, and high expression of HER2 is considered as the main risk factor [4]. This patient was identified as HER2 positive, hormone receptor negative, and regional lymphatic metastasis at N3 level during her visit to the hospital. Macromolecular monoclonal antibody trastuzumab was used as first-line drug for treatment, and so the development of BM might therefore not be occasional.
TKIs with relatively small molecular weight are important treatment options after failure of trastuzumab, especially for BC patients with BM [5]. Pyrotinib, which is developed in China, is a new generation oral, irreversible pan-ErbB TKI that potently inhibits EGFR/HER1, HER2 and HER4 [6]. Besides preclinical data, clinical data have proved the superiority of pyrotinib. Phase I studies of pyrotinib monotherapy [7] or in combination with capecitabine [8] reported it as a safe and highly effective drug in HER2-positive MBC patients. In a phase II clinical study (NCT02422199), the combination of pyrotinib and capecitabine was compared with the combination of lapatinib and capecitabine for the treatment of Chinese patients with HER2-positive relapsed or metastatic BC who previously received chemotherapy plus trastuzumab or not. The results showed that objective remission rates (ORR) of the two groups were 78.5% and 57.1%, respectively, and progression-free survival (PFS) time of 18.1 months vs. 7.0 months [9]. No significant difference in safety was shown between the two groups. The most common drug-related adverse events of pyrotinib included gastrointestinal reactions, such as diarrhea, nausea and vomiting, and were shown to be well-tolerated or controllable. In August 2018, pyrotinib received its first global conditional approval in China by its breakthrough data in phase II trial. It is used in combination with capecitabine for treatment of HER2-positive MBC patients who were previously treated with anthracycline and taxane chemotherapy, and is applicable to patients who previously received trastuzumab or not. Recent results of phase III PHENIX study from ASCO (2019) proved the safety and high efficacy of pyrotinib in both full population and the BM subgroup of BC patients [3]. During the use of pyrotinib plus capecitabine, the patient in this case showed poor tolerance to capecitabine, and thus received etoposide capsule as an alternative. This is because the moderate clinical effectiveness of oral etoposide has been demonstrated in MBC with options are limited but active treatment is still considered appropriate by a pooled analysis [10]. From the clinical efficacy and safety of etoposide during the patient’s later treatment course, we consider this drug a useful treatment option when combined with pyrotinib for patients with inability or inappropriateness to capecitabine drug usage.
Unexpectedly, half a year later, at the time of excellent control of parenchymal lesions in the brain, the patient developed devastating neurological symptoms, which were considered to be related with the progression caused by MM. Currently, the diagnosis of MM mainly depends on tumor history, symptom signs, gadolinium MRI of the brain and/or spinal cord and cytological examination of CSF [11]. Cytological identification of malignant cells in CSF remains the gold standard for diagnosing MM, but the sensitivity of it remained low during initial CSF examination and can be increased by repeated lumbar punctures [11]. Tumor history, clinical manifestations and imaging examination results supported the diagnosis of MM in the present case. There is a possibility that MM might be present during her first diagnosis of BPM, and it occurred with discrete symptoms at the time of presentation due to delayed diagnosis of MM with the currently available diagnostic techniques. However, the time of occurrence of clinical symptoms and repeated imaging findings in our patient supported that MM might be a new-onset. The prognosis of BC patients with symptomatic untreated MM remained dismal with a median survival time of 4-8 weeks [12]. Currently, there is no consensus regarding the choice of treatment for these patients. Treatment options consist of radiotherapy, intrathecal delivered chemotherapy [13] or targeted therapy [14], systemic chemotherapy, surgical treatment and supporting therapy. Our patient had numerous BPMs, and received WBRT before MM progression, resulting in poor clinical condition with impaired tolerance to systemic treatment, and intrathecal chemotherapy was thus started as per our protocol when applicable. Although the treatment was active, the patient’s disease condition was continuously deteriorated and died due to MM diagnosis at 1.2 months. We hypothesized that distinct pyrotinib responses between meningeal and parenchymal BMs might be related to the unique biology of BM, BC heterogeneity, and TKI drug response pattern, as BM from BC with different subtypes differs in their biological characteristics, metastatic patterns and treatment responses [15].
Taken together, new-type TKIs might cover the shortage of traditional macromolecule-sized anti-HER2 drugs, which is characterized by good permeability and multi-target blockade. The clinical trials focusing on pyrotinib are ongoing synchronously in China and the US, and the drug has become a promising member of small molecule EGFR/HER2 inhibitor family worldwide. This study supports the effectiveness and safety of pyrotinib use in HER2-positive BC patients with BPM. However, its efficacy still requires comprehensive and systematic evaluation in a larger group of BC patients bearing BM including BPM and MM in order to achieve a clear understanding with regard to the drug potentiality.
Acknowledgements
This work was supported by the First Affiliated Hospital of Bengbu Medical College Fund for Leading New Technology Project (No. 2018126) and Science Fund for Distinguished Young Scholars (No. 2019byyfyjq02), and the grant from Bengbu Medical College Key Project of Translational Medicine (No. BYTM2019009). Written informed consent was obtained from the family member of the patient for publication of this manuscript and accompanying images.
Disclosure of conflict of interest
None.
References
- 1.Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Goncalves A, Leheurteur M, Domont J, Gutierrez M, Cure H, Ferrero JM, Labbe-Devilliers C. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 2.Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, Silvestri K, Cotter CM, Componeschi KP, Marte JM, Connolly RM, Moy B, Van Poznak CH, Blackwell KL, Puhalla SL, Jankowitz RC, Smith KL, Ibrahim N, Moynihan TJ, O’Sullivan CC, Nangia J, Niravath P, Tung N, Pohlmann PR, Burns R, Rimawi MF, Krop IE, Wolff AC, Winer EP, Lin NU Translational Breast Cancer Research Consortium. TBCRC 022: a phase ii trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 2019;37:1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Z, Yan M, Hu X, Zhang Q, Ouyang Q, Feng J, Yin Y, Sun T, Tong Z, Wang X, Yao H, Zou J, Zhu X. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: a randomized phase III study. J. Clin. Oncol. 2019;37:1001–1001. doi: 10.1200/JCO.19.00108. [DOI] [PubMed] [Google Scholar]
- 4.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien AJ, Rugo HS. Tyrosine kinase inhibitors for human epidermal growth factor receptor 2-positive metastatic breast cancer: is personalizing therapy within reach? J. Clin. Oncol. 2017;35:3089–3091. doi: 10.1200/JCO.2017.73.5670. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, Chen X, Zhong D, Lou L, Tao W, Zhang L. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61. doi: 10.1016/j.ejps.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, Luo Y, Xing P, Lan B, Li M, Yi Z, Cai R, Yuan P, Zhang P, Li Q, Xu B. Phase I study and biomarker analysis of pyrotinib, a novel irreversible Pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2017;35:3105–3112. doi: 10.1200/JCO.2016.69.6179. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Guan X, Chen S, Yi Z, Lan B, Xing P, Fan Y, Wang J, Luo Y, Yuan P, Cai R, Zhang P, Li Q, Zhong D, Zhang Y, Zou J, Zhu X, Ma F, Xu B. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial. Clin Cancer Res. 2019;25:5212–5220. doi: 10.1158/1078-0432.CCR-18-4173. [DOI] [PubMed] [Google Scholar]
- 9.Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, Li H, Yu S, Feng J, Wang S, Hu X, Zou J, Zhu X, Xu B. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J. Clin. Oncol. 2019;37:2610–2619. doi: 10.1200/JCO.19.00108. [DOI] [PubMed] [Google Scholar]
- 10.Voutsadakis IA. A systematic review and pooled analysis of studies of oral etoposide in metastatic breast cancer. Eur J Breast Health. 2018;14:10–16. doi: 10.5152/ejbh.2017.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi H, Isogawa M. Management of breast cancer brain metastases. Chin Clin Oncol. 2018;7:30. doi: 10.21037/cco.2018.05.06. [DOI] [PubMed] [Google Scholar]
- 12.Scott BJ, Kesari S. Leptomeningeal metastases in breast cancer. Am J Cancer Res. 2013;3:117–126. [PMC free article] [PubMed] [Google Scholar]
- 13.Park MJ. Durable response of leptomeningeal metastasis of breast cancer to salvage intrathecal etoposide after methotrexate: a case report and literature review. Am J Case Rep. 2015;16:524–527. doi: 10.12659/AJCR.894007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park WY, Kim HJ, Kim K, Bae SB, Lee N, Lee KT, Won JH, Park HS, Lee SC. Intrathecal trastuzumab treatment in patients with breast cancer and leptomeningeal carcinomatosis. Cancer Res Treat. 2016;48:843–847. doi: 10.4143/crt.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witzel I, Oliveira-Ferrer L, Pantel K, Muller V, Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 2016;18:8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


